Abstract
Acute hypertension (HTN) in hospitalized children and adolescents occurs relatively frequently and in some cases, if not recognized and treated promptly, it can lead to hypertensive crisis with potentially significant morbidity and mortality. In contrast to adults, where acute HTN is most likely due to uncontrolled primary HTN, children and adolescents with acute HTN are more likely to have secondary HTN. This review will briefly cover evaluation of acute HTN and various age specific etiologies of secondary HTN and provide more in-depth discussion on treatment target, potential risks of acute HTN therapy, available pediatric data on intravenous and oral antihypertensive agents, and propose treatment schema including unique therapy of specific secondary HTN scenarios.
Keywords: Pediatric acute hypertension, hypertensive crisis treatment, hypertensive urgency, hypertensive emergency, nicardipine, labetalol, hydralazine, isradipine, clonidine
Introduction
Acute hypertension (HTN) in hospitalized children and adolescents occurs with relative frequency and can rarely result in medical emergency associated with significant morbidity and mortality, most commonly in the central nervous system [1]. Thus, prompt recognition, initiation of therapy, and continued monitoring to assess for HTN complications, treatment efficacy, and side effects are vital.
HTN in children and adolescents is defined as systolic blood pressure (BP) and/or diastolic BP ≥ the 95th percentile for age, gender and height on at least three separate occasions and is further classified as Stage 1 and Stage 2 [2]. Stage 1 HTN is defined as the systolic and/or diastolic BP ≥ the 95th percentile to 5 mmHg above the 99th percentile. Stage 2 HTN is defined as the systolic and/or diastolic BP ≥ 5 mmHg above the 99th percentile [2]. Hypertensive crisis is defined as a rapid increase in BP, usually far above the threshold for stage 2 HTN [3]. Traditionally, it has been divided into two categories: hypertensive emergency and urgency. However, this distinction may be arbitrary and relies on the judgment of the treating clinician [4]. Both require prompt pharmacologic intervention for BP reduction [5]. Hypertensive emergency is defined as acute severe symptomatic HTN with potentially life-threatening symptoms or target organ damage, while urgency is a similar level of HTN but without severe symptoms or target organ damage [6].
There are very limited published data on the prevalence of pediatric acute HTN in contrast to chronic HTN [7]. In one retrospective study performed in a tertiary care center, 35 of 246 children admitted to the hospital with sustained HTN had severe HTN with complications including encephalopathy and congestive heart failure [8]. Yang and colleagues reported a total of 55 children who presented with hypertensive crisis to their pediatric emergency department; of whom 84% were classified to have hypertensive urgency and 16% as hypertensive emergency [9].
In this review, we will briefly discuss the evaluation of pediatric patients with acute HTN including age specific secondary HTN etiologies as well as a more in-depth discussion of the therapy target and treatment options: both intravenous (IV) and oral agents. Furthermore, we present a therapeutic schema including a number of specific secondary HTN.
Evaluation
The first priority when approaching patients with suspected acute HTN is to confirm the BP measurement and to rapidly assess the severity of HTN. This is preferably done by manual auscultation using an appropriate size cuff. The Fourth report on high BP in children and adolescents provide guidelines on proper equipment and technique [2]. If hypertensive crisis is confirmed, therapy and evaluation should occur concurrently, not only in hypertensive emergency, but also in urgency to prevent patients from progressing to hypertensive emergency [1, 4, 10].
Targeted history and physical examination should seek the potential etiology of HTN and severity and duration of HTN. This includes assessing for target organ damage and contraindications to urgent initiation of therapy such as head trauma, stroke, intracranial mass and pain. In contrast to adults, where HTN crisis is most often due to uncontrolled primary HTN, children and adolescents are more likely to have secondary HTN. In neonates, most common etiologies are renovascular disease (including thrombi from umbilical artery lines and renal artery stenosis), coarctation of the aorta, autosomal recessive polycystic kidney disease, renal parenchymal disease and caffeine overdose. In children, most common etiologies are renal parenchymal disease (including acute glomerulonephritis, hemolytic uremic syndrome and reflux nephropathy), renovascular disease, coarctation of aorta and neuroendocrine tumors such as Pheochromocytoma. Lastly, adolescents have similar etiologies as children, and in addition, substance abuse including cocaine and amphetamines, preeclampsia, and drug overdose of pseudoephedrine, phenylpropanolamines, nonsteroidal anti-inflammatory drug and monoamine oxidase inhibitors must all be considered [3]. Thus, initial laboratory study should include serum chemistries, renal function, plasma rennin activity, aldosterone, urinalysis and if appropriate, plasma metanephrines or urine catecholamines.
When patients are more stable, evaluation should expand to imaging studies such as renal ultrasonography to screen for renal structural and parenchymal disease and echocardiography to evaluate for left ventricular hypertrophy. Renal ultrasound with Doppler study is not definitive for evaluating renal vascular disease [11], and thus, if suspicion for renal vascular disease is high, more definitive studies such as computed tomography angiography, magnetic resonance angiography and/or digital subtraction angiography are recommended. Dilated ophthalmologic exam should also take place. Brain MRI should be obtained if there is concern for posterior reversible encephalopathy syndrome. For more detailed discussions on the pathophysiology and evaluation of pediatric hypertensive crisis, readers are encouraged to seek these publications [3, 4, 7].
Treatment target
The rate of BP rise is as important as the severity of HTN, and therefore, after prompt recognition of hypertensive crisis, the immediate initiation of therapy is required. The key to treatment is to lower the BP promptly but gradually, since the duration of HTN is often difficult to determine upon presentation. The current recommendation is to reduce mean arterial pressure (MAP) by no more than 25% within the first 8-12 hours and then gradually normalizing in the next 48 to 72 hours [4, 12]. Care must be given not to lower the BP too rapidly, especially in patients with long-standing HTN, to avoid inducing organ ischemia including cerebral hypoperfusion and even death [4, 13]. In patients with chronic HTN, the cerebral autoregulation curve may be shifted to a higher BP range to protect the brain from hyperperfusion, as indicated by the dotted line in Figure 1 [4, 14]. Lowering the BP too rapidly may result in ischemic stroke [5].
Figure 1.

Altered cerebral perfusion auto-regulation (indicated by the dotted line) in chronic hypertension. Copyright permission obtained from reference [4]
Treatment options
Table 1 summarizes antihypertensive medications used to treat hospitalized children and adolescents with acute HTN, including their class, route, dosing and potential side effects. As in the case with many pediatric antihypertensive medications, a number of these agents do not have pediatric FDA approval for acute HTN, and the following discussion is based on published reports.
Table 1. Antihypertensive agents for hypertensive crisis.
| Drug | Class | Route | Dose | Comments |
|---|---|---|---|---|
| Nicardipine | Calcium channel blocker | IV bolus or infusion | Bolus: 30 µg/kg up to 2 mg/dose; Infusion: 0.5-4 µg/kg/min | May cause reflex tachycardia. |
| Labetalol | α and β adrenergic blocker | IV bolus or infusion | Bolus: 0.2-1 mg/kg/dose, up to 40 mg/dose; Infusion 0.25-3 mg/kg/hr | Contraindicated in asthma, heart failure and diabetics. May cause hyperkalemia and hypoglycemia. Does not cause reflex tachycardia |
| Hydralazine | Direct vasodilator | IV bolus or IM | IV: 0.2-0.6 mg/kg, maximum single dose 20 mg | May cause reflex tachycardia, fluid retention or headaches. When given as IV bolus, give every 4 hours |
| Esmolol | β1 adrenergic blocker | IV infusion | 100-500 µg/kg/min, up to 1000 µg/kg/min | May cause bradycardia. Contraindicated in asthma and heart failure. Very short-acting. |
| Enalaprilat | ACE inhibitor | IV bolus | 5-10 µg/kg/dose up to 1.2 mg/dose | May cause prolonged hypotension, hyperkalemia and acute renal failure |
| Fenoldopam | Dopamine receptor agonist | IV infusion | 0.2-0.8 µg/kg/min | Limited pediatric experience. |
| Sodium Nitroprusside | Direct vasodilator | IV infusion | 0.5-10 µg/kg/min | Associated with cyanide and thiocyanate toxicity. Monitor cyanide levels with prolonged use (>48 hours) or in hepatic or renal failure; or co-administer with sodium thiosulfate |
| Phentolamine | α blocker | IV bolus | 0.05-0.1 mg/kg/dose, up to 5 mg | May cause tachycardia |
| Clevidipine | Calcium channel blocker | IV infusion | 0.5-3.5 µg/kg/min (*limited pediatric data on dosing) | Contraindicated in those with egg and soy allergy as well as lipid disorders |
| Diazoxide | Direct vasodilator | IV bolus | 1-3 mg/kg every 5-15 min | Large boluses may result in severe hypotension |
| Isradipine | Calcium channel blocker | PO | 0.05-0.1 mg/kg/dose up to 5 mg/dose | Stable suspension can be compounded |
| Clonidine | Central α agonist | PO | 0.05-0.1 mg/dose, may be repeated up to 0.8 mg total | Side effects include dry mouth and sedation |
| Minoxidil | Direct vasodilator | PO | 0.1-0.2 mg/kg/dose up to 10 mg/dose | Most potent vasodilator; long-acting; long-term effects include fluid retention and hirsutism |
IV agents
Nicardipine and labetalol are the first line IV antihypertensive agents used. Esmolol is less frequently used with minimal published pediatric data. Previously, nitroprusside was commonly used; however, this has fallen out of favor due to problems with cyanide toxicity and tachyphylaxis that could develop with prolonged use. Hydralazine IV is an acceptable alternative to labetalol bolus. Enalaprilat is also occasionally used; however, it can induce acute kidney injury and must be used with caution in neonates and patients with chronic kidney disease and volume depletion. Newer IV agents like fenoldopam and clevidipine are not commonly used due to limited pediatric studies, but may be promising based on adult trials. Phentolamine may be helpful in catecholamine and cocaine induced hypertensive crisis.
Nicardipine is a second generation dihydropyridine calcium channel blocker and is considered as a first line agent in hypertensive crisis [2]. It induces vascular smooth muscle relaxation and peripheral vasodilation; however, it maintains some cardiac activity, making it capable of decreasing systemic vascular resistance without inducing extreme tachycardia [5]. It has a rapid onset of action, usually within 1-2 minutes with three hours duration of action after a single dose. These characteristics make this agent attractive for acute HTN due to various etiologies. Dosing starts at 0.5-1 µg/kg/min, and can be titrated up every 15 to 30 minutes, with a maximum dose of 4 µg/kg/min [4, 5]. Nicardipine was effective and safe in controlling hypertensive emergency in a case series of 7 pediatric patients. Two of the seven patients who received the infusion through a peripheral line developed superficial thrombophlebitis [15]. Flynn and colleagues published the largest pediatric cohort of patients who received nicardipine for severe HTN; 29 children were included aged 2 days of life to almost 18 years old [16]. The starting dose administered was 0.2-1.3 µg/kg/min, and BP target, identified as the 95th percentile for age and gender and height were achieved within 0.5 to 9 hours after the initiation of therapy with a mean dose of 0.3-4 µg/kg/min. None of the patients required additional antihypertensive agent. Adverse effects included hypotension, tachycardia, flushing and palpitations. Nicardipine is effective and safe in the management of hypertensive crisis; however, pediatric randomized controlled trial has not been performed to date.
Labetalol is an α1 and β adrenergic blocker that causes a reduction in peripheral vascular resistance with little effect on cardiac output that can be administered orally or intravenously. By blocking α1 receptors, it causes vasodilation. In hypertensive crisis, only IV route should be used. Because of its negative inotropic effects, it should not be used in individuals with bronchospasms or congestive heart failure. It may also mask the symptoms of hypoglycemia such as increased heart rate or tremors and therefore, it should not be used in diabetic patients. It has a rapid onset of action in 2-5 minutes, peaks at 5-15 minutes and its effect lasts up to 2-4 hours. It can be administered as a bolus dose of 0.2 – 1 mg/kg up to a maximum of 40 mg or as a continuous infusion of 0.25-3 mg/kg/h [2, 4, 6]. Thomas and colleagues reported 27 children <24 months of age who received 37 continuous infusions of either labetalol, nitroprusside or nicardipine for severe HTNs. All three agents led to significant reductions in BP within the first 6 hours of therapy. Time to achieve 20% reduction in mean systolic BP was comparable between the three agents. Dose response relationship demonstrated BP reduction with dosages up to 0.59 mg/kg/h, with doses > 0.6 mg/kg/h demonstrating minimal further reduction in BP. Patients with ischemic or traumatic brain injury were more likely to develop hypotension with labetalol in comparison to nitroprusside and nicardipine and required discontinuation of the infusion. Thus, the authors suggested caution in initiating labetalol in this patient population [17].
Hydralazine is a direct arteriolar smooth muscle vasodilator that decreases systemic vascular resistance whose mechanism of action may involve alteration of intracellular calcium metabolism. Its onset of action is within 10 minutes of administration, and it has maximal effects 10-80 minutes after administration; lasting for 2-4 hours [4]. When immediate IV access is not available, it can be given intramuscularly. By stimulating the sympathetic nervous system through its effects on arteriolar smooth muscle and its absence of opposing negative inotropic effects, it often causes reflex tachycardia, activation of the renin angiotensin system and sodium retention. Bolus dose is given at 0.2 to 0.6 mg/kg, with a maximum dose of 20 mg [2, 4]. Conversion to oral dosing is easily performed, given at 0.25 mg/kg [4]. There has been no pediatric published data to date on efficacy and safety of hydralazine for its use in acute HTN therapy.
Esmolol is an ultra-short acting cardioselective β1 adrenergic blocker with its onset of action occurring less than 1 minute and a half-life of 10-20 minutes. It is well-suited for use in individuals with multi-organ failure because its metabolism is independent of both hepatic and renal metabolism [6]. For hypertensive crisis secondary to catecholamine excess, esmolol should not be used as HTN is propagated by persistent α stimulation. At high doses, its β1 selectivity is lost and β2 receptors are activated in bronchioles and the peripheral vascular which may induce bronchoconstriction [5]. A loading dose is given at 100 to 500 µg/kg followed by infusion of 50 to 150 µg/kg/min, and it can be titrated every 10-15 minutes up to 1000 µg/kg/min [4, 5, 7]. Adverse effects include bradycardia, hypoglycemia, and potential for bronchoconstriction. Tabbutt and colleagues reported the results of a multicenter, randomized, double-blind trial that evaluated the efficacy of esmolol in 116 pediatric patients undergoing surgery for coarctation of aorta repair. Patients younger than 6 years old were randomized into three dosing groups which included bolus doses of 125 µg/kg, 250 µg/kg or 500 µg/kg followed by continuous infusions at the same dose per minute. All groups showed a significant decrease in systolic BP within the first 6 hours of administration with no statistically significant differences in the lowering of BP between age groups or between dosing groups. Reported adverse effects included hypotension, wheezing, bradycardia and reaction at the injection site [18]. Pediatric studies of esmolol in non-cardiac conditions have not been reported.
Enalaprilat is the only available IV angiotensin converting enzyme (ACE) inhibitor and may be very effective in patients with renin-mediated HTN. However, there has never been a systematic study on safety and efficacy of this agent, and published recommended dose is based on a small case series limited to neonates [19]. The onset of action is 15 minutes, with effects lasting up to 24 hours. A dose of 5 to 10 µcg/kg/dose is given up to 1.25 mg/dose [2, 3, 4]. In a case series of 10 premature infants who received enalaprilat dose of 7.4 to 22.9 µg/kg/24 hours, MAP decreased within 30 minutes of administration, and its effect on BP persisted for a median of 12 hours. Adverse effects included prolonged hypotension, oliguria, hyperkalemia, and increased serum creatinine [19]. With higher plasma renin activity and frequency of renovascular HTN in pediatrics, enalaprilat traditionally has been used infrequently.
Fenoldopam is a selective dopamine D1 receptor agonist that binds to α2 adrenoreceptors. When administered intravenously, it leads to peripheral, renal, cerebral and coronary artery vasodilation. The onset of action is within 5 minutes with maximal effect at 1 hour [4]. In a pediatric trial of fenoldopam, 77 children aged 1 month to 12 years of age undergoing controlled hypotension during surgery were randomly assigned to one of five treatment groups (placebo or 0.05, 0.2, 0.8 or 3.2 µg/kg/min) [20]. Dosages of 0.8 and 3.2 µg/kg/min significantly decreased BP, but doses greater than 1.2 µg/kg/min resulted in increased heart rate without additional reduction in BP. The recommended dose is 0.2 to 0.8 µg/kg/min [4]. No dosing adjustment is required for renal impairment. Side effects include tachycardia, flushing and headaches.
Clevidipine is an ultrashort-acting dihydropyridine calcium channel antagonist with potent arterial vasodilating activity to reduce systemic vascular resistance without having negative chronotropic or inotropic effects on the heart. Its onset of action is within 2 minutes with a rapid offset of 5-15 minutes and a half-life of less than 1 minute. It undergoes rapid metabolism by non-specific blood and tissue esterases, and therefore no dosing adjustment is required in those with renal or hepatic impairment [21]. Adult trials have demonstrated its efficacy in rapidly controlling BP in various clinical settings with a favorable adverse effect profile [22]. Because it is prepared in a lipid solution, it is contraindicated in those with egg and soy allergy as well as those with lipid disorders [3, 23]. One retrospective report included 14 pediatric patients, 11 months to 15 years old, undergoing surgery for congenital heart disease [24]. In 6 patients, it was administered as a continuous infusion for postoperative HTN; in 5 patients, a bolus dose for HTN during emergence from anesthesia; in 3 patients, it was given as continuous infusion for intraoperative BP control during cooling and cardiopulmonary bypass. For the 6 postoperative patients, dosing requirement ranged from 1 to 7 μg/kg/min to reach the target BP within 5 minutes. Two patients on higher dosing (5-7 μg/kg/min) required propranolol to treat tachycardia. . In the 5 patients with postoperative HTN, nine bolus doses (10–15 μg/kg/dose) were administered and resulted in a significant decrease in MAP without therapy needed for reflex tachycardia. Patients on cardiopulmonary bypass failed to reach the target MAP despite doses up to 10 μg/kg/min, and this was hypothesized to be secondary to inactivation of calcium channels during hypothermia.
Diazoxide is a direct vasodilator that increases the permeability of vascular smooth muscle membrane to potassium. In a multicenter study, McCrory and colleagues investigated its safety and efficacy in 36 children with severe symptomatic HTN aged 2 months to 18 years old. Diazoxide IV quickly reduced BP without significant adverse effects, and was effective at lowering BP in 94% of the patients [25]. Administering mini-boluses are recommended for injection since large boluses can result in severe hypotension. Recommended dosage is 1-3 mg/kg every 5-15 minutes [26]. This agent is unavailable in the United States since 2006 but may be available in other countries [personal correspondence with Merck].
Phentolamine is an α adrenergic antagonist that is used to treat catecholamine-induced HTN, as in pheochromocytoma, cocaine or pseudoephedrine overdose. Beta blockers can be added to prevent tachycardia once α blockade is achieved. Suggested dosing is 0.1 mg/kg with a maximum dose of 5 mg [3, 4, 6].
Sodium nitroprusside is a direct vasodilator of both venous and arteriolar smooth muscle cells with a rapid onset of action, 1-2 minutes, and a plasma half-life of less than 10 minutes. It reduces total peripheral resistance by releasing nitric oxide, which dilates venules and arterioles, thus reducing preload and afterload and making it useful in congestive heart failure from hypertensive crisis. Suggested dosing is 0.5-10 µg/kg/min [2, 4]. Toxicity is the result of the metabolism of nitroprusside to cyanide and thiocyanate and its risks increase after 24-48 hours or in patients with decreased renal function. Cyanide toxicity results in metabolic acidosis, methemoglobinemia, tachycardia, and altered mental status. Although thiocyanate has a less toxic profile than cyanide, its toxicity includes altered mental status, nausea, vomiting, psychosis, seizures, anorexia and coma. Co-administration with sodium thiosulfate can reduce elevated serum cyanide concentrations. Because of this toxicity potential, most recommend limiting nitroprusside use to situations where no other suitable agent is available [6].
Oral agents
Isradipine is a second generation dihydropyridine calcium channel antagonist with primary effects on L-type calcium channels, thereby causing vasodilation without a significant effect on myocardial function. It has an onset of action of 30-60 minutes, with its peak at 2-3 hours, and half-life is approximately 3-8 hours. Recommended starting dosing is 0.05 to 0.1 mg/kg per dose up to 5 mg. Miyashita and colleagues reported a retrospective single center experience of isradipine based on 282 hospitalized patients, aged 0.1 to 21.9 years with acute HTN [27]. All age groups were found to have a significant reduction in BP with doses of 0.06-0.11 mg/kg. Reported adverse events included nausea, emesis, headache, hypotension, dizziness, flushing and palpitations. The patients that experienced hypotension were concurrently taking azole antifungals, which inhibit the metabolism of isradipine. They recommended an initial dose 0.05 mg/kg for children < 2 years old because of higher rates of hypotension in this age group. Precise dosing can be achieved because a stable extemporaneous suspension may be compounded for infants and small children [28].
Clonidine is a centrally acting α2 adrenergic agonist which causes vasodilation by decreasing central sympathetic tone. It has an onset of action of approximately 15-30 minutes after ingestion. Its maximal effects are seen in 30-60 minutes [29]. Recommended dosing is 0.05-0.1 mg/dose in older children which can be repeated hourly up to eight hours (0.8 mg) [4]. It is minimally removed by hemodialysis, and therefore, it is very useful in dialysis patients with acute HTN [4, 29]. The most common adverse effects are dry mouth and sedation.
Minoxidil is a direct arterial vasodilator that causes potassium efflux by opening potassium channels in vascular smooth muscle cells, thus leading to relaxation and hyperpolarization [5, 30]. Its peak effect is within 1 hour with a half-life of 4 hours. Its effects may last up to 8-12 hours. Recommended dose is 0.1 to 0.2 mg/kg per dose up to 10 mg per dose. Because it is renally excreted, dose adjustment is necessary in patients with reduced renal function. It is removed by dialysis and should be dosed after each dialysis session. Use in children with severe chronic HTN refractory to other medications and for acute elevation in BP with chronic HTN has been reported [31, 32]. Long-term adverse effects are fluid retention and hirsutism. Minoxidil is not available in a stable oral suspension like isradipine and clonidine.
Traditionally, short-acting nifedipine, another calcium channel blocker, has been recommended for management of acute HTN in pediatrics [12, 33, 34]. However, its use is contraindicated in adults with acute HTN because of the risk of sudden and severe reduction in BP that can lead to ischemic events [6, 35, 36, 37]. The pediatric literature on short-acting nifedipine has been more controversial. While its efficacy has been supported by several case series, reports of adverse events after its use in children, including sudden severe reduction in BP, change in neurological status [36], cerebral ischemia [38], and ventricular arrhythmia [39] exist. Additionally, administration of short acting nifedipine to infants and small children is difficult and risky because of the high concentration of drug in the liquid within the capsule [4]. For these reasons, some experts have recommended that alternative agents be used, especially with the fact that there are other oral agents (such as isradipine) available that can be compounded to stable extemporaneous suspension [41, 42].
Treatment Schema
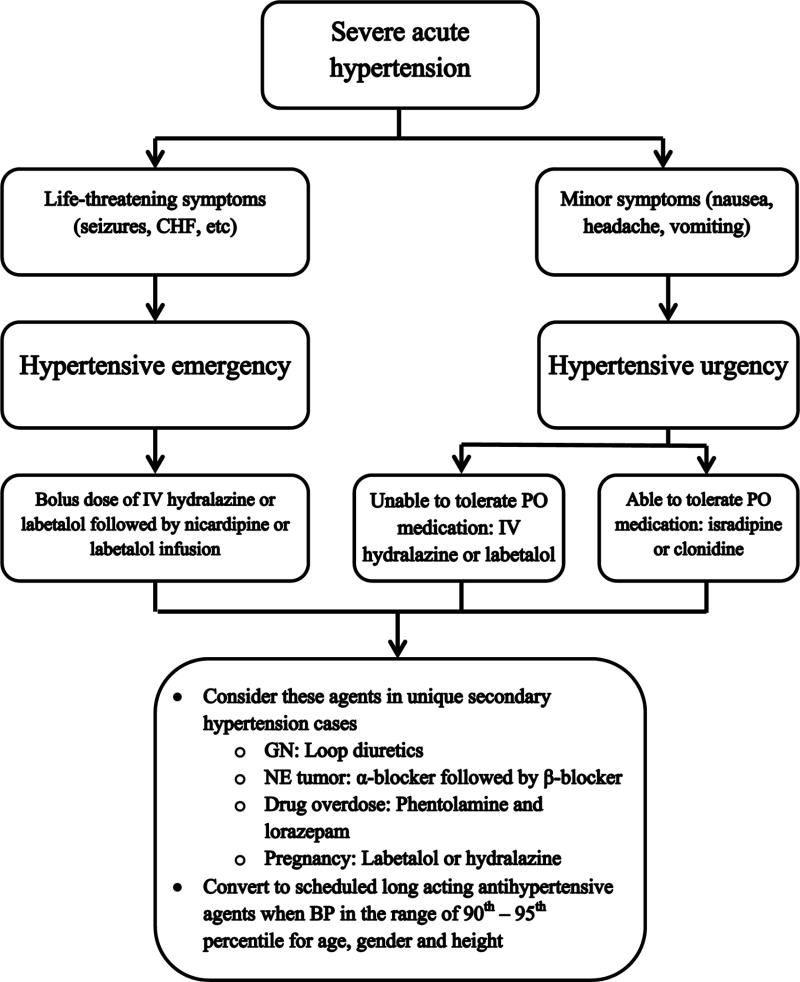
As illustrated in Figure 2, we recommend the following general approach to treatment of acute HTN in the hospital setting. After initial assessment, the provider must determine whether IV agent or oral agent is optimal to initiate therapy. Factors such as degree of HTN, type and severity of HTN symptoms, patient location within hospital, and tolerability of oral medication can be used to make this determination. In more severe hypertensive crisis cases where patients are in areas of the hospital such as the emergency unit and the general pediatric floor where continuous infusion of antihypertensive medication is not routinely performed, careful administration of an initial IV bolus of labetalol or hydralazine is recommended. These patients must have follow-up BP monitoring with oscillometric device programmed to measure repeated BP at short intervals until they are transferred to an intensive care unit. Once in the intensive care unit, continuous infusion of IV antihypertensive agents such as nicardipine and labetalol is recommended with the use of intra-arterial catheter to monitor BP reduction. In less severe hypertensive crisis cases, IV antihypertensive agents are appropriate if the urgency is secondary to an acute process with a rapid change in BP. Conversely, in more chronic conditions, the use of oral medications is indicated with the goal to lower blood pressure less rapidly. However, in cases where oral medication is not tolerated, the use of IV medication is accepted.
Figure 2.
Proposed algorithm for management of acute hypertension in children and adolescents. Copyright permission obtained and adapted from [4]. CHF: congestive heart failure, IV: intravenous, PO: oral, GN: glomerulonephritis, NE: neuroendocrine, BP: blood pressure
Once patients are out of hypertensive crisis and appear to require long-term antihypertensive therapy, our experience has been to start transitioning patients from short acting medication to long acting oral medications when their BP is around 90th to 95th percentile for their age, gender and height. We usually start long acting medication while short acting medications are being weaned off. By this time, evaluation of HTN may have revealed an etiology, and when possible, choice of agent should be dictated by addressing this underlying etiology.
Specific secondary Hypertension crisis
In the case of acute HTN secondary to acute glomerular diseases such glomerulonephritis (GN) with etiology of HTN being salt and volume retention, concurrent use of IV loop diuretics, such as furosemide, with agents discussed above will enhance antihypertensive efficacy. Because children and adolescents were most likely normotensive when they acquire acute GN, they experience very rapid rise in BP and are at high risk of posterior reversible encephalopathy syndrome and have very little time for resetting cerebral perfusion auto-regulation. Thus, these patients typically benefit from more aggressive antihypertensive therapy and tolerate treatment without experiencing organ ischemia.
Neuroendocrine causes of acute HTN such as pheochromocytoma caused by catecholamine excess should ultimately be treated with surgical resection; however, a medical regimen with antihypertensive agents should be initiated prior to surgical procedure to prevent post-operative complications from catecholamine release. Initially, alpha blockade is obtained with agents such as phenoxybenzamine or doxazosin, followed by beta blockade. Complete removal of the pheochromocytoma usually results in normalization of catecholamine secretion and BP within one week [43, 44, 45]. For more detailed discussion on preoperative pharmacological and volume management, readers are encouraged to refer to this review [46].
In drug overdose, such as cocaine and amphetamine, HTN secondary to indirect α adrenergic effects are often treated with phentolamine. HTN in these individuals is usually transient, often treated with lorazepam for HTN and agitation. Beta blockers are contraindicated in these patients due to its ability to cause unopposed α-adrenergic effects thus worsening HTN and myocardial ischemia [47].
Management of HTN in pregnancy must take into account both the mother and fetus. All antihypertensive agents cross the placenta, and there is limited comparative data on the efficacy and safety of these agents. Labetalol is first line therapy with hydralazine as an alternative. Calcium channel blockers such as nicardipine are also another option; however, experience with nicardipine is less than labetalol and hydralazine. Data on nicardipine showed that target BP was reached in less than 30 minutes in 70 percent of pregnant patients with severe HTN without severe fetal or maternal side effects [48]. ACE inhibitors and angiotensin receptor blockers are contraindicated at all stages of pregnancy because they cause significant fetal renal and cardiac abnormalities. Nitroprusside is also contraindicated because of potential cyanide toxicity.
Conclusion
Acute HTN is a relatively common encountered problem in hospitalized children and adolescents. With proper assessment, therapy and monitoring, morbidity of both HTN and over-treatment can minimized. Current treatment relies much on individual providers' experiences since published pediatric data on various antihypertensive agents is relatively sparse. Further research to elucidate acute HTN frequency in children and adolescents and large, prospective, comparative studies to examine the efficacy and safety of antihypertensive agents are needed.
Contributor Information
Tennille N. Webb, Email: tennille.webb@chp.edu, Pediatric Nephrology, University of Pittsburgh School of Medicine, Children's Hospital of Pittsburgh of UPMC, 4401 Penn Ave, Pittsburgh, PA 15206, USA, Phone: 412-692-5182, Fax: 412-692-7443.
Ibrahim F. Shatat, Email: shatat@musc.edu, Division of Pediatric Nephrology and Hypertension, Medical University of South Carolina, Children's Hospital, 96 Jonathan Lucas Street, CSB-428, Charleston, SC 29425, USA, Phone: 843-792-8904, Fax: 843-792-2033.
Yosuke Miyashita, Email: yosuke.miyashita@chp.edu, Pediatric Nephrology, University of Pittsburgh School of Medicine, Children's Hospital of Pittsburgh of UPMC, 4401 Penn Ave, Pittsburgh, PA 15206, USA, Phone: 412-692-5182, Fax: 412-692-7443.
References
- 1.Patel HP, Mitsnefes M. Advances in the pathogenesis and management of hypertensive crisis. Curr Opin Pediatr. 2005;17:210–4. doi: 10.1097/01.mop.0000150769.38484.b3. [DOI] [PubMed] [Google Scholar]
- 2.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The Fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–76. [PubMed] [Google Scholar]
- 3*.Chandar J, Zilleruelo G. Hypertensive crisis in children. Pediatr Nephrol. 2012;27:741–51. doi: 10.1007/s00467-011-1964-0. This review provides a detailed discussion on the pathogenesis and pathology of hypertensive crisis. The discussion includes etiology of hypertensive crisis based upon various age groups with suggested hypertensive agents. [DOI] [PubMed] [Google Scholar]
- 4*.Flynn JT, Tullus K. Severe hypertension in children and adolescents: pathophysiology and treatment. Pediatr Nephrol. 2009;24:1101–12. doi: 10.1007/s00467-008-1000-1. This review provides detailed assessment of the pathophysiology of acute hypertension, including discussion and illustration of cerebral perfusion auto-regulation. This review also provides many of the agent and their doses used to treat hypertensive crisis including some of the newer agents.. [DOI] [PubMed] [Google Scholar]
- 5*.Thomas CA. Drug treatment of hypertensive crisis in children. Pediatric Drugs. 2011;13(5):281–90. doi: 10.2165/11592130-000000000-00000. This review provides the pathogenesis and treatment goals of hypertensive crisis. This is also a good summary of antihypertensive agents used in hypertensive crisis including their mechanism of action, recommended doses and adverse effects. [DOI] [PubMed] [Google Scholar]
- 6.Marik PE, Varon J. Hypertensive Crises: Challenges and Management. Chest. 2007;131:1949–62. doi: 10.1378/chest.06-2490. [DOI] [PubMed] [Google Scholar]
- 7.Hari P, Sinha A. Hypertensive emergencies in children. Indian J Pediatr. 2011;78(5):569–75. doi: 10.1007/s12098-010-0297-5. [DOI] [PubMed] [Google Scholar]
- 8.Hari P, Bagga A, Srivastava RN. Sustained hypertension in children. Indian Pediatr. 2000;37:268–74. [PubMed] [Google Scholar]
- 9.Yang WC, Zhao LL, Chen CY, Wu YK, Chang YJ, Wu HP. First-attack pediatric hypertensive crisis presenting to the pediatric emergency department. BMC Pediatrics. 2012;12:200. doi: 10.1186/1471-2431-12-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh D, Akingbola O, Yosypiv I, El-Dahr S. Emergency management of hypertension in children. International Journal of Nephrology. 2012;2012:1–15. doi: 10.1155/2012/420247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tullus K, Roebuck DJ, McLaren CA, Marks SD. Imaging in the evaluation of renovascular disease. Pediatr Nephrol. 2010;25(6):1049–56. doi: 10.1007/s00467-009-1320-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suresh S, Mahajan P, Kamat D. Emergency Management of Pediatric Hypertension. Clin Pediatr. 2005;44:739–45. doi: 10.1177/000992280504400901. [DOI] [PubMed] [Google Scholar]
- 13.Marik P, Rivera R. Hypertensive emergencies: an update. Current Opinion in Critical Care. 2011;17:569–80. doi: 10.1097/MCC.0b013e32834cd31d. [DOI] [PubMed] [Google Scholar]
- 14.Rose JC, Mayer SA. Optimizing blood pressure in neurological emergencies. Neurocrit Care. 2004;1(3):287–99. doi: 10.1385/NCC:1:3:287. [DOI] [PubMed] [Google Scholar]
- 15.Tenney F, Sakarcan A. Nicardipine is a safe and effective agent in pediatric hypertensive emergencies. Am J Kidney Dis. 2000;35(5):E20. doi: 10.1016/s0272-6386(00)70285-x. [DOI] [PubMed] [Google Scholar]
- 16**.Flynn JT, Mottes TA, Brophy PD, Kershaw DB, Smoyer WE, Bunchman TE. Intravenous nicardipine for treatment of severe hypertension in children. J Pediatr. 2001;139:38–43. doi: 10.1067/mpd.2001.114030. This retrospective study provides the largest cohort of pediatric patients who received nicardipine for severe hypertension in a hospital setting. [DOI] [PubMed] [Google Scholar]
- 17**.Thomas CA, Moffett BS, Wagner JL, Mott AR, Feig DI. Safety and efficacy of intravenous labetalol for hypertensive crisis in infants and small children. Pediatr Crit Care Med. 2011;12(1):28–32. doi: 10.1097/PCC.0b013e3181e328d8. This retrospective analysis is a comparative study between labetalol, nitroprusside and nicardipine for severe hypertension in children and adolescents. [DOI] [PubMed] [Google Scholar]
- 18.Tabbutt S, Nicolson SC, Adamson PC, Zhang X, Hoffman ML, Well W, Backer CL, McGowan FX, Tweddell JS, Bokesch P, Schreiner M. The safety, efficacy, and pharmacokinetics of esmolol for blood pressure control immediately after repair of coarctation of the aorta in infants and children: a multicenter, double-blind, randomized trial. J Thorac Cardiovasc Surg. 2008;136(2):321–8. doi: 10.1016/j.jtcvs.2007.09.086. [DOI] [PubMed] [Google Scholar]
- 19.Wells TG, Bunchman TE, Kearns GL. Treatment of neonatal hypertension with enalaprilat. J Pediatr. 1990;117(4):664–7. doi: 10.1016/s0022-3476(05)80711-5. [DOI] [PubMed] [Google Scholar]
- 20.Hammer GB, Verghese ST, Drover DR, Yasater M, Tobin JR. Pharmacokinetics and pharmacodynamics of fenoldopam mesylate for blood pressure control in pediatric patients. BMC Anesthesiol. 2008;8:6–15. doi: 10.1186/1471-2253-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deeks ED, Keating GM, Keam SJ. Clevidipine: a review of its use in management of acute hypertension. Am J Cardiovasc Drugs. 2009;9(2):117–34. doi: 10.2165/00129784-200909020-00006. [DOI] [PubMed] [Google Scholar]
- 22.Tobias JD, Tulman DB, Bergese SD. Clevidipine for perioperative blood pressure control in infants and children. Pharmaceuticals. 2013;6:70–84. doi: 10.3390/ph6010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Towe E, Tobias JD. Preliminary experience with clevidipine in the pediatric population. J Intesive Care Med. 2010;25:349–52. doi: 10.1177/0885066610377977. [DOI] [PubMed] [Google Scholar]
- 24**.Tobias JD, Schechter ES, Phillips A, Weinstein S, Michler R, Berkenbosch JW, Montoya C. Clevidipine for perioperative blood pressure control in infants and children undergoing cardiac surgery for congenital heart disease. J Pediatr Pharmacol Ther. 2011;16:55–60. This retrospective review evaluates perioperative use of clevidipine in pediatric patients undergoing surgery for congenital heart disease. Clevidipine was found to be effective in perioperative blood pressure control but was not effective during cooling and cardiopulmonary bypass. [PMC free article] [PubMed] [Google Scholar]
- 25.McCrory WW, Kohaut ED, Lewy JE, Lieberman E, Travis LB. Safety of IV diazoxide in children with severe hypertension. Clin Pediatr. 1979;18:661–7,671. doi: 10.1177/000992287901801102. [DOI] [PubMed] [Google Scholar]
- 26.Grossman E, Ironi AN, Messerli FH. Comparative tolerability profile of hypertensive crisis treatments. Drug Saf. 1998;19:99–122. doi: 10.2165/00002018-199819020-00003. [DOI] [PubMed] [Google Scholar]
- 27**.Miyashita Y, Peterson D, Rees J, Flynn J. Isradipine for treatment of acute hypertension in hospitalized children and adolescents. J Clin Hypertens. 2010;12(11):850–5. doi: 10.1111/j.1751-7176.2010.00347.x. This retrospective observational study evaluates the efficacy and safety of isradipine in treating acute hypertension in hospitalized pediatric patients. All age groups were found to have significant reduction in blood pressure, with those younger than 2 years old with the most significant decrease, therefore recommending a lower starting dose for these individuals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacDonald JL, Johnson CE, Jacobson P. Stability of isradipine in an extemporaneously compounded oral liquid. Am J Hosp Pharm. 1994;51:2409–2411. [PubMed] [Google Scholar]
- 29.Sica DA. Centrally acting antihypertensive agents; an update. J Clin Hypertens. 2007;9:399–405. doi: 10.1111/j.1524-6175.2007.07161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirsten K, Nelson K, Kirsten D, Heintz B. Clinical pharmacokinetics of vasodilators. Par I. Clin Pharmacokinet. 1998;34:457–82. doi: 10.2165/00003088-199834060-00003. [DOI] [PubMed] [Google Scholar]
- 31.Pennisi AJ, Takahashi M, Bernstein BH, Singsen BH, Uittenbogaart C, Ettenger RB, Malekzadeh MH, Hanson V, Fine RN. Minoxidil therapy in children with severe hypertension. J Pediatr. 1977;90:813–9. doi: 10.1016/s0022-3476(77)81260-2. [DOI] [PubMed] [Google Scholar]
- 32.Strife CF, Quinlan M, Waldo FB, Fryer CJ, Jackson EC, Welch TR, McEnery PT, West CD. Minoxidil for control of acute blood pressure elevation in chronically hypertensive children. Pediatrics. 1986;78:861–5. [PubMed] [Google Scholar]
- 33.Patel HP, Mitsnefes M. Advances in the pathogenesis and management of hypertensive crisis. Curr Opin Pediatr. 2005;17(2):210–4. doi: 10.1097/01.mop.0000150769.38484.b3. [DOI] [PubMed] [Google Scholar]
- 34.Constantine E, Linakis J. The assessment and management of hypertensive emergencies and urgencies in children. Pediatr Emerg Care. 2005;21(6):391–6. doi: 10.1097/01.pec.0000166733.08965.23. [DOI] [PubMed] [Google Scholar]
- 35.Blaszak RT, Savage JA, Ellis EN. The use of short-acting nifedipine in pediatric patients with hypertension. J Pediatr. 2001;139(1):34–7. doi: 10.1067/mpd.2001.114699. [DOI] [PubMed] [Google Scholar]
- 36.Egger DW, Deming DD, Hamada N, Perkin RM, Sahney S. Evaluation of the safety of short-acting nifedipine in children with hypertension. Pediatr Nephrol. 2002;17:35–40. doi: 10.1007/s004670200006. [DOI] [PubMed] [Google Scholar]
- 37.Yiu V, Orrbine E, Rosychuk RJ, MacLaine P, Goodyer P, Girardin C, Gowrishankar M, Ogborn M, Midgley J, Filler G, Harley F. The safety and use of short-acting nifedipine in hospitalized hypertensive children. Pediatr Nephrol. 2004;19(6):644–50. doi: 10.1007/s00467-004-1444-x. [DOI] [PubMed] [Google Scholar]
- 38.Sasaki R, Hirota K, Masuda A. Nifedipine-induced transient cerebral ischemia in a child with Cockayne syndrome. Anaesthesia. 1997;52(12):1236. [PubMed] [Google Scholar]
- 39.Castaneda MP, Walsh CA, Woroniecki RP, Del Rio M, Flynn JT. Ventricular arrhythmia following short-acting nifedipine administration. Pediatr Nephrol. 2005;20(7):1000–2. doi: 10.1007/s00467-005-1854-4. [DOI] [PubMed] [Google Scholar]
- 40.Flynn JT, Pasko DA. Calcium channel blockers: pharmacology and place in therapy of pediatric hypertension. Pediatr Nephrol. 2000;15(3–4):302–16. doi: 10.1007/s004670000480. [DOI] [PubMed] [Google Scholar]
- 41.Flynn JT. Nifedipine in the treatment of hypertension in children. J Pediatr. 2002;40(6):787–8. doi: 10.1067/mpd.2002.124972. [DOI] [PubMed] [Google Scholar]
- 42.Truttmann AC, Zehnder-Schlapbach S, Bianchetti MG. A moratorium should be placed on the use of short-acting nifedipine for hypertensive crises. Pediatr Nephrol. 1998;12(3):259. [PubMed] [Google Scholar]
- 43.Fonkalsrud EW. Pheochromocytoma in childhood. Prog Pediatr Surg. 1991;26:103. doi: 10.1007/978-3-642-88324-8_13. [DOI] [PubMed] [Google Scholar]
- 44.Caty MG, Coran AG, Geagen M, Thompson NW. Current diagnosis and treatment of pheochromocytoma in children. Experience with 22 consecutive tumors in 14 patients. Arch Surg. 1990;125(8):978. doi: 10.1001/archsurg.1990.01410200036004. [DOI] [PubMed] [Google Scholar]
- 45.Perel Y, Schlumberger M, Marguerite G, Alos N, Revillon Y, Sommelet D, De Lumley L, Flamant F, Dyon JF, Lutz P, Heloury H, Lemerle J. Pheochromocytoma and paraganglioma in children: a report of 24 cases of the French Society of Pediatric Oncology. Pediatr Hematol Oncol. 1997;14(5):413. doi: 10.3109/08880019709028771. [DOI] [PubMed] [Google Scholar]
- 46.Van der Horst-Schrivers ANA, Kerstens MN, Wolffenbuttle BHR. Preoperative pharmacological management of phaeochromocytoma. Neth J Med. 2006;64:290–5. [PubMed] [Google Scholar]
- 47.Hollander JE. Cocaine intoxication and hypertension. Ann Emerg Med. 2008;51(3 Suppl):S18. doi: 10.1016/j.annemergmed.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 48.Nij Bijvank SW, Duvekot JJ. Nicardipine for the treatment of severe hypertension in pregnancy: a review of the literature. Obstet Gynecol Surv. 2010;65(5):341. doi: 10.1097/OGX.0b013e3181e2c795. [DOI] [PubMed] [Google Scholar]