Abstract
BACKGROUND:
The high level of matrix metalloproteinase 9 (MMP9) is thought to slow down the healing of diabetic foot ulcers. Whether it can influence the biological behaviors of skin fibroblasts and affect wound healing is still unclear. The present study aimed to observe changes in the biological behaviors of rat dermal fibroblasts induced by high expression of MMP9 and to clarify the possible mechanisms of wound healing for diabetic foot.
METHODS:
A cell model of skin fibroblast with high expression of MMP9 was established by co-culture of high glucose (22.0 mmol/L) and homocysteine (100 μmol/L). A control group was incubated with normal glucose (5.5 mmol/L). Realtime PCR, ELISA and gelatin zymography were used to detect the MMP9 mRNA, protein expression and activity of MMP9. Flow cytometry, CCK-8, ELISA assay, scratch test and transwell were used to detect cell proliferation, viability, collagen (hydroxyproline) secretion, horizontal migration and vertical migration of cells. The data were expressed as mean±SD. P value less than 0.05 was considered statistically significant.
RESULTS:
The expression of MMP9 mRNA, protein levels and the activity of MMP9 were much higher in the high MMP9 group than in the control group (7.05±1.02 vs. 1.00±0.00, 206.9±33.6 pg/mL vs. 40.4±5.9 pg/mL, and 1.47±0.13 vs. 0.57±0.12, respectively, P<0.01). The proportion of S-phase cells, proliferation index, cell viability, collagen (hydroxyproline) secretion, horizontal migration rate and the number of vertical migration cells were lower in the high MMP9 group than in the control group (P<0.01).
CONCLUSION:
Fibroblasts with a high expression of MMP9 decreased proliferation, activity, secretion and migration of collagens, suggesting that MMP9 may inhibit the biological behaviors of fibroblasts.
KEY WORDS: Matrix metalloproteinase 9, Fibroblast, Biological behaviors, Diabetic foot
INTRODUCTION
Matrix metalloproteinase 9 (MMP9) is thought to be closely related to diabetic foot ulcers.[1] Before the skin was injured, a “hidden damage” in diabetic rats was correlated with the level of MMP9,[2] compared with non-diabetic rats. The level of MMP9 was increased significantly in the skin during the wound healing.[3,4] It is recognized that the high level of MMP9 slows down the healing of diabetic foot ulcers by the excessive degradation of extracellular matrix, growth factors, growth factor receptors, integrins and their receptors, and increase the local inflammatory response to the wound.[5,6] Whether MMP9 can influence the biological behaviors of skin fibroblasts and affect the wound healing is still obscure. Skin fibroblast as an important effector cell in wound repair can affect its biological properties and ultimately the wound healing. This study aimed to determine the changes of the biological behaviors of rat dermal fibroblasts induced by a high expression of MMP9, and to clarify the possible mechanisms of wound healing of diabetic foot.
METHODS
Cell and reagents
Skin fibroblast cell line CRL-1213 of Sprague-Dawley rats was purchased from American Type Culture Collection (ATCC, USA).
DMEM (Gibco, USA), homocysteine (Fluka, Italy), fetal bovine serum (Hyclone, USA), TRIZol (Invitrogen, USA), realtime PCR kit (TaKaRa, Japan), rat MMP9 ELISA kit (Uscn Life Science Inc., China), CCK-8 kit (Dojindo Laboratorise, Japan), rat hydroxyproline ELISA kit (R&D Systems, USA), and transwell 24-well plates (Corning, USA) were used.
Cell culture[7] and grouping
Fibroblasts were grown in DMEM containing 10% fetal bovine serum in a CO2 incubator (37 °C, 5% CO2). Subcultures were digested with 0.25% trypsin when the cells were 90% fusion. The cells were inoculated to 6-well plates according to the density of (1.5–2.0) ×105 cells for each hole. When the cells were 70% fusion and in the logarithmic growth phase, they were replaced with 0.5% fetal bovine serum medium for starvation. After 24-hour serum deprivation, fibroblasts were cultured in DMEM containing 10% FBS at two different conditions (according to the grouping) for 6 hours.
This study consisted of a control group using DMEM with normal glucose concentration (5.5 mmol/L) and a high MMP9 group using DMEM with high glucose (22.0 mmol/L) and hyperhomocysteine (100 μmol/L) co-culture.[8–10]
Determination of MMP9 mRNA[11]
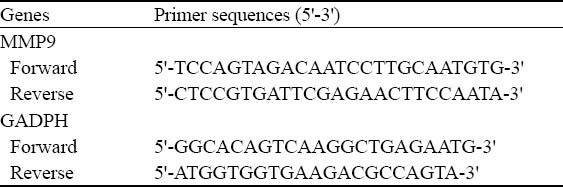
MMP9 mRNA levels were determined by realtime PCR. GAPDH was used as the internal control (Table 1). A total volume of reverse reaction was 20.0 μL at 37 °C for 15 minutes or at 85 °C for 5 seconds. It was amplified according to the instructions for the realtime PCR kit. The PCR reactions took place with denaturation at 95 °C for 30 seconds, then 40 cycles at 95 °C for 5 seconds followed by elongation at 60 °C for 20 seconds. The relative value of MMP9 mRNA was shown as the ratio of MMP9/GADPH.
Table 1.
Realtime PCR primer sequences of MMP9 and GADPH genes
Determination of MMP9 protein
MMP9 protein was measured by ELISA assay. At the end of cell culture the supernatant was collected, and double-antibody sandwich ABC-ELISA was used to detect protein expression of MMP9. The whole test was done according to the manufacturer's protocol.
Determination of MMP9 activity
MMP9 activity was assessed by gelatin zymography. Equal aliquots of conditioned culture media from an equal number of cells were fractionated using precast zymogram gel containing 1% gelatin. After electrophoresis, the gel was incubated in eluate containing 2.5% Triton X-100 to elution SDS, and Triton X-100 was washed out by lotion, incubated in buffer containing Ca2+ and Zn2+ for 48 hours at 37 °C, and stained with 0.5% Coomassie Brilliant Blue solution for 4 hours. The gel was placed into the bleaching gel solution until the digested band appeared, and then the gel was photographed for analysis.
Proliferation of cells[12]
Proliferation of cells was assessed by flow cytometry. The cells were collected into the dedicated tube for flow cytometry, and washed by 1 mL PBS once. They were digested with EDTA-free trypsin, and the remaining cells were washed again with 1 mL PBS. The cells were centrifuged at 2 500 r/min for 6 minutes, and the supernatant was discarded. The cells were washed with PBS again and resuspended with 1 mL PBS; 300 μL cell suspension was added into 700 μL ice-cold ethanol drop by drop at 4 °C and fixed in dark overnight. Then the cells were centrifuged at 2 500 r/min for 10 minutes, and the supernatant was discarded. The cells were washed twice with PBS, resuspended with 500 μL PBS combined with 100 U/mL RNase, and incubated at 37 °C for 30 minutes. Two mg/mL ethidium bromide was added to a final concentration of 50 μg/ mL, and the cells were incubated in dark for 30 minutes. Cell cycle was detected by standard procedures of flow cytometry, the S-phase cell ratio and proliferation index were calculated at the same time. S-phase cell ratio = S/(Go/Gj+S+G/M); proliferation index = (S+G2/M) / (G0/GJ+S+G2/M).
Viability of cells
Viability of cells was assessed by CCK-8. The cells were inoculated into 96-well plates according to the density of (2.0–3.0)×103 cells for each hole. CCK-8 and médium were mixed according to the ratio of 10 μL: 100 μL per well in a clean EP tube. The medium was discarded in 96-well plates, and washed once with PBS. Premixed CCK-8 and medium were added into 96-well plates, and incubated at 37 °C for 0.5–1 hour. The values of A450 were obtained using an automatic detector.
Collagen (hydroxyproline) secretion
Collagen (hydroxyproline) secretion was detected by ELISA. Hydroxyproline was measured according to the instructions for the rat hydroxyproline ELISA kit. The concentration of hydroxyproline was calculated from the A450 value obtained with an automatic detector.
Horizontal migration of cells
Horizontal migration was assessed by a scratch test. The scratch was done at the bottom of 6-well plates with a small tip along the ruler. The scratch area was washed repeatedly with PBS until the cells were removed thoroughly. The purpose medium was added and cultured for 6 hours. Five different horizons were selected under an inverted microscope, and the distance between cells at 0 and 6 hours was measured after the scratch while calculating the average cell migration rate. The cell migration rate = (0 hour scratch width – 6-hour scratch width) / 0 hour scratch width×100%.
Vertical migration of cells
Transwell was used to evaluate the vertical migration of cells. The cells in the logarithmic growth phase were suspended by purpose medium containing 0.5% FBS after conventional digestion. 100 μL cell suspension (cell density 1.0×105/mL) was added into the upper chamber, 500 μL purpose medium containing 10% FBS was added into the lower chamber. After 16 hours of culture, the upper chamber was removed, and washed by PBS for 3 times. The cells were wiped gently with cotton in the upper membrane, fixed with 4% paraformaldehyde for 30 minutes, and stained with crystalline violet for 15 minutes. Then four visions (×200) randomly selected to count the number of cells were moved to the lower membrane under an inverted microscope for calculating the number of vertical migration cells.
Statistical analysis
The results of the study were expressed as means ±SD. Student's t test was used to compare the two groups. Data analysis was performed using SPSS 12.0 software for Windows. A P value <0.05 was considered statistically significant.
RESULTS
Establishment of a fibroblast cell model of high MMP9 expression
MMP9 mRNA level, protein expression, and protease activity in the high glucose and hyperhomocysteine groups were 7.05±1.02, 206.9±33.6 pg/mL and 1.47±0.13 respectively, which were much greater than those in the normal glucose control group (1.00±0.00, 40.4±5.9 pg/mL and 0.57±0.12, P<0.01) (Figures 1 and 2). The results suggested that the fibroblast cell model of high MMP9 expression was created successfully.
Figure 1.
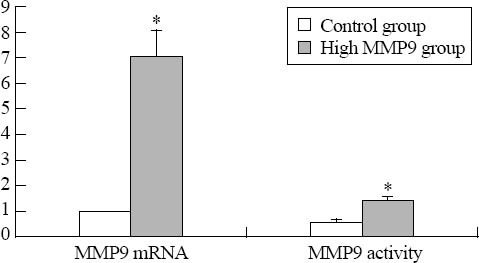
The expression of MMP9 mRNA and protease activity (P<0.01).
Figure 2.
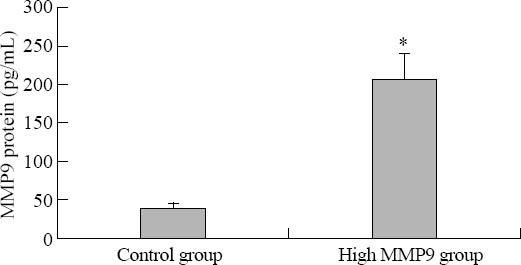
The expression of MMP9 protein (P<0.01).
Changes in biological behaviors of rat dermal fibroblasts induced by high expression of MMP9
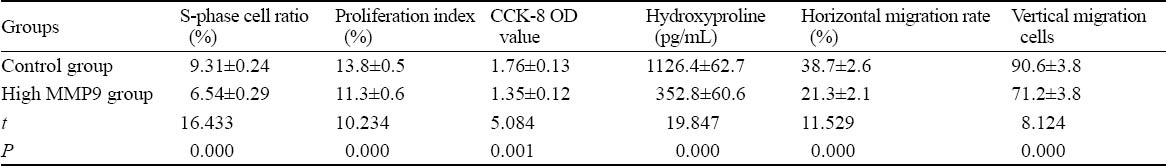
Proliferation, viability, secretion and migration of cells are important biological behaviors of fibroblasts. The cells in the high expression MMP9 group decreased proliferation, activity, collagen secretion and horizontal and vertical migration abilities (P<0.01, Table 2), suggesting that MMP9 may inhibit the biological behaviors of fibroblasts.
Table 2.
Changes in biological behaviors of rat dermal fibroblasts induced by a high expression of MMP9 (mean±SD)
DISCUSSION
Fibroblast is the major repair cell in skin, giving a rate of 40% to 60% in total cells. The biological effects of fibroblast play a vital role in wound healing of skin.[13] The finding that skin fibroblast DNA synthesis was significantly decreased but apoptosis was increased in patients with diabetes, indicates that the proliferation of fibroblast was inhibited in the pathological state of diabetes.[14] The same result was found in humans.[15] Whether the changes in biological behavior of fibroblast are related to the high level of MMP9 expression in diabetes is not clear. In this study we found that the expression of MMP9 mRNA, protein level and the activity of MMP9 in the high MMP9 group were much higher than those in the control group. Moreover, the S-phase cell ratio, proliferation index and cell viability in the high MMP9 group were lower than those in the control group, as reported previously.[15] This finding suggests that the inhibition of fibroblast proliferation and viability was likely to be related to the high level of MMP9 expression.
Wound healing is dependent on the proliferation and migration of fibroblasts, formation of granulation tissue, collagen secretion, and collagen-based scar formation, which need the participation of different cells, extracellular matrix (ECM) and soluble components.[16] Fibroblasts have a negative effect on collagen synthesis and wound healing. In the present study the amount of hydroxyproline was lower in the high expression MMP9 group than in the control group. This finding suggests that in the diabetic environment, the high activity of MMP9 increases the degradation of ECM but the number and activity of fibroblasts and collagen synthesis are decreased. The negatively balanced collagen metabolism could delay the healing of diabetic foot wounds.
Migration is another biological property of fibroblasts. In the phase of solidification and inflammation of wound healing, platelets and inflammatory cells release a large amount of inflammatory cytokines which promote fibroblast migration to the wound.[17] Fibroblast has two kinds of migration, horizontal and vertical. In this study, the results of scratch test and transwell showed that the horizontal and vertical migration abilities of fibroblasts were significantly decreased in the high expression MMP9 group. These changes prevent fibroblasts crawling to the wound in time to play their roles. And because the inhibition of cell proliferation, viability and collagen synthesis, even if fibroblasts migrate to the wound, they can not function normally, i.e., they can not proliferate efficiently and produce enough ECM and cell factors. Thus the normal cycle of wound healing is broken, resulting in disorder of wound healing.
In summary, fibroblasts with a high expression of MMP9 decrease the proliferation, activity, secretion and migration of collagens, suggesting that MMP9 may inhibit the biological behaviors of fibroblasts and influence the healing of diabetic foot.
ACKNOWLEDGEMENTS
We are grateful to Dr Ping Zhu and Xiao-ying Xie for their contribution to part of the study.
Footnotes
Funding: This work was supported by grants from the National Natural Science Foundation of China (81070660), the Science and Technology Project Foundation of Guangdong Province (2008A030201012), and Medical Science and Technology Research Foundation of Guangdong Province (A2012183), and the Science and Technology Project Foundation of Guangdong Province (2009B091300128).
Ethical approval: Not needed.
Conflicts of interest: The authors have no competing interests relevant to the present study.
Contributors: Xue SN proposed the study and wrote the paper. All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
- 1.Muller M, Trocme C, Lardy B, Morel F, Halimi S, Benhamou PY. Matrix metalloproteinases and diabetic foot ulcers: the ratio of MMP-1 to TIMP-1 is a predictor of wound healing. Diabet Med. 2008;25:419–426. doi: 10.1111/j.1464-5491.2008.02414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan L, Zhu P, Chen LH, Yang C, Lao GJ, Du J, et al. Imbalance of MMP-9/TIMP-1 during the cutaneous ‘underlying diorder’in diabetic rats. Chin J Endocrinol Metab. 2008;24:533–537. [Google Scholar]
- 3.Yang C, Zhu P, Yan L, Chen L, Meng R, Lao G. Dynamic changes in matrix metalloproteinase 9 and tissue inhibitor of metalloproteinase 1 levels during wound healing in diabetic rats. J Am Podiatr Med Assoc. 2009;99:489–496. doi: 10.7547/0990489. [DOI] [PubMed] [Google Scholar]
- 4.Wu CT, Wang ZH, Li ZQ, Wang LF. Effect of spironolactone on cardiac remodeling after acute myocardial infarction. World J Emerg Med. 2013;4:48–53. doi: 10.5847/wjem.j.issn.1920-8642.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu SC, Driver VR, Wrobel JS, Armstrong DG. Foot ulcers in the diabetic patient, prevention and treatment. Vasc Health Risk Manag. 2007;3:65–76. [PMC free article] [PubMed] [Google Scholar]
- 6.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 7.Song MF, Peng KL, Wang C, Liu Y, Lu CR. The effects of ammonium perchlorate (AP) on pulmonary fibrosis. Chin J Emerg Med. 2006;15:402–405. [Google Scholar]
- 8.Chen LH, Xie XY, Yang C, Yan L, Zhu P, Lao GJ, et al. Effects of high glucose and homocysteine on expression of MMP-9 in rat fi broblasts. Chin J Pathophysiol. 2010;26:1839–1843. [Google Scholar]
- 9.Xue SN, Lei J, Lin DZ, Zhu P, Yan L. Establishment of a cell model with high expression of MMP9. Chin J Health Laboratory Technology. 2011;21:82–84. [Google Scholar]
- 10.Solini A, Santini E, Nannipieri M, Ferrannini E. High glucose and homocysteine synergistically affect the metalloproteinasestissue inhibitors of metalloproteinases pattern, but not TGFB expression, in human fi broblasts. Diabetologia. 2006;49:2499–2506. doi: 10.1007/s00125-006-0377-2. [DOI] [PubMed] [Google Scholar]
- 11.Liu QY, Yong-ming Yao YM. Inflammatory response and immune regulation of high mobility group box-1 protein in treatment of sepsis. World J Emerg Med. 2010;1:93–98. [PMC free article] [PubMed] [Google Scholar]
- 12.Chen XD, Jiang Q, Wu BY, Wang SB, Huang LY, Wang ZC. Effects of Aloe coarse polysacchande on proliferation and cell cycle of the cultured keratinocytes. Chin J Emerg Med. 2006;15:406–409. [Google Scholar]
- 13.Andreea SI, Marieta C, Anca D. AGEs and Glucose Levels Modulate Type Iand III Procollagen mRNA Synthesis in Dermal Fibroblasts Cells Culture. Exp Diabetes Res. 2008;2008:473603. doi: 10.1155/2008/473603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niu Y, Xie T, Ge K, Lin Y, Lu S. Effects of extracellular matrix glycosylation on proliferation and apoptosis of human dermal fibroblasts via the receptor for advanced glycosylated end products. Am J Dermatopathol. 2008;30:344–351. doi: 10.1097/DAD.0b013e31816a8c5b. [DOI] [PubMed] [Google Scholar]
- 15.Wang MJ, Lu SL, Qing C, Hua LN, Shi GY, Sheng ZY, et al. Effects of different courses of disease on the biological behavior of dermal fi broblasts in diabetic rats. Acta Universitatis Medicinalis Secondae Shanghai. 2005;25:459–462. 466. [Google Scholar]
- 16.Toriseva M, Kähäri VM. Proteinases in cutaneous wound healing. Cell Mol Life Sci. 2009;66:203–222. doi: 10.1007/s00018-008-8388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366:1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]


