Abstract
BACKGROUND:
The intestine is not only the main target attacked by sepsis but also the vital organ which mediated sepsis. The recovery of the damaged intestinal barrier structure and function is related to the occurrence and outcome of multiple organ dysfunction syndrome (MODS). How to protect and reduce the damage of the intestinal mucosa and how to promote the reconstruction of the intestinal mucosa have been the important topics in sepsis for many years. This study aimed to investigate the influential factors of intestinal mucosal reconstruction after intestinal epithelial injury in vivo in a mouse model of sepsis.
METHODS:
Mice were subjected to cecal ligation and puncture (CLP) for induction of sepsis to assess intestinal mucosal damage, epithelial cell apoptosis, and transformed number of goblet cells, and to detect the concentration of TNF-α, IL-1 and TGF-β1 and TFF3 (trefoil factor 3) expression in the small intestinal mucosa. All above were performed by HE staining, western blot, ELISA and immunohistochemistry respectively. The experimental animals were divided into a sepsis group and a sham-operation group. The animals with sepsis were separately killed at 6 (7 animals), 24 (7 animals) and 48 hours (7 animals) after CLP.
RESULTS:
Injured intestinal mucosa was observed in the 3 groups under a light microscope, in which damage scores in the 24-hour and 48-hour groups were higher than in the 6-hour group and no difference was found between the two groups. Moreover, less of goblet cells or other epithelial cells adjacent to the injured surface migrated into the wound to cover the denuded area. The number of goblet cells was substantially decreased in the three CLP groups compared with the sham-operation group. Protein levels of IL-1 and TNF-α were significantly increased by 3–4 fold at all time points when compared with the sham-operation group, and cleaved caspase-3 by 4 fold. Although TFF3 expression was modestly increased for 6 hours after the onset of CLP, it appeared to decline at 24 hours and 48 hours as shown by Western blot. A similar tendency was observed upon TGF-β1, i.e. the protein level was not elevated at 24 hours and 48 hours, but increased modestly at 6 hours.
CONCLUSIONS:
Sepsis from CLP shows less restitution on the surface of injured intestinal mucosa. There is evidence that both constant inflammatory reaction and epithelial cell apoptosis may affect mucosal reestablishment of the intestine at the onset of sepsis. Mucosa after severe sepsis showed the state of high inflammation, and declined goblet cell function and mucosal reconstruction, which affected the repair of damaged intestinal barrier. Constant inflammatory reaction, and declined goblet cell function and mucosal reconstruction ability may affect the reestablishment of intestinal mucosa at the onset of sepsis.
KEY WORDS: Sepsis, Cecal ligation and puncture, Intestinal mucosa, Restitution, Goblet cells, Intestinal trefoil factor 3, Transforming growth factor β1, Cysteine-containing aspartate-specific proteases
INTRODUCTION
Sepsis and sepsis-related multiple organ failure remain as major challenges for clinicians. In spite of the extensive research in the past, the pathophysiology of sepsis is still poorly understood, and hospitalization and mortality of septic patients increase year by year. To study the underlying mechanisms of sepsis and the associated systemic inflammatory response, several experimental animal models have been developed to mimic pathophysiologic changes in septic patients. Cecal ligation and puncture in rodents has become a widely used model for sepsis and is currently regarded as the gold standard in research. The intestine is not only the main target attacked by sepsis but also the vital organ which mediated the sepsis. The recovery of the damaged intestinal barrier structure and function is related to the occurrence and outcome of multiple organ dysfunction syndrome (MODS).[1-3] How to protect and reduce the damage of the intestinal mucosa, and how to promote the reconstruction of the intestinal mucosa have been the important topics of research in sepsis for many years. In this study, we investigated the influential factors of reconstruction of the intestinal mucosa for epithelial injury in vivo by a mouse model of sepsis.
METHODS
Experimental animals and grouping
Kunming mice, weighing 20 to 25 g, which obtained from the Experimental Animal Center of Sun Yat-sen University in Guangzhou, China, were randomly divided into a sham-operation group and a sepsis group. In the sham-operation group, mice were only anesthetized and operated on, but were not subjected to cecal ligation and puncture (CLP). In the sepsis group, mice were subdivided into three groups according to the different time points after the sham-operation, namely, a 6-hour group, a 24-hour group, and a 48-hour group (n=7). In addition, other 30 mice that underwent CLP were used for calculating the mortality rate. The experiment was completed in the pathophysiology laboratory of the Sun Yat-sen Institute of Cardiopulmonary Cerebral Resuscitation.
Experimental model of sepsis
Sepsis was induced by CLP.[4] After an overnight fasting, the mice were anesthetized with sodium pentobarbital intraperitoneally (100 mg/kg). Through a midline laparotomy, the cecum was exposed and ligated by No. 4 silk on 2/3. The distal end of the cecum was punctured for two times with No. 18 syringe needles, and was squeezed softly to make little intestinal contents into the abdomen. Two strips of 7# silk were left through the cecum. To avoid prolapse, we knotted the silk with two ends. Then the abdomen was closed to finish the operation. The mice were killed respectively at 6, 24, 48 hours after the operation. Distal ileum specimens were collected and washed by PBS. They were treated with 4% paraformaldehyde (pH 7.4), frozen in liquid nitrogen, and stored at –8 °C.
Histopathological examination
Ileum pathology
Paraformaldehyde fixed, paraffin embedded distal ileum specimens were cut into 4–5 μm thick sections, deparaffinized in xylene, and rehydyated in graded ethanol. The sections were then stained with hematoxylin-eosin (HE) for histological observation under a light microscope.
Goblet cells in the mucosa
The number of goblet cells in the sections obtained at different time points were counted. Ten crypts of the intestinal villus in each section were selected, and three sections per animal were taken on average.
Assessment of damaged ileal mucosa
Damage of the mucosa was observed as follows:[5,6] Grade 0: normal structure of the intestinal mucosa; Grade 1: the subepithelial gap broaden at the apex of the villus, which accompanied by capillary blood congestion; Grade 2: the subepithelial gap further broaden and epithelial layers separated from the lamina propria; Grade 3: a lot of epithelial layers separated from both sides of villi and a few villi exfoliated; Grade 4: lamina propria exposed because lots of villi exfoliated; Grade 5: the lamina propria digested and disintegrated, leading to ulceration.
Western blot
The expression of caspase-3 in the intestinal mucosa was determined by Western blot. The intestinal mucosa was scraped, and pre-cooled RIPA protein lysate was added into the intestinal mucosa and grinded. It was cracked for 30 minutes on ice with lysis buffer, then the supernatant was collected after the lysate was centrifuged at 20 000 g for 30 minutes at 4 ºC. Protein concentration in the lysate was measured by PIERCE BCATM Protein Assay kit (Thermo Fisher Scientific Inc. Rockford, USA), then the protein was saved in a –80 ºC refrigerator. Protein (20 μg) samples were mixed with sample loading buffer, heated for 5 minutes at 95 °C ,and separated by SDS-PAGE. Proteins were then transferred to Hybond ECL nitrocellulose membrane (Amersham Pharmacia Biotech Europe GmbH, Freiburg, Germany). Nonspecific binding sites were blocked in 5% nonfat milk and 0.1% Tween-20 in TBS for one hour at room temperature, after which the membrane was incubated in primary antibody buffer overnight by shaking gently at 4 ºC, followed by washing three times in 0.1% TBST. Afterward, the membrane was incubated with peroxidase-labeled secondary antibodies at room temperature for 1 hour and again washed three times in 0.1% TBST. The blots were then detected using Lumi-Light (Roche, Mannheim, Germany) with a Kodak Imager. The bands corresponding to the detected protein were analyzed using Adobe Photoshop 7.0 software.
Before electrophoresis, the protein was mixed with sample buffer at a 3:1 ratio, and denaturated at 100 ºC for 5 minutes. Twenty μg protein samples were taken during electrophoresis with 12% polyacrylamide gel. After transfer to NC membrane after electrophoresis, the samples were blocked for one hour, and incubated at 4 ºC with first antibodies (casase-3 antibodies from Cell Signaling Technology, USA; TFF3 purchased from Abcam), overnight. Then the HRP-labeled specific secondary antibody (U.S. Biological, USA) was added and the samples were incubated at room temperature for one hour and ECL reagent was exposed. The NC membrane was blocked again after elution, and the rabbit anti-mouse GAPDH antibody (Novus Biologicals) was used for blot reaction, scanning pictures and analyzing the band optical density.
ELISA
TNF-α, IL-1 and TGF-β1 in the mucosa were analyzed with ELISA kit which was purchased from the Dakota Bio-technology Company. The Human Quantikine TGF-β1 kit was bought from R&D Systems.
Statistical analysis
The data were expressed as mean±standard error. And comparisons were performed between groups of data using one-way ANOVA. A P value less than 0.05 was considered statistically significant. All data were analyzed by SPSS 13.0 statistical software.
RESULTS
Analysis of mortality rate
According to Figure 1, the mortality rate increased with the development of sepsis. The mortality rate was 3.3% (1/30) within 12 hours, and 63.3% (19/30) within 48 hours; the total mortality rate was 83.3%. No mice died after 72 hours, and none of the mice in the sham-operation group was dead.
Figure 1.
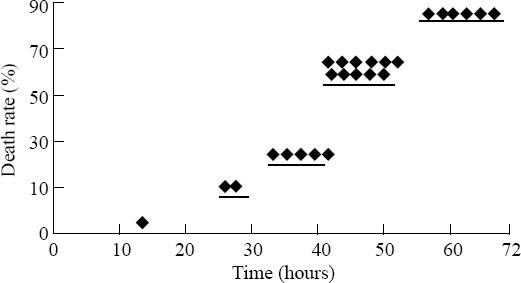
Analysis of the mortality rate of 30 mice with sepsis.
Pathological changes of the ileal mucosa
Under a light microscope (Figures 2 and 3), seriously damaged ileal mucosa was observed at three time points. The damaged mucosal scores were 2.24, 3.01 and 3.13 respectively in the 6-, 24- and 48-hour groups, and they were statistically different as compared to that of the sham-operation group (0.56) (P<0.01). The 24- and 48-hour groups showed more serious damage than the 6-hour group. No obvious goblet cells were accumulated in the intestinal villi or deciduous epithelial cells. The counts of goblet cells of the 6-, 24- and 48-hour groups were significantly lower than that of the sham-operation group. Moreover, those of the 24- and 48-hour groups were significantly lower than that of the 6-hour group.
Figure 2.
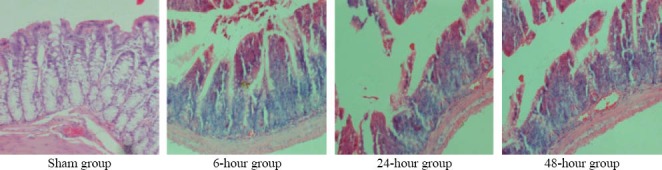
Representative pictures of damaged intestinal mucosa after CLP (HE, original magnification×200).
Figure 3.
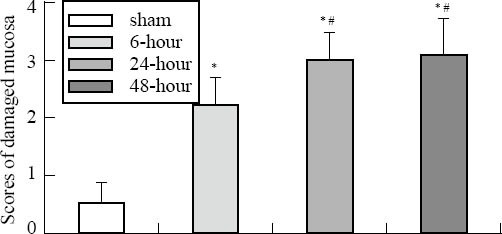
Scores of damaged mucosa in the small intestine after CLP. Compared with the sham-operation group, *P<0.01; compared with the 6-hour group, #P<0.05.
ELISA
The levels of IL-6 and TNF-α of the three experimental groups were 3–4 fold higher than those of the sham-operation group (P<0.05). And the level of TGF-β1 of the 6-hour group was higher than that of the sham-operation group; but the levels of the 24-hour and 48-hour groups were decreased more significantly than those of the 6-hour group (Table 1).
Table 1.
The levels of inflammatory mediators and number of goblet cells in intestinal mucosa after CLP (mean±SD, n=7)

Western blot
The protein levels of caspase-3 in the intestinal mucosa of the 6-, 24-, 48-hour groups were 2–4 fold higher than those of the sham-operation group, but no statistical significance was seen in the groups. The TFF3 protein level in the mucosa of the 6-hour group was slightly increased compared with that of the sham-operation group; but the TFF3 protein levels of the 24-, 48-hour groups were significantly lower than those of the sham-operation group (Figures 4 and 5).
Figure 4.
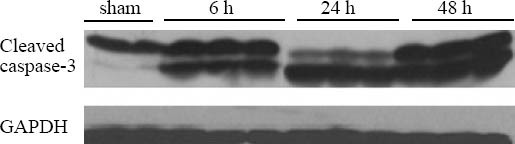
Western blot showing caspase-3 expression in damaged intestinal mucosa after CLP (n=3).
Figure 5.
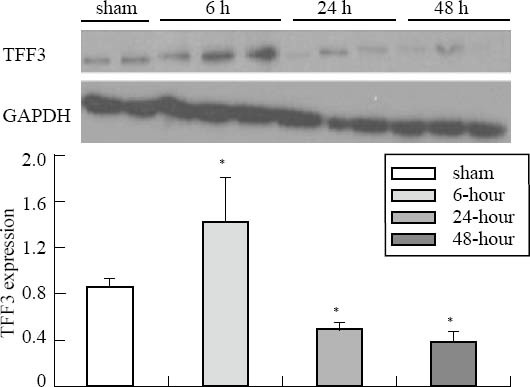
TFF3 expression of intestinal mucosa in the sham-operation, 6-, 24- and 48-hour groups. Compared with the sham-operation group, *P<0.05.
DISCUSSION
Some studies have shown that damaged intestinal mucosa has the ability to repair itself rapidly, and can establish the continuity of surface epithelial cells in a short period of time. In our study, however, we did not find obvious rapid reconstruction of the intestinal mucosa in mice with cecal ligation and puncture (CLP)-induced sepsis. The goblet cells which were the major player in the reconstruction of the intestinal epithelia were not a large gathering around the damaged villi, but reduced instead. The expression of TFF3 and TGF-β1 which play an important role in the reconstruction of the intestinal mucosa also showed a progressive reduction. Accompanied by increased pro-inflammatory cytokines and pro-apoptotic caspse-3 protein levels, these findings suggested that persistent mucosal inflammation, cell apoptosis of the intestinal epithelia and reduced ability of reconstruction were the influencing factors for the reconstruction of damaged intestinal mucosa.
Repair of the intestinal epithelia has at least three different and overlapping mechanisms and is subjected to complex and tight regulation.[7] The epithelial cells near the damaged site cover the surfaces of the villi or mucosa through migration, and rebuild a new continuous villus surface. This repair without cell proliferation is called reconstruction, which happens in a few minutes to several hours after a damage to the mucosa, usually within 24 to 48 hours. Moreover, a physical barrier is required to prevent bacteria and endotoxin translocation. Then cell proliferation is moblized to complement the reduced cell number and proliferation of cells through differentiation. The mucosa will eventually meet the biological function of the mucosal epithelium.[8,9] Hence the first step for the repair of intestinal mucosal injury is the reconstruction of epithelial cells, in which goblet cells are particularly important. In the current study, goblet cells were not involved significantly in the reconstruction. TFF3, an important product of goblet cells, plays a key role in protecting the intestinal mucosa and promoting the reconstruction of damaged mucosa. Dignass et al[10] reported that the involvement of TFF3 will increase the ability of goblet cells by 3–6 times in the reconstruction. Our studies[11,12] have also confirmed that the rapid reconstruction of damaged intestinal mucosa in hemorrhagic shock animals was accompanied by increased TFF3 expression.
Like TFF3, TGF-β1 promotes the repair of damaged intestinal epithelia. TGF-β1 can also promote the migration of intestinal epithelial cells to the damaged mucosa.[10,13] In our study, TGF-β1 in the 6-hour group showed a transient increase, followed by a rapid decline, and then approached to the level of the sham group. Since damaged cells can release part of TGF-β1, its true expression may be lower. In addition to supporting cell renewal, TGF-β1 can regulate the inflammation of the mucous membrane by inhibiting the TNF-α and IFN-γ expression of proinflammatory cytokines and increasing the IL-10 expression of antiinflammatory mediators.[14,15] Another study found that a number of other factors involving in tissue repair such as IL-1, IL-2 and IFN-beta, and growth hormone (EGF and FGF) were activated by the TGF-β1-dependent pathway. Thus the low level of TGF-β1 expression not only failed to reduce the inflammatory response, but also increased the injury of the intestinal epithelia and delayed healing after the damage.[16-18]
The levels of inflammatory cytokines 6, 24, and 48 hours after CLP were several times higher than those of the control group. This finding proved that there was a continuing inflammation in the mucosa. Inflammatory cytokines highly expressed in the intestinal mucosa can lead to apoptosis of a large number of intestinal epithelial cells, including goblet cells. The death of cells may be due to the decline of promoting repair factors or the inhibition of mucosal reconstruction or repair.
Sepsis leads to intestinal mucosal injury, bacterial translocation, and further aggravation of intestinal and remote organ injury. Apoptosis of a large number of intestinal epithelial cells may damage the intestinal barrier. The decline in goblet cells and the expression of TFF3 and TGF-β1, which can protect the intestinal mucosa and promote its repair after damage, affect the repair of the damaged mucosa.
Footnotes
Funding: The study was supported by a grant from National Natural Science Foundation of China (81071761) and Guangdong National Natural Science Foundation (10151008901000135).
Ethical approval: The present study was approved by the Animal Care and Use Committee of Sun Yat-sen Memorial Hospital of Sun Yat-sen University, Guangzhou, China.
Conflicts of interest: The authors have no competing interests relevant to the present study.
Contributors: Chang RM proposed and wrote the paper. All authors edited the final version of the paper.
REFERENCES
- 1.Deitch EA. Gut lymph and lymphatics: a source of factors leading to organ injury and dysfunction. Ann N Y Acad Sci. 2010;1207:E103–11. doi: 10.1111/j.1749-6632.2010.05713.x. [DOI] [PubMed] [Google Scholar]
- 2.Coopersmith CM, Stromberg PE, Dunne WM, Davis CG, Amiot DM, 2nd, Buchman TG, et al. Inhibition of intestinal epithelial apoptosis and survival in a murine model of pneumonia-induced sepsis. JAMA. 2002;287:1716–1721. doi: 10.1001/jama.287.13.1716. [DOI] [PubMed] [Google Scholar]
- 3.Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock. 2001;15:1–10. doi: 10.1097/00024382-200115010-00001. [DOI] [PubMed] [Google Scholar]
- 4.Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, 3rd, Bland KI, et al. Cecal ligation and puncture. Shock. 2005;24:52–57. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Estivariz C, Gu LH, Gu L, Jonas CR, Wallace TM, Pascal RR, et al. Trefoil peptide expression and goblet cell number in rat intestine: effects of KGF and fasting-refeeding. Am J Physiol Regul Integr Comp Physiol. 2003;284:R564–573. doi: 10.1152/ajpregu.00428.2002. [DOI] [PubMed] [Google Scholar]
- 6.Nadler EP, Dickinson E, Knisely A, Zhang XR, Boyle P, Beer-Stolz D, et al. Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. J Surg Res. 2000;92:71–77. doi: 10.1006/jsre.2000.5877. [DOI] [PubMed] [Google Scholar]
- 7.Yin K, Dang SC, Zhang JX. Relationship between expression of triggering receptor-1 on myeloid cells in intestinal tissue and intestinal barrier dysfunction in severe acute pancreatitis. World J Emerg Med. 2011;2:216–221. doi: 10.5847/wjem.j.1920-8642.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Potten CS, Kellett M, Rew DA, Roberts SA. Proliferation in human gastrointestinal epithelium using bromodeoxyuridine in vivo: data for different sites, proximity to a tumour, and polyposis coli. Gut. 1992;33:524–529. doi: 10.1136/gut.33.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nusrat A, Delp C, Madara JL. Intestinal epithelial restitution. Characterization of a cell culture model and mapping of cytoskeletal elements in migrating cells. J Clin Invest. 1992;89:1501–1511. doi: 10.1172/JCI115741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dignass A, Lynch-Devaney K, Kindon H, Thim L, Podolsky DK. Trefoil peptides promote epithelial migration through a transforming growth factor beta-independent pathway. J Clin Invest. 1994;94:376–383. doi: 10.1172/JCI117332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang C, Gao GR, Jiang HY, Lv CG, Zhang BL, Xie MS, et al. Effects of environmental hypothermia on hemodynamics and oxygen dynamics in a conscious swine model of hemorrhagic shock. World J Emerg Med. 2012;3:128–134. doi: 10.5847/wjem.j.issn.1920-8642.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciacci C, Lind SE, Podolsky DK. Transforming growth factor beta regulation of migration in wounded rat intestinal epithelial monolayers. Gastroenterology. 1993;105:93–101. doi: 10.1016/0016-5085(93)90014-4. [DOI] [PubMed] [Google Scholar]
- 13.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, et al. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakuraba H, Ishiguro Y, Yamagata K, Munakata A, Nakane A. Blockade of TGF-beta accelerates mucosal destruction through epithelial cell apoptosis. Biochem Biophys Res Commun. 2007;359:406–412. doi: 10.1016/j.bbrc.2007.05.117. [DOI] [PubMed] [Google Scholar]
- 15.Sturm A, Dignass AU. Epithelial restitution and wound healing in inflammatory bowel disease. World J Gastroenterol. 2008;14:348–353. doi: 10.3748/wjg.14.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi HP, Deitch EA, Da Xu Z, Lu Q, Hauser CJ. Hypertonic saline improves intestinal mucosa barrier function and lung injury after trauma-hemorrhagic shock. Shock. 2002;17:496–501. doi: 10.1097/00024382-200206000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Zhang ZJ, Peng LB, Luo YJ, Zhou CY. Prospective experimental studies on the renal protective effect of ulinastatin after paraquat poisoning. World J Emerg Med. 2012;3:299–304. doi: 10.5847/wjem.j.issn.1920-8642.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanayama N, el Maradny E, Yamamoto N, Tokunaga N, Maehara K, Terao T. Urinary trypsin inhibitor: a new drug to treat preterm labor: a comparative study with ritodrine. Eur J Obstet Gynecol Reprod Biol. 1996;67:133–138. doi: 10.1016/0301-2115(96)02454-2. [DOI] [PubMed] [Google Scholar]


