Abstract
P-21 activated kinases, or PAKs, are serine–threonine kinases that serve a role in diverse biological functions and organ system diseases. Although PAK signaling has been the focus of many investigations, still our understanding of the role of PAK in inflammation is incomplete. This review consolidates what is known about PAK1 across several cell types, highlighting the role of PAK1 and PAK2 in inflammation in relation to NADPH oxidase activation. This review explores the physiological functions of PAK during inflammation, the role of PAK in several organ diseases with an emphasis on cardiovascular disease, and the PAK signaling pathway, including activators and targets of PAK. Also, we discuss PAK1 as a pharmacological anti-inflammatory target, explore the potentials and the limitations of the current pharmacological tools to regulate PAK1 activity during inflammation, and provide indications for future research. We conclude that a vast amount of evidence supports the idea that PAK is a central molecule in inflammatory signaling, thus making PAK1 itself a promising prospective pharmacological target.
Keywords: PAK1, PAK2, NADPH oxidase, Cardiac, Cytoskeletal dynamics, Cell migration
1. Introduction
P-21 activated kinases, or PAKs, are serine–threonine kinases activated by the small GTP binding proteins Cdc42 and Rac1 [1,2], and they serve a role in diverse biological functions and organ system diseases [3]. So far, the PAK isoforms identified in mammalian cells are characterized into group I (PAK1–3) and group II (PAK4–6). The first group of PAKs shares a high sequence homology and is highly evolutionarily conserved [3].
Originally discovered in brain tissue [4], PAKs are important regulators of the inflammatory response. To the best of our knowledge, only PAK1 and PAK2, but not PAK3, have been thus far associated with inflammation, immunity, and infective diseases [5–9]. Additionally, PAK1 and PAK2 are the two most abundantly expressed PAK isoforms in white blood cells, including T lymphocytes, neutrophils, macrophages, and mast cells [10–14]. One way in which PAK1 and PAK2 regulate the molecular mechanisms of inflammation is through activation of their downstream target nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase) in neutrophils [15,16]. In the above mentioned cell types, PAK1 is implicated in the regulation of NADPH oxidase activity through several direct and indirect mechanisms, which are described hereafter.
In this review, we describe the role of PAKs in inflammation, with an emphasis on PAK1 and PAK2. PAK signaling has been the focus of many investigations under many different experimental models and conditions. This review aims to piece together what is known about PAK1 across several cell types, highlighting the role of PAK1 and PAK2 in inflammation in relation to NADPH oxidase activation. Also, we will discuss the role of PAK1 as a therapeutic anti-inflammatory target, explore the potentials and the limitations of the current drugs that regulate PAK1 activity during inflammation, and provide indications for future research.
2. PAK structure and activation
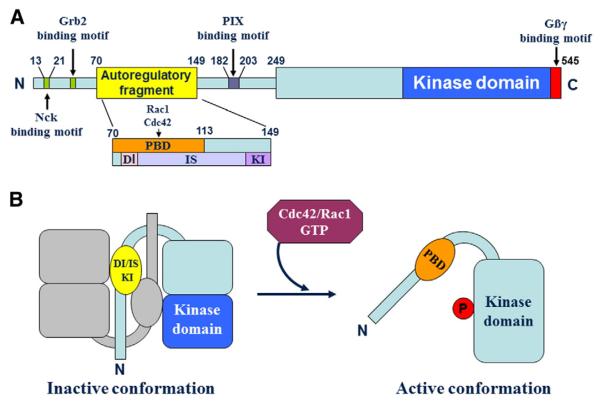
P21-activated kinases are a family of enzymes that are central in regulating intracellular signaling and cellular functions [13,17,18]. The actions of PAKs in the context of different cellular functions [19] are tightly regulated and are often achieved as a result of conformational changes in PAK molecular domains. Thus, a clear knowledge of the structure of PAKs is fundamental to understand their regulation and functions. In Fig. 1, we illustrate the primary structure of PAK1, and we show one of the most studied mechanisms of its activation.
Fig. 1.
PAK1 structure and mechanism of activation. PAK1 primary structure is illustrated in the top panel (A). The N-terminus of PAK1 structure is characterized by the autoregulatory fragment, as well as sites for the binding of Nck, Grb2, and PIX. The N-terminal end of PAK also comprises a series of well-conserved proline residues that are functionally important for PIX-mediated activation of PAK1. The autoregulatory fragment is a complex amino acid stretch that comprises the p21-binding domain (PBD) for Rac1 and Cdc42, as well as some functional sites, namely the dimerization segment (DI), the inhibitor switch domain (IS), and the kinase-inhibiting segment (KI). The C-terminus end of the PAK1 protein comprises the bilobal catalytic kinase domain. PAK1 quaternary structure is illustrated in the bottom panel (B). When inactive, PAK1 homodimerizes with another PAK1 protein, a dimeric complex that is stabilized in its inactive conformation by the KI segment, which prevents ATP from accessing its binding pocket in the PAK1 structure. The inactive state of PAK1 is further stabilized by the IS domain, which by interacting with the kinase domain, further stabilizes the interaction of the KI domain with the kinase domain. Following the binding of GTP-bound Cdc42/Rac1 association with PBD, the IS and the KI domains undergo a conformational change, which results in the activation of the kinase domain, uncoupling of the PAK1 dimer, and (auto)phosphorylation of PAK1, a series of events that leads to the stabilization of the monomeric active conformation. Figure adapted from [163,164].
The entire PAK structure is incompletely understood, although the crystal structure of PAK1 has been partly resolved. So far, it is known that the N-terminus of Group I PAKs shares a unique proline-rich motif located between amino acids 182–203, which is the binding site of the SH3 domain of PIX, an essential activator of PAK1/2/3 (Fig. 1 A). Thus far, only some parts of the auto-inhibitory domain, located in the N-terminal domain, and the kinase domain, located in the C-terminal domain, of some members of the PAK family have been elucidated [19].
One important key to understanding PAK structure and function is to understand its mechanism of activation. When in the inactive state, PAK1 homodimerizes with another PAK1 molecule through the dimerization segment (DI), the inhibitor switch domain (IS), the kinase-inhibiting segment (KI) domain, and an amino acid segment across the kinase domain (Fig. 1 B). The IS domain overlaps with the protein binding domain (PBD), which binds the GTPases Rac1/cdc42 and the auto-inhibitory domain (AID). The binding of GTPases to the PBD induces a conformational change in the kinase inhibitor (KI) fragment located within the AID. This is first followed by the dissociation of the IS from the kinase domain, then by PAK autophosphorylation of the T-loop, resulting in the activation of PAK1.
An ATP binding pocket (where ATP is the native ligand) exists and lies between the N-lobe and the C-lobe of the PAK1 kinase domain. This niche has been recently exploited to achieve pharmacological modulation of PAK1. A molecular analysis of the kinase domain structure using a Q-site finder technique has illustrated that molecular niches exist that could nest small pharmacological molecules. In fact, most PAK non-native ligands (e.g. 3Q53 [20], 3FXZ [21], 3FY0 [21], and 2HY8 [22]) bind to the ATP binding pocket, nurturing hopes that modulation of PAKs can be pharmacologically achieved.
Additional studies are required to fully understand the mechanism of PAK activation, especially across multiple organs and cell types. There remains a limited understanding of the effects of PAK regulation of the inflammatory process. A better understanding of both the role of PAK in multiple systems, as well as the relationship between the PAK structure and function, will provide the necessary knowledge to design activators or inhibitors that bind to PAK and modulate its functions in the inflammatory process.
3. Mechanisms of PAK activation
Besides activation via the small Rho GTPase Rac1, PAK1 can alternatively or synergistically be activated by other molecules, including membrane lipids [23] or receptor agonists [24]. Recently, we have understood that a vast number of important regulators of the inflamma-tory process act upon PAK1 as a downstream target and exert their effects through PAK1. In this section and in Fig. 2, we describe some recent advances in the understanding of signaling mechanisms involved in the activation of PAK during inflammation.
Fig. 2.
PAK1 at the center of inflammatory signaling. P-21 activated kinase 1 (PAK1) is targeted by upstream mediators of inflammation, such as advanced glycation end products (AGEs), high mobility group box 1 protein (HMGB1), receptor for advanced glycation endproducts (RAGE), and chemokine (C-X-C motif) ligand 1 (CXCL1). PAK1 targets downstream effectors, such as NADPH oxidase and extracellular-signal-regulated kinases 1/2 (ERK1/2), leading to a variety of pathological conditions, including cardiac dysfunction. Other abbreviations: protein kinase C alpha (PKCα), nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB), mitogen-activated protein kinase (MAPK), reactive oxygen species (ROS), chemokine (C-X-C motif) receptor 2 (CXCR2), and protein phosphatase 2a (PP2A). References: a [31], b [47], c [165], d [35], e [36], f [71], g [32], h [77], i [166], j [37], k [1], l [167], m [132], n [34], o [41].
3.1. Lipid-mediated and receptor tyrosine kinase-mediated activation of PAK1 and NADPH oxidase
Long chain sphingoid bases, sphingosine, phosphatidic acid, and phosphatidyl inositol produce stimulatory effects on PAK1 through a domain that is similar to the GTP ase binding domain [25]. On the contrary, other lipids, such as ceramide, have no effect on its activation [25]. These lipid-mediated stimulatory effects of PAK1 have been tested in vitro by measuring phosphorylation on a p47phox substrate. In in vivo systems, both sphingosine and sphingoid bases regulate phagocyte oxidant production [26]. Additionally, lipids have also been implicated in cytoskeletal remodeling [27,28], an important mechanism that facilitates the translocation of the cytosolic subunits of the NADPH oxidase to the plasma membrane [26].
3.2. PAK and RAGE
The receptor for advanced glycation endproducts (RAGE) is a transmembrane receptor involved in the activation and regulation of inflammation. RAGE activation has been implicated in sterile inflammation, cancer, diabetes, and Alzheimer's disease [29,30]. RAGE is an upstream activator of several other important regulators in the inflammatory process, including NADPH oxidase [31,32], Cdc42/Rac1 [33], protein kinase C alpha (PKCα) [31], p21-ras [34], and nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) [35] (Fig. 2). These mediators in turn trigger the activation of PAK1 by Cdc42/Rac1 [36].
3.3. PAK and HMGB1
Another mechanism leading to chemotaxis of leukocytes involves the RAGE receptor ligand, high mobility group box 1 protein (HMGB1) [37]. HMGB1 is a nuclear protein released from cells during periods of stress, including cell death [29,38]. HMGB1 activates innate immunity [38] and is one activator of RAGE during necrosis [39]. HMGB1 can act as a chemoattractant [37]. PAKs are activated in neutrophils following administration of a variety of chemoattractants [40]. Other ligands of RAGE include advanced glycation end products (AGEs), which have been shown to result in an increase in ROS production [41]. AGEs are often implicated in age-related disorders such as Alzheimer's disease [42], cardiovascular disease [43], retinal disease [44], atherosclerosis [45], and diabetes [45]. AGEs have been demonstrated to be able to act as upstream regulators of PAK1 signaling [46]. However, these signaling pathways are incompletely understood, and remain the focus of many research groups.
3.4. PAK and CXCL1
The signaling cascade involving the chemokine (C-X-C motif) ligand 1 (CXCL1) is known to play a major role in inflammation and wound healing [47]. In addition to the above mentioned regulators of inflammatory mechanisms, the signaling cascade involving CXCL1 can lead to the chemotaxis of leukocytes. The downstream effects of CXCL1 signaling intertwine with the downstream effects of the RAGE and Cdc42/Rac1 signaling cascade. One study demonstrated how CXCL1 regulates chemotaxis via PAK1 and also leads to the activation of NF-κB via the Ras-MEKK1-MEK4/6-p38MAPK cascade [47]. NF-κB is a downstream target of several regulators, including p21-ras [34], RAGE [35], and ROS [48]. It is a protein complex involved in the up-regulation of major proinflammatory mediators in diseases, such as atherosclerosis [49], and it is involved in the regulation of cell survival, making it an important potential target in cancer therapy [50].
In conclusion, there appears to be a vast amount of evidence supporting the idea that PAK is a central molecule in inflammatory signaling, thus making PAK1 itself a promising prospective pharmacological target. Discovering how to regulate the activity of PAK1 downstream of RAGE and CXCL1 could provide an indirect therapeutic approach to governing the activity of PAK1's upstream activators in a wide variety of pathological conditions. One example of this could be in cardiac ischemia and reperfusion injury, in which RAGE activation alters cardiac contractility [51]. In this example, targeting PAK downstream of RAGE may provide a therapeutic tool, since PAK acts on the myofilament proteins [1,52], which are the final determinants in the regulation of contractile force [53].
4. NADPH oxidase in cardiovascular and inflammatory diseases
As discussed earlier in this review, PAK1 and PAK2 are upstream regulators of the NADPH oxidase (NOX) in neutrophils [15,16]. The NOX is a multi-subunit enzyme complex which generates reactive oxygen species (ROS), such as and H2O2, by catalyzing electron transfer from NADPH to molecular oxygen (O2) [54]. For a long time, the NOX has been believed to be an enzyme located exclusively in the plasma membrane and in the phagosomes of professional phagocytic cells, with a role in the activation of the respiratory burst and the killing of engulfed microorganisms. Phagocyte NADPH oxidase consists of the cytosolic subunits p47phox, p67phox, p40phox, the small GTPase Rac, and the flavocytochrome b558 complex, which is comprised of two membrane-bound subunits, gp91phox and p22phox (Fig. 3 A and B).
Fig. 3.
(A) Structure of NADPH oxidase (NOX). Catalytic Nox subunits (Nox1, Nox2, Nox3, Nox4, Nox5) and Duox1/2 are shown. Nox2 (gp91phox), originally found in phagocytes, is a prototype of Noxs. In plasma or endosomal membranes, Nox1, Nox2, Nox3 and Nox4 couple with the regulatory subunit p22phox. Nox1, Nox2, and Nox3 associate with a cytosolic small G protein Rac, and a cytosolic “activator” subunit (p67phox or NOXA1) as well as an “organizer” subunit (p47phox or NOXO1), which is required for NOX activation. Nox4 does not require cytosolic subunits for its activation. In addition, Nox5 and Duox1/2 do not need other subunits and are activated by Ca2+ via Ca2+-binding EF-hand motifs, while Duox1/2 contain a peroxidase domain in the extracellular space. (B) Regulation of NADPH oxidase by PAK. Upon agonist stimulation, the small G protein Rac1 translocates from the cytosol to the membranes and associates with the cytosolic “activator” subunit p67phox. Rac1 then activates PAK, which in turn induces phosphorylation of cytosolic organizer subunit p47phox. This results in the assembly of the Nox2 (gp91phox)-based oxidase complex and increased O2− production.
In the last decade, advances in the understanding of the NOX family have resulted in a current classification of seven NOX family members, which are structurally and functionally quite similar, yet different and unique in their catalytic subunit (Fig. 3 A) [55]. Each member of the NADPH oxidase family comprises a specific catalytic subunit, which carries the same name as the corresponding NADPH oxidase isoform, namely, NOX1, NOX2 (gp91phox), NOX3, NOX4, NOX5, DUOX1, and DUOX2 (see Table 1) [56–58]. Ultra-structural studies have revealed that most of the members of the NADPH oxidase family are membrane-bound enzymes whose catalytic subunits require the cytosolic “organizer” and “regulator” subunit components to achieve full functioning activity [59–62]. All of these oxidases are capable of transporting electrons across the plasma membranes to generate ROS.
Table 1.
The catalytic subunit NOX1 binds to and requires stabilization partner p22phox for activity, in addition to the binding of the organizer/regulatory subunit NOXO1 (or its homolog p47phox), and the activating subunit NOXA1 (or its homolog p67phox), which interacts with Tks4 and Tks5 (two regulators of NOX1). Similarly, both the cytosolic p47phox and p67phox are necessary for NOX2 activity, along with Rac1 (or Rac2), p40phox, and p22phox. NOX3 is functionally very similar to NOX1 and 2. NOX1, NOX2 and p22phox are membrane-bound subunits that assemble together with the flavocytochrome b558 (cyt b558) of FADH to form the catalytic core of the NADPH oxidase. NOX4 and NOX5 are structurally less complex, the former requiring p22phox, and the latter requiring Ca2+ and calmodulin to bind the N-terminal EF hand Ca2+-binding sites, in order to be constitutively active. DUOX1 and DUOX2 are evolutionarily related to the NOX5 isoform. However, DUOX1/2 differ from NOX5 by the number of N-terminal transmembrane domains and the presence of extracellular peroxidase homology domains. (CaM = Calmodulin; EF hand motif = helix-loop-helix structural domain; PLCγ2 = Phospholipase Cγ2; PLD = Phospholipase D)
| NADPH oxidase isoform | Catalytic Core Subunits of the Complex | Stabilization Partners | Regulatory Cytosolic Subunits | Other Factors | Citations |
|---|---|---|---|---|---|
| NOX1 | NOX1 | p22phox | Rac, Noxal (p67phox), Noxol (p47phox), Tks4/5 | FADH, NADPH | [153, 154] |
| NOX2 | NOX2 | p22phox | Rac, p40phox, p47phox, p67phox | FADH, NADPH | [154, 155] |
| NOX3 | NOX3 | p22phox | Rac, Noxal (p67phox), Noxo 1 (p47phox), | NADPH | [156] |
| NOX4 | NOX4 | p22phox | Poldip 2, Tks 4/5 | FADH, NADPH | [157, 158] |
| NOX5 | NOX5 | EF hand motif, CaM, HSP90 | [157, 158] | ||
| DUOX1 | DUOX1 | Duoxa 1 | PLCγ2/PLD | [159-161] | |
| DUOX2 | DUOX2 | Duoxa 2 | PLCγ2/PLD | [161, 162] |
NOXs are now recognized to have specific subcellular localizations, which is required for localized H2O2 production and activation of specific redox signaling pathways to mediate various functions [63,64]. The expression levels of NOXs vary significantly between organ and tissue types [55,65,66]; NOXs are expressed at high levels only in phagocytes and widely expressed at low concentrations in a variety of organs and cell types, including heart, blood vessel, endothelial cells, vascular smooth muscle cells, and adventitia [55,65]. NOXs have been implicated in numerous physiological processes, such as the immune response [56,67] and cellular development, migration, proliferation, differentiation, and gene expression [56,68]. However, overexpression and/or activation of NOX contribute to various pathophysiologies, including hypertension [1,56,69–72], thyroid disease [56,73,74], atherosclerosis [75], myocardial infarction and reperfusion injury [76], cardiac hypertrophy [77], cancer [78] and inflammation [79]. These findings suggest that NOXs could be a suitable target for tissue-specific pharmacological inhibition with an implication in a wide variety of diseases [80,81].
On one particular note, PAK1 has been shown to be involved in cardiac ischemia/reperfusion injury [52,82] and hypertrophy [1,83]. Given that PAKs regulate NOX activation via p47phox phosphorylation (Fig. 3 B) as described in detail below, it is likely that PAKs are important therapeutic targets for various NOX-dependent cardiovascular and inflammatory diseases. The role of PAK1 in cardiovascular diseases is expanded upon below in Section 7.
5. Role of PAK1 in NADPH oxidase activation
NADPH oxidase activity is finely regulated by PAK1 and PAK2 [15,84]. In human neutrophils, PAK1 has been shown to co-localize with and phosphorylate p47phox and to directly bind to p22phox [15], ascertaining that PAK1 interacts with these two NADPH oxidase subunits. In addition, another study reported that PAK2 phosphorylates p67phox, another NADPH oxidase component [85]. Since it is known that PAK1 is regulated by the NADPH oxidase subunit Rac1, it stands to reason that Rac1 activation of PAK1, which in turn phosphorylates and activates p47phox, provides a mechanism through which NADPH oxidase components can communicate [84]. This provides a mechanism by which the NADPH oxidase can self-regulate via Rac1-PAK1-p47phox signaling [84]. Finally, PAK1 is also a major regulator of the actin cytoskeleton [86], which is important for NADPH oxidase assembly and function [87–90].
5.1. PAK1 phosphorylation of p47phox
One of the most important mechanisms of NADPH oxidase activation is mediated by phosphorylation of the NADPH oxidase subunit p47phox. PAK1 mediates p47phox phosphorylation at Ser303/304/320/328 [84], which occurs both in vitro and in vivo [10,15]. Phosphorylated p47phox favors the coupling of the p47phox/p67phox heterodimer, which migrates from the cytosol to the membrane to interact with cyt b558. Also, p47phox phosphorylation induces exposure of key binding sites that favor p47phox binding to p22phox on the membrane [91,92] through membrane phosphoinositol lipids [11,93] to achieve full activation of the NADPH oxidase. This role for PAK1 has been investigated in Ra2 cells transduced with wild type PAK1, PAK1 mutants, or the auto-inhibitory fragment p21-binding domain (PBD) of PAK1 [84]. In a study by Roepstorff et al., when the PBD or the dominant-negative (K299A) PAK1 mutant was transduced, no significant effects on the respiratory burst or superoxide production were observed in Ra2 cells treated with N-formyl-methionyl-leucyl-phenylalanine (fMLP) [84]. However, transduction of active forms of PAK1 (mutants H83L/H86L and PAK1-T423E) resulted in p47phox phosphorylation in vivo [84]. Similarly, phosphomimetic mutants of p47phox (S303/304/328D) in Ra2 cells resulted in a significant increase in superoxide production compared to baseline [84]. Likewise, PAK1 serves as a key kinase that mediates the activation of the NADPH oxidase and the respiratory burst in activated neutrophils [11]. In conclusion, in order to obtain full activation of p47phox, several Ser residues need to be phosphorylated, including Ser303/304/320, and particularly Ser328 [94]. This mechanism is sufficient to induce a conformational change in p47phox that favors its binding to p22phox and to the phosphatidylinositol lipids present on the membrane [84].
5.2. PAK1 regulation of Rac1, a component of NADPH oxidase
Small GTPases, such as Rac1 or Rac2, Vav1 and P-Rex, all of which are guanine exchange factors (GEF), are also important enzymes that regulate NADPH oxidase during inflammation [84,95–97]. Specifically, Rac1 migrates to the membrane independently of p47phox/p67phox to regulate NADPH oxidase activity both in vivo and in vitro [98]. Rac1 functions are mediated by several downstream effectors, including PAK1 [4,10,99]. During inflammation, Rac1 is crucial for NADPH oxidase function, as supported by the evidence that NADPH oxidase activation is inhibited in Rac1 knockout mice [100]. In fact, hearts of Rac1 knockout mice, which have experimentally developed diabetes, are characterized by lack of NADPH oxidase activation, reduced expression of its subunits, and loss of ROS production [100]. Better myocardial function in these diabetic Rac1 knockout mice has been attributed to reduced myocardial collagen deposition and attenuated development of myocardial hypertrophy [100].
5.3. PAK1-mediated cytoskeletal remodeling and NADPH oxidase activation
The role of PAK1 in the regulation of the cell cytoskeleton has been investigated in several blood cells involved in the inflammatory response [10,101–103]. PAK1-mediated cytoskeletal remodeling plays a key role in NADPH oxidase assembly and promotes NADPH oxidase activation [102], possibly through activation of cortactin and/or filamin [104,105]. Several studies have shown that the actin cytoskeleton is implicated in NADPH oxidase regulation, possibly by binding p47phox and possibly other members of the NADPH oxidase complex [106–108]. It is believed that p47phox and p40phox bind to moesin a member of the ERM (Ezrin/Radixin/Moesin) family known to bind actin [109], and then are translocated to the membrane. However, the precise mechanism of the NADPH oxidase translocation to the membrane remains elusive and further studies in this direction are warranted.
5.4. PAK1-mediated regulation of PGAM-B and NADPH oxidase
Another mechanism by which PAK1 influences the activity of the NADPH oxidase in white blood cells is by regulating phosphoglycerate mutase (PGAM)-B activity, an enzyme of the glycolytic pathway [110]. During activation of the immune response, PAK1 is activated, an event that leads to the association of PAK1 with PGAM-B and subsequent phosphorylation of this enzyme on Ser23 and Ser118, thus resulting in the inhibition of endogenous PGAM-B and the glycolytic pathway [110]. Inhibition of the glycolytic pathway results in a reduced breakdown of glucose to generate ATP and NADH for the energetic needs of the cell. Glucose is therefore diverted from an energy production pathway to the pentose phosphate pathway, a pathway that leads primarily to the production of NADPH, a cofactor that is necessary for proper NADPH oxidase functioning. Fueling of NADPH oxidase with the cofactor NADPH leads to a sustained oxidative burst in the phagocytes during the inflammatory response [111].
6. Physiological functions of PAK during inflammation
6.1. PAK and neutrophils
The results of the studies illustrated so far implicate a clear role for PAK1 in the innate immunity. PAK1 and PAK2 have been implicated in remodeling of the actin cytoskeleton in activated human neutrophils [14,101]. Under these experimental conditions, PAK1 appears to colocalize with F-actin at the membrane ruffles and lamellipodia present at the leading edge of polarized cells [14], where PAKs may serve as a link between upstream molecules (such as Rho GTPases) and downstream cytoskeletal proteins. PAK1 also has a role in the formation of pseudopodia and phagocytic invaginations, thus contributing to the formation of cup-shaped membrane structures which envelop and enclose exogenous particles [14]. Even though PAK1 is involved in the engulfment of bacteria, it is not involved in the internalization process [14]. Thus, it appears that PAK kinases are important regulators of chemotaxis of human neutrophils, thus accounting for neutrophil directionality, spreading, and migration speed [101]. PAK mediated effects on neutrophil migration are the results of changes in the localization of active RhoA and the formation of aberrant vinculin-rich complexes [101]. While PAK1 is important in regulating the directional migration of myeloid cells towards a chemoattractant, PAK1−/− macrophages show a preserved Colony Stimulated Factor-1-mediated chemotaxis [102]. In myeloid cells, PAK1 is an important scaffold protein, which by docking Gβγ and PIX, promotes activation of Cdc42 to further activate PAK1 itself and promote chemotaxis [112]. The chemokine CXCL1, which attracts leukocytes to damage tissue during inflammation, induces chemotaxis by activation of the Cdc42–PAK1 cascade in CXCR2-expressing HEK293 cells, independent of ERK1/2 activation [47].
6.2. PAK and macrophages
In macrophages, PAK1 regulates cytoskeletal dynamics with the net result of lamellipodia formation, which serve as the motor during cell migration [102]. T lymphocytes depend on PAK1 for lamellipodia formation and gene activation [113]. PAK1−/− mice display defects in lamellipodia formation and a lack of response of macrophage and ERK activation in response to growth stimuli. Analysis of macrophages obtained from PAK1−/− mice indicated lower stability of lamellipodia, in association with reduced Erk phosphorylation and activation. PAK1 plays a pivotal role in the regulation of cytoskeletal dynamics through Erk, as demonstrated by inhibitory effects on lamellipodia dynamics and cell locomotion by administration of Erk inhibitors [102]. In nonmyeloid cells, PAK1 influences the immune response mediated by T cells via Erk and NFAT [13]. Subdomains of SLP-76 and BLNK (SH2 domain-containing leukocyte phosphoprotein of 76 kDa and B cell linker protein, respectively) act as scaffold proteins to promote multiprotein assembly [114]. After phosphorylation of N-terminal tyrosines, both SLP-76 and BLNK bind to Vav and Nck, which in turn bind Cdc42, PAK1, and WASP, to finally activate Rho-GTPases [115,116]. This complex mechanism appears to be critical for T-cell receptor- and B-cell receptor-induced actin cytoskeletal rearrangement and cell activation [117].
6.3. Pak1 and mast cells
Degranulation of mast cell intracellular vesicles is largely mediated by PAK1 and PP2A [118]. Recently, it has been demonstrated that activation of PAK1 promotes PP2A subunit assembly and then activation [118], which in turn dephosphorylates threonine 567 of Ezrin/Radixin/Moesin (ERM) molecules [119]. Dephosphorylation of ERM results in changes of the F-actin and vesicle dynamics, followed by release of heparin and other inflammation mediators [119]. PAK1 knock-out mice are characterized by reduced systemic histamine release, while primary mast cells in which Ezrin expression is disrupted are characterized by impaired actin dynamics and reduced vesicle degranulation [119].
6.4. PAK and endothelial cells
Endothelial cells serve as an active barrier function, and they are involved in neutrophil recruitment and hemostasis. In this respect, various studies have investigated myosin light chain (MLC) as a target of PAK1. PAK1 activation induces MLC dephosphorylation through activation of PP2A in human endothelial cells in an in vitro model of thrombin-induced endothelial barrier dysfunction [120]. The expression in endothelial cells of activated PAK1 or unphosphorylatable MLC20 resulted in less thrombin-induced endothelial barrier dysfunction, implicating active PAK1 in regulating endothelial cell permeability [121,122]. However, in a model of acute cardiac ischemia and reperfusion, MLC phosphorylation was reduced in the hearts from PAK1 knock-out mice compared to wild-type. Gamma-PAK phosphorylates non-muscle myosin II regulatory light chain [123]. PAK4 phosphorylates myosin regulatory light chain and contributes to Fcγ receptor-mediated phagocytosis [124]. PAK1 activation can increase or decrease MLC phosphorylation in primary human intestinal smooth muscle depending on the circumstances, such as mechanical stretch, via regulation of MLC phosphatase targeting subunit [125]. Rac induces an increase in phosphorylation of myosin regulatory light chain in HeLa cells [126]. Therefore, PAK regulation of MLC phosphorylation differs between various cell types and under different experimental conditions.
6.5. PAK and platelets
Platelets are cells that are primarily involved in hemostasis, in the inflammation process, in cytokine signaling, and phagocytosis [127]. In the presence of thrombin, cofilin present in platelets undergoes dephosphorylation and activation [128]. Recently, the class II of PAKs has been implicated as signal molecules that link intracellular Ca2+ changes to cofilin dephosphorylation and activation, thus leading to degranulation of thrombin-activated platelets [129]. This study demonstrated that the Ca2+-dependent pathway leads to the secretion of granule content through activation of calcineurin and Rac1, which in turn activate the class II PAKs and cofilin [129].
7. Role of PAK in organ diseases
PAK1 is widely distributed in the human body, although it is more abundantly expressed in the brain (subthalamic nucleus, occipital lobe) [130], the cardiovascular system [130], and the blood [14]. Expression levels of PAK isoforms in other cell types has been recently reviewed elsewhere [13]. In multiple cell types, PAK1 has been shown to interact with NADPH oxidase. In recent years, our understanding of the interaction between PAK1 and NADPH oxidase has recently progressed, both in cardiovascular and inflammatory diseases.
7.1. PAK1, NADPH oxidase, and the cardiovascular system
PAK1 is an important modulator of cardiac excitation–contraction coupling, which functionally operates at the level of the excitable membranes and at the level of the myofilament proteins. Recently, it has been demonstrated that PAK1 is a key protein that mediates the functional stability of the transverse tubule, a functional sarcolemmal invagination, in cardiac myocytes. In fact, in one study, PAK1 deficient mouse hearts were characterized by underdeveloped transverse tubule structural integrity, associated with decreased amplitude, prolonged rise time and delayed recovery time of transmembrane Ca2+ transients [131]. However, on the contrary, additional studies have demonstrated that Ca2+ transients do not change in PAK1-deficient ventricular myocytes (VM) when subjected to short periods of simulated ischemia. Under these conditions, an overload of intracellular Ca2+ was measured due to an increase in the Na+/Ca2+ exchanger (NCX) activity [132]. Under these conditions, it has been proposed that PAK1 suppresses NOX2 activity and reduces ROS production in the cardiac cells. In the absence of PAK1 (as in PAK1−/− VM) or in VM in which PAK1 activity was pharmacologically achieved by administering the drug IPA3, excessive ROS production was measured as the result of increased NOX2 activity, leading to NCX-mediated Ca2+ intracellular accumulation. PAK1/NOX2-mediated production of ROS was reduced in VM in which NOX2 activity deficient or inhibited. To our knowledge, this is the first evidence that PAK1 acts as a negative regulator of NOX2 in the cardiac cells, thus supporting the hypothesis that PAK1 itself could be critical for NOX2 assembly and function in the heart.
7.2. PAK1, NADPH oxidase, and inflammatory diseases
Similarly, PAK1 is involved in the regulation of NADPH oxidase in diseases associated with inflammatory activation, proliferation and angiogenesis of endothelial cells [133]. In human umbilical vein endothelial cells, it has been demonstrated that thrombin leads to the activation of phosphodiesterase 2 (PDE2) and subsequently Rac1, PAK1, and NOX2, which finally leads to ROS production, endothelial proliferation, and angiogenesis [133]. Along these lines, PAK1 activation is responsible for initiation of inflammation and atherosclerosis in endothelial cells of the aortic arch [5], through a mechanism that implicates NF-kB, leucocyte recruitment, and fibronectin deposition. However, while NADPH oxidase has been heavily implicated in the development of atherosclerosis [134], the possible involvement of NADPH oxidase in the activation of inflammatory pathways is yet to be experimentally verified.
Despite the vast number of studies investigating PAK regulation of the NADPH oxidase, it still remains poorly understood how PAK regulates NADPH oxidase in different cell types, and what overall effect this has on organ function, both normally and during a diseased state. A better understanding is required of the mechanism of PAK regulation of inflammation in different cell types. Future studies are warranted to discover the potential pharmaceutical interventions to regulate PAK in specific disease states.
8. PAKs as a pharmacological target
It has been previously demonstrated that the ATP-binding pocket in the kinase domain of PAK1 is characterized by a specific size and shape, in a way that is different from the other PAK family members, thus making this pocket a suitable target for specific pharmacological inhibition. Recently, a selective inhibitor with greater affinity for PAK1 than for PAK4-6 has been developed, thus confirming that ATP-binding pockets between different PAK isoforms significantly differ in their atomic structure [135,136]. More recently, the co-crystallization of PAK1 with the selective and potent inhibitor FL172, obtained from a small library of 48 ruthenium complexes, represents an important pharmacological advancement. FL172 works as an allosteric inhibitor that targets the PAK1 transition state in mammalian cells [137]. Therefore, PAK is a good candidate for selective pharmacological targeting by small molecules because of its intrinsic structural properties. However, it is also clear that the novel small molecules targeted to inhibit PAK1 should have IC50 values in the nanomolar range of PAK1 to achieve a more selective inhibition. So far, to the best of our knowledge, only a few molecules (FRAX486 developed by Afraxix, and the PAK1 inhibitor ST-2001, a derivative of staurosporine) are potent enough to inhibit PAK1 at nanomolar concentrations [137–139].
PAK1 is an interesting target for affinity reagents. It naturally binds SH3 adaptor proteins such as PIX and NCK, making PAK itself a good binding partner for competitive inhibitory proteins. It is known that PAK1 structure naturally provides three potential binding sites for pharmacologically-active small proteins. The first binding site is located in the PBD, which corresponds to the Cdc42/Rac interactive-binding motif (residues 75–90). The second and the third site for protein binding are the kinase inhibitory tail (residues 137–139) and the inhibitory switch (IS) (which overlaps the GTPase binding region of PAK1), two PAK domains that are suitable for binding of peptides or small proteins that can recognize specific PAK amino acid sequences. Recently, novel pharmacological strategies have been developed by computational design to target activated PAK1 with affinity reagents. Modifications of the IS have been developed to design better peptides that would bind the C-lobe of the PAK1 kinase domain with increased stability, solubility and binding affinity [140,141]. The development of new computational protocols used to design novel peptides can be used to engineer molecular probes that could detect the “open/closed” state and activity of protein enzymes, and also potentially work as therapeutics by manipulating their activities.
Notably, most of the aforementioned PAK pharmacological inhibitors are currently under investigation in cancer biology. It is known that PAKs have a clear role in the pathogenesis and progression of cancer, and have been implicated in the mechanisms that are responsible for cancer resistance to drugs. However, to the best of our knowledge none of these compounds have been investigated for their anti-inflammatory potential through the inhibition of PAK activity.
One major objective when designing specific PAK inhibitors is to target exclusively group I PAKs, since to the best of our knowledge, only PAK1 and PAK2 are involved in the inflammation process. PAK group I contains highly conserved C- and N-domains, which are not present in group II, a characteristic that confers drug specificity. Moreover, a better understanding of the molecular interaction of the PAK structure and the identification of the missing regions in the known crystal structure could help us design potent selective inhibitors that fit better in the PAK three-dimensional structure. Finally, PAK inhibitors should be specifically targeted to organs and/or cells involved in the inflammation process (e.g. white cells and the endothelium [5]), thus sparing other organs (e.g. the heart) and limiting potential side effects that could be driven by PAK inhibition, such as cardiac hypertrophy [1,52,83].
To the best of our knowledge, none of the drugs designed so far have been tested in the context of inflammation. Currently, recent studies have demonstrated that the inhibition of PAKs might be beneficial in several human conditions such as Neurofibromatosis Type 2 [135,142], fragile X syndrome [136], thyroid cancer [143], other cancers [144,145], and ulcerative colitis [9]. Yet, in spite of the large body of evidence that PAK1/2 regulate NADPH oxidase activity, no PAK inhibitors have been implicated in the regulation of inflammation. The reason for the delay in this specific area of investigation may stem from the yet incomplete characterization of the functional roles of PAK in neutrophil chemotaxis, oxidative burst, and other anti-microbial functions (e.g. phagocytosis, degranulation and neutrophil extracellular traps). Moreover, some PAK inhibitors recently developed failed to selectively inhibit PAK, or they rapidly degraded in the intracellular space (e.g. IPA-3) [146]. However, while small-molecule inhibitors are advantageous since they are generally hydrosoluble and orally bioavailable, other forms of inhibition could be achieved by using peptide inhibitors or monoclonal antibodies. Novel investigations in these directions are certainly merited.
9. Future directions
A promising area for PAK1-targeted therapeutics is allergen disorders [118,147]. Some relevant studies have confirmed a primary role for PAK1 in orchestrating the cross-talk between inflammation, immunity, and allergy. In addition, recent expert opinions further support the idea for PAK1 involvement in these disorders and that PAK1 is a suitable target for pharmacological inhibition [148]. Moreover, a robust amount of literature indicates that NADPH oxidase is heavily implicated in the development of allergic reactions [149–152]. In spite of this initial evidence, it is still unclear in which specific allergic disorders PAK1 is involved, and what could be the role of PAK1 in regulating NADPH oxidase under these conditions. Our understanding of the relationship between PAK1 and NADPH oxidase should therefore be explored in a series of conditions, including allergic reactions, asthma, and anaphylactic shock. Novel anti-allergic drugs that would target PAK would represent a novel approach to the treatment of allergies, which would add anti-Pak drugs to the current pharmacological armamentarium. (See Table 2 for a concise list of future directions and open questions of PAK signaling, and Fig. 2 for a summary of some of the above mentioned studies, as well as a proposed possible signaling pathway.)
Table 2.
Future directions and open questions for investigations of PAK signaling. P21-activated kinase in inflammatory and cardiovascular disease. Domenico M. Taglieri, Masuko Ushio-Fukai, Michelle M. Monasky.
| Future directions and open questions: |
|---|
| Novel PAK inhibitors should target only PAK1 and PAK2 and achieve specific inhibition in inflammatory cells only. |
| A better understanding of the missing regions in the crystal structure of PAK1 and PAK2 will help design better selective inhibitors. |
| Old and new PAK1 and PAK2 inhibitors should be tested in the context of inflammation. |
| The role of PAK1 and PAK2 is still unclear in many aspects of cell inflammation. |
| Alternative means of PAK1 and PAK2 inhibition such as peptide inhibitors or monoclonal antibodies should be investigated. |
| Is there a role for PAK inhibition in allergy disorders? |
| Are there alternative ways to achieve PAK inhibition other than preventing PAK phosphorylation/activation? |
10. Conclusions
In conclusion, it should be kept in mind that PAK-mediated phosphorylation of the NADPH oxidase is only one of many ways by which the inhibition of the NADPH oxidase can be achieved. In fact, other mechanisms (such as the assembly of the NADPH oxidase complex, translocation of NADPH oxidase subunits to the membrane, post-translational modifications, changes in calcium transients and membrane potentials, as well as changes in the NADPH oxidase gene expression) may significantly affect the NADPH oxidase activity and its potential inhibition. To add complexity to this matter, the different levels of PAK isoform expression in different bodily tissues and organs, as well as its subcellular localization and function may vary significantly. Therefore, additional studies should be oriented towards the investigation of other means of NADPH oxidase inhibition, and/or should aim at the identification and characterization of drugs that target unique PAK1/2 motives and/or that target specific intracellular PAK microdomains.
Acknowledgments
This work was supported by grants R01HL116976 and R21HL112293 from the National Institutes of Health (to M. U.-F.) and by a Marie Curie International Incoming Fellowship, PIIF-GA-2013-626429 (to M. M. M.).
Abbreviations
- AID
Auto-inhibitory domain
- ATP
Adenosine-triphosphate
- B-cell
B lymphocytes
- BLNK
B cell linker protein
- CaM
Calmodulin
- Cdc
Cell division control protein
- cyt b558
Flavocytochrome b558
- DUOX1/2
Dual oxidase 1/2
- EF hand motif
Helix-loop-helix structural domain
- ERK
Extracellular-signal-regulated kinase
- ERM
Ezrin/Radixin/Moesin
- Fc
Fragment crystallizable
- FcεRI
Fc epsilon RI, high-affinity IgE receptor
- fMLP
N-formyl-methionyl-leucyl-phenylalanine
- GEF
Guanine exchange factors
- GTP
Guanosine-5′-triphosphate
- GTPases
Guanosine-5′-triphosphate hydrolase
- Gβγ
G protein beta-gamma complex
- IC50
Half maximal inhibitory concentration
- IgE
Immunoglobulin E
- IS
Inhibitory switch domain
- KI
Kinase inhibitor fragment
- LIMK-1
LIM domain kinase 1
- MLC
Myosin Light Chain
- NADH
Nicotinamide adenine dinucleotide
- NADPH oxidase
Nicotinamide adenine dinucleotide phosphate-oxidase
- Nck
Non-catalytic region of tyrosine kinase adaptor protein
- NFAT
Nuclear factor of activated T-cells
- NOX1/2/3/4/5
NADPH oxidase 1/2/3/4/5
- PAK1/2/3
p-21 activated kinase 1/2/3
- PAKs
p-21 activated kinases
- PBD
p21-binding domain
- PDE2
Phosphodiesterase 2
- PGAM
Phosphoglycerate mutase
- PIX
Pak interactive exchange factor
- PLCγ2
Phospholipase Cγ2
- PLD
Phospholipase D
- PP2A
Protein phosphatase 2
- Ra2 cells
Microglial cell line
- Rac1/2
Ras-related C3 botulinum toxin substrate 1/2
- Rho
Rho family of GTPases
- RhoA
Ras homolog gene family, member A
- ROS
Reactive oxygen species
- SH3
SRC Homology 3
- SLP-76
SH2 domain-containing leukocyte phosphoprotein
- T-cell
T lymphocytes
- Vav1
Proto-oncogene vav
- WASp
Wiskott–Aldrich syndrome protein
References
- [1].Taglieri DM, Monasky MM, Knezevic I, Sheehan KA, Lei M, Wang X, Chernoff J, Wolska BM, Ke Y, Solaro RJ. J. Mol. Cell. Cardiol. 2011;51(6):988–996. doi: 10.1016/j.yjmcc.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Knaus UG, Wang Y, Reilly AM, Warnock D, Jackson JH. J. Biol. Chem. 1998;273(34):21512–21518. doi: 10.1074/jbc.273.34.21512. [DOI] [PubMed] [Google Scholar]
- [3].Jaffer ZM, Chernoff J. Int. J. Biochem. Cell Biol. 2002;34(7):713–717. doi: 10.1016/s1357-2725(01)00158-3. [DOI] [PubMed] [Google Scholar]
- [4].Manser E, Leung T, Salihuddin H, Zhao ZS, Lim L. Nature. 1994;367(6458):40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- [5].Jhaveri KA, Debnath P, Chernoff J, Sanders J, Schwartz MA. BMC Cardiovasc. Disord. 2012;12:55. doi: 10.1186/1471-2261-12-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gadepalli R, Kotla S, Heckle MR, Verma SK, Singh NK, Rao GN. J. Biol. Chem. 2013;288(43):30815–30831. doi: 10.1074/jbc.M113.463414. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [7].Mann J, Patrick CN, Cragg MS, Honeychurch J, Mann DA, Harris M. J. Immunol. 2005;175(10):6560–6569. doi: 10.4049/jimmunol.175.10.6560. [DOI] [PubMed] [Google Scholar]
- [8].Yurdagul A, Jr., Chen J, Funk SD, Albert P, Kevil CG, Orr AW. Mol. Biol. Cell. 2013;24(3):398–408. doi: 10.1091/mbc.E12-07-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Khare V, Lyakhovich A, Dammann K, Lang M, Borgmann M, Tichy B, Pospisilova S, Luciani G, Campregher C, Evstatiev R, Pflueger M, Hundsberger H, Gasche C. Biochem. Pharmacol. 2013;85(2):234–244. doi: 10.1016/j.bcp.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Knaus UG, Morris S, Dong HJ, Chernoff J, Bokoch GM. Science. 1995;269(5221):221–223. doi: 10.1126/science.7618083. [DOI] [PubMed] [Google Scholar]
- [11].Ding J, Knaus UG, Lian JP, Bokoch GM, Badwey JA. J. Biol. Chem. 1996;271(40):24869–24873. doi: 10.1074/jbc.271.40.24869. [DOI] [PubMed] [Google Scholar]
- [12].Lian JP, Badwey JA. FEBS Lett. 1997;404(2–3):211–215. doi: 10.1016/s0014-5793(97)00134-8. [DOI] [PubMed] [Google Scholar]
- [13].Pacheco A, Chernoff J. Int. J. Biochem. Cell Biol. 2010;42(1):13–16. doi: 10.1016/j.biocel.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dharmawardhane S, Brownson D, Lennartz M, Bokoch GM. J. Leukoc. Biol. 1999;66(3):521–527. doi: 10.1002/jlb.66.3.521. [DOI] [PubMed] [Google Scholar]
- [15].Martyn KD, Kim MJ, Quinn MT, Dinauer MC, Knaus UG. Blood. 2005;106(12):3962–3969. doi: 10.1182/blood-2005-03-0859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Chang LC, Lin RH, Huang LJ, Chang CS, Kuo SC, Wang JP. Eur. J. Pharmacol. 2009;615(1–3):207–217. doi: 10.1016/j.ejphar.2009.04.050. [DOI] [PubMed] [Google Scholar]
- [17].Kumar A, Molli PR, Pakala SB, Bui Nguyen TM, Rayala SK, Kumar R. J. Cell. Biochem. 2009;107(4):579–585. doi: 10.1002/jcb.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Arias-Romero LE. J. Chernoff, Biol. Cell. 2008;100(2):97–108. doi: 10.1042/BC20070109. [DOI] [PubMed] [Google Scholar]
- [19].Lei M, Lu W, Meng W, Parrini MC, Eck MJ, Mayer BJ, Harrison SC. Cell. 2000;102(3):387–397. doi: 10.1016/s0092-8674(00)00043-x. [DOI] [PubMed] [Google Scholar]
- [20].Wang J, Wu JW, Wang ZX. Structure. 2011;19(12):1752–1761. doi: 10.1016/j.str.2011.10.013. [DOI] [PubMed] [Google Scholar]
- [21].Maksimoska J, Feng L, Harms K, Yi C, Kissil J, Marmorstein R, Meggers E. J. Am. Chem. Soc. 2008;130(47):15764–15765. doi: 10.1021/ja805555a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lu H, Lei M, Schulze-Gahmen U. Crystal Structure of the Complex Between Human Pak1-kinase and 3-Hydroxystaurosporine. http://www.rcsb.org/pdb/explore/explore.do?structureId=2hy8.
- [23].Malecka KA, Szentpetery Z, Peterson JR. J. Biol. Chem. 2013;288(13):8887–8897. doi: 10.1074/jbc.M112.428904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Futosi K, Fodor S, Mocsai A. Int. Immunopharmacol. 2013;17(4):1185–1197. doi: 10.1016/j.intimp.2013.11.010. [DOI] [PubMed] [Google Scholar]
- [25].Bokoch GM, Reilly AM, Daniels RH, King CC, Olivera A, Spiegel S, Knaus UG. J. Biol. Chem. 1998;273(14):8137–8144. doi: 10.1074/jbc.273.14.8137. [DOI] [PubMed] [Google Scholar]
- [26].Nakamura T, Abe A, Balazovich KJ, Wu D, Suchard SJ, Boxer LA, Shayman JA. J. Biol. Chem. 1994;269(28):18384–18389. [PubMed] [Google Scholar]
- [27].Merrill AH, Jr., Liotta DC, Riley RT. Trends Cell Biol. 1996;6(6):218–223. doi: 10.1016/0962-8924(96)10021-0. [DOI] [PubMed] [Google Scholar]
- [28].Seufferlein T, Rozengurt E. J. Biol. Chem. 1994;269(44):27610–27617. [PubMed] [Google Scholar]
- [29].Sims GP, Rowe DC, Rietdijk ST, Herbst R, Coyle AJ. Annu. Rev. Immunol. 2010;28:367–388. doi: 10.1146/annurev.immunol.021908.132603. [DOI] [PubMed] [Google Scholar]
- [30].Yamagishi S. Exp. Gerontol. 2011;46(4):217–224. doi: 10.1016/j.exger.2010.11.007. [DOI] [PubMed] [Google Scholar]
- [31].Thallas-Bonke V, Thorpe SR, Coughlan MT, Fukami K, Yap FY, Sourris KC, Penfold SA, Bach LA, Cooper ME, Forbes JM. Diabetes. 2008;57(2):460–469. doi: 10.2337/db07-1119. [DOI] [PubMed] [Google Scholar]
- [32].Grossin N, Wautier MP, Wautier JL. Biorheology. 2009;46(1):63–72. doi: 10.3233/BIR-2009-0519. [DOI] [PubMed] [Google Scholar]
- [33].Ramasamy R, Yan SF, Schmidt AM. J. Leukoc. Biol. 2009;86(3):505–512. doi: 10.1189/jlb.0409230. [DOI] [PubMed] [Google Scholar]
- [34].Lander HM, Tauras JM, Ogiste JS, Hori O, Moss RA, Schmidt AM. J. Biol. Chem. 1997;272(28):17810–17814. doi: 10.1074/jbc.272.28.17810. [DOI] [PubMed] [Google Scholar]
- [35].Chen G, Ward MF, Sama AE, Wang H. J. Interferon Cytokine Res. 2004;24(6):329–333. doi: 10.1089/107999004323142187. [DOI] [PubMed] [Google Scholar]
- [36].Ke Y, Lei M, Solaro RJ. Prog. Biophys. Mol. Biol. 2008;98(2–3):238–250. doi: 10.1016/j.pbiomolbio.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Venereau E, Casalgrandi M, Schiraldi M, Antoine DJ, Cattaneo A, De Marchis F, Liu J, Antonelli A, Preti A, Raeli L, Shams SS, Yang H, Varani L, Andersson U, Tracey KJ, Bachi A, Uguccioni M, Bianchi ME. J. Exp. Med. 2012;209(9):1519–1528. doi: 10.1084/jem.20120189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Andersson U, Tracey KJ. Annu. Rev. Immunol. 2011;29:139–162. doi: 10.1146/annurev-immunol-030409-101323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zheng Y, Gardner SE, Clarke MC. Arterioscler. Thromb. Vasc. Biol. 2011;31(12):2781–2786. doi: 10.1161/ATVBAHA.111.224907. [DOI] [PubMed] [Google Scholar]
- [40].Huang R, Lian JP, Robinson D, Badwey JA. Mol. Cell. Biol. 1998;18(12):7130–7138. doi: 10.1128/mcb.18.12.7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yan HD, Li XZ, Xie JM, Li M. Chin. Med. J. (Engl.) 2007;120(9):787–793. [PubMed] [Google Scholar]
- [42].Srikanth V, Maczurek A, Phan T, Steele M, Westcott B, Juskiw D, Munch G. Neurobiol. Aging. 2011;32(5):763–777. doi: 10.1016/j.neurobiolaging.2009.04.016. [DOI] [PubMed] [Google Scholar]
- [43].Simm A, Wagner J, Gursinsky T, Nass N, Friedrich I, Schinzel R, Czeslik E, Silber RE, Scheubel RJ. Exp. Gerontol. 2007;42(7):668–675. doi: 10.1016/j.exger.2007.03.006. [DOI] [PubMed] [Google Scholar]
- [44].Glenn JV, Stitt AW. Biochim. Biophys. Acta. 2009;1790(10):1109–1116. doi: 10.1016/j.bbagen.2009.04.016. [DOI] [PubMed] [Google Scholar]
- [45].Baynes JW, Thorpe SR. Free Radic. Biol. Med. 2000;28(12):1708–1716. doi: 10.1016/s0891-5849(00)00228-8. [DOI] [PubMed] [Google Scholar]
- [46].Gallicchio MA, Bach LA. Biochim. Biophys. Acta. 2013;1833(12):2922–2932. doi: 10.1016/j.bbamcr.2013.05.024. [DOI] [PubMed] [Google Scholar]
- [47].Wang D, Sai J, Carter G, Sachpatzidis A, Lolis E, Richmond A. Biochemistry. 2002;41(22):7100–7107. doi: 10.1021/bi025902m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chandel NS, Trzyna WC, McClintock DS, Schumacker PT. J. Immunol. 2000;165(2):1013–1021. doi: 10.4049/jimmunol.165.2.1013. [DOI] [PubMed] [Google Scholar]
- [49].Monaco C, Andreakos E, Kiriakidis S, Mauri C, Bicknell C, Foxwell B, Cheshire N, Paleolog E, Feldmann M. Proc. Natl. Acad. Sci. U. S. A. 2004;101(15):5634–5639. doi: 10.1073/pnas.0401060101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Escarcega RO, Fuentes-Alexandro S, Garcia-Carrasco M, Gatica A, Zamora A. Clin. Oncol. (R. Coll. Radiol.) 2007;19(2):154–161. doi: 10.1016/j.clon.2006.11.013. [DOI] [PubMed] [Google Scholar]
- [51].Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, Kaya Z, Buss S, Autschbach F, Pleger ST, Lukic IK, Bea F, Hardt SE, Humpert PM, Bianchi ME, Mairbaurl H, Nawroth PP, Remppis A, Katus HA, Bierhaus A. Circulation. 2008;117(25):3216–3226. doi: 10.1161/CIRCULATIONAHA.108.769331. [DOI] [PubMed] [Google Scholar]
- [52].Monasky MM, Taglieri DM, Patel BG, Chernoff J, Wolska BM, Ke Y, Solaro RJ. Am. J. Physiol. Heart Circ. Physiol. 2012;302(1):H224–H230. doi: 10.1152/ajpheart.00612.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Monasky MM, Varian KD, Davis JP, Janssen PM. Pflugers Arch. 2008;456(2):267–276. doi: 10.1007/s00424-007-0394-0. [DOI] [PubMed] [Google Scholar]
- [54].Patriarca P, Basford RE, Cramer R, Dri P, Rossi F. Biochim. Biophys. Acta. 1974;362(2):221–232. doi: 10.1016/0304-4165(74)90215-3. [DOI] [PubMed] [Google Scholar]
- [55].Bedard K, Krause KH. Physiol. Rev. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- [56].Lassegue B, San Martin A, Griendling KK. Circ. Res. 2012;110(10):1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Brandes RP, Weissmann N, Schroder K. Free Radic. Biol. Med. 2010;49(5):687–706. doi: 10.1016/j.freeradbiomed.2010.04.030. [DOI] [PubMed] [Google Scholar]
- [58].Sirker A, Zhang M, Shah AM. Basic Res. Cardiol. 2011;106(5):735–747. doi: 10.1007/s00395-011-0190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].DeLeo FR, Quinn MT. J. Leukoc. Biol. 1996;60(6):677–691. doi: 10.1002/jlb.60.6.677. [DOI] [PubMed] [Google Scholar]
- [60].Groemping Y, Rittinger K. Biochem. J. 2005;386(Pt 3):401–416. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Groemping Y, Lapouge K, Smerdon SJ, Rittinger K. Cell. 2003;113(3):343–355. doi: 10.1016/s0092-8674(03)00314-3. [DOI] [PubMed] [Google Scholar]
- [62].Sumimoto H. Febs J. 2008;275(13):3249–3277. doi: 10.1111/j.1742-4658.2008.06488.x. [DOI] [PubMed] [Google Scholar]
- [63].Ushio-Fukai M. Sci. STKE. 2006;349:re8. doi: 10.1126/stke.3492006re8. [DOI] [PubMed] [Google Scholar]
- [64].Ushio-Fukai M. Antioxid. Redox Signal. 2009;11(6):1289–1299. doi: 10.1089/ars.2008.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Nauseef WM. J. Biol. Chem. 2008;283(25):16961–16965. doi: 10.1074/jbc.R700045200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Szanto I, Rubbia-Brandt L, Kiss P, Steger K, Banfi B, Kovari E, Herrmann F, Hadengue A, Krause KH. J. Pathol. 2005;207(2):164–176. doi: 10.1002/path.1824. [DOI] [PubMed] [Google Scholar]
- [67].Segal BH, Grimm MJ, Khan AN, Han W, Blackwell TS. Free Radic. Biol. Med. 2012;53(1):72–80. doi: 10.1016/j.freeradbiomed.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Coyoy A, Valencia A, Guemez-Gamboa A, Moran J. Free Radic. Biol. Med. 2008;45(8):1056–1064. doi: 10.1016/j.freeradbiomed.2008.06.027. [DOI] [PubMed] [Google Scholar]
- [69].Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. J. Clin. Invest. 2003;111(8):1201–1209. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Lijnen PJ, van Pelt JF, Fagard RH. Cardiovasc. Ther. 2012;30(1):e1–e8. doi: 10.1111/j.1755-5922.2010.00205.x. [DOI] [PubMed] [Google Scholar]
- [71].Lovelock JD, Monasky MM, Jeong EM, Lardin HA, Liu H, Patel BG, Taglieri DM, Gu L, Kumar P, Pokhrel N, Zeng D, Belardinelli L, Sorescu D, Solaro RJ, Dudley SC., Jr. Circ. Res. 2012;110(6):841–850. doi: 10.1161/CIRCRESAHA.111.258251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Jeong EM, Monasky MM, Gu L, Taglieri DM, Patel BG, Liu H, Wang Q, Greener I, Dudley SC, Jr., Solaro RJ. J. Mol. Cell. Cardiol. 2013;56:44–54. doi: 10.1016/j.yjmcc.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wang X, Sun Z. J. Cell. Mol. Med. 2010;14(1–2):368–380. doi: 10.1111/j.1582-4934.2008.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Weyemi U, Caillou B, Talbot M, Ameziane-El-Hassani R, Lacroix L, Lagent-Chevallier O, Al Ghuzlan A, Roos D, Bidart JM, Virion A, Schlumberger M, Dupuy C. Endocr. Relat. Cancer. 2010;17(1):27–37. doi: 10.1677/ERC-09-0175. [DOI] [PubMed] [Google Scholar]
- [75].Gray SP, Di Marco E, Okabe J, Szyndralewiez C, Heitz F, Montezano AC, de Haan JB, Koulis C, El-Osta A, Andrews KL, Chin-Dusting JP, Touyz RM, Wingler K, Cooper ME, Schmidt HH, Jandeleit-Dahm KA. Circulation. 2013;127(18):1888–1902. doi: 10.1161/CIRCULATIONAHA.112.132159. [DOI] [PubMed] [Google Scholar]
- [76].Webster KA. Futur. Cardiol. 2012;8(6):863–884. doi: 10.2217/fca.12.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Maulik SK, Kumar S. Toxicol. Mech. Methods. 2012;22(5):359–366. doi: 10.3109/15376516.2012.666650. [DOI] [PubMed] [Google Scholar]
- [78].Coso S, Harrison I, Harrison CB, Vinh A, Sobey CG, Drummond GR, Williams ED, Selemidis S. Antioxid. Redox Signal. 2012;16(11):1229–1247. doi: 10.1089/ars.2011.4489. [DOI] [PubMed] [Google Scholar]
- [79].Sareila O, Kelkka T, Pizzolla A, Hultqvist M, Holmdahl R. Antioxid. Redox Signal. 2011;15(8):2197–2208. doi: 10.1089/ars.2010.3635. [DOI] [PubMed] [Google Scholar]
- [80].Williams HC, Griendling KK. J. Cardiovasc. Pharmacol. 2007;50(1):9–16. doi: 10.1097/FJC.0b013e318063e820. [DOI] [PubMed] [Google Scholar]
- [81].Cifuentes-Pagano E, Csanyi G, Pagano PJ. Cell. Mol. Life Sci. 2012;69(14):2315–2325. doi: 10.1007/s00018-012-1009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Egom EE, Ke Y, Musa H, Mohamed TM, Wang T, Cartwright E, Solaro RJ, Lei M. J. Mol. Cell. Cardiol. 2010;48(2):406–414. doi: 10.1016/j.yjmcc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Liu W, Zi M, Naumann R, Ulm S, Jin J, Taglieri DM, Prehar S, Gui J, Tsui H, Xiao RP, Neyses L, Solaro RJ, Ke Y, Cartwright EJ, Lei M, Wang X. Circulation. 2011;124(24):2702–2715. doi: 10.1161/CIRCULATIONAHA.111.048785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Roepstorff K, Rasmussen I, Sawada M, Cudre-Maroux C, Salmon P, Bokoch G, van Deurs B, Vilhardt F. J. Biol. Chem. 2008;283(12):7983–7993. doi: 10.1074/jbc.M708281200. [DOI] [PubMed] [Google Scholar]
- [85].Ahmed S, Prigmore E, Govind S, Veryard C, Kozma R, Wientjes FB, Segal AW, Lim L. J. Biol. Chem. 1998;273(25):15693–15701. doi: 10.1074/jbc.273.25.15693. [DOI] [PubMed] [Google Scholar]
- [86].Sells MA, Knaus UG, Bagrodia S, Ambrose DM, Bokoch GM. J. Chernoff, Curr. Biol. 1997;7(3):202–210. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- [87].El Benna J, Faust RP, Johnson JL, Babior BM. J. Biol. Chem. 1996;271(11):6374–6378. doi: 10.1074/jbc.271.11.6374. [DOI] [PubMed] [Google Scholar]
- [88].Fontayne A, Dang PM, Gougerot-Pocidalo MA, El-Benna J. Biochemistry. 2002;41(24):7743–7750. doi: 10.1021/bi011953s. [DOI] [PubMed] [Google Scholar]
- [89].Inanami O, Johnson JL, McAdara JK, Benna JE, Faust LR, Newburger PE, Babior BM. J. Biol. Chem. 1998;273(16):9539–9543. doi: 10.1074/jbc.273.16.9539. [DOI] [PubMed] [Google Scholar]
- [90].Tsai YR, Huang LJ, Lin HY, Hung YJ, Lee MR, Kuo SC, Hsu MF, Wang JP. Eur. J. Pharmacol. 2013;701(1–3):114–123. doi: 10.1016/j.ejphar.2013.01.015. [DOI] [PubMed] [Google Scholar]
- [91].Sumimoto H, Hata K, Mizuki K, Ito T, Kage Y, Sakaki Y, Fukumaki Y, Nakamura M, Takeshige K. J. Biol. Chem. 1996;271(36):22152–22158. doi: 10.1074/jbc.271.36.22152. [DOI] [PubMed] [Google Scholar]
- [92].Kawahara T, Ritsick D, Cheng G, Lambeth JD. J. Biol. Chem. 2005;280(36):31859–31869. doi: 10.1074/jbc.M501882200. [DOI] [PubMed] [Google Scholar]
- [93].Zhan Y, Virbasius JV, Song X, Pomerleau DP, Zhou GW. J. Biol. Chem. 2002;277(6):4512–4518. doi: 10.1074/jbc.M109520200. [DOI] [PubMed] [Google Scholar]
- [94].Hoyal CR, Gutierrez A, Young BM, Catz SD, Lin JH, Tsichlis PN, Babior BM. Proc. Natl. Acad. Sci. U. S. A. 2003;100(9):5130–5135. doi: 10.1073/pnas.1031526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Zhao X, Carnevale KA, Cathcart MK. J. Biol. Chem. 2003;278(42):40788–40792. doi: 10.1074/jbc.M302208200. [DOI] [PubMed] [Google Scholar]
- [96].Dorseuil O, Reibel L, Bokoch GM, Camonis J, Gacon G. J. Biol. Chem. 1996;271(1):83–88. doi: 10.1074/jbc.271.1.83. [DOI] [PubMed] [Google Scholar]
- [97].Nie B, Cheng N, Dinauer MC, Ye RD. Cell. Signal. 2010;22(5):770–782. doi: 10.1016/j.cellsig.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Heyworth PG, Bohl BP, Bokoch GM, Curnutte JT. J. Biol. Chem. 1994;269(49):30749–30752. [PubMed] [Google Scholar]
- [99].Martin GA, Bollag G, McCormick F, Abo A. EMBO J. 1995;14(9):1970–1978. doi: 10.1002/j.1460-2075.1995.tb07189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Li J, Zhu H, Shen E, Wan L, Arnold JM, Peng T. Diabetes. 2010;59(8):2033–2042. doi: 10.2337/db09-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Itakura A, Aslan JE, Kusanto BT, Phillips KG, Porter JE, Newton PK, Nan X, Insall RH, Chernoff J, McCarty OJ. PLoS One. 2013;8(9):e73063. doi: 10.1371/journal.pone.0073063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Smith SD, Jaffer ZM, Chernoff J, Ridley AJ. J. Cell Sci. 2008;121(Pt 22):3729–3736. doi: 10.1242/jcs.027680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Aslan JE, Itakura A, Haley KM, Tormoen GW, Loren CP, Baker SM, Pang J, Chernoff J, McCarty OJ. Arterioscler. Thromb. Vasc. Biol. 2013;33(7):1544–1551. doi: 10.1161/ATVBAHA.112.301165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Vadlamudi RK, Li F, Adam L, Nguyen D, Ohta Y, Stossel TP, Kumar R. Nat. Cell Biol. 2002;4(9):681–690. doi: 10.1038/ncb838. [DOI] [PubMed] [Google Scholar]
- [105].Maceyka M, Alvarez SE, Milstien S, Spiegel S. Mol. Cell. Biol. 2008;28(18):5687–5697. doi: 10.1128/MCB.00465-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].van Bruggen R, Anthony E, Fernandez-Borja M, Roos D. J. Biol. Chem. 2004;279(10):9097–9102. doi: 10.1074/jbc.M309284200. [DOI] [PubMed] [Google Scholar]
- [107].Nauseef WM, Volpp BD, McCormick S, Leidal KG, Clark RA. J. Biol. Chem. 1991;266(9):5911–5917. [PubMed] [Google Scholar]
- [108].el Benna J, Ruedi JM, Babior BM. J. Biol. Chem. 1994;269(9):6729–6734. [PubMed] [Google Scholar]
- [109].Wientjes FB, Reeves EP, Soskic V, Furthmayr H, Segal AW. Biochem. Biophys. Res. Commun. 2001;289(2):382–388. doi: 10.1006/bbrc.2001.5982. [DOI] [PubMed] [Google Scholar]
- [110].Shalom-Barak T, Knaus UG. J. Biol. Chem. 2002;277(43):40659–40665. doi: 10.1074/jbc.M206650200. [DOI] [PubMed] [Google Scholar]
- [111].Stanton RC. IUBMB Life. 2012;64(5):362–369. doi: 10.1002/iub.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Li Z, Hannigan M, Mo Z, Liu B, Lu W, Wu Y, Smrcka AV, Wu G, Li L, Liu M, Huang CK, Wu D. Cell. 2003;114(2):215–227. doi: 10.1016/s0092-8674(03)00559-2. [DOI] [PubMed] [Google Scholar]
- [113].Yablonski D, Kane LP, Qian D, Weiss A. EMBO J. 1998;17(19):5647–5657. doi: 10.1093/emboj/17.19.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Bubeck Wardenburg J, Pappu R, Bu JY, Mayer B, Chernoff J, Straus D, Chan AC. Immunity. 1998;9(5):607–616. doi: 10.1016/s1074-7613(00)80658-5. [DOI] [PubMed] [Google Scholar]
- [115].Li W, She H. Histol. Histopathol. 2000;15(3):947–955. doi: 10.14670/HH-15.947. [DOI] [PubMed] [Google Scholar]
- [116].Bustelo XR. Mol. Cell. Biol. 2000;20(5):1461–1477. doi: 10.1128/mcb.20.5.1461-1477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Brownlie RJ, Zamoyska R. Nat. Rev. Immunol. 2013;13(4):257–269. doi: 10.1038/nri3403. [DOI] [PubMed] [Google Scholar]
- [118].Allen JD, Jaffer ZM, Park SJ, Burgin S, Hofmann C, Sells MA, Chen S, Derr-Yellin E, Michels EG, McDaniel A, Bessler WK, Ingram DA, Atkinson SJ, Travers JB, Chernoff J, Clapp DW. Blood. 2009;113(12):2695–2705. doi: 10.1182/blood-2008-06-160861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Staser K, Shew MA, Michels EG, Mwanthi MM, Yang FC, Clapp DW, Park SJ. Exp. Hematol. 2013;41(1):56–66. e52. doi: 10.1016/j.exphem.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Ke Y, Lum H, Solaro RJ. Can. J. Physiol. Pharmacol. 2007;85(3–4):281–288. doi: 10.1139/y06-100. [DOI] [PubMed] [Google Scholar]
- [121].Birukova AA, Xing J, Fu P, Yakubov B, Dubrovskyi O, Fortune JA, Klibanov AM, Birukov KG. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010;299(5):L652–L663. doi: 10.1152/ajplung.00202.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Hinoki A, Kimura K, Higuchi S, Eguchi K, Takaguri A, Ishimaru K, Frank GD, Gerthoffer WT, Sommerville LJ, Autieri MV, Eguchi S. Hypertension. 2010;55(1):161–165. doi: 10.1161/HYPERTENSIONAHA.109.143057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Chew TL, Masaracchia RA, Goeckeler ZM, Wysolmerski RB. J. Muscle Res. Cell Motil. 1998;19(8):839–854. doi: 10.1023/a:1005417926585. [DOI] [PubMed] [Google Scholar]
- [124].Bright MD, Frankel G. Int. J. Biochem. Cell Biol. 2011;43(12):1776–1781. doi: 10.1016/j.biocel.2011.08.015. [DOI] [PubMed] [Google Scholar]
- [125].Chu J, Pham NT, Olate N, Kislitsyna K, Day MC, LeTourneau PA, Kots A, Stewart RH, Laine GA, Cox CS, Jr., Uray K. J. Biol. Chem. 2013;288(2):1200–1213. doi: 10.1074/jbc.M112.370718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Brzeska H, Szczepanowska J, Matsumura F, Korn ED. Cell Motil. Cytoskeleton. 2004;58(3):186–199. doi: 10.1002/cm.20009. [DOI] [PubMed] [Google Scholar]
- [127].Movat HZ, Weiser WJ, Glynn MF, Mustard JF. J. Cell Biol. 1965;27(3):531–543. doi: 10.1083/jcb.27.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Pandey D, Goyal P, Bamburg JR, Siess W. Blood. 2006;107(2):575–583. doi: 10.1182/blood-2004-11-4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Pandey D, Goyal P, Dwivedi S, Siess W. Blood. 2009;114(2):415–424. doi: 10.1182/blood-2008-10-183582. [DOI] [PubMed] [Google Scholar]
- [130].Burbelo PD, Kozak CA, Finegold AA, Hall A, Pirone DM. Gene. 1999;232(2):209–215. doi: 10.1016/s0378-1119(99)00110-9. [DOI] [PubMed] [Google Scholar]
- [131].DeSantiago J, Bare DJ, Ke Y, Sheehan KA, Solaro RJ, Banach K. J. Mol. Cell. Cardiol. 2013;60:121–128. doi: 10.1016/j.yjmcc.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Desantiago J, Bare DJ, Xiao L, Ke Y, Solaro RJ, Banach K. J. Mol. Cell. Cardiol. 2014;67:77–85. doi: 10.1016/j.yjmcc.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Diebold I, Djordjevic T, Petry A, Hatzelmann A, Tenor H, Hess J, Gorlach A. Circ. Res. 2009;104(10):1169–1177. doi: 10.1161/CIRCRESAHA.109.196592. [DOI] [PubMed] [Google Scholar]
- [134].Madamanchi NR, Runge MS. Am. J. Physiol. Heart Circ. Physiol. 2010;298(1):H1–H2. doi: 10.1152/ajpheart.01020.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Licciulli S, Maksimoska J, Zhou C, Troutman S, Kota S, Liu Q, Duron S, Campbell D, Chernoff J, Field J, Marmorstein R, Kissil JL. J. Biol. Chem. 2013;288(40):29105–29114. doi: 10.1074/jbc.M113.510933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Dolan BM, Duron SG, Campbell DA, Vollrath B, Shankaranarayana Rao BS, Ko HY, Lin GG, Govindarajan A, Choi SY, Tonegawa S. Proc. Natl. Acad. Sci. U. S. A. 2013;110(14):5671–5676. doi: 10.1073/pnas.1219383110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Yi C, Maksimoska J, Marmorstein R, Kissil JL. Biochem. Pharmacol. 2010;80(5):683–689. doi: 10.1016/j.bcp.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Nheu TV, He H, Hirokawa Y, Tamaki K, Florin L, Schmitz ML, Suzuki-Takahashi I, Jorissen RN, Burgess AW, Nishimura S, Wood J, Maruta H. Cancer J. 2002;8(4):328–336. doi: 10.1097/00130404-200207000-00009. [DOI] [PubMed] [Google Scholar]
- [139].Cai XZ, Wang J, Li XD, Wang GL, Liu FN, Cheng MS, Li F. Cancer Biol. Ther. 2009;8(14):1360–1368. doi: 10.4161/cbt.8.14.8720. [DOI] [PubMed] [Google Scholar]
- [140].Jha RK, Wu YI, Zawistowski JS, MacNevin C, Hahn KM, Kuhlman B. J. Mol. Biol. 2011;413(2):513–522. doi: 10.1016/j.jmb.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Jha RK, Leaver-Fay A, Yin S, Wu Y, Butterfoss GL, Szyperski T, Dokholyan NV, Kuhlman B. J. Mol. Biol. 2010;400(2):257–270. doi: 10.1016/j.jmb.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Yi C, Wilker EW, Yaffe MB, Stemmer-Rachamimov A, Kissil JL. Cancer Res. 2008;68(19):7932–7937. doi: 10.1158/0008-5472.CAN-08-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Ma Y, McCarty SK, Kapuriya NP, Brendel VJ, Wang C, Zhang X, Jarjoura D, Saji M, Chen CS, Ringel MD. J. Clin. Endocrinol. Metab. 2013;98(8):E1314–E1322. doi: 10.1210/jc.2012-3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Ong CC, Jubb AM, Jakubiak D, Zhou W, Rudolph J, Haverty PM, Kowanetz M, Yan Y, Tremayne J, Lisle R, Harris AL, Friedman LS, Belvin M, Middleton MR, Blackwood EM, Koeppen H, Hoeflich KP. J. Natl. Cancer Inst. 2013;105(9):606–607. doi: 10.1093/jnci/djt054. [DOI] [PubMed] [Google Scholar]
- [145].Ghosh A, Awasthi S, Peterson JR, Hamburger AW. Br. J. Cancer. 2013;108(3):557–563. doi: 10.1038/bjc.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Simmons DL. Drug Discov. Today. 2006;11(5–6):210–219. doi: 10.1016/S1359-6446(05)03721-9. [DOI] [PubMed] [Google Scholar]
- [147].McFawn PK, Shen L, Vincent SG, Mak A, Van Eyk JE, Fisher JT. Am. J. Physiol. Lung Cell. Mol. Physiol. 2003;284(5):L863–L870. doi: 10.1152/ajplung.00068.2002. [DOI] [PubMed] [Google Scholar]
- [148].Kichina JV, Goc A, Al-Husein B, Somanath PR, Kandel ES. Expert Opin. Ther. Targets. 2010;14(7):703–725. doi: 10.1517/14728222.2010.492779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Moon JH, Kim TH, Lee HM, Lee SH, Choe W, Kim HK, Lee JH, Oh KH. Am. J. Rhinol. Allergy. 2009;23(4):370–376. doi: 10.2500/ajra.2009.23.3340. [DOI] [PubMed] [Google Scholar]
- [150].Sevin CM, Newcomb DC, Toki S, Han W, Sherrill TP, Boswell MG, Zhu Z, Collins RD, Boyd KL, Goleniewska K, Huckabee MM, Blackwell TS, Peebles RS., Jr. Am. J. Respir. Cell Mol. Biol. 2013;49(3):396–402. doi: 10.1165/rcmb.2012-0442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Banerjee ER, Henderson WR., Jr. Allergy Asthma Clin. Immunol. 2013;9(1):6. doi: 10.1186/1710-1492-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Banerjee ER, Henderson WR., Jr. Clin. Mol. Allergy. 2012;10(1):2. doi: 10.1186/1476-7961-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Debbabi M, Kroviarski Y, Bournier O, Gougerot-Pocidalo MA, El-Benna J, Dang PM. Faseb J. 2013;27(4):1733–1748. doi: 10.1096/fj.12-216432. [DOI] [PubMed] [Google Scholar]
- [154].Dahan I, Issaeva I, Gorzalczany Y, Sigal N, Hirshberg M, Pick E. J. Biol. Chem. 2002;277(10):8421–8432. doi: 10.1074/jbc.M109778200. [DOI] [PubMed] [Google Scholar]
- [155].Belambri SA, Hurtado-Nedelec M, Senator A, Makni-Maalej K, Fay M, Gougerot-Pocidalo MA, Marie JC, Dang PM, El-Benna J. Am. J. Blood Res. 2012;2(3):187–193. [PMC free article] [PubMed] [Google Scholar]
- [156].Cheng G, Ritsick D, Lambeth JD. J. Biol. Chem. 2004;279(33):34250–34255. doi: 10.1074/jbc.M400660200. [DOI] [PubMed] [Google Scholar]
- [157].von Lohneysen K, Noack D, Wood MR, Friedman JS, Knaus UG. Mol. Cell. Biol. 2010;30(4):961–975. doi: 10.1128/MCB.01393-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Kawahara T, Jackson HM, Smith SM, Simpson PD, Lambeth JD. Biochemistry. 2011;50(12):2013–2025. doi: 10.1021/bi1020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Dupuy C, Ohayon R, Valent A, Noel-Hudson MS, Deme D, Virion A. J. Biol. Chem. 1999;274(52):37265–37269. doi: 10.1074/jbc.274.52.37265. [DOI] [PubMed] [Google Scholar]
- [160].De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F. J. Biol. Chem. 2000;275(30):23227–23233. doi: 10.1074/jbc.M000916200. [DOI] [PubMed] [Google Scholar]
- [161].Singh DK, Kumar D, Siddiqui Z, Basu SK, Kumar V, Rao KV. Cell. 2005;121(2):281–293. doi: 10.1016/j.cell.2005.02.036. [DOI] [PubMed] [Google Scholar]
- [162].Banfi B, Molnar G, Maturana A, Steger K, Hegedus B, Demaurex N, Krause KH. J. Biol. Chem. 2001;276(40):37594–37601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- [163].Parrini MC, Matsuda M, de Gunzburg J. Biochem. Soc. Trans. 2005;33(Pt 4):646–648. doi: 10.1042/BST0330646. [DOI] [PubMed] [Google Scholar]
- [164].Pirruccello M, Sondermann H, Pelton JG, Pellicena P, Hoelz A, Chernoff J, Wemmer DE, Kuriyan J. J. Mol. Biol. 2006;361(2):312–326. doi: 10.1016/j.jmb.2006.06.017. [DOI] [PubMed] [Google Scholar]
- [165].Volz HC, Kaya Z, Katus HA, Andrassy M. Semin. Thromb. Hemost. 2010;36(2):185–194. doi: 10.1055/s-0030-1251503. [DOI] [PubMed] [Google Scholar]
- [166].Bokoch GM. Immunol. Res. 2000;21(2–3):139–148. doi: 10.1385/IR:21:2-3:139. [DOI] [PubMed] [Google Scholar]
- [167].Funayama A, Shishido T, Netsu S, Narumi T, Kadowaki S, Takahashi H, Miyamoto T, Watanabe T, Woo CH, Abe J, Kuwahara K, Nakao K, Takeishi Y, Kubota I. Cardiovasc. Res. 2013;99(4):657–664. doi: 10.1093/cvr/cvt128. [DOI] [PMC free article] [PubMed] [Google Scholar]