Abstract
BACKGROUND:
Intravenous transplantation has been regarded as a most safe method in stem cell therapies. There is evidence showing the homing of bone marrow stem cells (BMSCs) into the injured sites, and thus these cells can be used in the treatment of acute myocardial infarction (MI). This study aimed to investigate the effect of intravenous and epicardial transplantion of BMSCs on myocardial infarction size in a rabbit model.
METHODS:
A total of 60 New Zealand rabbits were randomly divided into three groups: control group, epicardium group (group I) and ear vein group (group II). The BMSCs were collected from the tibial plateau in group I and group II, cultured and labeled. In the three groups, rabbits underwent thoracotomy and ligation of the middle left anterior descending artery. The elevation of ST segment >0.2 mV lasting for 30 minutes on the lead II and III of electrocardiogram suggested successful introduction of myocardial infarction. Two weeks after myocardial infarction, rabbits in group I were treated with autogenous BMSCs at the infarct region and those in group II received intravenous transplantation of BMSCs. In the control group, rabbits were treated with PBS following thoracotomy. Four weeks after myocardial infarction, the heart was collected from all rabbits and the infarct size was calculated. The heart was cut into sections followed by HE staining and calculation of infarct size with an image system.
RESULTS:
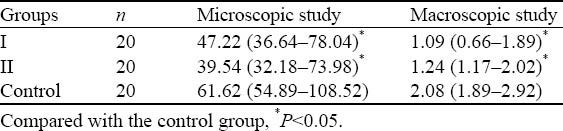
In groups I and II, the infarct size was significantly reduced after transplantation with BMSCs when compared with the control group (P<0.05). However, there was no significant difference in the infarct size between groups I and II (P>0.05).
CONCLUSION:
Transplantation of BMSCs has therapeutic effect on MI. Moreover, epicardial and intravenous transplantation of BMSCs has comparable therapeutic efficacy on myocardial infarction.
KEY WORDS: Bone marrow stem cells, Acute myocardial infarction, Epicardial transplantation, Intravenous transplantation, Infarct size, Rabbit
INTRODUCTION
Acute myocardial infarction (MI) is a common disease, but it is a dangerous illness with high mortality. The clinical treatments of MI include conservative therapy, thrombolytic therapy, interventional therapy, coronary artery bypass grafting, heart transplantation and others. The former four treatments can’t repair the necrotic myocardium. Although the transplanted heart can replace the injured heart, the source is scare. Apart from immune rejection, high medical cost and ethical issues are practical in heart transplantation. Thus, heart transplantation is extremely limited in clinical practice. In recent years, bone marrow stem cells (BMSCs) in the treatment of MI have been extensively investigated.
BMSCs can be transplanted via the epicardium, endocardium, coronary artery or veins.[1] Intravenous transplantation of BMSCs has been regarded as a most safe method. There is evidence showing the homing of BMSCs into the injured sites,[2–4] and thus these cells can be used in the treatment of acute MI. In the present study, epicardial and intravenous transplantation of BMSCs was performed to treat acute MI in a rabbit model, and the survival and differentiation of these BMSCs were observed. At the same time, the infarct size was measured macroscopically and microscopically to compare the therapeutic efficacy of BMSCs transplantation.
METHODS
Experimental animals
A total of 60 healthy New Zealand rabbits, aged 2–4 months, weight 2–3 kg, were purchased from the Experimental Animal Center of Dalian Medical University.
Reagents
Fetal bovine serum (FBS; PAA, Germany), low-glucose DMEM (Hyclone, USA), trypsin, mouse monoclonal antibody (GIBCO, Alstralia), BrdU (Sigma, USA), lymphocyte separation solution (Tianjin Xinhaoyang Biotech Co., Ltd) and secondary antibodies (Zhongshan Golden Bridge Biotech Co., Ltd) were used in the present study.
Grouping
The 60 New Zealand rabbits were randomly assigned into three groups (n=20 per group): control group, epicardium group (group I), and ear vein group (group II). In group I, rabbits received epicardial transplantation of BMSCs; in group II, rabbits were treated by transplantation of BMSCs via the ear vein.
Preparation of autologous BMSCs
The rabbits were anesthetized with 3% sodium pentobarbital via the ear vein. Following local anesthesia with 1% lidocaine, bone marrow aspiration was done at the tibial plateau of the knee with a 10 mL syringe which was connected to a bone marrow puncture needle and contained 1 mL of heparin and 1 mL of complete medium. A total of 8 mL of bone marrow was aspired and then mixed with PBS at a volume ratio of 1:1. The mixture was added to the lymphocyte separation solution at a volume ratio of 2:1. Centrifugation was done at 3 000 r/min for 20 minutes. The cloudy layer (lymphocytes) was harvested and washed in serum-free DMEM twice by centrifugation at 1000 r/min for 10 minutes. The cells were subsequently suspended in complete medium. The cell suspension of appropriate volume was diluted and mixed with 1 mL of 0.4% trypan blue for detection of the cell viability. The cell density was adjusted to 105/L and seeded into a 25 cm2 flask followed by incubation in complete medium in a humidified environment with 5% CO2 at 37 ºC (HEPA Class 100, Thermo).
Culture and passaging of BMSCs
The BMSCs were cultured for 5 days and then observed under an inverted microscope (OLYMPUS, Japan). When 90% of cells were spindle-shaped and adherent to the wall, the medium was refreshed completely and thereafter once every 3 days. At 2 weeks after culture, when the cell confluence reached about 90%, 0.25% trypsin and EDTA were added followed by digestion at 37 ºC for 1–2 minutes. When the cells became round and shedding under a light microscope, complete medium was added to stop the digestion. Then, the cells were washed in D-Hanks twice and cell suspension was prepared. Passaging was done at a ratio of 1:3 (P1). The cells of passage 3 (P3) were used in the following experiments.
Establishment of acute MI animal model
Rabbits were anesthetized with 3% sodium pentobarbital at 1 mL/kg via the ear vein and electrocardiography was performed. The electrocardiogram in lead II was recorded. Then, tracheotomy and mechanical ventilation (DW2000 ventilator; Shanghai Jiapeng Tech Co., Ltd) were performed. The animals were fixed on a table, the hair was removed and the skin was sterilized. An incision was made at the left 2nd intercostal space which was then opened. The pericardium was open to expose the heart. Ligation was made at the middle left anterior descending coronary artery (ADCA). The elevation of ST segment of >0.2 mV for longer than 30 minutes in lead II and III and the myocardium supported by the ADCA becoming dark red suggested the successful establishment of myocardial infarction. Post-operatively, the animals were intramuscularly treated with penicillin for infection prophylaxis for 3 days.
Labeling and detection of BMSCs
When the confluence of BMSCs of P3 reached 70%– 80%, these cells were harvested, and used to prepare the cell suspension, and then seeded into coverslip-coated dishes. BMSCs were maintained in dishes. The coverslip was taken out of the dishes, washed in PBS, fixed in 4% paraformaldehyde and washed in PBS. Following treatment with 2 mol/L HCL at room temperature, the coverslips were washed in PBS and treated with 0.3% tritonx-100. After washing in PBS, these coverslips were treated with 3% H2O2 in deionized water. After washing in PBS, the coverslips were blocked in normal goat serum and then with primary antibody (1:100) at 4 ºC overnight. After washing in PBS, these coverslips were incubated with biotin conjugated secondary antibody. Following washing in PBS, mounting was performed. Cells were observed under an immunofluorescence microscope (OLYMPUS, Japan) at a magnification of 200 times. The staining intensity of nuclei was determined. Five fields were randomly selected from each section, and the number of positive cells (green fluorescence) and negative cells was counted.
Transplantation of BMSCs
Two weeks after MI, BMSCs were used to prepare the cell suspension. In the control group, thoracotomy was performed, and PBS (30 μL) was injected at the epicardium. In group I, thoracotomy was performed, and BMSCs were transplanted at the upper, middle and lower parts of the anterior and posterior edge of infarct region (30 μL; 5×106/100 μL). In group II, BMSCs were transplanted via the ear vein (30 μL; 5×106/100 μL).
Determination of infarct size
Four weeks after transplantation, the heart was collected and the infarct size was calculated. First, the macroscopic infarct size was calculated. Then, the heart was frozen and cut into sections (6 mm) followed by HE staining. The sections were adherent to the slides which were treated with Harris hematoxylin for 5 minutes, washed in water for 1 minute, treated with 75% ethanol for 30 seconds, rinsed in water, treated with ammonia for 30 seconds, washed in water for 1 minute, treated with acidified eosin in ethanol for 12 minutes and rapidly washed in water. After dehydration in ethanol and transparentization in xylene, mounting was done with neutral gum. The infarct size was determined under a microscope with an image analysis system (Table 1).
Table 1.
Infarct size in different groups
Statistical analysis
Statistical analysis was made with SPSS version 17.0. Data were expressed as median and range interquartile (25th interquartile, 75th interquartile). Comparisons were done with rank-sum test and a value of P<0.05 was considered statistically significant.
RESULTS
At 5 days after culture, cells were closely adherent to the wall of a flask and the cell confluence reached 80%–90%. Then, digestion for passaging and purification were done. Following passaging, the cells were further cultured for 3 days with cell confluence reaching about 100%, and they became spindle-shaped (Figure 1).
Figure 1.
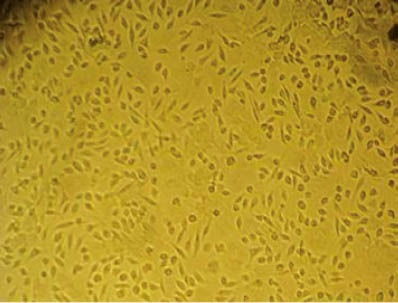
Bone marrow stem cells (original magnification×100).
BrdU staining and identification of BMSCs were performed. Results showed that the proportion of BMSCs was as high as 95% (Figure 2).
Figure 2.
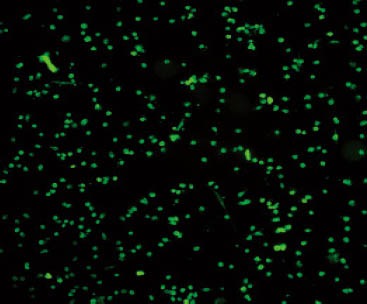
BrdU staining of bone marrow stem cells (original magnification×40).
Following MI, BMSCs was transplanted. Four weeks later, scars were found in the heart, dark grey could be easily differentiated from the surrounding normal tissues. However, the borderline between infarct size and normal tissues was blurred. HE staining of the heart specimens showed myocardial fibers were irregularly arranged and obvious borderline was found between infarct size and normal myocardium. In the infarct region, myocardial cells were absent or degraded and replaced with scars (Figures 3 and 4).
Figure 3.
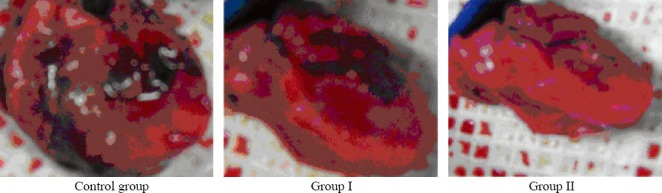
Macroscopic hearts in different groups.
Figure 4.

HE staining of the heart specimens in different groups.
DISCUSSION
Cardiovascular diseases, especially acute MI, have been considered as major causes of death in humans. Following acute MI, loss of myocardial cells, myocardial fibrosis, and scarring may take place. These changes are factors affecting heart function and the pathological basis of refractory heart failure.[5] Because of acute MI, the infarct myocardium enlarges or changes in shape, which may persistently influence the contractibility and electric activity of the ventricles and finally result in ventricular remodeling. To date, no effective treatments have been developed to treat the necrotic myocardium. Although pharmacotherapy, thrombolic therapy, interventional therapy and coronary artery bypass grafting can improve myocardial ischemia and heart failure, they have failed to repair necrotic myocardium and regenerate myocardial cells. Heart transplantation, scare source and immune rejection after heart transplantation have limited its wide application. Thus, to promote the regeneration of myocardial cells in the infarct region, to prevent ventricular remodeling and to improve the long-term life quality of patients with MI have become key points in the treatment of acute MI.
Transplantation of autogenous BMSCs has been a novel treatment of acute MI, which can regenerate myocardial cells in the infarct region. Soonpaa et al[6] transplanted mouse embryonic cardiomyocytes into adult mice with MI, and found the long-term survival of these embryonic cardiomyocytes. Moreover, the cardiac function of adult mice was markedly improved, and the cardiomyocytes formed intercalated discs with host cells. This finding suggests the regeneration of cardiomyocytes. The feasibility and advantages of transplantation of stem cells have been extensively investigated in the treatment of acute MI since the first study was published 20 years ago. The evidence included the following. 1) Myocardial microenvironment may support the growth of MSCs and can promote the differentiation of MSCs into myocardial cells.[7] Following transplantation, MSCs can migrate via the circulation to repair different interstitial tissues. Moreover, MSCs in different tissues may also express different tissue-specific proteins.[8] The special homing and the expression of tissue-specific proteins of these MSCs suggest that microenvironment plays an important role in the differentiation of MSCs. 2) The transplanted MSCs have favorable elasticity and thus may strengthen the stress of the ventricular wall, which significantly limits the over-extension of scar tissues after MI.[9] 3) Transplanted MSCs can differentiate normal myocardial cells and form new myocardial tissues with host cells,[10] which promotes the absorption of scar tissues. 4) MSCs, a group of multipotent stem cells, can be differentiated into the myocardium like tissues and vascular cells. The generation of vascular cells may promote angiogenesis in scar tissues after MI, while facilitating the survival and growth of newly generated myocardial cells.[11] In addition, transplanted MSCs can secret vascular endothelial growth factors promoting the angiogenesis.[12]
The mechanisms underlying the therapeutic effect of BMSCs on acute MI are as follows: 1) BMSCs can differentiate into cardiomyocyte-like cells which possess the ability to contract and express some contraction-related proteins. In addition, the newly generated myocardial cells can also form intercalated discs with normal myocardial cells involving in the synchronous contraction and reducing the area of scar tissues.[13] 2) The BMSCs can differentiate into vascular endothelial cells and smooth muscle cells.[14] Moreover, BMSCs can secret some cell active substances via the paracrine including fibroblast growth factor, vascular endothelial growth factor and stem cell homing factor.[15,16] These factors may further assist the establishment of collateral circulation and improve the myocardial perfusion. 3) The BMSCs can regulate the metabolism of extracellular matrix, which may inhibit the thickening of the infarct ventricular wall and the enlargement of the left ventricle, increase the local movement of the ventricular wall and improve the relaxation in the diastolic phase, which prevent the detrimental ventricular remodeling.[17] 4)The transplanted BMSCs in the heart can secret some substances which may inhibit the production of inflammatory cytokines such as tumor necrosis factor, interleukin-1 and interleukin-6 and suppress the accumulation of type I and II collagens, thus reducing the area of scar tissues.[18] These mechanisms are still in the exploratory stage, and the present study was to confirm the above mechanisms that the transplanted BMSCs could migrate into the infarct myocardium and differentiate into myocardial cells, which could further inhibit the enlargement of infarct region, reduce the infarct size, improve the heart function, and prevent the subsequent vascular remodeling. BMSCs can be transplanted via the epicardium, endocardium, coronary artery and veins. But the transplantation of BMSCs via the local vein is a new way. Theoretically, the transplantation of BMSCs via the vein is the most safe way, and can promote angiogenesis, increase local myocardial perfusion and strengthen left ventricular function.[19] Some investigators emphasized the safety of intravenous transplantation of BMSCs, the number of transplanted BMSCs and the mechanisms underlying the therapeutic effect of BMSC transplantation on MI. Following intravenous injection, the BMSCs can migrate into multiple organs and systems via the circulation. When there is lesion in a specific organ or system, the organ or system may generate signals for homing of stem cells. The BMSCs can differentiate into multiple cells. In the presence of above signals, the transplanted BMSCs migrate into the injured organs or system exerting effect. Thus, a lot of BMSCs, rather than a few BMSCs, migrate into the infarct myocardium. Epicardial transplantation of BMSCs has a precise location to transplant these stem cells into the infarct region. All BMSCs are injected into the infarct region, which assures the therapeutic efficacy. In the present study, the infarct size was measured to compare the therapeutic efficacy of epicardial and intravenous transplantation of MSCs. Our results demonstrated that intravenous transplantation of BMSCs had definite therapeutic effect on MI and had comparable therapeutic efficacy on the epicardial transplantation of BMSCs. Thus, we speculate that intravenous transplantation of BMSCs can replace the epicardial transplantation of BMSCs to treat cardiovascular diseases, especially MI.
It has been regarded that transplanted BMSCs can migrate into the infarct myocardium and differentiate into myocardial cells and blood vessels, which increase blood supply and reperfusion, prevent the enlargement of infarct size, promote the absorption of infarct myocardium and improve heart function. This is a major mechanism underlying the therapeutic effect of BMSC transplantation on acute MI. With the development of in-depth studies, some different views are proposed on the therapeutic effect of MSCs. Toma et al[20] proposed that paracrine and electric and mechanical stimulation of BMSCs play determinant roles in the differentiation of stem cells in the myocardium. However, Wang et al[21] proposed that myocardial tissues could provide an appropriate amount of matrix. At the same time, in the presence of specific growth factors and signals for the differentiation, transplanted BMSCs could differentiate into myocardial cells to improve the symptoms of MI. In addition, some investigators speculated that the improvement of heart function following BMSC transplantation is dependent on paracrine, but not the previously presumed differentiation of BMSCs into myocardial cells to regenerate myocardial cells. Thus, there is no consensus on the therapeutic effect of BMSCs on MI (improvement of myocardial ischemia and heart function). To find consistent mechanism may provide a breakthrough for the cell therapy of acute MI.
Previous animal experiments and ongoing clinical trials have demonstrated that BMSCs have definite therapeutic effect on acute MI. However, they only confirmed the short-term therapeutic effect of BMSCs. Thus, except the short-term improvement of heart function following BMSC transplantation, whether the therapeutic effect of BMSC transplantation may last for a long time is controversial. Yousef et al[22] published a study in which the patients with acute MI receiving treatment with BMSCs were followed up for 5 years. Their results showed treatment with BMSCs could effectively improve the left ventricular ejection fraction (LVEF), quality of life and reduce the mortality of patients with acute MI. A double blind, multicenter clinical trial from Germany[23] also revealed that coronary artery transplantation of BMSCs could reduce the incidence of major cardiovascular events and persistently improve heart function. Although intravenous transplantation of BMSCs is the most safe treatment with stem cells, traditionally intravenous transplantation of BMSCs had no therapeutic effect on MI, and transplanted BMSCs might be obstructed at different sites during migration in the circulation. However, our findings demonstrated that transplanted BMSCs could migrate into the injured site. Following intravenous transplantation of BMSCs, numerous cells migrated into the infarct myocardium exerting therapeutic effect.
Great progress has been made in the treatment of acute MI with BMSCs, but there are increasing issues. Further investigations on the application of BMSC transplantation in the treatment of MI may provide evidence for stem cell therapy as a novel strategy.
Footnotes
Funding: The study was supported by grants from the Scientific Research Plan Project of Liaoning Province (20092250096) and Scientific Research Plan Project of Dalian (2010E15SF178).
Ethical approval: The study was approved by the Animal Care and Use Committee of Dalian Central Hospital, Dlian, China.
Conflicts of interest: The authors declare that there is no conflict of interest.
Contributors: Ji LL designed the research, analyzed the data, and wrote the paper. All authors read and approved the final version.
REFERENCES
- 1.Rasmussen JG, Frøbert O, Holst-Hansen C, Kastrup J, Baandrup U, Zachar V, et al. Comparison of human adipose-derived stem cells and bone marrow-derived stem cells in a myocardial infarction model. Cell Transplant. 2012 Dec 4; doi: 10.3727/096368912X659871. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 2.Vulliet PR, Greeley M, Halloran SM, MacDonald KA, Kittleson MD. Intra-coronaryarterial injection of mesenchymal stromal cells and microinfarction in dogs. Lancet. 2004;363:783–784. doi: 10.1016/S0140-6736(04)15695-X. [DOI] [PubMed] [Google Scholar]
- 3.Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, et al. Systemic delivery of bone marrow— derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 4.Wang L, Li Y, Chen X, Chen J, Gautam SC, Xu Y, et al. MCP1,MIP-1,IL-8 and ischemic cerebral tissue enhance human bone marrow stromal cell migerationin interface culture. Hematology. 2002;7:113–117. doi: 10.1080/10245330290028588. [DOI] [PubMed] [Google Scholar]
- 5.Yang SM, Lo CM. Transition to computed radiography: can emergency medicine doctors accurately predict the need of film printing to facilitate optimal patient care? World J Emerg Med. 2011;2:33–37. [PMC free article] [PubMed] [Google Scholar]
- 6.Soonpaa MH, Koh GY, Klug MG, Field LJ. Formation of nascent in tercalated disks between grafted fetal cadiomyocytes and host myocardium. Science. 1994;264:98–101. doi: 10.1126/science.8140423. [DOI] [PubMed] [Google Scholar]
- 7.Gersh BJ, Simari RD, Behfar A, Terzic CM, Terzic A. Cardiac cell repair therapy: a clinical perspective. Mayo Clin Proc. 2009;84:876–892. doi: 10.4065/84.10.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horwitz EM. Stem cell plasticity: a new image of the bone marrow stem cell. Curr Opin Pediatr. 2008;15:32–37. doi: 10.1097/00008480-200302000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Kudo M, Wang Y, Wani MA, Xu M, Ayub A, Ashraf M. Implantation of bone marrow stem cells reduces the infarction and fibrosis in ischemic mouse heart. J Mol Cell Cardiol. 2003;35:1113–1119. doi: 10.1016/s0022-2828(03)00211-6. [DOI] [PubMed] [Google Scholar]
- 10.Chamsi Pasha H. Genetics and heart disease. Saudi Med J. 2003;24:1–18. [PubMed] [Google Scholar]
- 11.Davani S, Marandin A, Mersin N, Royer B, Kantelip B, Hervé P, et al. Mesenchymal progenitor cells differentiate into an endothelial phenotype, enhance vascular density,and improve heart function in a rat cellular cardiomyoplasty model. Circulation. 2003;108:253–258. doi: 10.1161/01.cir.0000089186.09692.fa. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Liang JL, Wang S, Wang H, Zheng XZ, Yang Z, et al. Autologous bone marrow stromal cell transplantation with application of granulocyte colony-stimulating factor improves rabbit cardiac performance after acute myocardial infarction. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27:43–5. 48. [PubMed] [Google Scholar]
- 13.Greenberg SB, Murphy GS, Vender JS. Current use of the pulmonary artery catheter. Curr Opin Crit Care. 2009;15:249–253. doi: 10.1097/MCC.0b013e32832b302b. [DOI] [PubMed] [Google Scholar]
- 14.Zhang HS, Fang JP, Su HB, Yang M. Culture in vitro and lentivirus transfection of rat mesenchymal stem cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2011;19:1472–1476. [PubMed] [Google Scholar]
- 15.Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996;166:585–592. doi: 10.1002/(SICI)1097-4652(199603)166:3<585::AID-JCP13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Tang YL, Zhao Q, Qin X, Shen L, Cheng L, Ge J, et al. Paracrine action enhances the efects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg. 2005;80:229–236. doi: 10.1016/j.athoracsur.2005.02.072. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Yang W, Wang GN, Li J, Li XR, Zhang J, et al. Circulating microRNAs, novel biomarkers of acute myocardial infarction: a systemic review. World J Emerg Med. 2012;3:257–260. doi: 10.5847/wjem.j.issn.1920-8642.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinnaird T, Stabile E, Burnett MS, Shou M, Lee CW, Barr S, et al. Local delivery of marrow-derived stromal cells augments collateral perfusion through pararin mechanisms. Circulation. 2004;109:1543–1749. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 19.Zeng R, Chen YC, Zeng Z, Liu WQ, Liu XX, Liu R, et al. Different angiogenesis effect of mini-TyrRS/mini-TrpRS by systemic administration of modified siRNAs in rats with acute myocardial infarction. Heart Vessels. 2010;25:324–332. doi: 10.1007/s00380-009-1200-z. [DOI] [PubMed] [Google Scholar]
- 20.Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate tocardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 21.Wang JS, Shum-Tim D, Chedrawy E, Chiu RC. The coronary delivery of marrow stromal cells for myocardial regeneration:pathophysiologic and therapeutic implications. J Thorac Cardiovasc Surg. 2001;122:699–705. doi: 10.1067/mtc.2001.116317. [DOI] [PubMed] [Google Scholar]
- 22.Yousef M, Schannwell CM, Köstering M, Zeus T, Brehm M, Strauer BE. The BALANCE Study: clinical benefit and long-term outcome after intracoronary autologous bone marrow cell transplantation in patients with acute myocardial infarction. J Am Coll Cardiol. 2009;53:2262–2269. doi: 10.1016/j.jacc.2009.02.051. [DOI] [PubMed] [Google Scholar]
- 23.Assmus B, Rolf A, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, et al. Clinical outcome 2 years after intrancoronary administration of bone marrow-derived progenitor cells in acute muocardial infarction. Circ Heart Fail. 2009;3:89–96. doi: 10.1161/CIRCHEARTFAILURE.108.843243. [DOI] [PubMed] [Google Scholar]


