Abstract
BACKGROUND:
Current studies on CD62P have focused mainly on cardiovascular diseases, while only few studies have evaluated the effects of CD62P on the development of sepsis and the association between endothelial cell injury with inflammation and coagulation. This study attended to explore the association between endothelial cell injury with inflammation and coagulation by evaluating the expression of soluble CD62P (s-CD62P) in plasma and its mechanism in patients with sepsis, thus to provide the evidence of effective treatment of sepsis with anti-adhesion therapy targeted CD62P.
METHODS:
A total of 70 critically ill patients with systemic inflammatory response syndrome (SIRS) admitted to intensive care unit (ICU) between September 2009 and February 2010 were enrolled for a prospective and control study. According to the diagnostic criteria of sepsis/SIRS, the patients were divided into two groups: a sepsis group (n=38) and a SIRS group (n=32). Another 20 healthy volunteers served as a control group. Patients in the sepsis group and SIRS group were matched by clinical signs of high blood pressure, diabetes and its complications. The demographics of the patients including age, sex, body mass index (BMI), smoking and alcohol addict were compared among the groups. Six mL peripheral blood samples were collected within 24-hour admission in ICU for enzymelinked immunosorbent assay (ELISA) to detect the plasma levels of s-CD62P, TNF-α, and hs-CRP. And variables of coagulation function such as platelet (PLT), prothrombin (PT), activated partial thromboplastin time (APTT), D-dimer and antithrombin-III (AT-III) were analyzed during 24 hours after admission to ICU. Meanwhile sequential organ failure assessment (SOFA) score of critically ill patients was evaluated. Data were expressed as mean±standard deviation and were statistically analyzed by using SPSS 17.0 statistical software. The differences in plasma levels of s-CD62P of patients in each group were analyzed by ANOVA and the Kruskal-Wallis test. The relations between s-CD62P and inflammatory cytokines as well as with coagulation were determined by Pearson’s product moment correlation coefficient analysis. Changes were considered as statistically significant if P value was less than 0.05.
RESULTS:
Compared with the control group and SIRS group, the sepsis group demonstrated significantly higher levels of s-CD62P, TNF-α and highly sensitive C-reactive protein (hs-CRP) (P<0.05). The plasma levels of D-dimer, PT, and APTT in the sepsis and SIRS groups were significantly higher than those in the control group, while the platelet count and the activity of AT-III were obviously lower (P<0.05). In the sepsis group, the plasma levels of hs-CRP and TNF-α were positively correlated with PT, APTT, and D-dimer, and negatively correlated with AT-III and PLT (P<0.05). The plasma levels of s-CD62P were significantly correlated with the plasma levels of TNF-α, hs-CRP, D-dimer, PT, and APTT, whereas they were correlated negatively well with PLT and AT-III (P<0.05).
CONCLUSIONS:
The concentration of plasma s-CD62P is elevated as a early biomarker in patients with sepsis, and it serves as one of the pathogenic factors responsible for endothelial cell damage. Coagulation and mediators of inflammation promote each other, aggravating the severity of sepsis. Plasma s-CD62P may be an important factor for the development of coagulation and inflammatory reaction.
KEY WORDS: Sepsis, Endothelial cell injury, Plasma soluble CD62P, Inflammatory cytokine, Coagulation
INTRODUCTION
Currently, sepsis is considered as a disease process involving progressive endothelial cell injury resulting from disorders of systemic inflammatory and antiinflammatory balance, activation of leukocyte and platelet, damage of the coagulation system, which ultimately induces multiple organ failure.[1] Granule membrane protein (GMP) CD62P, as an important member of the selectin family of adhesion molecules, is a marker of endothelial cell injury and platelet activation,[2] as well as an important component for triggering and maintaining inflammatory responses. Current studies on CD62P have mainly focused on cardiovascular diseases, whereas few studies have evaluated the effects of CD62P on the development of sepsis and the association between endothelial cell injury with inflammation and coagulation. This study aimed to explore the association between endothelial cell injury with inflammation and coagulation by evaluating the expression of soluble CD62P (s-CD62P) in plasma and its mechanism in patients with sepsis, thus providing the evidence of effective treatment of sepsis with anti-adhesion therapy targeted CD62P.
METHODS
Patients
Seventy patients with systemic inflammatory response syndrome (SIRS), aged 49.1±19.1 years, 54 males and 16 females, admitted to the intensive care unit (ICU) of the General Hospital of Ningxia Medical University from September 2009 to February 2010, were included in this study. Thirty eight patients were diagnosed with sepsis (sepsis group), with primary diseases of trauma in 19 patients, pulmonary infection in 5 patients, and abdominal surgery complicated with critical illness in 12 patients. Among the 32 SIRS patients (SIRS group), primary disease contained trauma in 17 patients, abdominal surgery complicated with critical illness in 10 patients, and selective surgery in 5 patients. Another 20 healthy subjects who underwent physical examination in our hospital served as the control group.
The percentages of patients with hypertension, diabetes and complications in the sepsis and SIRS groups were similar (P>0.05). Also, there were no significant differences in sex, age, smoking, drinking, and clinical characteristics including body mass index (BMI) (P>0.05 for all) between the three groups (Table 1).
Table 1.
General characteristics between the sepsis, SIRS and control groups
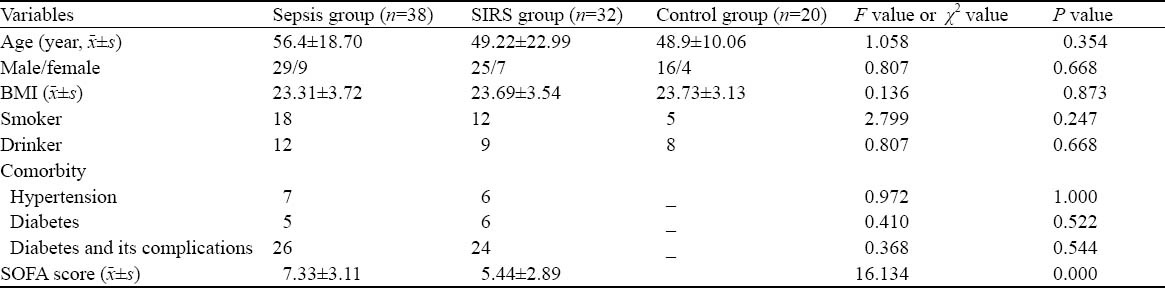
Patients with chronic diseases or dysfunction of liver (cirrhosis, portal hypertension), kidney (longterm dialysis) or patients with immune suppression due to immunosuppressive therapy, radio-chemotherapy, leukemia, lymphoma, and acquired immunodeficiency syndrome (AIDS) were excluded from the study. Moreover, patients allergic to anticoagulant drugs, patients with malignancy, peripartum patients, and patients younger than 14 years old were all excluded from the study.
This study was approved by the Ethics Committee of the General Hospital of Ningxia Medical University and informed consents were obtained from all subjects before enrollment.
Laboratory examination
Clinical data of the patients were collected to assess the Sequential Organ Failure Assessment (SOFA) score. Six mL fasting venous blood was collected from the patients in the morning within 24 hours after admission to the ICU and was injected equally into two negative pressure vacuum anticoagulant tubes containing 0.109 mmol/L sodium citrate to obtain a mixture of natrium citricum/whole blood at a ratio of 1:9. The tubes were centrifuged for 10 minutes at 3 000 r/min after mixing. The separated plasma obtained from tube 1 was stored at –80 ºC and ready for examination in batch to measure the levels of s-CD62P and TNF-α by double-sandwich ABC enzyme-linked immunosorbent assay (ELISA) with kits (U.S. R&D company) according to the instructions. The separated plasma obtained from tube 2 was immediately used to measure the coagulation markers which could be stored at room temperature for no more than 2 hours or at 2–8 ºC for no more than 4 hours.
Statistical analysis
Quantitative data were presented as mean±SD. Non-normal distribution data were presented as median. Student’s t test was used for comparison of two groups, variance analysis for comparison of multiple groups, nonparametric rank-sum test (i.e. the Kruskal-Wallis test) for non-paired data with heterogeneous variance, Pearson’s product-moment correlation coefficient test for data from normal distribution to assess the relationship. Two-tailed P<0.05 was considered statistically significant. All statistical analysis was performed using SPSS software (version 17.0).
RESULTS
Plasma level of s-CD62P
The s-CD62P levels of patients in the sepsis group were significantly higher (P<0.05). The s-CD62P levels of patients in the SIRS group increased with the severity of inflammatory response compared to those of patients in the normal control group as shown by mean value, although there was no significant difference between them (P>0.05) (Table 2).
Table 2.
Comparison of s-CD62P, hs-CRP, and TNF-α between the sepsis group, SIRS group, and control group (mean±SD)
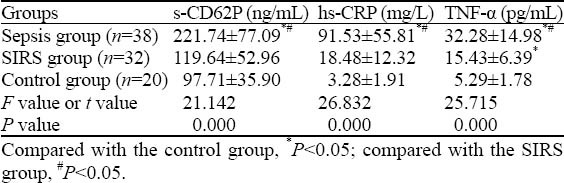
Plasma levels of hs-CRP, TNF-α and SOFA score
The levels of hs-CRP and TNF-α in patients of the sepsis group were significantly higher (P<0.05). Also, the SOFA score of the sepsis group was significantly higher than that of the SIRS group (P<0.05) (Table 2).
Count of platelet, PT, APTT, level of D-dimer and activity of AT-III
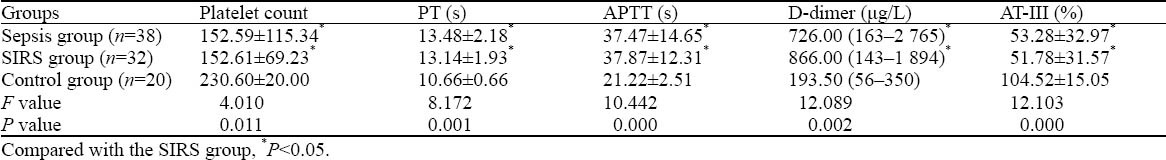
The patients in the sepsis group and SIRS group showed a higher level of D-dimer and longer PT and APTT (P<0.05); but they had significantly decreased platelet count and AT-III activity (P<0.05) (Table 3).
Table 3.
Comparison of platelet count, PT, APTT, D-dimer, and AT-III between the sepsis group, SIRS group, and control
Relationship of inflammatory responses with coagulation function/markers
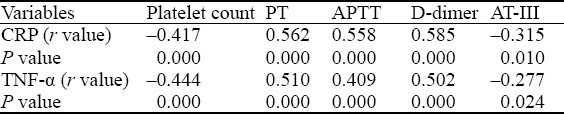
The levels of hs-CRP and TNF-α were positively correlated with D-dimer level, PT, and APTT in patients with critical illness respectively, and were negatively correlated with platelet count and AT-III activity (Table 4).
Table 4.
The correlation between plasma levels of inflammatory factor and coagulation indexes Variables Platelet count PT APTT D-dimer AT-III
Relationship of plasma level of s-CD62P with inflammatory factor and coagulate markers
There was a significant positive relationship between the plasma level of s-CD62P and the levels of hs-CRP and TNF-α in patients with critical illness (P<0.05) (Table 5). Similarly, the level of s-CD62P was positively correlated with D-dimer level, PT, and APTT, respectively (P<0.05), and was negatively correlated with platelet count and AT-III activity (P<0.05).
Table 5.
The correlation between plasma levels of s-CD62P, inflammatory factor and coagulation indexes

DISCUSSION
An increasing amount of evidence has shown that endothelial cell injury and activation of platelets induced by the interactions of leukocyte with endothelial cells or platelets play an important role in sepsis development. Activated endothelial cells or platelets induced by tissue hypoxia and/or stimulation of inflammatory mediators first expressed GMP CD62P, mainly found in Weibel– Palade bodies of endothelial cells and α-granules of resting platelet, which can mediate initial endothelial/ platelet-leukocyte adhesion by binding with P-selectin glycoprotein ligand-1 (PSGL-1) on the surface of these cells and also involve in adhesion and immigration cascades of leukocyte on the surface of the endothelium.[3,4] Then, endothelial cell injury may be induced by secretion of myeloperoxidase, cathepsin G, elastase, and generation of reactive oxygen species of neutrophils after adhesion,[5,6] which could be indirectly evaluated by measuring the levels of these indicators or their metabolic products in the laboratory.[7]
The results of this study demonstrated that the plasma level of s-CD62P of the sepsis group was significantly higher than that of the SIRS group and normal control group. The s-CD62P levels of patients in the SIRS group increased with the severity of SIRS compared with those of patients in the normal control group as shown by mean values, although there was no significant difference between them. This indicated that CD62P was involved in inflammatory response and mediated the development of sepsis, and could be considered as an early biomarker of endothelial cell injury. It has been shown that endothelial cell injury characterized by significantly increased adhesion molecule of α-granule membrane protein 140 (i.e. CD62P) occurred simultaneously with the development of coagulation/fibrinolysis disorders in patients with critical illness.[8] Clinically, endothelial cell injury can be detected by combined measurement of several biomarkers of endothelial injury including circulating endothelial cells (cECs), endothelial cell specific molecule-1 (ESM-1), adhesion molecule of CD62P and E-selectin, soluble thrombomodulin (sTM), angiopoietin-2 (Ang-2).[9] However, it is difficult to distinguish endothelial cell injury caused by stimulating endothelial cells from vascular endothelial dysfunction caused with detection of these biomarkers, so the application of endothelium-derived active substances measurement in clinical practice has certain limitations.
This study showed that patients in the sepsis and SIRS groups had significantly increased D-dimer level, and prolonged PT and APTT, but significantly decreased AT-III activity compared to the normal control group. Levi et al[10] also confirmed a decreased AT level during severe inflammatory reactions. These results indicated that inflammation would induce the activation of the coagulation system as well as down regulation of physiological anticoagulant mechanisms and inhibition of the fibrinolytic system with the development of sepsis.[11] In addition, our data showed that D-dimer, AT-III, PT and APTT were all significantly related with TNF-α and hs-CRP in patients with critical illness. Plessier et al[12] reported the severity of coagulation dysfunction was closely related to the severity of sepsis in patients with infection. These results further confirmed the close relationship between coagulation system and inflammatory response, the interaction of inflammation and coagulation/fibrinolysis, which will promote the development of sepsis in combination.[13]
The results of our study indicated that CD62P is not only involved in inflammatory response by its relationship with hs-CRP and TNF-α, but also involved in platelet activation and subsequent widespread microthrombosis in microvessels via its relationship with D-dimer, AT-III activity, PT and APTT and that CD62P is an important initiating factor for inflammatory response and coagulation/fibrinolysis dysfunction in sepsis. Mosad et al[14] found in 176 sepsis patients complicated with DIC, CD62P level was positively correlated with the level of DIC, fibrinogen consumption, D-dimer, and markers of thrombin activation. CD62P can not only initiate the inflammatory cascade but also trigger the coagulation cascade. CD62P may initiate the coagulation process by mediating the adhesion of platelets with endothelial cells and leukocytes, which subsequently activate nuclear factor κB (NF-κB) and increase the expression and release of tissue factor (TF) of monocytes, and by subsequent fusion with the membrane of platelets[15] and induce a hypercoagulable state by activating the extrinsic coagulation pathway to produce fibrin.[16] Activated leukocytes can accelerate thrombosis by releasing cathepsin and elastase, hydrolyzing tissue factor pathway inhibitor (TFPI), and further activating platelets and coagulation factor V, thus releasing reactive oxygen species and leading to direct or indirect activation or damage of vascular endothelium.[16] Currently, the role of CD62P in inflammatory response/coagulation and organ dysfunction in sepsis has been a research topic. Animal experiments found that endothelial injury could increase the expression of adhesion molecules including CD62P, resulting in widespread microvascular inflammation and thrombosis as well as increased mortality of sepsis.[2]
In conclusion, endothelial injury, inflammatory response, and coagulation dysfunction are involved in the whole process of sepsis development, and have interaction with each other triggered by a variety of factors. CD62P and its ligand play an important role in the development of sepsis and organ dysfunction. In an animal study, therapies against CD62P or blocking PSGL-1 were demonstrated to be able to improve organ function and prognosis; simvastatin could reduce the leukocyte aggregation and endotoxemia-induced liver damage by indirect decrease of P-selectin expression.[17] Further studies are needed on the relationship between sepsis, CD62P and clinical treatment, and anti-adhesion therapy targeted on CD62P will provide a new approach to prevent and treat sepsis and complicated organ dysfunction.
Footnotes
Funding: None.
Ethical approval: This study was approved by the Ethics Committeeof the General Hospital of Ningxia Medical University; informedconsents were obtained from all subjects before enrollment.
Conflicts of interest: None.
Contributors: Ding H proposed the study, analyzed the data and wrote the first draft. All authors contributed to the design and interpretation of the study and to further drafts.
REFERENCES
- 1.Hoesel LM, Gao H, Ward PA. New insights into cellularmechanisms during sepsis. Immunol Res. 2006;34:133–141. doi: 10.1385/IR:34:2:133. [DOI] [PubMed] [Google Scholar]
- 2.Vachharajani V, Russell JM, Scott KL, Conrad S, Stokes KY, Tallam L, et al. Obesity exacerbates sepsis-induced inflammation and microvascular dysfunction in mouse brain. Microcirculation. 2005;12:183–194. doi: 10.1080/10739680590904982. [DOI] [PubMed] [Google Scholar]
- 3.Davì G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 4.Yang J, Furie BC, Furie B. The biology of P-selectin glycoprotein ligand-1: its role as a selectin counterreceptor in leukocyte-endothelial and leukocyte-platelet interaction. Thromb Haemost. 1999;81:1–7. [PubMed] [Google Scholar]
- 5.Zhou SH, Sun YF, Wang G. Effects of hyperbaric oxygen on intestinal mucosa apoptosis caused by ischemia-reperfusion injury in rats. World J Emerg Med. 2012;3:135–140. doi: 10.5847/wjem.j.issn.1920-8642.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bevilacqua MP. Endothelial-leukocyte adbesion molecules. Annu Rev Immunol. 1993;11:767–804. doi: 10.1146/annurev.iy.11.040193.004003. [DOI] [PubMed] [Google Scholar]
- 7.Martínez-Dolz L, Almenar L, Reganon E, Vila V, Chamorro C, Andrés L, et al. Follow-up study on the utility of von Willebrand factor levels in the diagnosis of cardiac allograft vasculopathy. J Heart Lung Transplant. 2008;27:760–766. doi: 10.1016/j.healun.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 8.King DR, Namias N, Andrews DM. Coagulation abnormalities following thermal injury. Blood Coagul Fibrinolysis. 2010;21:666–669. doi: 10.1097/MBC.0b013e32833ceb08. [DOI] [PubMed] [Google Scholar]
- 9.Reinhart K, Bayer O, Brunkhorst F, Meisner M. Markers of endothelial damage in organ dysfunction and sepsis. Crit Care Med. 2002;30:S302–312. doi: 10.1097/00003246-200205001-00021. [DOI] [PubMed] [Google Scholar]
- 10.Levi M, van der Poll T, Büller HR. Bidirectional relation between inflammation and coagulation. Circulation. 2004;109:2698–2704. doi: 10.1161/01.CIR.0000131660.51520.9A. [DOI] [PubMed] [Google Scholar]
- 11.Qiu QM, Li ZW, Tang LM, Sun Q, Lu ZQ, Liang H, et al. Expression of high mobility group protein B1 in the lungs of rats with sepsis. World J Emerg Med. 2011;2:302–306. doi: 10.5847/wjem.j.1920-8642.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plessier A, Denninger MH, Consigny Y, Pessione F, Francoz C, Durand F, et al. Coagulation disorders in patients with cirrhosis and severe sepsis. Liver Int. 2003;23:440–448. doi: 10.1111/j.1478-3231.2003.00870.x. [DOI] [PubMed] [Google Scholar]
- 13.Dhainaut JF, Shorr AF, Macias WL, Kollef MJ, Levi M, Reinhart K, et al. Dynamic evolution of coagulopathy in the first day of severe sepsis: relationship with mortality and organ failure. Crit Care Med. 2005;33:341–348. doi: 10.1097/01.ccm.0000153520.31562.48. [DOI] [PubMed] [Google Scholar]
- 14.Mosad E, Elsayh KI, Eltayeb AA. Tissue factor pathway inhibitor and P-selectin as markers of sepsis-induced non-overt disseminated intravascular coagulopathy. Clin Appl Thromb Hemost. 2011;17:80–87. doi: 10.1177/1076029609344981. [DOI] [PubMed] [Google Scholar]
- 15.Levi M, Van der Poll T. Two-way interactions between inflammation and coagulation. Trends Cardiovasc Med. 2005;15:254–259. doi: 10.1016/j.tcm.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Palabrica T, Lobb R, Furie BC, Aronovitz M, Benjamin C, Hsu YM, et al. Leukocyte accumulation promoting fibrin deposition is mediated in vivo by P-selectin on adherent platelets. Nature. 1992;359:848–851. doi: 10.1038/359848a0. [DOI] [PubMed] [Google Scholar]
- 17.Slotta JE, Laschke MW, Schilling MK, Menger MD, Jeppsson B, Thorlacius H. Simvastatin attenuates hepatic sensitization to lipopolysaccharide after partial hepatectomy. J Surg Res. 2010;162:184–192. doi: 10.1016/j.jss.2009.03.057. [DOI] [PubMed] [Google Scholar]


