Abstract
BACKGROUND:
The effect of pituitary adenylate cyclase activating polypeptide (PACAP) during traumatic brain injury (TBI) and whether it can modulate secondary injury has not been reported previously. The present study evaluated the potential protective effects of ventricular infusion of PACAP in a rat model of TBI.
METHODS:
Male Sprague Dawley rats were randomly divided into 3 treatment groups (n=6, each): sham-operated, vehicle (normal saline)+TBI, and PACAP+TBI. Normal saline or PACAP (1 μg/5 μL) was administered intracerebroventricularly 20 minutes before TBI. Right parietal cortical contusion was produced via a weight-dropping method. Brains were extracted 24 hours after trauma. Histological changes in brains were examined by HE staining. The numbers of CD4+ and CD8+ T cells in blood and the spleen were detected via flow cytometry.
RESULTS:
In injured brain regions, edema, hemorrhage, inflammatory cell infiltration, and swollen and degenerated neurons were observed under a light microscope, and the neurons were disorderly arrayed in the hippocampi. Compared to the sham group, average CD4+ CD8– lymphocyte counts in blood and the spleen were significantly decreased in rats that received TBI+vehicle, and CD4– CD8+ were increased. In rats administered PACAP prior to TBI, damage was attenuated as evidenced by significantly increased CD4+, and decreased CD8+, T lymphocytes in blood and the spleen.
CONCLUSION:
Pretreatment with PACAP may protect against TBI by influencing periphery T cellular immune function.
KEY WORDS: Traumatic brain injury, Pituitary adenylate cyclase activating polypeptide, CD4+ T lymphocyte, CD8+ T lymphocyte, Rat, Spleen, Blood, Flow cytometry
INTRODUCTION
Besides the initial primary insult in traumatic brain injury (TBI), secondary injuries are also possible. Secondary injuries are mainly caused by local inflammation and immune suppression, as well as dysregulation of the neuroendocrine system.[1] The prevention and treatment of secondary brain injury has become a focus for neuroscience studies.
Pituitary adenylate cyclase-activating polypeptide (PACAP) has been considered as a neuromodulator and trophic factor, and has demonstrated protective effects in brain ischemia and Parkinson’s disease.[2,3] It was also found to participate in immune regulation, and be able to suppress antigen-presenting cell function in an autoimmune encephalomyelitis model.[4] Yet the effect of PACAP during TBI and whether it can modulate secondary injury has not been reported previously. The present study aimed to evaluate the potential protective effects of ventricular infusion of PACAP in a rat model of TBI.
METHODS
Rats
Eighteen adult male Sprague Dawley rats (220–240 g) were provided by the Experimental Center of Xuzhou Medical College (license: SYXK [su] 2002–0038). The rats were randomly assigned to 3 groups (n=6 each): sham-operated (skull opening only), saline and TBI (NS+TBI; 5 µL saline injection into the ventricle 20 minutes prior to TBI), and PACAP and TBI (PACAP+TBI; 1 µg PACAP [St. Louis, US] in 5 µL saline infusion into the ventricle 20 minutes prior to TBI). The stereotaxic coordinates for ventricular infusion were AP 1.0, L 1.5 and H 4.5 mm. The infusion lasted for 2 minutes and the needle was left for 10 minutes before withdrawal.
The rat TBI model was set up as previously described.[6] A 20 g weight was dropped from a height of 30 cm (600 g/cm) onto a metal plate over the 4-mm hole in the skull (dura was left intact before the hit). Then the incision was closed and the animals were left for free recovery. All procedures were in accordance with the local Ethics Committee for Animal Experimental Research and the project was approved by the institution.
Histology
Twenty-four hours after the surgery and induced trauma, the rats were euthanized after transcardial perfusion with 4% paraformaldehyde (in 0.1 mol phosphate buffered saline). Their brains were removed, post-fixed in 4% paraformaldehyde for 48 hours, and then processed for 5-µm paraffin sections, followed by HE staining to assess histological changes after TBI.
Flow cytometry
Blood samples (5 mL) in each rat were obtained from the heart ventricle after the rats were euthanized, with heparin added for anti-coagulation. Additionally, some spleen tissues were homogenized. Fifty microliters of blood or spleen tissue solution were then mixed with 1.25 µL CD4 monoclonal antibody (phycoerythrinlabeled) and 1 µL CD8 monoclonal antibody (fluorescein isothiocyanate-labeled; BioLegend, San Diego, US) for a 15-minute incubation in the dark at room temperature. Then 450 µL hemolysin was added to the mixture in the dark for another 15 minutes. The solution was examined with fluorescence-activated cell sorting (FACS) calibur flow cytometry (BD, US), and the results were analyzed with CellQuest Pro software.
Statistical analyses
Data were presented as mean ± standard deviation and were analyzed with SPSS 16.0 software (Chicago, US). Analysis of variance (ANOVA) was used for comparisons among the 3 treatment groups, and Scheffe’s test was used for comparisons between 2 groups. P<0.05 was considered statistically significant.
RESULTS
General changes in brain morphology
Right parietal cortical contusion was produced via a weight-dropping method. Sites of injury (diameter 5 mm) were found in local cortical tissues, with ischemic and necrotic regions (Figure 1).
Figure 1.
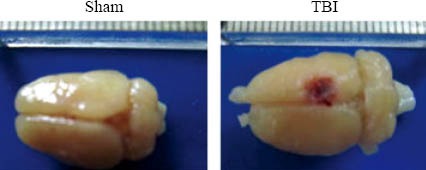
Schematic representation of the cortical contusion area induced by weight-dropping trauma.
Effects of PACAP on cortical and hippocampal injury
In the TBI group, HE staining of injured brain regions revealed the presence of edema, hemorrhage, inflammatory cell infiltration, and swollen and degenerated neurons. In the hippocampi, neurons were disorderly arrayed. PACAP infusion into the ventricle prior to induced trauma reduced edema in the brain tissue and alleviated neuronal swelling and necrosis (Figure 2).
Figure 2.
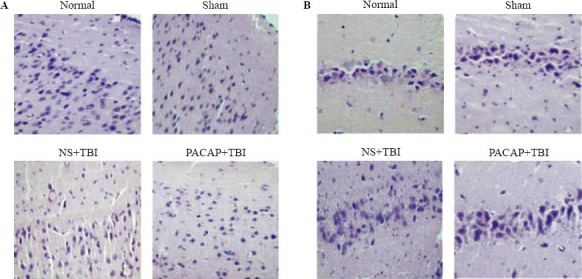
Photomicrographs showing the effects of microinjection of PACAP into the ventricle on cerebral cortex neurons adjacent to the contused area and hippocampal neurons following TBI in rats. A: cortical tissues adjacent to the injury site; B: the hippocampus of the injury side. HE staining (original magnification×200).
Effects of PACAP on circulating CD4+ and CD8+ T cells
Flow cytometry showed that, compared with the sham-operated rats, there were significantly fewer CD4+ T cells in the blood of NS+TBI rats (P=0.000). However, ventricular infusion of PACAP prior to induced trauma partially inhibited this response: there were significantly more CD4+ T cells in the PACAP+TBI group, compared with the NS+TBI group (P=0.019; Table 1). There were significant differences in the numbers of CD4+ T cells among the three treatment groups (F=26.702, P=0.000).
Table 1.
The effects of microinjection of PACAP into the brain lateral ventricle on CD4+ and CD8+ T cell numbers in the blood of TBI rats (n =6, mean±SD)
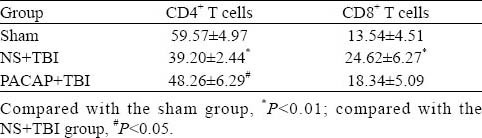
On the other hand, compared with the sham-operated rats there were significantly more CD8+ T cells in the blood of NS+TBI rats (P=0.01). Although PACAP treatment prior to trauma decreased the numbers of CD8+ T cells compared with the NS+TBI rats, the difference was not significant (P=0.162; Table 1, Figure 3). There were significant differences in the number of CD8+ T cells among the three groups (F=6.452, P=0.010).
Figure 3.
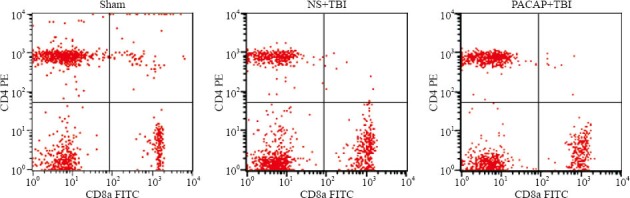
Effects of microinjection of PACAP into the lateral ventricle on CD4+ and CD8+ T cell numbers in the blood of TBI rats.
Effects of PACAP infusion on CD4+ and CD8+ T cells in spleen tissues
Flow cytometry showed that, similar to the results for circulating CD4+ T cells, compared with the sham-operated group there were significantly fewer CD4+ T cells in the spleen tissues of NS+TBI rats (P=0.005). However, there were no differences in the numbers of CD4+ T cells between the PACAP+TBI and NS+TBI groups (P=0.839; Table 2, Figure 4). There were significant differences among the three groups (F=8.987, P=0.003).
Table 2.
The effects of microinjection of PACAP into the ventricle on CD4+ and CD8+ T cell numbers in the spleens of TBI rats (n=6, mean±SD).

Figure 4.
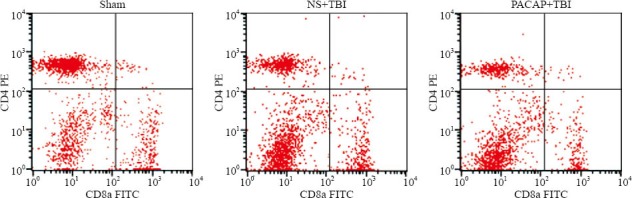
Effects of microinjection of PACAP into the ventricle on CD4+ and CD8+ T cell numbers in the spleen of TBI rats.
The number of CD8+ T cells increased in the NS+TBI group after induced trauma, compared with the sham-operated rats (P=0.034), and PACAP infusion did not decrease these numbers (P=0.199, compared with NS+TBI; Table 2, Figure 4). There were significant differences among the three groups (F=4.413, P=0.031).
DISCUSSION
TBI is currently the third leading factor of the central nervous system, and is the main cause of mortality in males between 20 and 45 years of age. TBIs are both primary and secondary. Secondary injuries include pathological changes due to immune reactions and blood flow changes in the local brain area. These lead to dysfunction in metabolism and the release of excitatory amino acids, calcium increase, oxidative stress, and immune responses.[7–9] Both local immune responses and post-injury immune suppression have roles in the development of secondary TBI.[1]
PACAP was discovered in 1989 by Miyata et al[10,11] in the hypothalamus, and was found to activate pituitary adenylate cyclase. PACAP includes PACAP38 and PACAP27, and its functions include neuromodulation, neurotrophic effect, and neuroprotection.[2] In one study, PACAP inhibited the JNK and p38 signaling pathways to prevent neuronal death in rat hippocampi following brain ischemia, and protected against ethanol-induced neuronal death via PAC1 signaling.[12] Moreover, PACAP could alleviate diffuse axonal injury after TBI.[13] The results of the present study concur with these findings.
The administration of PACAP prior to contusion injury prevented neuronal death and brain tissue edema afterward, which is highly specific for these brain areas.
PACAP was found to ameliorate experimental autoimmune encephalomyelitis,[14] and inhibit IL-12 expression via cAMP-dependent and non-dependent pathways. It also could upregulate the expression of Il10 and its release. In the present study we examined the effects of PACAP on TBI-induced immune responses. TBI induced T cell-mediated immune suppression,[16] in both animal and clinical studies. The percentage of CD4+ and CD8+ T cells could be considered indices of a functional immune system. CD4+ T cells recognized the antigen presented by major histocompatibility complex (MHC) class II molecules (Fas signaling),[17] while CD8+ T cells recognized the antigen presented by MHC class I molecules.[18,19] Our results were consistent with these data, and we found that, post-contusive injury, CD4+ T cells decreased while CD8+ T cells increased, implying suppression of the immune response. With PACAP treatment, CD4+ T cells increased and CD8+ decreased. Possibly, PACAP inhibits the expression of IL-12 thereby preventing T cell proliferation, or PACAP inhibits FasL expression suppressing the apoptosis of CD4+ T cells.[20–22]
In conclusion, our results indicate that PACAP is neuroprotective after TBI, partly through the regulation of circulating T cells. Ventricular infusion of PACAP with local efficiency may suggest new possibilities in clinical therapies.
Footnotes
Funding: None.
Ethical approval: The study was approved by the Animal Ethical Committee of the 97th Hospital of PLA, Xuzhou, China.
Conflicts of interest: The authors declare that there is no conflict of interest.
Contributors: Hua R proposed the study and wrote the paper. All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
- 1.Yong VW, Rivest S. Taking advantage of the systemic immune system to cure brain diseases. Neuron. 2009;64:55–60. doi: 10.1016/j.neuron.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 2.Bourgault S, Vaudry D, Dejda A, Doan ND, Vaudry H. Fournier Pituitary adenylate cyclase-activating polypeptide: focus on structure-activity relationships of a neuroprotective Peptide. Curr Med Chem. 2009;16:4462–4480. doi: 10.2174/092986709789712899. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez A, Chiriva-Internati M, Grammas P. Transduction of PACAP38 protects primary cortical neurons from neurotoxic injury. Neurosci Lett. 2008;448:52–55. doi: 10.1016/j.neulet.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato H, Ito A, Kawanokuchi J, Jin S, Mizuno T, Ojika K, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) ameliorates experimental autoimmune encephalomyelitis by suppressing the functions of antigen presenting cells. Mult Scler. 2004;10:651–659. doi: 10.1191/1352458504ms1096oa. [DOI] [PubMed] [Google Scholar]
- 5.Paxinos G, Watson C. 2nd ed. Sydney: Academic Press; 1986. The rat brain in sterotaxic coordinates; pp. F23–26. [Google Scholar]
- 6.Feeney DM, Boyeson MG, Linn RT, Murray HM, Dail WG. Responses to cortical injury: I. Methodology and local effects of contusions in the rat. Brain Res. 1981;211:67–77. doi: 10.1016/0006-8993(81)90067-6. [DOI] [PubMed] [Google Scholar]
- 7.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 8.Liu SL, Wang ZP, Zeng YM, Jiang S, Wang SQ. Relation between GLu-R and the protective effect of hypothermia on oxygen and glucose deprivation injury in hippocampal slice or rat. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2005;21:127–132. [PubMed] [Google Scholar]
- 9.Schibler A, Humphreys S. Increased brain tissue oxygen tension in children with traumatic brain injury using temperature-corrected guided ventilation during prophylactic hypothermia. Crit Care Resusc. 2012;14:20–24. [PubMed] [Google Scholar]
- 10.Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, et al. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- 11.Vaudry D, Falluel-Morel A, Bourgault S, Basille M, Burel D, Wurtz O, et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol Rev. 2009;61:283–357. doi: 10.1124/pr.109.001370. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez A, Chiriva-Internati M, Grammas P. Transduction of PACAP38 protects primary cortical neurons from neurotoxic injury. Neurosci Lett. 2008;448:52–55. doi: 10.1016/j.neulet.2008.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kovesdi E, Tamas A, Reglodi D, Farkas O, Pál J, Tóth G, et al. Posttraumatic administration of pituitary adenylate cyclase activating polypeptide in central fluid percussion injury in rats. Neurotox Res. 2008;13:71–78. doi: 10.1007/BF03033558. [DOI] [PubMed] [Google Scholar]
- 14.Kato H, Ito A, Kawanokuchi J, Jin S, Mizuno T, Ojika K, et al. Pituitary adenylate cyclase-activating polypeptide (PACAP) ameliorates experimental autoimmune encephalomyelitis by suppressing the functions of antigen presenting cells. Mult Scler. 2004;10:651–659. doi: 10.1191/1352458504ms1096oa. [DOI] [PubMed] [Google Scholar]
- 15.Lv B, Tang Y, Chen F, Xiao X. Vasoactive intestinal Peptide and pituary adenylate cyclase-activating polypeptide inhibit tissue factor expression in monocyte in vitro and in vivo. Shock. 2009;31:185–191. doi: 10.1097/SHK.0b013e31817d423a. [DOI] [PubMed] [Google Scholar]
- 16.Mrakovcic-Sutic I, Tokmadzic VS, Laskarin G, Mahmutefendic H, Lucin P, Zupan Z, et al. Early changes in frequency of peripheral blood lymphocyte subpopulations in severe traumatic brain-injured patients. Scand J Immunol. 2010;72:57–65. doi: 10.1111/j.1365-3083.2010.02407.x. [DOI] [PubMed] [Google Scholar]
- 17.Delgado M, Ganea D. VIP and PACAP inhibit Fas ligand-mediated bystander lysis by CD4(+) T cells. J Neuroimmunol. 2001;112:78–88. doi: 10.1016/s0165-5728(00)00414-8. [DOI] [PubMed] [Google Scholar]
- 18.Hobbs TR, O’Malley JP, Khouangsathiene S, Dubay CJ. Comparison of lactate, base excess, bicarbonate, and pH as predictors of mortality after severe trauma in rhesus macaques (Macaca mulatta) Comp Med. 2010;60:233–229. [PMC free article] [PubMed] [Google Scholar]
- 19.Siveen KS, Kuttan G. Effect of Aerva lanata on cell-mediated immune responses and cytotoxic T-lymphocyte generation in normal and tumor-bearing mice. J Immunotoxicol. 2012;9:25–33. doi: 10.3109/1547691X.2011.609191. Epub 2011 Nov 30. [DOI] [PubMed] [Google Scholar]
- 20.Ganea D, Rodriguez R, Delgado M. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: players in innate and adaptive immunity. Cell Mol Biol (Noisy-le-grand) 2003;49:127–142. [PubMed] [Google Scholar]
- 21.Ganea D, Delgado M. The neuropeptides VIP/PACAP and T cells: inhibitors oractivators? Curr Pharm Des. 2003;9:997–1004. doi: 10.2174/1381612033455116. [DOI] [PubMed] [Google Scholar]
- 22.Delgado M, Ganea D. Anti-inflammatory neuropeptides: a new class of endogenous immunoregulatory agents. Brain Behav Immun. 2008;22:1146–1151. doi: 10.1016/j.bbi.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


