Abstract
BACKGROUND:
Early withdrawal of invasive mechanical ventilation (IMV) followed by noninvasive MV (NIMV) is a new strategy for changing modes of treatment in patients with acute exacerbations of chronic obstructive pulmonary disease (AECOPD) with acute respiratory failure (ARF). Using pulmonary infection control window (PIC window) as the switch point for transferring from invasive to noninvasive MV, the time for early extubation can be more accurately judged, and therapy efficacy can be improved. This study aimed to prospectively investigate the clinical effectiveness of fiberoptic bronchscopy (FOB) in patients with AECOPD during sequential weaning of invasive-noninvasive MV.
METHODS:
Since July 2006 to January 2011, 106 AECOPD patients with ARF were treated with comprehensive medication and IMV after hospitalization. Patients were randomly divided into two groups according to whether fiberoptic bronchoscope is used (group A, n=54) or not (group B, n=52) during sequential weaning from invasive to noninvasive MV. In group A, for sputum suction and bronchoalveolar lavage (BAL), a fiberoptic bronchoscope was put into the airway from the outside of an endotracheal tube, which was accompanied with uninterrupted use of a ventilator. After achieving PIC window, patients of both groups changed to NIMV mode, and weaned from ventilation. The following listed indices were used to compare between the groups after treatment: 1) the occurrence time of PIC, the duration of MV, the length of ICU stay, the success rate of weaning from MV for the first time, the rate of reventilation and the occurrence rate of ventilator-associated pneumonia (VAP); 2) the convenience and safety of FOB manipulation. The results were compared using Student’s t test and the Chi-square test.
RESULTS:
The occurrence time of PIC was (5.01±1.49) d, (5.87±1.87) d in groups A and B, respectively (P<0.05); the duration of MV was (6.98±1.84) d, (8.69±2.41) d in groups A and B, respectively (P<0.01); the length of ICU stay was (9.25±1.84) d, (11.10±2.63) d in groups A and B, respectively (P<0.01); the success rate of weaning for the first time was 96.30%, 76.92% in groups A and B, respectively (P<0.01); the rate of reventilation was 5.56%, 19.23% in groups A and B, respectively (P<0.05); and the occurrence rate of VAP was 3.70%, 23.07% in groups A and B, respectively (P<0.01). Moreover, it was easy and safe to manipulate FOB, and no side effect was observed.
CONCLUSIONS:
The application of FOB in patients with AECOPD during sequential weaning of invasive-noninvasive MV is effective in ICU. It can decrease the duration of MV and the length of ICU stay, increase the success rate from weaning MV for the first time, reduce the rate of reventilation and the occurrence rate of VAP. In addition, such a method is convenient and safe in patients of this kind.
KEY WORDS: Acute exacerbations of chronic obstructive pulmonary disease, Acute respiratory failure, Mechanical ventilation, Sequential weaning of invasive-noninvasive ventilation, Fiberoptic bronchscopy, Bronchoalveolar lavage, Pulmonary infection control window, Side effect, Success rate
INTRODUCTION
Noninvasive positive pressure ventilation (NPPV)is commonly used to treat acute exacerbation of chronic obstructive pulmonary disease (AECOPD)[1] and is considered as the first-line therapy.[2] NPPV is not suitable for those patients who have severe bronchial pulmonary infection and a lot of sputum, haven’t enough strength to cough, and/or are unconscious due to severe hypercapnia. This was considered as a relative contraindication to apply NPPV in acute respiratory failure (ARF).[2,3] Under these circumstances, an artificial airway and invasive mechanical ventilation (IMV) is needed to facilitate sputum drainage and to guarantee enough respiratory support. During IMV, however, due to implementation of an invasive artificial airway, ventilator associated pneumonia (VAP) requiring repeated treatment often occurs,[4,5] because bacteria may migrate into the bronchi along with the tube and the sediment upon the cuff may flow downwards. Once VAP occurs, the patient’s condition become worse, the duration of MV will be prolonged, and weaning is difficult to be performed.[2,3] The key to reduce VAP is to shorten the duration of artificial airway placement.[3] Early withdrawal of IMV followed by noninvasive MV (NIMV) is a new strategy for treatment. Several controlled studies have demonstrated the safety of using sequential NIMV following short-term IMV in AECOPD patients.[6-8] It is key to choose an appropriate time to transfer from IMV to NIMV to successfully perform sequential weaning, but the appropriate switch time hasn’t been defined. Researchers have recommended to use pulmonary infection control window (PIC) as the switch point, but the clinical criteria for the appearance of PIC were still in debate.[7,8] We found that studies have reported that the diagnostic fibreoptic bronchoscope (FOB) may be helpful in detecting suspected pneumonia even in AECOPD with ARF.[6,9] They found that the early suction of secretions with FOB may increase the success rate of NPPV, and this may be a potential alternative option to endotracheal intubation. We hypothesize that assisted with FOB under IMV, AECOPD patients with ARF can have earlier PIC and easier extubation and weaning from IMV to NIMV than those treated without FOB. From July 2006 to April 2011, we used this method to treat hospitalized AECOPD patients during the weaning from MV.
METHODS
General information
Totally 106 patients were enrolled in this study. Case selection criteria were as follows: a) receiving IMV in ICU because of hypercapnic respiratory failure and generous sputum; b) age ≤85 years; c) meeting the criteria of COPD guidelines constituted by the Chinese Medical Association in 2002;[10] d) being capable of self-care in the past year; e) aggravation of COPD due to severe pulmonary infection; f) being willing to use FOB and BAL due to a lot of sputum without enough strength to cough.
The patients were excluded if they had: a) severe cerebral, heart, hepatic and renal failure; b) severe malnutrition; c) severe water and electrolyte disturbance difficult to be normalized; d) upper airway or face injury preventing mask ventilation; and e) intolerance to NPPV.
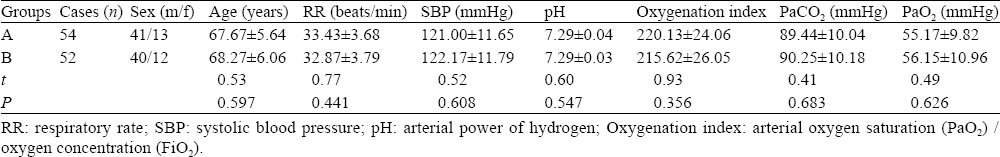
The 106 patients were selected and randomly divided into treatment group (group A) and control group (group B). Patients in group A were treated with sequential weaning of invasive-noninvasive MV supplemented by bedside FOB suction and BAL. Patients in group B, however, were treated with simple application of sequential weaning only. There was no significant difference between sex, age, severity of illness between the two groups (P>0.05) (Table 1).
Table 1.
Comparisons of general data between the two groups at hospital admission (mean±SD)
Routine examination and treatment
The patients of the two groups were treated with a ventilator, active infection control, water, electrolyte and acid-base balance maintance, liver and kidney function protection and nutritional support after admission. HR, BP, SaO2, TeCO2, ECG and other parameters were monitored. CT scans or regular chest X-ray (daily) was performed. Airway secretions were sucked with an ordinary suction tube or FOB, and sputum specimens were obtained for pathogenic examination. Nasogastric feeding was given when the patients had artificial airway, but peros eating was taken under NPPV.
MV and sequential weaning
According to the Diagnosis and Treatment Guide for Chronic Obstructive Pulmonary Disease with Acute Exacerbation in Patients with Mechanical Ventilation (2007),[3] all patients were intubated after admission. Irritable patients were given appropriate sedation. C-SIMV mode was adopted at the early stage of MV, and P-SIMV mode was adopted when the condition was improved. When PIC[11,12] appeared, patients were extubated, NPPV-CPAP mode was adopted, and weaning was decided according to the patient condition.
FOB-assisted therapy
According to the Diagnosis and Treatment of Fiberoptic Bronchoscope,[13] FOB suction and bronchoalveolar lavage (BAL) were performed in the patients of group A within 24 hours after intubation, followed by once every 1-2 days, until lung infection reached PIC.
Before FOB, routine chest X-ray or CT scans were done; routine hematocyte and platelet count, clotting time, liver and kidney function, ECG, blood gas and blood electrolytes, etc. were determined. Family members of patients were told the effects and risk of this treatment, and signed an informed consent. Patients were fasted for 4-6 hours before FOB.
After the pillow was removed, patients were adopted with supine position and head hypsokinesis. Oxygen concentration for the ventilator was increased appropriately, and multi-function monitor was used to monitor BP, RR, ECG, PaO2 and TeCO2. The patients were anesthetized by intravenous injection of 3-5 mL of 1% propofol and repeated once every 10-15 minutes or 5-10 mL per hour by pumping, maintaining loss of eyelash reflex. One percent tetracaine was used for anesthesia in the nasal cavity and throat mucosa, and 1% ephedrine for nasal spray, with an interval of 2-5 minutes and repeated for 2-3 times. The outside wall of a fiberoptic bronchoscope was fully lubricated with sterile liquid paraffin. After the successful anesthesia, the bilateral nasal cavity was first gently probed with the bronchoscope; and the larger side of the nasal aisle was selected, through which the bronchoscope slowly reached to the glottis along the side of the posterior nares. Secretion was sucked thoroughly above the airbag of the endotracheal tube. After entry of the bronchoscope beyond the fissure of the glottis, the endotracheal tube airbag was deflated, and the bronchoscope entered the trachea from the outside of the endotracheal tube. The airbag was inflated with gas again so that MV would not leak but FOB will not be interfered. About 300 mg of 2% lidocaine was injected from the biopsy hole of the bronchoscope for trachea and bronchus mucosal anesthesia. The trachea and bronchus were observed and sputum was sucked thoroughly. BAL was operated for severe pulmonary infection. Finally, a diluted solution of budesonide, terbutaline and sensitive antibiotics was injected into the severe lung disease section. During the FOB operation, if SaO2<80%, SBP<90 mmHg, manipulation should be suspended until the SaO2>90%, SBP>90 mmHg, otherwise stopped. After the operation, blood gas analysis was made again after 30 minutes and the patients were fasted for 2-3 hours.
Instruments
The following instruments were used: U.S. vela ventilator, Beter endotracheal tube (ID 7.0-8.0 mm), Truphatek laryngoscope, Olympus BF type30 fiberoptic bronchoscope, Mindray Beneview T5 patient monitor, and Radiometer ABL80 blood gas analyzer.
Observation index
Indices listed below were compared after treatment: a) the occurrence time of PIC, the duration of mechanical ventilation, the length of ICU stay, the success rate of weaning from MV for the first time, the rate of re-ventilation and the occurrence rate of VAP; b) the convenience and safety of FOB manipulation.
Successful weaning for the first time
AECOPD patients successfully weaned from MV for more than 48 hours after admission.
Weaning failure
a) re-endotracheal intubation and/or IMV needed within 48 hours extubation; b) re-endotracheal intubation and/or IMV needed in the process of NIMV.
One of the indications for re-endotracheal intubation
a) pH≤7.20, and progressive increase of PaCO2 after extubation; b) hypoxia (in sufficient oxygen therapy PaO2<50 mmHg) difficult to be corrected; c) coma, drowsiness or delirium and other serious disturbance of consciousness; d) respiratory or cardiac arrest; e) weak shallow breathing (RR<8 times/min) or severe dyspnea (RR>40 times/min).
PIC criteria of group A
Airway secretions significantly reduced so that lumen obstruction was not observed under FOB; secretions became white or lighter, and viscosity was II degrees,[14] easy to be sucked; mucosal congestion significantly reduced without edema; infiltration was partially absorbed and accompanied by one of the followings: the body temperature lower than 38 °C, peripheral blood leukocytes count ≤10×109/L or its decreasion ≥2×109/L.
PIC criteria of group B
The PIC criteria of group B were similar to the criteria of group A, except the finding under FOB.
VAP diagnostic criteria
VAP diagnostic criteria were consistent with those described in the “Guideline for Mechanical Ventilation in Patients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease (2006)”.[12]
Statistical analysis
Measurement data were expressed as mean ± standard deviation, and count data expressed as percentage. The results were compared using Student’s t test or the Chi-square test. P<0.05 was considered statistically significant. SPSS17.0 software was used for data processing.
RESULTS
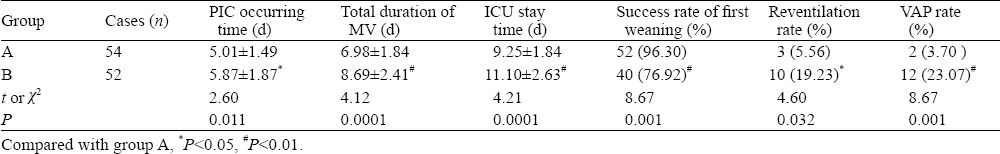
The occurrence time of PIC was earlier in group A than in group B; the duration of mechanical ventilation and the length of ICU stay were shorter in group A than in group B; the success rate of weaning from ventilation for the first time was higher in group A than in group B. The rate of re-ventilation and the occurrence rate of VAP were lower in group A than in group B. Two patients in group A failed to wean for the first time because of unstable hemodynamics in one patient and fully unconscious in another patient. The difference between the groups was statistically significant (Table 2).
Table 2.
Comparison of results after treatment (mean±SD)
In group A, 54 patients were treated with FOB for 218 times, the average frequency was 3.5±1.5 times, and the operation time was 20-30 (25±5) minutes each time. The propofol dosage was 6-20 (13±7) mL each time. During FOB, SpO2 slightly decreased in 20 patients, and SaO2 quickly returned to over 90% by adjusting the ventilator oxygen concentration or by suspension of operations a short while. No patients had SBP<90 mmHg. Neither serious complications as cardiac arrhythmia, hemoptysis and pneumothorax nor significant changes in biochemical tests developed.
DISCUSSION
In AECOPD patients with similar clinic conditions in this study, PIC occurred earlier in group A than in group B. The total duration of MV and ICU stay were shorter in group A than in group B. The successful rate of weaning for the first time was higher but the re-ventilation rate lower in group A than in group B. The findings indicated that FOB treatment was an effective method to increase the successful rate of weaning and reduce weaning difficulties, and PIC judged by FOB was a more accurate index for successful weaning. VAP occurred in 2 patients of group A and 12 patients of group B, showing that FOB effectively prevented the occurrence of VAP in these patients. This may be related to short time of intubation and IMV time and more efficient drainage of sputum in group A. Also intravenous anesthesia significantly reduced the irritation caused by MV and FOB, and this increased patients’ compliance, and protected ventilation and aeration, as reported elsewhere.[14-17]
Under normal conditions, successful weaning from MV in AECOPD patients is largely dependent on the effective drainage of mucus and the administration of sensitive antibiotics.[3] The commonly used suction tube can remove part of bronchial airway secretions, but it is difficult to clean them because of blind suction. In addition, hypoxia and CO2 retention will be aggravated because of bronchospasm and mucosal injury. FOB, on the other hand, can efficiently suck secretions both from subglottic and superficial parts visually. Combined with BAL and local application of a systemic vasodilator or bronchodilator, FOB could effectively remove deep sputum, relieve local atelectasis and bronchial spasm, improve ventilation, and correct hypoxia and CO2 retention.
A study[7] revealed that the appearance of PIC window indicates that ventilatory insufficiency is the major problem in patients and drainage of sputum is a minor one. At this time, patient condition can be stable and improved if supported with ventilation. This finding suggests that timely extubation followed by IMV on the appearance of the PIC window can relieve patient’s fatigue of respiratory muscles and ventilatory insufficiency, and decrease the risk of lower airway infection and VAP. If IMV continues after the appearance of the PIC window, VAP will occur, patients’ condition will be aggravated and the duration of MV be prolonged. This will result in ventilator dependence and consequent failure to wean. So it is essential to accurately predict the beginning of the PIC window for sequential weaning.
The difference between this study and others lies in the criteria of judging PIC as a switching point from IMV to NIMV. Studies[7,8] used PIC as the guide for weaning, but PIC criteria mainly adopted secretion changes as well as chest X-ray, accompanied by the body temperature and/or peripheral blood leukocytes count. The major defect of the studies is the ignorance of lumen obstruction, mucosal congestion, and even edema. Plain chest X-ray is routinely used to confirm pneumonia. When infiltrates occur, it is difficult to differentiate among cardiogenic pulmonary edema, noncardiogenic pulmonary edema, pulmonary contusion, atelectasis and pneumonia. Because atelectasis is common in ICU patients, repeated chest X-ray after pulmonary physiotherapy is helpful to differentiate infiltrates caused by atelectasis from those due to infection.[18] Some studies examined the accuracy of portable chest radiograph in the ICU.[19-23] A postmortem study reported that a sensitivity of 69% and a specificity of 75% in diagnosis of new and persistent infiltrates by chest radiographs are dependent on two of the following three criteria: a) fever >38.3 °C; b) leukocytosis>12×109/mL, and/or c) purulent tracheobronchial secretions.[24] Fàbregas et al[25] also reported that clinical diagnosis of VAP was associated with about 30%-35% false-negative and 20%-25% false-positive results. In this study we modified the PIC criteria by adding observations with FOB to decrease the hysteresis of X-ray display. Through the bronchoscope, more intuitive understanding of the lung conditions, more accurate determination of PIC, and better sequential weaning were achieved.
The limitations of the present study include the following: a) In group A, the onset time of the first FOB was not strictly uniform, thus affecting the PIC determination; b) further analysis of the relation between pathogenic sputum test results, clinical medication, treatment efficacy, and prognosis was not performed; c) we failed to analyze the respective role of bronchoscopy and chest radiograph in PIC determination.
Footnotes
Funding: None.
Ethical approval: The present study was approved by the Ethical Committee of Tongzhou People’s Hospital.
Conflicts of interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Contributors: Song RR proposed the study, and wrote the first draft. All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
- 1.Nava S, Hill N. Non-invasive ventilation in acute respiratory failure. Lancet. 2009;374:250–259. doi: 10.1016/S0140-6736(09)60496-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrosino N, Vagheggini G. Nonivasive positive pressure ventilation in the acute care setting:where are we? Eur Respir J. 2008;31:874–886. doi: 10.1183/09031936.00143507. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Qing-yuan Z. Guideline for mechanical ventilation in patients with acute exacerbation of chronic obstructive pulmonary disease(2007) Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2007;19:513–518. [PubMed] [Google Scholar]
- 4.Wang C, Shang M, Huang K, Tong Z, Kong W, Jiang C, et al. Sequential non-invasive mechanical ventilation following short-term invasive mechanical ventilation in COPD induced hypercapnic respiratory failure. Chin Med J (Engl) 2003;116:39–43. [PubMed] [Google Scholar]
- 5.MacIntyre NR. Evidence-based ventilator weaning and discontinuation. Respir Care. 2004;49:830–836. [PubMed] [Google Scholar]
- 6.Collaborating Research Group for Noninvasive Mechanical Ventilation of Chinese Respiratory Society. Pulmonary infection control window in treatment of severe respiratory failure of chronic obstructive pulmonary diseases: a prospective, randomized controlled, multi-centred study. Chin Med J (Engl) 2005;118:1589–1594. [PubMed] [Google Scholar]
- 7.Wang C, Shang M, Huang K. Sequential non-invasive following short-term invasive mechanical ventilation in COPD induced hypercapnic respiratory failure. Zhonghua Jie He He Hu Xi Za Zhi. 2000;23:212–216. [PubMed] [Google Scholar]
- 8.Du LL, Han H, Zhang XJ, Wei L. Randomized control study of sequential non-invasive following short-term invasive mechanical ventilation in the treatment of acute respiratory distress syndrome as a result of existing pulmonary diseases in elderly patients. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2009;21:394–396. [PubMed] [Google Scholar]
- 9.Da Conceiçao M, Genco G, Favier JC, Bidallier I, Pitti R. Fiberoptic bronchoscopy during noninvasive positive-pressure ventilation in patients with chronic obstructive lung disease with hypoxemia and hypercapnia. Ann Fr Anesth Reanim. 2000;19:231–236. doi: 10.1016/s0750-7658(00)00213-6. [DOI] [PubMed] [Google Scholar]
- 10.Nagai A. Guidelines for the diagnosis and management of chronic obstructive pulmonary disease: 3rd edition. Nihon Rinsho. 2011;69:1729–1734. [PubMed] [Google Scholar]
- 11.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/ American Thoracic Society. consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44:S27–72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collaborating Research Group for Sequential Invasive to Noninvasive Ventilation. Application of pulmonary infection control window as switching point for sequential invasive to noninvasive ventilation in treatment of severe respiratory failure of chronic obstructive pulmonary disease: a randomized controlled study. Zhonghua Jie He He Hu Xi Za Zhi. 2006;29:14–18. [PubMed] [Google Scholar]
- 13.Liu CT. Beijing: Beijing University Medical Press; 2003. The diagnosis and therapeutics of fibrobronchoscope; pp. 43–45. [Google Scholar]
- 14.Lu Q Clinical Research Coordination Group of Guaifenesin Compound Pseudoephedrine Hydrochloride Oral Solution. A prospective multicenter randomized controlled clinical study on the efficacy and safety of Guaifenesin compound pseudoephedrine hydrochloride oral solution. Zhonghua Er Ke Za Zhi. 2010;48:204–207. [PubMed] [Google Scholar]
- 15.Reinhardt AK. Non-invasive ventilation for acute exacerbations of COPD. Br J Hosp Med (Lond) 2009;70:524–527. doi: 10.12968/hmed.2009.70.9.43870. [DOI] [PubMed] [Google Scholar]
- 16.Bem RA, van Woensel JB, Bos AP, Koski A, Farnand AW, Domachowske JB, et al. Mechanical ventilation enhances lung inflammation and caspase activity in a model of mouse pneumovirus infection. Am J Physiol Lung Cell Mol Physiol. 2009;296:L46–56. doi: 10.1152/ajplung.00467.2007. Epub 2008 Nov 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez R, De-La-Rosa-Ramirez I, Maldonado-Hernandez A, Dominguez-Cherit G. Should patients undergoing a bronchoscopy be sedated? Acta Anaesthesiol Scand. 2003;47:411–415. doi: 10.1034/j.1399-6576.2003.00061.x. [DOI] [PubMed] [Google Scholar]
- 18.Vaideeswar P, Bavdekar SB, Biswas P, Sarangarajan R. Bhosale Viral ventilator-associated pneumonia: uncovering tip of the iceberg. Indian J Pathol Microbiol. 2011;54:339–343. doi: 10.4103/0377-4929.81633. [DOI] [PubMed] [Google Scholar]
- 19.Wunderink RG, Woldenberg LS, Zeiss J, Day CM, Ciemins J, Lacher DA. The radiologic diagnosis of autopsy-proven ventilator-associated pneumonia. Chest. 1992;101:458–463. doi: 10.1378/chest.101.2.458. [DOI] [PubMed] [Google Scholar]
- 20.Meduri GU, Mauldin GL, Wunderink RG, Leeper KV, Jr, Jones CB, Tolley E, et al. Causes of fever and pulmonary densities in patients with clinical manifestations of ventilator-associated pneumonia. Chest. 1994;106:221–235. doi: 10.1378/chest.106.1.221. [DOI] [PubMed] [Google Scholar]
- 21.Winer-Muram HT, Jennings SG, Wunderink RG, Jones CB, Leeper KV., Jr Ventilator-associated Pseudomonas aeruginosa pneumonia: radiographic findings. Radiology. 1995;195:247–252. doi: 10.1148/radiology.195.1.7892480. [DOI] [PubMed] [Google Scholar]
- 22.Winer-Muram HT, Rubin SA, Ellis JV, Jennings SG, Arheart KL, Wunderink RG, et al. Pneumonia and ARDS in patients receiving mechanical ventilation: diagnostic accuracy of chest radiography. Radiology. 1993;188:479–485. doi: 10.1148/radiology.188.2.8327701. [DOI] [PubMed] [Google Scholar]
- 23.Lefcoe MS, Fox GA, Leasa DJ, Sparrow RK, McCormack DG. Accuracy of portable chest radiography in the critical care setting. Diagnosis of pneumonia based on quantitative cultures obtained from protected brush catheter. Chest. 1994;105:885–887. doi: 10.1378/chest.105.3.885. [DOI] [PubMed] [Google Scholar]
- 24.Torres A, el-Ebiary M, Padró L, Gonzalez J, de la Bellacasa JP, Ramirez J, et al. Validation of different techniques for the diagnosis of ventilator-associated pneumonia. Comparison with immediate postmortem pulmonary biopsy. Am J Respir Crit Care Med. 1994;149:324–331. doi: 10.1164/ajrccm.149.2.8306025. [DOI] [PubMed] [Google Scholar]
- 25.Fàbregas N, Ewig S, Torres A, El-Ebiary M, Ramirez J, de La Bellacasa JP, et al. Clinical diagnosis of ventilator associated pneumonia revisited: comparative validation using immediate post-mortem lung biopsies. Thorax. 1999;54:867–873. doi: 10.1136/thx.54.10.867. [DOI] [PMC free article] [PubMed] [Google Scholar]


