Abstract
BACKGROUND:
Platelet endothelial cell adhesion molecule-1 (PECAM-1), also known as CD31, is mainly distributed in vascular endothelial cells. Studies have shown that PECAM-1 is a very significant indicator of angiogenesis, and has been used as an indicator for vascular endothelial cells. The present study aimed to explore the relationship between the expression of PECAM-1 and the degree of acute lung injury (ALI) and fibrosis in paraquat (PQ) induced lung injury in rabbits.
METHODS:
Thirty-six adult New Zealand rabbits were randomly divided into three groups (12 rabbits in each group) according to PQ dosage: 8 mg/kg (group A), 16 mg/kg (group B), and 32 mg/kg (group C). After PQ infusion, the rabbits were monitored for 7 days and then euthanized. The lungs were removed for histological evaluation. Masson staining was used to determine the degree of lung fibrosis (LF), and semi-quantitative immune-histochemistry analysis to determine the expression of PECAM-1. Pearson’s product-moment correlation analysis was performed to evaluate the relationship between the expression of PECAM-1 and the extent of lung injuries expressed by ALI score and degree of LF.
RESULTS:
Rabbits in the three groups showed apparent poisoning. The rabbits survived longer in group A than in groups B and C (6.47±0.99 days vs. 6.09±1.04 days vs. 4.77±2.04 days) (P<0.05). ALI score was lower in group A than in groups B and C (8.33±1.03 vs. 9.83±1.17 vs. 11.50±1.38) (P<0.05), and there was statistically significant difference between group B and group C (P=0.03). LF was slighter in group A than in groups B and C (31.09%±2.05 % vs. 34.37%±1.62 % vs. 36.54%±0.44%) (P<0.05), and there was statistically significant difference between group B and group C (P=0.026). The PEACAM-1 expression was higher in group A than in groups B and C (20.31%±0.70% vs. 19.34%±0.68% vs. 18.37%±0.46%) (P<0.05), and there was statistically significant difference between group B and group C (P=0.017). Pearson’s correlation analysis showed that the expression of PECAM-1 was negatively correlated to both ALI score (Coe=–0.732, P=0.001) and degree of LF (Coe=–0.779, P<0.001).
CONCLUSIONS:
The PECAM-1 expression significantly decreases in New Zealand rabbits after PQ poisoning, and the decrease is dose-dependent. The PECAM-1 expression is negatively correlated with ALI score and LF, showing a significant role in the development of lung injuries induced by PQ.
KEY WORDS: Platelet endothelial cell adhesion molecule-1, Paraquat, Acute lung injury, Lung fibrosis
INTRODUCTION
In recent years, there has been a growing interest in paraquat (PQ) induced injury to vascular (or pulmonary) endothelial cells of the lung. Researchers have discovered that PQ can cause loss of viability in the lung’s capillaries’ endothelial cells, dysfunction in cell membranes, reduction of coenzyme II (NADPH), and inhibition of the anti-oxidation defense system’s enzyme activity.[1-4] Platelet endothelial cell adhesion molecule-1 (PECAM-1), also known as CD31, is mainly distributed in vascular endothelial cells. Studies have shown that PECAM-1 is a very significant indicator of angiogenesis, and has been used as an indicator for vascular endothelial cells. Using PECAM-1 as the object of observation, this study was to investigate its relationship with the process of PQ poisoning, the severity of damage in lung vascular endothelial cells, and the severity of acute lung injury (ALI).
METHODS
Experimental animals
Thirty-six adult New Zealand rabbits were used in the experiment. Eighteen of them were male and the rest were female, each rabbit weighing (to be consistent with the table listed later) 2.1-2.3 kg. This experiment was approved by the Zhongshan University Committee of Experimental Animal Ethics and conducted at the Center for Experimental Animals, Zhongshan University.
Grouping and treatment
PQ solution of 20% volume fraction (Beijing Syngenta Investment Co., Ltd., China) was injected into the stomach of the New Zealand rabbits. The rabbits were randomly divided into three groups (12 in each group) according to the following dosages: 8 mg/kg (group A), 16 mg/kg (group B), and 32 mg/kg (group C). Before the treatment, the rabbits had water, but fasted from food for 12 hours. After gastric injections of different dosages of PQ solution, the rabbits were put back to their cages, free to eat and drink. After 7 days of poisoning, 60 mg/kg of pentobarbital sodium (Sigma Company, Germany) was injected into the ear vein to euthanize the experimental animals.
Pathological examination of lung tissue and ALI scoring
Specimens from the lower right lobe of the lung were washed in PBS buffer solution (pH 7.3, 0.1 mol/L), fixed in 10% neutral formaldehyde solution for 48 hours, dehydrated, dipped in wax, embedded, and sliced. After cutting paraffin wax into 5 μm and HE staining, pathological changes in the tissue were observed under a microscope. The scoring of lung injury is based on the following four parameters: a) alveolar congestion; b) bleeding; c) gap or infiltration/accumulation of neutrophils in blood vessel walls; d) alveolar septal thickening or hyaline membrane formation. A score of 0-4 points was assigned to each parameter per pathological severity, and the 4 individual scores were summed up for a cumulative pathological ALI score.[5-7]
Masson staining appraisal of fibrosis degree of the lung
Five μm slices of paraffin wax were collected and dewaxed to water: a) stained in Masson compound dye for 5 minutes; b) lightly washed in 0.2% acetic acid solution; c) placed in 5% phosphotungstic acid for 5-10 minutes; d) dip in 0.2% acetic acid solution 2 times; e) stained in bright green dye for 5 minutes, then washed in 0.2% acetic acid 2 times; f) dehydrated in ethanol, xylene, and cement in neutral gum. In each group, 6 different high power fields (40×) were taken for semi-quantitative analysis using the image processing software of Image-Proplus 6.0.
Expression of PECAM-1 in lung tissue
The immune-histochemical labeled Strept Avidin Biotin method was applied to conduct staining. Under a microscope, yellowish brown granular formation in the cell membrane and cytoplasm was seen as positive expression. In each group, 6 different high power fields (40×) were randomly taken for semi-quantitative analysis using the image processing software of Image-Proplus 6.0. Anti-PECAM-1 was purchased from the Wuhan Boster Company (China).
Statistical analysis
All data were input into the SPSS13.0 statistics software package. They were expressed as mean ± standard deviation or the quartile. According to the normality test (Kolmogorov-Smirnov) results, ANOVA (one-way ANOVA), Wilcoxon’s rank-sum test or the Mann-Whitney U test was used. Post Hoc test was used for multiple comparisons, Pearson’s method was used for the analysis of correlation between ALI score, LF degree and PECAM-1 expression. P < 0.05 was considered statistically significant.
RESULTS
Physiological variables and survival of each group’s animals
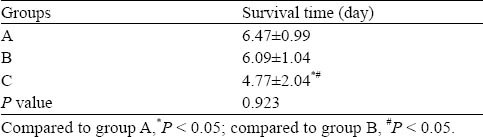
There were no significant differences among the three groups in physiological variables (Table 1). At 4 hours after the gastric injection of PQ solution, the rabbits were sluggish and less active. At 1 day, the above symptoms were aggravated, and other symptoms, including shedding, squinting, arched back, cyanosis of the lip, staggering gaits and unresponsiveness, appeared. At 3-7 days, the above poisoning symptoms were most severe. In the dying rabbits, deep respiration appeared, respiratory rate increased, and cyanosis appeared in the entire body. Nine rabbits in group A survived for 7 days, 6 rabbits in group B survived for 7 days, and 3 rabbits in group C survived for 7 days. There were significant differences between the three groups (Table 2).
Table 1.
The physiological variables of rabbits in each group
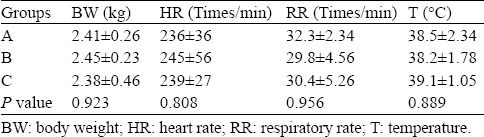
Table 2.
The survival time of rabbits in each group
ALI score and LF
Under the naked eye, hyperemia and edema of the lung tissue were seen. Under a microscope, pulmonary consolidation, infiltration of inflammatory cells in the pulmonary interstitum and alveolar space and diffuse pulmonary hemorrhage were found. Partial lung interval was broken, and this presented as typical bulla and fibroblast proliferation, and the aforementioned symptoms were aggravated with the increase of PQ dosages (Figure 1).
Figure 1.
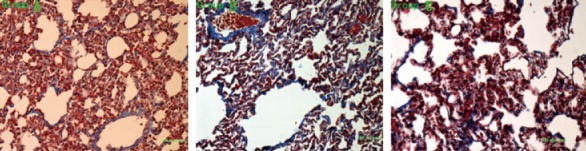
The injury and fibrosis of the lungs in each group.
Seven days after PQ poisoning, lung injury and fibrosis developed in each group. The ALI score was significantly lower in group A (8.33±1.03) than in group B (9.83±1.17) (P=0.047) and in group C (11.50±1.38) (P=0.03). The degree of lung fibrosis was lower in group A (31.09 ±2.05)% than in group B (34.37±1.62)% (P=0.002) and in group C (36.54±0.44)% (P<0.01). There was a statistically significant difference between group B and group C (P=0.026).
Expression of PECAM-1 in lung tissue
After lung injury, the expression of PECAM-1 in lung tissue decreased significantly (Figure 2). The expression was higher in group A (20.31±0.70)% than in group B (19.34±0.68)% (P=0.16) and in group C (18.37±0.46)% (P<0.01). There was a significant difference between group B and group C (P=0.017).
Figure 2.
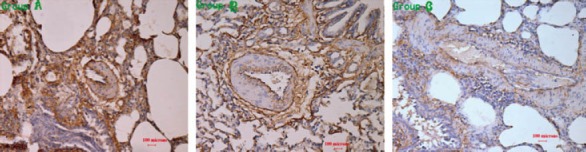
The expression of PECAM-1 in the lungs in each group.
Correlation between the PECAM-1 expression and ALI score and LF
PQ poisoning caused ALI and significant reduction of PECAM-1 expression in lung tissue, and these changes were dose-dependent, and strongly correlated with lung injury and lung fibrosis (Figure 3). It was demonstrated that vascular endothelial cell damage played an important role in the occurrence and development of PQ-induced lung injury.
Figure 3.
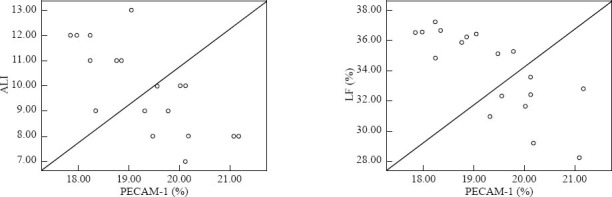
The correlation analysis of the PECAM-1 expression and ALI score and lung fibrosis.
Pearson’s product-moment correlation analysis showed that the PECAM-1 expression had a negative correlation with ALI score (Coe= –0.732, P=0.001) and LF (Coe= –0.779, P<0.001).
DISCUSSION
Since its introduction in agriculture in 1962, paraquat (PQ) used as a desiccant and defoliant has caused thousands of deaths in humans from both accidental and voluntary exposure. The lethal oral dose for an adult is 30-40 mg/kg, and the high intake of PQ can cause quick death mainly because of multiple organ dysfunction/failure syndrome (MODS/MOF) induced by lung injury. PQ poisoning has a high mortality in China, but there are no effective antidotes, and its mechanism remains unclear. PQ poisoning can lead to multi-system, multi-organ, multi-level injury, and mainly affect the lung, characterized by acute alveolitis and interstitial lung fibrosis as the main causes of death.[8-12]
ALI shows inflammation and increased pulmonary capillary permeability. During acute lung injury, histopathological changes in lung vascular endothelial cells occurred about 48 hours after PQ poisoning. At 48 hours after intraperitoneal injection of PQ, lung vascular endothelial cell injury was seen in the rats, and this was characterized by a significant increase of pinocytic vesicles. After 72-96 hours, endothelial cells began to break down and continuity disintegrated, cell junctions were widened, and hydration abnormalities occurred. Besides the increased permeability and edema, vascular endothelial cell damage could cause aggregation of polymorphonuclear leukocytes, alveolar hemorrhage, pulmonary capillary thrombosis, and occasional occurrence of ischemic necrosis. These can lead to further development of lung disease, membrane formation, and lung fibrosis. Therefore, lung vascular endothelial injury is the basis of edema and lung fibrosis in mid to late stage of lung injury.
In recent years, studies have shown that PECAM-1 is an important indicator of angiogenesis, mainly distributed in the junction between endothelial cells, and participates in the maintenance of monolayer integrity of lung vascular endothelial cells. PECAM-1 is considered as a specific marker of endothelial cells.[13] The conjunction between endothelial cells can maintain vascular integrity, regulate vascular permeability and leukocyte exudation.[14] PECAM-1 not only regulates functions of endothelial cell barrier and platelet, but also participates in leukocyte migration. It inhibits apoptosis, and mediates adhesion molecules of signal transduction. PECAM-1 is necessary for the maintenance of endothelial integrity and prevention of apoptosis during septic shock and for STAT3-mediated acute phase responses that promote survival during septic shock.[15] In the process of inhibiting apoptosis, PECAM-1 activates or inhibits the signaling proteins in signaling pathways to achieve its purpose.[16] Moreover, the expression of PECAM-1 may play an important role in PECAM-1’s regulatory functions.[17] The lung is the major target organ for PQ poisoning. In this study, we found that the expression of PECAM-1 on the surface of lung tissue vascular endothelial cells was decreased, endothelial cells and basic membranes were swelling, and the intercellular space of endothelial cells was increased, especially at 7 days after PQ poisoning. This finding was similar to that reported by Stalmans.[18] After PQ poisoning, the expression of PECAM-1 was significantly decreased in the lung tissue, and was dose-dependent, and this was closely correlated with the degree of lung injury and lung fibrosis. This indicates that the decrease of PECAM-1expression can aggravate lung inflammation and lung fibrosis.
In summary, the expression of PECAM-1 is significantly decreased in PQ-induced lung injury in rabbit models, and its decrease is closely related to PQ dosage, lung injury, and lung fibrosis.
ACKNOWLEDGMENTS
We thank Hope Xu from Princeton University USA for her help in the recondition and instruction of the manuscript.
Footnotes
Funding: The study was supported by grants from Guangdong Medical Research Fund (2010501) and Guangzhou Pharmaceutical Health Science Fund (2009-YB-111).
Ethical approval: Not needed.
Conflicts of interest: The authors declare that there is no conflict of interest.
Contributors: Shi J wrote the paper. All authors read and approved the final version of the manuscript.
REFERENCES
- 1.Tsukamoto M, Tampo Y, Sawada M, Yonaha M. Paraquat-induced oxidative stress and dysfunction of the glutathione redox cycle in pulmonary microvascular endothelial cells. Toxicol Appl Pharmacol. 2002;178:82–92. doi: 10.1006/taap.2001.9325. [DOI] [PubMed] [Google Scholar]
- 2.Takizawa M, Komori K, Tampo Y, Yonaha M. Paraquat-induced oxidative stress and dysfunction of cellular redox systemsincluding antioxidative defense enzymes glutathione peroxidase and thioredoxinreductase. Toxicol In Vitro. 2007;21:355–363. doi: 10.1016/j.tiv.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Tsukamoto M, Tampo Y, Sawada M, Yonaha M. Paraquat-induced membrane dysfunction in pulmonary microvascular endothelialcells. Pharmacol Toxicol. 2000;86:102–109. doi: 10.1034/j.1600-0773.2000.d01-19.x. [DOI] [PubMed] [Google Scholar]
- 4.Takizawa M, Komori K, Tampo Y, Yonaha M. Paraquat-induced oxidative stress and dysfunction of cellular redox systemsincluding antioxidative defense enzymes glutathione peroxidase and thioredoxinreductase. Toxicol In Vitro. 2007;21:355–363. doi: 10.1016/j.tiv.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad I, Kumar A, Shukla S, Prasad PH, Singh C. The involvement of nitric oxide in maneb-and paraquat-induced oxidative stressin rat polymorphonuclear leukocytes. Free Radic Res. 2008;42:849–862. doi: 10.1080/10715760802513733. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad I, Shukla S, Kumar A. Maneb and paraquat-induced modulation of toxicant responsive genes in the ratliver: comparison with polymorphonuclear leukocytes. Chem Biol Interact. 2010;188:566–579. doi: 10.1016/j.cbi.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Kumar A, Ahmad I, Shukla S, Singh BK, Patel DK, Pandey HP, et al. Effect of zinc and paraquat co-exposure on neurodegeneration: Modulation ofoxidative stress and expression of metallothioneins, toxicant responsive andtransporter genes in rats. Free Radic Res. 2010;44:950–965. doi: 10.3109/10715762.2010.492832. [DOI] [PubMed] [Google Scholar]
- 8.Suntres ZE. Role of antioxidants in paraquat toxicity. Toxicology. 2002;180:65–77. doi: 10.1016/s0300-483x(02)00382-7. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Sheibani N. Expression pattern of alternatively spliced PECAM-1 isoforms in hematopoieticcells and platelets. J Cell Biochem. 2002;87:424–438. doi: 10.1002/jcb.10321. [DOI] [PubMed] [Google Scholar]
- 10.Lou MF. Redox regulation in the lens. Prog Retin Eye Res. 2003;22:657–682. doi: 10.1016/s1350-9462(03)00050-8. [DOI] [PubMed] [Google Scholar]
- 11.Wilhelm J, Smistik Z, Mahelkova G, Vytasek R. Redox regulation of proliferation of lens epithelial cells in culture. Cell Biochem Funct. 2007;25:317–321. doi: 10.1002/cbf.1390. [DOI] [PubMed] [Google Scholar]
- 12.Bonilla E, Medina-Leendertz S, Villalobos V, Molero L, Bohorquez A. Paraquat-induced oxidative stress in drosophila melanogaster: effects ofmelatonin, glutathione, serotonin, minocycline, lipoic acid and ascorbic acid. Neurochem Res. 2006;31:1425–1432. doi: 10.1007/s11064-006-9194-8. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Ahmad I, Shukla S, Singh BK, Patel DK, Pandey HP, et al. Endothelial cell activation and blood coagulation in critically ill patients withlung injury. Wien Klin Wochenschr. 2002;114:853–858. [PubMed] [Google Scholar]
- 14.Marszalek A, Daa T, Kashima K, Nakayama I, Yokoyama S. Ultrastructural and morphometric studies related to expression of the celladhesion molecule PECAM-1/CD31 in developing rat lung. J Histochem Cytochem. 2000;48:1283–1289. doi: 10.1177/002215540004800911. [DOI] [PubMed] [Google Scholar]
- 15.Carrithers M, Tandon S, Canosa S, Michaud M, Graesser D, Madri JA. Enhanced susceptibility to endotoxic shock and impaired STAT3 signaling inCD31-deficient mice. Am J Pathol. 2005;166:185–196. doi: 10.1016/S0002-9440(10)62243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown S, Heinisch I, Ross E, Shaw K, Buckley CD, Savill J. Apoptosis disables CD31-mediated cell detachment from phagocytes promotingbinding and engulfment. Nature. 2002;418:200–203. doi: 10.1038/nature00811. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Sheibani N. Expression pattern of alternatively spliced PECAM-1 isoforms in hematopoieticcells and platelets. J Cell Biochem. 2002;87:424–438. doi: 10.1002/jcb.10321. [DOI] [PubMed] [Google Scholar]
- 18.Stalmans I. Role of the vascular endothelial growth factor isoforms in retinal angiogenesisand DiGeorge syndrome. Verh K Acad Geneeskd Belg. 2005;67:229–276. [PubMed] [Google Scholar]


