Abstract
BACKGROUND:
Few studies have reported the effect of aldosterone receptor antagonist (ARA) on myocardial remodeling after acute myocardial infarction (AMI). This study was undertaken to investigate the preventive effect of ARA on myocardial remodeling after AMI.
METHODS:
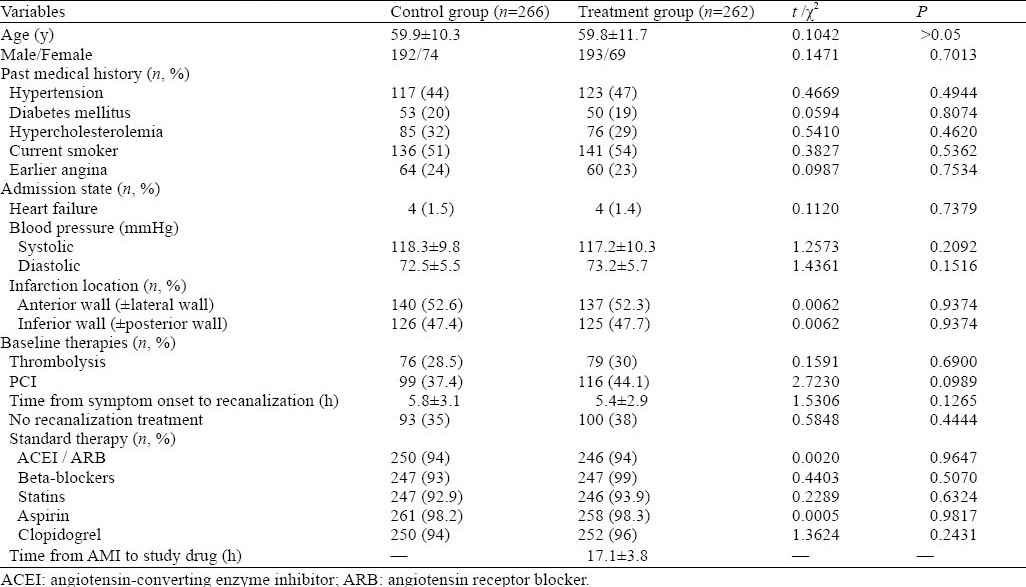
A total of 616 patients who had been admitted into the CCU of the First Affiliated Hospital of Harbin Medical University from January 2008 to January 2010 were studied prospectively. Only 528 patients were observed completely, including 266 of the control group and 262 of the treatment group. There was no statistical difference in age, gender, medical history, admission situation, and treatment between the two groups (P>0.05). The preventive effects of spironolactone on cardiac remodeling, left ventricular function, renal function and blood levels of potassium were evaluated by echocardiography, serum potassium and serum creatinine at one-month and one-year follow-up.
RESULTS:
The echocardiography indicators such as LVESD, LVEDD, LVEF, LAD-ML and LAD-SI were significantly improved in the treatment group compared with the control group at one year (P<0.05). In the treatment group, LVESD, LVEDD, LVPWT, LVEF, LAD-ML and LAD-SI were more significantly improved at one year than one month (P<0.05, P=0.007 to LVEF), and in the control group LVEF was more significantly improved at one year than one month (P=0.0277). There were no significant differences in serum potassium and serum creatinine levels between the two groups.
CONCLUSION:
On the basis of conventional treatment, the early combination of low-dose spironolactone (20 mg/d) could inhibit cardiac remodeling at late stage and prevent heart failure.
KEY WORDS: Myocardial infarction, acute, Ventricular remodeling, Atrial remodeling, Aldosterone, Aldosterone blockade, Spironolactone, Cardiac function, Prognosis
INTRODUCTION
Excessive activation of the neuroendocrine system can promote ventricular remodeling after acute myocardial infarction (AMI).[1] As an important part of the renin-angiotensin-aldosterone system (RAAS), the pathophysiology of aldosterone in the cardiovascular system and aldosterone escape determined crucial effect of aldosterone receptor antagonist (ARA) in treating cardiovascular diseases.[2,3] But few studies have reported the effect of ARA on myocardial remodeling after AMI. This study aimed to investigate the preventive effect of ARA on myocardial remodeling after AMI.
METHODS
Patients
A total of 616 patients with first-onset STEMI, who had been admitted into the CCU of the First Affiliated Hospital of Harbin Medical University within 24 hours from onset from January 2008 to January 2010, were enrolled in the study.
Diagnostic criteria[4]
A patient with active chest pain took a 12-lead electrocardiogram showing: 1) ST-segment elevation ≥1 mm (0.1 mV) in 2 or more adjacent limb leads (from aVL to III, including -aVR); 2) ST-segment elevation ≥1 mm (0.1 mV) in precordial leads V4 through V6; 3) ST-segment elevation ≥2 mm (0.2 mV) in precordial leads V1 through V3; or 4) new left bundle-branch block. Positive tests for cardiac enzymes troponin and creatinine kinase isoenzyme MB are helpful, but not essential. Therapy should not be delayed while awaiting results. Reciprocal depressions (ST depressions in the leads corresponding to the opposite side of the heart) make the diagnosis of STEMI more specific.
Exclusion criteria
Excluded patients were those with 1) non-STEMI; 2) only right ventricular infarction; 3) old myocardial infarction; 4) cardiac function Killip class IV or hypotension; 5) renal dysfunction (serum creatinine > 221 µmol/L); 6) serum potassium >5.0 mmol/L; 7) the time from onset to admission longer than 24 hours; 8) age of more than 75 years.
Groups
All patients were randomly divided into a control group (n=308) with standard therapy and a treatment group (n=308) with standard therapy plus oral administration of spironolactone 20 mg per day for one year, and administration of spironolactone after admission to the hospital within 17.1±3.8 hours. Standard therapy was prescribed with one-year oral administration of clopidogrel, aspirin, statins, β-blocker, angiotension converting enzyme inhibitors, and angiotensin II receptor blocker.
The patients were followed up for one month and one year from onset respectively. During the follow-up, 26 patients (10 patients in the treatment group and 16 in the control group) died. Eight patients died from acute cerebrovascular disease, 10 from gastrointestinal bleeding, 4 from cancer, and 4 from traffic accidents. Twenty-five patients were lost to follow-up, 30 had re-infarction, and 7 male patients were withdrown from treatment because they were tolerable to breast pain. Therefore, only 528 patients were observed and treated completely within one year, with 266 in the control group and 262 in the treatment group. Between the two groups, there was no significant difference in age, gender, medical history, admission status, and treatment (Table 1).
Table 1.
Characteristics of the study population at baseline
Data collection
During the follow-up, those who died, were lost to follow-up, had re-infarction, or completed the treatment because of side-effect after administration of spironolactone, were excluded from the study. The effect of spironolactone on cardiac remodeling, left ventricular function, renal function and blood levels of potassium were evaluated by echocardiography, serum potassium and serum creatinine at one-month and one-year follow-up, respectively. Echocardiographic indicators included left ventricular end-diastolic diameter (LVEDD), left ventricular end systolic diameter (LVESD), interventricular septum thickness (IVST), left ventricular posterior wall thickness (LVPWT), left ventricular ejection fraction (LVEF), left atrial transverse diameter (LAD-ML), left atrial vertical diameter (LAD-SI), right atrial transverse diameter (RAD-ML), and right atrial vertical diameter (RAD-SI).
Statistical analysis
Statistical analysis was performed using the PASW 18.0 statistical software. The data were presented as mean±standard deviation. The differences in the intra-group and inter-group were determined using Student’s t test for variance nonhomogeneity. The categorical data were performed using the Chi-square test. P<0.05 was considered statistically significant.
RESULTS
Echocardiographic results
Intra-group comparison
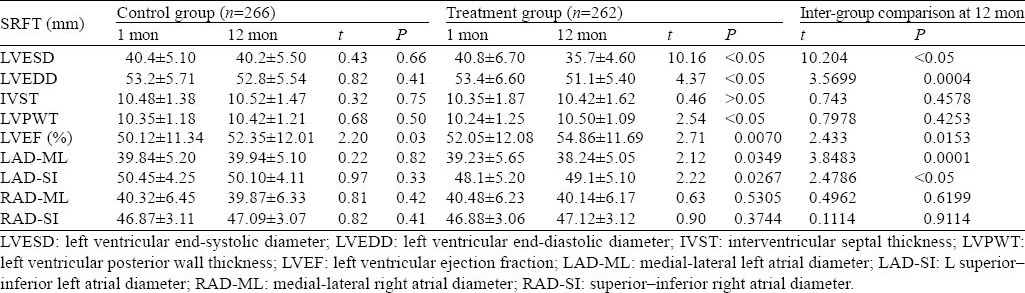
There was no statistical difference in the results of echocardiography between the two groups at one month (P>0.05). The intra-group comparison in the treatment group showed that there was a significant difference in the results of echocardiography such as LVEDD, LVESD, LVPWT, LVEF, LAD-ML, LAD-SI (P<0.05, and P=0.007 for LVEF) between one month and one year, but there was no difference in IVST, RAD-ML and RAD-SI. The intra-group comparison in the control group showed that there was a significant difference in LVEF at one year (P=0.0277) (Table 2).
Table 2.
Correlation analysis of chest compression quality indexes
Inter-group comparison
There was a significant difference in LVESD, LVEF, and LAD-SI between the two groups at one year (P<0.05) (Figures 1–3). There was a significant difference in LVEDD and LAD-ML between the two groups at one year (P<0.01) (Figures 4 and 5).
Figure 1.
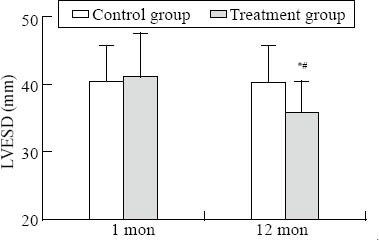
Comparison of LVESD. Intra-group comparison, *P<0.05; inter-group comparison, #P<0.05.
Figure 3.
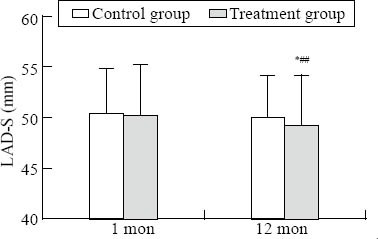
Comparison of LAD-S. Intra-group comparison, *P<0.05; inter-group comparison, #P<0.05.
Figure 4.
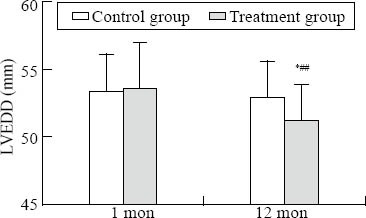
Comparison of LVEDD. Intra-group comparison, *P<0.05; inter-group comparison, ##P<0.05.
Figure 5.
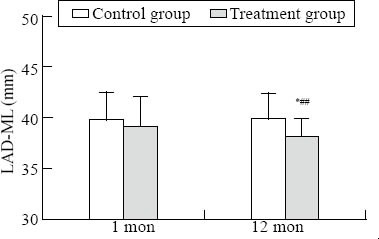
Comparison of LAD-ML. Intra-group comparison, *P<0.05; inter-group comparison, ##P<0.01.
Figure 2.
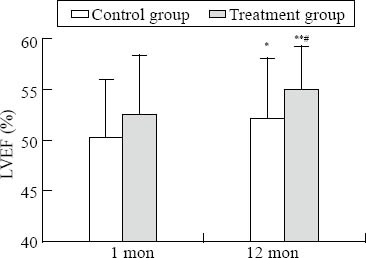
Comparison of LVEF. Intra-group comparison in the control group, *P<0.05; intra-group comparison in the treatment group, **P<0.05; inter-group comparison, #P<0.05.
Laboratory examination
There was no significant difference in serum creatinine or serum potassium (Table 3). The application of spironolactone had no effect on renal function, which did not cause hyperkalemia.
Table 3.
Plasma concentrations of potassium and creatinine at 1-month and 12-month follow-up respectively

DISCUSSION
Ventricular remodeling after AMI refers to the changes in size, shape, structure and physiology of the heart after injury to the myocardium. After the insult occurs, a series of histopathological and structural changes occur in the left ventricular myocardium, leading to progressive decline in left ventricular performance. Ultimately, ventricular remodeling may result in diminished contractile (systolic) function and reduced stroke volume.[5] Cardiac myocyte is the major cell involved in the remodeling. Fibroblasts, collagen, the interstitium, and the coronary vessels to a lesser extent also play a role.[6] The aldosterone extracted from the infarcted myocardium could stimulate cardiomyocyte apoptosis and promote myocardial interstitial and perivascular fibrosis. Aldosterone is thought to generate in the adrenal zone through the classical renin-angiotensin-aldosterone system, and it is also synthesized by myocardial RAAS itself, including infarcted myocardium.[7,8] Meanwhile, angiotensin II could promote the synthesis of aldosterone in the myocardium. Some studies[9,10] demonstrated that the myocaridium could uptake aldosterone to induce ventricular remodeling in the acute phase of myocadial infarction. Theoretically, myocardial remodeling can be prevented by blocking all links of the RAAS pathway. After an acute fall of aldosterone in response to administration of an ACE inhibitor or ARB, the level of aldosterone rises again, a phenomenon known as ’aldosterone escape’.[11] The combination of aldosterone receptor antagonist with ACEI or ARB can prevent myocardial hypertrophy caused by aldosterone escape. It is significant for myocardial remodeling and the treatment of heart failure. In our study, patients in both groups were conventionally given with ACEI or ARB, and spironolactone was added in the treatment group, in which myocardial remodeling was inhibited significantly.
In this study, no significant difference was observed in echocardiographic results in both groups at one month, indicating that there was no obvious cardiac remodeling, which was ascribed to early revascularization after admission and standard treatment. But myocardial remodeling could be detected using echocardiography at one year. It is well known that aldosterone can directly induce collagen production and promote the accumulation of extracellular matrix and fibrosis by balancing the synthesis and degradation of matrix collagens.[12] The infarcted myocardial scarring completes at 2 months after AMI. However, because of the phenomenon of “aldosterone escape”, plasma aldosterone in 3-month use of AECI/ARB increases again to promote cardiac remodeling further. In this study, the echocardiographic results including LVEDD, LVPWT, and LVEF at one year were obviously different from the results at one month in the treatment group, and there was a significant difference between the two groups and within each group (P<0.05). There was also a significant difference in LVEF before and after treatment in the control group (P=0.0277), possibly because of the application of ACEI/ARB, β-blocker and statins. While the results in the treatment group were more significant than in the control group (P=0.007). This indicates that aldosterone antagonists could inhibit the late remodeling after AMI and long-term application of spironolactone is necessary after AMI.
Greco et al[13] mostly focused on the efficacy of aldosterone antagonists in preventing heart failure, and guidelines recommended spironolactone to be applied in heart failure patients with left ventricular ejection fraction <40% after the application of ACEI. The patients in the treatment group of our study were given with a small dose of spironolactone regularly, setting aside the LVEF. The results demonstrated that spironolactone inhibited significantly cardiac remodeling after AMI.
Ventricular remodeling could affect biatrium.[14] Spironolactone was found to improve the process of atrial remodeling after AMI in rats,[15] but little clinical data were available on the effect of aldosterone antagonists on atrial remodeling after AMI. The left atrium could play an important auxiliary pump function, which is significant to maintain cardiac output for patients with AMI.[16] Thus it is necessary to treat atrial remodeling after myocardial infarction. However, atrial remodeling depends on ventricular remodeling, especially in patients with severe heart failure. Although spironolactone plays a positive role in left ventricular dysfunction, there is little knowledge about its preventive effect on myocardial infarction.[17]
In a recent study,[18] 110 patients were treated with spironolactone. After a six-month follow-up, atrial remodeling after AMI was detected with echocardiography. The result indicated that spironolactone was less effective for left atrial remodeling and atrial electrical mechanical properties in AMI patients, and it was not recommended for the AMI patients with normal cardiac function.
In our study, atrial remodeling was detected with echocardiography in 528 STEMI patients. In the two groups, there was no statistical significance in right atrial measurements. In the treatment group, however, it was statistically significant in left remodeling. Hence, it is uncertain whether it is associated with primary cardiac function or follow-up duration, and further research is needed to clarify the mechanism.
Footnotes
Funding: The study was supported by a grant from Science and Technology Planning Project of Heilongjiang Province, China (GB08C402-01).
Ethical approval: The study was approved by the Ethical Committee of First Affiliated Hospital, Harbin Medical University, Harbin, China.
Competing interest: The authors declare that there is no conflict of interest.
Contributors: Wu CT proposed the study, analyzed the data and wrote the first drafts. All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
- 1.Furlan D, Sahnane N, Mazzoni M, Pastorino R, Carnevali I, Stefanoli M, et al. Diagnostic utility of MS-MLPA in DNA methylation profiling of adenocarcinomas and neuroendocrine carcinomas of the colon-rectum. Virchows Arch. 2012 Dec 9; doi: 10.1007/s00428-012-1348-2. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 2.Bousquet E, Zhao M, Ly A, Leroux Les Jardins G, Goldenberg B, et al. The aldosterone-mineralocorticoid receptor pathway exerts anti-inflammatory effects in endotoxin-induced uveitis. PLoS One. 2012;7:e49036. doi: 10.1371/journal.pone.0049036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roscioni SS, de Zeeuw D, Bakker SJ, Lambers Heerspink HJ. Management of hyperkalaemia consequent to mineralocorticoid-receptor antagonist therapy. Nat Rev Nephrol. 2012;8:691–699. doi: 10.1038/nrneph.2012.217. [DOI] [PubMed] [Google Scholar]
- 4.No authors listed. Myocardial infarction redefined – a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the Redefinition of Myocardial Infarction. Eur Heart J. 2000;21:1502–1513. doi: 10.1053/euhj.2000.2305. [DOI] [PubMed] [Google Scholar]
- 5.Li WX, Kong X, Zhang JX, Yang JR. Long-term intake of sesamin improves left ventricular remodelling in spontaneously hypertensive rats. Food Funct. 2012 Dec 14; doi: 10.1039/c2fo30220a. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Jugdutt BI. Matrix metalloproteinases as markers of adverse remodeling after myocardial infarction. Card Fail. 2006;12:73–76. doi: 10.1016/j.cardfail.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Vatankulu MA, Bacaksız A, Sonmez O, Alıhanoglu Y, Koc F, Demır K, et al. Does Spironolactone Have a Dose-Dependent Effect on Left Ventricular Remodeling in Patients with Preserved Left Ventricular Function After an Acute Myocardial Infarction? Cardiovasc Ther. 2012 Sep 11; doi: 10.1111/1755-5922.12006. [DOI] [PubMed] [Google Scholar]
- 8.Nagata K. Mineralocorticoid antagonism and cardiac hypertrophy. Curr Hypertens Rep. 2008;10:216–221. doi: 10.1007/s11906-008-0041-y. [DOI] [PubMed] [Google Scholar]
- 9.Fiebeler A, Schmidt F, Müller DN, Park JK, Dechend R, Bieringer M, et al. Mineralocorticoid receptor affects AP-1 and nuclear factor-kappab activation in angiotensin II-induced cardiac injury. Hypertension. 2001;37(2 Part 2):787–793. doi: 10.1161/01.hyp.37.2.787. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi M, Tsutamoto T, Wada A, Tsutsui T, Ishii C, Ohno K, et al. Immediate administration of mineralocorticoid receptor antagonist spironolactone prevents post-infarct left ventricular remodeling associated with suppression of a marker of acute myocardial collagen synthesis in patients with first anterior acute myocardial infarction. Circulation. 2003;107:2559–2265. doi: 10.1161/01.CIR.0000068340.96506.0F. [DOI] [PubMed] [Google Scholar]
- 11.Struthers AD. The clinical implications of aldosterone escape in congestive heart failure. Eur J Heart Fail. 2004;6:539–545. doi: 10.1016/j.ejheart.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Kawazu T, Nishino T, Obata Y, Furusu A, Miyazaki M, Abe K, et al. Production and degradation of extracellular matrix in reversible glomerular lesions in rat model of habu snake venom-induced glomerulonephritis. Med Mol Morphol. 2012;45:190–198. doi: 10.1007/s00795-011-0559-y. [DOI] [PubMed] [Google Scholar]
- 13.Greco C, Castelli G, Crea F, Gavazzi A, Gensini GF, Scherillo M, et al. New evidences on the use of aldosterone receptor antagonists in left ventricular dysfunction. From myocardial infarction to heart failure. G Ital Cardiol (Rome) 2012;13:809–816. doi: 10.1714/1188.13164. [DOI] [PubMed] [Google Scholar]
- 14.Giallauria F, Galizia G, Lucci R, D’Agostino M, Vitelli A, Maresca L, et al. Favourable effects of exercise-based cardiac rehabilitation after acute myocardial infarction on left atrial remodeling. Int J Cardiol. 2009;136:300–306. doi: 10.1016/j.ijcard.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 15.Milliez P, Deangelis N, Rucker-Martin C, Leenhardt A, Vicaut E, Robidel E, et al. Spironolactone reduces fibrosis of dilated atria during heart failure in rats with myocardial infarction. Eur Heart J. 2005;26:2193–2199. doi: 10.1093/eurheartj/ehi478. [DOI] [PubMed] [Google Scholar]
- 16.Danzmann LC, Bodanese LC, Köhler I, Torres MR. Left atrioventricular remodeling in the assessment of the left ventricle diastolic function in Patients with heart failure: a review of the currently studied echocardiographic variables. Cardiovasc Ultrasound. 2008;6:56. doi: 10.1186/1476-7120-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savoye C, Equine O, Tricot O, Nugue O, Segrestin B, Sautière K, et al. Left ventricular remodeling after anterior wall acute myocardial infarction in modern clinical practice (from the REmodelage VEntriculaire [REVE] study group) Am J Cardiol. 2006;98:1144–1149. doi: 10.1016/j.amjcard.2006.06.011. Epub 2006 Aug 31. [DOI] [PubMed] [Google Scholar]
- 18.Kayrak M, Bacaksiz A, Vatankulu MA, Ayhan SS, Ari H, Kaya Z, et al. The effects of spironolactone on atrial remodeling in patients with preserved left ventricular function after an acute myocardial infarction: a randomized follow-up study. Coron Artery Dis. 2010;21:477–485. doi: 10.1097/MCA.0b013e32833fd243. [DOI] [PubMed] [Google Scholar]


