Abstract
BACKGROUND:
Ulinastatin (UTI) is a urinary trypsin inhibitor extracted and purified from urine of males. This study aimed to explore the effects of UTI on paraquat-induced-oxidative stress in human type II alveolar epithelial cells.
METHODS:
The human type II alveolar epithelial cells, A549 cells, were cultured in vitro. The A549 cells were treated with different concentrations of paraquat (200, 400, 600, 800, 1 000, 1 200 µmol/L) and ulinastatin(0, 2 000, 4 000, 6 000, 8 000 U/mL) for 24 hours, the cell viability was measured by cell counting kit-8 and the median lethal concentration was selected. In order to establish an in vitro model of paraquat intoxication and to determine the safe dose of ulinastatin, we calculated LD50 using cell counting kit-8 to determine the survival rate of the cells. A549 cells were divided into normal control group, paraquat group and paraquat+ulinastatin group. The levels of malondialdehyde (MDA) and myeloperoxidase (MPO) were detected by biochemistry colorimetry, while the level of reactive oxygen spies (ROS) was detected by DCFH-DA assay.
RESULTS:
The survival rate of A549 cells treated with different concentrations of paraquat decreased in a concentration-dependent manner. Whereas there was no decrease in the survival rate of cells treated with 0–4 000 U/mL ulinastatin. The levels of MDA, MPO, and ROS were significantly higher in the paraquat group than in the normal control group after 24-hour-exposure. And the survival rate of the paraquat+ulinastatin group was higher than that of the paraquat group, but lower than that of the normal control group. The levels of MDA, MPO, and ROS were lower than those of the paraquat group.
CONCLUSION:
Ulinastatin can alleviate the paraquat-induced A549 cell damage by reducing oxidative stress.
KEY WORDS: Ulinastatin, Paraquat, Oxidative stress, A549 cell
INTRODUCTION
Paraquat (PQ) is a fast-acting contact herbicide. It is highly lethal upon entry into human body with the lungs being its major target organ.[1–4] After entering the alveolar epithelial cells, PQ undergoes oxidative reaction and subsequently triggers the reactive oxygen cascade.[5–7] The numerous reactive oxygen spies (ROS) released from the cascade oxidize the unsaturated fatty acids in various biomembranes, resulting in damages of the capillary endothelial and alveolar epithelial cells, thus leading to pulmonary injury.[8–10] Ulinastatin (UTI) is a urinary trypsin inhibitor extracted and purified from urine of males. Multiple studies have confirmed its effect on stabilization of lysosome membranes, decrease of inflammatory mediatorrelease and so on.[11–13] Clinical studies have showed promising therapeutic effect of UTI on PQ poisoning, but the exact mechanism remains unclear.[14,15] We suspect that UTI alleviates the clinical manifestation of PQ poisoning by reducing the level of oxidative stress induced by PQ. Thus, we designed this experiment where type II alveolar epithelium exposed to PQ was treated with UTI and recorded the effect of UTI on intracellular biomarkers for oxidative stress.
METHODS
Material and reagents
A549 cells were kept in the Central Laboratory of Second Military Medical University. Others were used as follows: standardized PQ 100 mg (Sigma Co. USA); UTI (Guangdong Techpool Pharmaceutical Co.); bovine fetal serum, DMEM (hyperglycemic) media, trypsin (Gibco Co. USA); CCK-8, MDA, DCFH-DA, BCA kits (Beyotime Institute of Biotechnology); MPO kit (Nanjing Jiancheng Bioengineering Institute); centrifuge type 75005440 (Thermo, USA); Microplate Reader Synergy 2 (BioTek, USA), photographed with Gen5 1.10 software.
Culture of A549 cells
The A549 cells were cultured in DMEM (hyperglycemic) media which contained 10% FBS. The media were put into a 5% CO2 incubator at 37 °C, with fluid changed every other day. The cells were digested with 0.25% trypsin when they covered 80%–90% of the surface area, and divided into subcultures with a ratio of 1:2. The cells in the log phase were selected to undergo the experiment procedures.
Measurement of cell viability
CCK-8 is a fast and sensitive kit for measurement of cell proliferation and intoxication using WST-8, which could be reduced to formazan by intramitochondria dehydrogenase in the presence of electron coupling agents. Formazan is of an orange color, and will get lighter as the cells get more intoxicated. The cells were put into 96- pole plates with a concentration of 5×105/L, incubated till they covered 70% area, and subsequently treated with media that contain different concentrations of PQ and UTI (0, 200, 400, 600, 800, 1 000, 1 200 µmol/L and 0, 2 000, 4 000, 6 000, 8 000 U/mL respectively). There were four-double poles in every group. After 24-hour incubation, 20 µL CCK-8 agent was added to each pole, and further incubated for 1 hour, when absorbance (A) at 450 nm wave-length was measured with a microplate reader. The cell survival rate (%) = (the mean of A in experiment group / the mean of A in control group) ×100%.
Groups
The cells were put into a 96-pole plate with a concentration of 5×105/L, incubated till they covered 70% area, treated with media that contain different concentrations of PQ and UTI, and examined with CCK-8. Different groups in this study included control group, PQ-treated group, and groups treated with UTI of different concentrations.
Changes of intracellular malondialdehyde (MDA)
MDA is the end product of polyunsaturated fatty acid peroxide degeneration. It could react with TBA in a relatively high temperature and low pH, producing a red MDA-TBA compound, which could be detected with colorimetry. The cells were kept in lysate on ice for 10 minutes, taken out with a spatula, mixed, and centrifuged at 4 °C 14 000 g for 15 minutes. The supernatant was then treated with BCA kit to detect its protein concentration. Subsequently, 100 µL protein sample was mixed with 200 µL operative solution, bathed in boiling water for 15 minutes, cooled, then centrifuged with 1 000 g for 10 minutes under room temperature. 200 µL supernatant was used to measure its absorbance at 532 nm using a 96-pole plate and a microplate reader. The concentration of MDA (µmol/mg) was calculated according to the standard curve.
Change in the activity of intracellular myeloperoxidase (MPO)
MPO is secreted by neutrophils, mononucleocytes and macrophages in some tissues. It contains heme cofactor and has the ability to reduce peroxide. It could react with O-anisidine and produce a yellow compound. The cells in all groups were digested by pancreatic enzyme after respective procedures, then bathed in 37 °C water with homogenate agent, after which chromogenic agent was added and the solution was put in 60 °C water-bath for 10 minutes. The activity of the enzyme (U/g) was calculated based on the absorbance of the solution at 460 nm.
Level of intracellular reactive oxygen (ROS) using DCFH-DA
DCFH-DA has no fluorescence as for itself, but it can freely cross the cytoplasm membrane and be lysed by intracellular esterase and become DCFH which cannot cross the cell membrane. Now the indicator is located inside the cell, it can be oxidized by intracellular ROS to produce DCF which is fluorogenic. The digested cells were added to 1 mL agent with 10 µmol/L DCFH-DA, incubated for 30 minutes, and washed three times. The level of ROS was indicated by average cell fluorescence measured by a microplate reader with the excitation wavelength of 488 nm and the emission wavelength of 525 nm.
Statistical analysis
All data were analyzed with SPSS 11.0 software. Measurement data were expressed as mean ± standard deviation. Student's t test was used for comparison between the two groups. α was set to be 0.05, with P<0.05 indicating that the difference was statistically significant.
RESULTS
Effect of PQ and UTI on the survival rate of A549 cells
After incubation for 24 hours in PQ solutions with concentrations of 200, 400, 600, 800, 1 000 and 1 200 µmol/L, the survival rate of the cells decreased as the concentration increased. As indicated by Figure 1, the survival rate in groups of 400, 600, 800, 1 000, 1 200 µmol/L were significantly different from the control group (P<0.05). For the purpose of this experiment, we artificially set the PQ concentration of 800 µmol/L where the survival rate was 57.01%, as the intervention point. At the UTI concentration of 2 000 and 4 000 U/mL, the cell survival rates were 94.9%±4.3%, 91.1%±1.6% respectively. This indicated there was no damage to the cells at these concentrations, whereas the survival rate decreased significantly at the concentrations of 6 000 and 8 000 U/mL (75.6%±3.2% and 56.5%±1.1% respectively). Thus we chose 2 000 and 4 000 U/mL as the operating concentration in our study.
Figure 1.
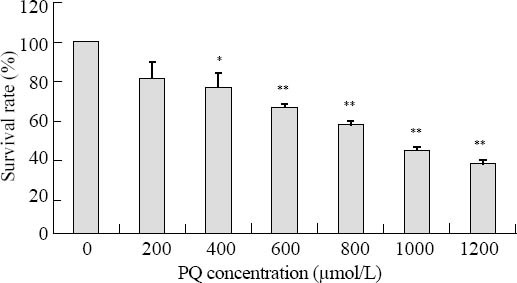
Effect of different PQ concentrations on the cell survival rate. The survival rate of the cells decreased as the concentration increased. Compared to the control group, *P<0.05; compared to the control group, **P<0.01.
UTI reduced the PQ-induced decrease of A549 survival rate
The cells were pretreated with UTI for 1 hour, and then 800 µmol/L PQ was added for 24 hours. The result showed that the survival rate in the UTI group was higher than that in the group treated with PQ only. The difference is statistically significant (Figure 2).
Figure 2.
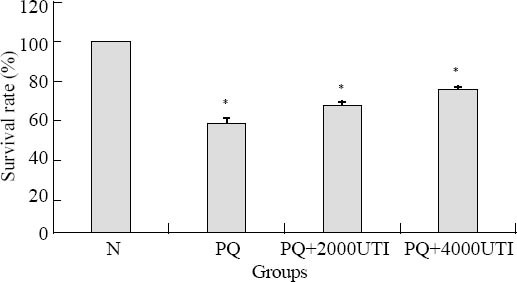
Effect of UTI on PQ-induced decrease of survival rate. Compared to the control group, *P<0.01.
Changes of intracellular MDA, MPO activity, and ROS
After treatment with 800 µmol/L PQ for 24 hours, the levels of intracellular MDA, MPO activity, and ROS increased significantly, whereas 1 hour pretreatment with 2 000 or 4 000 U/mL UTI before PQ significantly decreased the intracellular MDA level, MPO activity, and ROS (Table 1).
Table 1.
Comparison of intracellular fluorescence intensity, MPO activity and MDA level (mean±SD)

DISCUSSION
In this study, we found that the survival rate of A549 cells treated with PQ for 24 hours were decreased as the concentration increased. There was no damage of cells when the concentration of UTI was lower than 4 000 U/mL, whereas the survival rate decreased with solutions of 6 000 and 8 000 U/mL. Thus the operating concentrations were set to be 2 000 and 4 000 U/mL. The A549 cells pretreatment with UTI for 1 hour significantly decreased the PQ-induced cell death. This proved that UTI could protect A549 cells from cellular toxicities induced by PQ.
After entering the pulmonary alveolar epithelium, PQ undergoes oxidative-reductive reactions and activate ROS cascade, producing massive ROS, which could induce apoptosis by pathways such as peroxidation of cellular lipids, damage of DNA molecular, modulate apoptosis-related genes.[16–18] The results of this study showed that massive ROS was produced after exposure to PQ, which indicated strong oxidative stress on the cells. UTI could significantly reduce the increase of ROS induced by PQ, and the effect was stronger as the concentration of UTI increased. MDA is a lipidperoxide produced during the metabolism of oxygen free radicals. The intracellular level of MDA reflects the precedence of lipid peroxide free radicals and the extension of cellular lipid peroxidation.[19,20] MPO is an important peroxidase, which participates in the oxidative stress reaction. Combined with MDA, it could help to better indicate the level of oxidative stress.[21,22] In this study the intracellular levels of MDA and MPO were both significantly elevated after treatment with PQ compared to the control group, and UTI could significantly lower the extension of that increase induced by PQ, with some concentration-dependent feature. The above results led us to the conclusion that the pretreatment of UTI could significantly reduce the oxidative stress on the cells, and the effect was dose-dependent.
UTI is a wide-spectrum hydrolase inhibitor composed of 143 amino acids. It is purified from male urine.[23–25] As it is a human body protein, it is not immunogenic, and this is why it could be used safely and wildly. Multiple studies have suggested that UTI has a protective effect for pulmonary cells.[26–29] Possible mechanisms include inhibition of inflammatory mediator release, elimination of oxygen free radicals, anti-oxidation and so on. Ito et al[30] reported that UTI could inhibit the rapid increase of pulmonary TNF-α and MPO in mice with acute pulmonary injury, thus significantly reducing the infiltration of inflammatory cells in the alveolar walls and improving symptoms such as edema and hemorrhage. Our study also confirmed the protective effect of UTI for type II human alveolar epithelium A549.
PQ poisoning could lead to a strong oxidative stress on cells. UTI could protect cells against PQ-induced cell damage, probably through mechanisms such as elimination of oxygen free radicals, reduction of lipid peroxidation, and subsequent inhibition of oxidative stress reaction.
Footnotes
Funding: The study were supported by grants from National Natural Science Foundation(81272071) and Techpool Foundation(01201111).
Ethical approval: The present study was approved by the Animal Care and Use Committee of Shanghai First People's Hospital, School of Medicine, Shanghai Jiaotong University, Shanghai, China.
Conflicts of interest: The authors have no competing interests relevant to the present study.
Contributors: Meng XX proposed the study and wrote the draft of paper.
REFERENCES
- 1.Lee SK, Ameno K, In SW, Yang JY, Kim KU, Koo KS, et al. Levels of paraquat in fatal intoxications. Int J Legal Med. 1999;112:198–200. doi: 10.1007/s004140050233. [DOI] [PubMed] [Google Scholar]
- 2.Lin JL, Lin-Tan DT, Chen KH, Huang WH, Hsu CW, Hsu HH, et al. Improved survival in severe paraquat poisoning with repeated pulse therapy of cyclophosphamide and steroids. Intensive Care Med. 2011;37:1006–1013. doi: 10.1007/s00134-010-2127-7. [DOI] [PubMed] [Google Scholar]
- 3.Tong F, Liu FR, Zhang JJ. The study on expression of TNF-α in acute lung injury caused by paraquat arid the protection effect of rhubarb. Chin J Emerg Med. 2009;3:241–246. [Google Scholar]
- 4.Wang RL, Tang X, Wu X, Xu R, Yu KL, Xu K. The relationship between HIF-1α expression and the early lung fibrosis in rats with acute paraquat poisoning. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2012;30:273–277. [PubMed] [Google Scholar]
- 5.Faner R, Rojas M, Macnee W, Agustí A. Abnormal lung aging in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;186:306–313. doi: 10.1164/rccm.201202-0282PP. [DOI] [PubMed] [Google Scholar]
- 6.Jones DP. Extracellular redox state: refining the definition of oxidative stress in aging. Rejuvenation Res. 2006;9:169–181. doi: 10.1089/rej.2006.9.169. [DOI] [PubMed] [Google Scholar]
- 7.Xie H, Wang R, Tang X, Xiong Y, Xu R, Wu X. Paraquat-induced pulmonary fibrosis starts at an early stage of inflammation in rats. Immunotherapy. 2012;4:1809–1815. doi: 10.2217/imt.12.122. [DOI] [PubMed] [Google Scholar]
- 8.Dinis-Oliveira RJ, Duarte JA, Sánchez-Navarro A, Remião F, Bastos ML, Carvalho F. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol. 2008;38:13–71. doi: 10.1080/10408440701669959. [DOI] [PubMed] [Google Scholar]
- 9.Lee EY, Hwang KY, Yang JO, Hong SY. Predictors of survival after acute paraquat poisoning. Toxicol Ind Health. 2002;18:201–206. doi: 10.1191/0748233702th141oa. [DOI] [PubMed] [Google Scholar]
- 10.Mustafa A, Gado AM, Al-Shabanah OA, Al-Bekairi AM. Protective effect of aminoguanidine against paraquat-induced oxidative stress in the lung of mice. Comp Biochem Physiol C Toxicol Pharmacol. 2002;132:391–397. doi: 10.1016/s1532-0456(02)00095-9. [DOI] [PubMed] [Google Scholar]
- 11.Bingyang J, Jinping L, Mingzheng L, Guyan W, Zhengyi F. Effects of urinary protease inhibitor on inflammatory response during on-pump coronary revascularisation. Effect of ulinastatin on inflammatory response. J Cardiovasc Surg (Torino) 2007;48:497–503. [PubMed] [Google Scholar]
- 12.Inoue K, Takano H, Yanagisawa R, Sakurai M, Shimada A, Sato H, et al. Antioxidative role of urinary trypsin inhibitor in acute lung injury induced by lipopolysaccharide. Int J Mol Med. 2005;16:1029–1033. [PubMed] [Google Scholar]
- 13.Huang RH, Wan XY. Effects of ulinastatin on toll-like receptor 4 signaling pathway in lung tissue of rats after lipopolysaccharide insult. Chin J Emerg Med. 2012;11:1226–1229. [Google Scholar]
- 14.Inoue K, Takano H, Shimada A, Yanagisawa R, Sakurai M, Yoshino S, et al. Urinary trypsin inhibitor protects against systemic inflammation induced by lipopolysaccharide. Mol Pharmacol. 2005;67:673–680. doi: 10.1124/mol.104.005967. Epub 2004 Dec 2. [DOI] [PubMed] [Google Scholar]
- 15.Di M, Li L, Lan C, Sun CH, Zhang R, Gao YX. Effects of ulinastatin on myocardial injury induced by acute paraquat poisoning. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2012;24:342–345. [PubMed] [Google Scholar]
- 16.Skalli O, Pelte MF, Peclet MC, Gabbiani G, Gugliotta P, Bussolati G, et al. Alpha-smooth muscle actin, a differentiation marker of smooth muscle cells, is present in microfilamentous bundles of pericytes. J Histochem Cytochem. 1989;37:315–321. doi: 10.1177/37.3.2918221. [DOI] [PubMed] [Google Scholar]
- 17.Faner R, Rojas M, MacNee W, Agustí A. Abnormal Lung Aging in Chronic Obstructive Pulmonary Disease and Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2012;186:306–313. doi: 10.1164/rccm.201202-0282PP. [DOI] [PubMed] [Google Scholar]
- 18.Clejan L, Cederbaum AI. Synergistic interactions between NADPH-cytochrome P-450 reductase, paraquat, and iron in the generation of active oxygen radicals. Biochem Pharmacol. 1989;38:1779–1786. doi: 10.1016/0006-2952(89)90412-7. [DOI] [PubMed] [Google Scholar]
- 19.Gaweł S, Wardas M, Niedworok E, Wardas P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad Lek. 2004;57:453–455. [PubMed] [Google Scholar]
- 20.Ma YT, Tian YP, Shi HW, Lv CH, Liu JH, Sun ZP. Effects of high dose ambroxol on lung injury induced by paraquat in rats. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2007;25:523–526. [PubMed] [Google Scholar]
- 21.Inoue K, Takano H, Yanagisawa R, Sakurai M, Shimada A, Sato H, et al. Antioxidative role of urinary trypsin inhibitor in acute lung injury induced by lipopolysaccharide. Int J Mol Med. 2005;16:1029–1033. [PubMed] [Google Scholar]
- 22.Zhi Q, Sun H, Qian X, Yang L. Edaravone, a novel antidote against lung injury and pulmonary fibrosis induced by paraquat? Int Immunopharmacol. 2011;11:96–102. doi: 10.1016/j.intimp.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Sato H, Kajikawa S, Kuroda S, Horisawa Y, Nakamura N, Kaga N, et al. Impaired fertility in female mice lacking urinary trypsin inhibitor. Biochem Biophys Res Commun. 2001;281:1154–1160. doi: 10.1006/bbrc.2001.4475. [DOI] [PubMed] [Google Scholar]
- 24.Yu JB, Yao SL. Protective effects of hemin pretreatment combined with ulinastatin on septic shocks in rats. Chin Med J (Engl) 2008;121:49–55. [PubMed] [Google Scholar]
- 25.Sumi H, Takada Y, Takada A. Studies on human urinary trypsin inhibitor 1. Its modification on treatment of urine with acid. Thromb Res. 1977;11:747–754. doi: 10.1016/0049-3848(77)90103-7. [DOI] [PubMed] [Google Scholar]
- 26.Yeh ST, Guo HR, Su YS, Lin HJ, Hou CC, Chen HM, et al. Protective effects of N-acetylcysteine treatment post acute paraquat intoxication in rats and in human lung epithelial cells. Toxicology. 2006;223:181–190. doi: 10.1016/j.tox.2006.03.019. Epub 2006 Apr 6. [DOI] [PubMed] [Google Scholar]
- 27.Yang Q, Liu X, Liu M, Zhang L, Guan Y. Ulinastatin-mediated protection against zymosan-induced multiple organ dysfunction in rats. Biologicals. 2010;38:552–556. doi: 10.1016/j.biologicals.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Okuhama Y, Shiraishi M, Higa T, Tomori H, Taira K, Mamadi T, et al. Protective effects of ulinastatin against ischemiareperfusion injury. J Surg Res. 1999;82:34–42. doi: 10.1006/jsre.1998.5496. [DOI] [PubMed] [Google Scholar]
- 29.Shen J, Gan Z, Zhao J, Zhang L, Xu G. Ulinastatin reduces pathogenesis of phosgene-induced acute lung injury in rats. Toxicol Ind Health. 2012 Oct 16; doi: 10.1177/0748233712463776. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Ito K, Mizutani A, Kira S, Mori M, Iwasaka H, Noguchi T. Effect of Ulinastatin, a human urinary trypsin inhibitor, on the oleic acid-induced acute lung injury in rats via the inhibition of activated leukocytes. Injury. 2005;3:387–394. doi: 10.1016/j.injury.2004.06.018. [DOI] [PubMed] [Google Scholar]


