Abstract
Doxorubicin (Dox) is an antineoplastic agent that can cause cardiomyopathy in humans and experimental animals. As an inducer of reactive oxygen species and a DNA damaging agent, Dox causes elevated expression of p21WAF1/Cip1/Sdi1 (p21) gene. Elevated levels of p21 mRNA and p21 protein have been detected in the myocardium of mice following Dox treatment. With chronic treatment of Dox, wild type (WT) animals develop cardiomyopathy evidenced by elongated nuclei, mitochondrial swelling, myofilamental disarray, reduced cardiac output, reduced ejection fraction, reduced left ventricular contractility, and elevated expression of ANF gene. In contrast, p21 knockout (p21KO) mice did not show significant changes in the same parameters in response to Dox treatment. In an effort to understand the mechanism of the resistance against Dox induced cardiomyopathy, we measured levels of antioxidant enzymes and found that p21KO mice did not contain elevated basal or inducible levels of glutathione peroxidase and catalase. Measurements of 6 circulating cytokines indicated elevation of IL-6, IL-12, IFNγ and TNFαin Dox treated WT mice but not p21KO mice. Dox induced elevation of IL-6 mRNA was detected in the myocardium of WT mice but not p21KO mice. While the mechanism of the resistance against Dox induced cardiomyopathy remains unclear, lack of inflammatory response may contribute to the observed cardiac protection in p21KO mice.
Keywords: Cell Cycle Inhibitor, antioxidant enzymes, inflammatory response, cardiomyopathy
Introduction
Doxorubicin (Dox) is an anthracycline quinone frequently used during chemotherapy for hematopoietic cancer and a wide range of solid tumors, including breast carcinoma, small-cell lung carcinoma and metastatic thyroid carcinoma. At high doses, Dox causes acute cardiac toxicity manifested by fatal arrhythmia. To reduce cardiac toxicity, formula and administration protocols have been improved and patients are closely monitored in clinics during Dox administration. However, chronic cardiotoxicity develops in certain individuals months or years after initial Dox administration. Such cardiotoxicity is manifested by ventricular dysfunction and dilated cardiomyopathy (Friedman et al., 1978; Lipshultz et al., 1991). Morphological and ultrastructural analyses of Dox induced cardiomyopathy reveal loss of myofibrils, dilation of sarcoplasmic reticulum, cytoplasmic vacuolization, mitochondrial swelling and increased number of lysosomes in cardiomyocytes (Lefrak et al., 1973; Bristow et al., 1978). Dox also induces inflammatory reactions in the heart, leading to thrombosis in the atria and myocarditis (Bristow et al., 1978; Fujihira et al., 1993; Gaudin et al., 1993). Increasing evidence suggests that inflammation is a key element in the progression of heart failure regardless of the type of initial insult (Suleiman et al., 2006; Heymans et al., 2009).
Cardiac specific toxicity of Dox is associated with the fact that cardiomyocytes have the highest content of mitochondria among all cell types. About 40% cell volume is occupied by mitochondria in cardiomyocytes (Barth et al., 1992). When accepting electrons from oxoreductive enzymes in the mitochondria, Dox forms semiquinone free radicals, which can initiate a chain of redox reactions (Myers et al., 1977). With mitochondria isolated from cardiac tissue, incubation with Dox in vitro leads to generation of superoxide anion and H2O2 (Doroshow and Davies, 1986). Administration of pharmacological agents with antioxidant properties or overexpressing antioxidant proteins or enzymes have been shown to reduce cardiac toxicity of Dox (Kotamraju et al., 2000; Ludke et al., 2009; Kang et al., 1996; Sun et al., 2001). In addition to producing reactive oxygen species (ROS), Dox can intercalate DNA, inhibit topoisomerase II and block cell proliferation.
Like most DNA damaging agents or ROS inducers, Dox elicits stress response at the cellular level, inducing the expression of p21WAF1/Cip1/Sdi1 (p21) gene. Although cardiomyocytes are mostly non-proliferative cells, elevated levels of p21 have been observed under several disease conditions, including myocardial infarction (Kuhn et al., 2007). Whereas p21 suppresses cell proliferation due to its function as a cyclin-dependent kinase inhibitor, little is known about the role of p21 in nonproliferative cells such as cardiomyocytes. Expecting Dox to cause an elevation of p21 in the myocardium, we examined whether p21 is involved in Dox induced cardiomyopathy.
Materials and Methods
Animals
All animal studies were reviewed and approved by the University of Arizona Institutional Animal Care and Use Committee. The breeding pairs of wild type (B6; 129 SF1) or p21KO mice (B6; 129 S2 Cdkn1atm1Tyj) were purchased from the Jackson laboratory. The p21KO mice were back crossed with wild type animals to generate offspring of wild type or homozygous, which were bred in parallel so the same generation was used for experimental comparisons. The mice used for experiments were 2 to 5 generations from the heterozygous parents.
Dox Treatment
Wild type (WT) or p21KO animals (male, 5-7 weeks old, 18-22 g) were treated with Dox, (Sigma, St Louis, MO) according to Sun et al. (Sun et al., 2001). Animals were dosed with 4 mg/kg Dox (i.p. 10 ml/kg in saline, controls received the same volume of saline without Dox) twice a week for a total of 10 injections. p21KO mice were slightly smaller than WT and were selected for bigger sizes within the same litter for treatment of an equal concentration of Dox, i.e. 85 μg per injection, as WT animals. The animals were not treated for 2 weeks between the first 4 injections and last 6 injections to allow recovery from bone marrow depression. Various measurements were taken 2 weeks after the final injection. After collecting the blood from the abdominal vena cava, serum was prepared by centrifugation at 2000×g for 5 min at 4°C. Upon excision, the hearts were immediately frozen in liquid nitrogen and were ground in liquid nitrogen bath using a mortar and pestle. The resulting tissue powders were divided into 3 parts for dissolving in 1) TRIzol for RNA isolation; 2) Phosphate buffered saline (PBS, pH 7.4) for enzymatic activity assays; and 3) Lysis buffer (Tris 0.05M, pH 6.8, Sodium Dodecyl Sulfate 2%, Glycerol 40%) for Western blots.
Hemodynamic Measurements
Two weeks after final Dox injection, mice were anesthetized with urethane (1 g/kg, i.p.) and α-chloralose (50 mg/kg, i.p, Calbiochem, CA.) and placed in a supine position on a temperature-controlled surgical mat for a closed chest protocol (Lips et al, 2004). Animals were ventilated via a cannula connected to a pressure-control respirator (Harvard Apparatus, Holliston, MA) after tracheotomy. Upon exposure and isolation from the internal jugular vein and the vagus nerve, the right common carotid artery was clamped with a microvessel clip (FST, Foster city, California) to the caudal end and sutured to the cranial end. The artery was incised with a 27 Gauge needle and the incision was enlarged with microscissors for insertion of a Millar 1.4 Fr catheter (SPR-719, ADInstruments, Colorado Springs, CO). The catheter containing four conductance electrodes was introduced into the left ventricle via the carotid artery and aortic valve. The external jugular vein was cannulated for volume administration with a maximum 300 μl of 50% albumin in saline. In order to acquire the pressure-volume loops, the respirator was paused for 5-6 sec (Nemoto et al., 2003). The conductance of catheter was expressed in Volts− by the Millar system (Millar MCS-100, ADInstruments, Colorado Springs, CO), which quantifies the voltage difference between two sensor electrodes by converting the resistance to voltage. The system was calibrated as described (Yang et al., 1999). The pressure-volume loop data were obtained with PVAN software (Conductance Technologies, San Antonio, TX) following data acquisition with a Powerlab DAQ system (ADinstruments, Colorado Springs, CO). Regression analyses of multiple isochronal pressure-volume loops were produced by compression of the Inferior Vena Cava in order to decrease the preload volume for calculation of the End Systolic Pressure-Volume Relationship index (slope) and the End Diastolic pressure-volume Relationship index (slope).
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA) from ground myocardial tissues. cDNAs were synthesized with Moloney Murine Leukemia Virus reverse transcriptase (Invitrogen, Carlsbad, CA) at 37°C for 1.5 hours from 2 μg of total RNA in a 35 μl reaction mixture. Following reverse transcription, 3 μl products were used for 25 μl PCR with Taq polymerase (Takara Bio, Japan) and primer sets as listed in Table 1. The PCR products were detected by 1% agarose gel electrophoresis and ethidium bromide staining.
Table 1.
PCR Primers and Conditions
| Gene | Primer | PCR condition |
|---|---|---|
| p21 | 5’AGTGTGCCGTTGTCTCTTCG 5’ACACCAGAGTGCAAGACAAGC |
94°C 30’, 60°C 30’, 72°C 30’, 30 cycles |
| EC-SOD | 5’CTGAGGACTTCCCAGTGAGC 5’GGTGAGGGTGTCAGAGTGGT |
94°C 30’, 60°C 30’, 72°C 30’, 30 cycles |
| catalase | 5’GCAGATACCTGTGAACTGTC 5’GTAGAATGTCCGCACCTGAG |
94°C 30’, 60°C 30’, 72°C 30’, 30 cycles |
| GPxl | 5’CCTCAAGTACGTCCGACCTG 5’CAATGTCGTTGCGGACACC |
94°C 30’, 60°C 30’, 72°C 30’, 30 cycles |
| NQO1 | 5’CATTCTGAAAGGCTGGTTTGA 5’CTAGCTTTGATCTGGTTGTCAG |
94°C 30’, 60°C 30’, 72°C 30’, 30 cycles |
| ANF | 5’GTGTACAGTGCGGTGTCCAA 5’ACCTCATCTTCTACCGGCATC |
94°C 30’, 55°C 60’, 72°C 60’, 40 cycles |
| GAPDH | 5’ACCCCTTCATTGACCTCAACTACA 5’AGTGATGGCATGGACTGTGGTCAT |
94°C 30’, 60°C 30’, 72°C 30’, 30 cycles |
| IL-6 | 5’ATGAAGTTCCTCTCTGCAAGAGACT 5’CACTAGGTTTGCCGAGTAGATCTC |
94°C 30’, 60°C 30’, 72°C 60’, 30 cycles |
| IL-10 | 5’CACTACCAAAGCCACAAAGC 5’CATGGCCTTGTAGACACCTT |
94°C 30’, 55°C 60’, 72°C 60’, 30 cycles |
| INFγ | 5’CACACTGCATCTTGGCTTTGC 5’CCTTGCTGTTGCTGAAGAAGGTAG |
94°C 30’, 60°C 30’, 72°C 60’, 30 cycles |
| TNFα | 5'CCA GAC CCT CAC ACT CAG AT 5’AAC ACC CAT TCC CTT ACA AG |
94°C 30’, 60°C 30’, 72°C 60’, 30 cycles |
Light and Electronic Microscopy
After anesthesia, the hearts were fixed in situ by vascular perfusion of saline for 10 mins followed by 10 mins perfusion of 2% glutaraldehyde plus 2% paraformaldehyde (pH 7.4, Karnovsky solution). For histology analyses, the hearts were excised and the left ventricles were embedded in paraffin for thin (5 micron) transversal sectioning and staining with Hematoxylin and Eosin. For electron microscopy, the left ventricular tissues were cut into 3 mm blocks and fixed 3 days in Karnovsky solution before fixation in osmium, dehydration in ethanol and resin embedment for standard procedures of electron microscopy.
Immunoblotting
An equal amount (20-40 μg) of proteins from each sample was separated by SDS polyacrylamide gel electrophoresis for overnight transfer at 30 volts onto a PVDF membrane as described (Chen et al., 2005). The primary monoclonal antibodies bound to p21, α-Smooth Muscle Actin or Actin were recognized by Horseradish Peroxidase conjugated-secondary antibody (Zymed, South San Francisco, CA) for detection by enhanced chemiluminescence.
Enzymatic Assay
Superoxide dismutase (SOD), catalase and glutathione peroxidase (GPx) activity assays were performed using assay kits from Cayman Chemicals. Samples were prepared according to the manufacture protocol for detection by a plate reader (Alexa, CA) of SOD at 450 nm, catalase at 540 nm and GPx at 340 nm.
Flow Cytometry
Mouse Inflammation Kit was used for quantifications of multiple soluble cytokines using serum samples per instruction (BD Biosciences). The calibrators were six cytokines ranging in concentration from 0 to 5 ng/ml for the assay system containing capture antibody beads and PE detection reagent. Thus six standard curves were obtained from one set of calibrators and the levels of serum cytokines were calculate based on these standard curve using BD Cytometric Bead Array software.
Quantification and Statistic Analysis
The intensities of bands from RT-PCR or Western blots were quantified using NIH Image J 1.32. All values were expressed as means ± standard deviations. Statistical significances were determined using Stata 8.2 software (Statacorp, Texas, USA). Comparisons were made by Student`s t test for two samples or by ANOVA with post hoc Bonferroni test for multiple samples.
RESULTS
p21 Knockout Mice Are Resistant to Dox Induced Cardiomyopathy
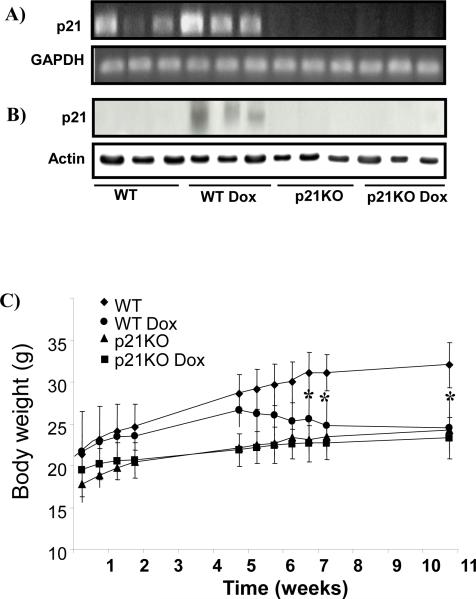
Dox is known to induce p21 gene expression in various cell types. To address whether Dox induced p21 in the myocardium, we measured levels of p21 mRNA or protein. Dox treatment resulted in elevated levels of p21 mRNA and protein in the myocardia of WT animals (Fig 1A&B). Towards the end of Dox administration, an average 16% or 13% decrease of body weight was observed for WT or p21KO animals (Fig 1C). WT mice treated with Dox were highly sensitive to handling, anesthesia and the surgical procedure necessary for measurement of cardiac function, with a combined procedural mortality rate of 52% (Table 2). In contrast, p21KO animals treated with Dox had 19% procedural mortality rate (Table 2). The ratios of heart to body weight, a sign of cardiac hypertrophy, showed over 10% increase in WT but no increase in p21KO animals treated with Dox (Table 2). The protocol of Dox administration has been shown to induce cardiac specific injury (Sunet al., 2001). Blood glucose and serum alanine aminotransferase and aspartate aminotransferase were measured to exclude a role of Dox in disturbance of general metabolism or causing liver injury (Table 2).
Figure 1. Dox Induced p21 mRNA and Protein in the Myocardium of Wild Type Mice.
Mice were divided into 4 groups: wild type control (WT, n=15), WT treated with Dox (n=12), p21KO control (n=13) and p21KO treated with Dox (n=13). Larger p21KO mice were chosen for Dox injection so the dose could be kept the same as with WT mice. Myocardial tissues were collected at the end of 10 weeks of treatment for measurements of p21 mRNA by RT-PCR (A) or p21 protein by Western blot (B). Animals were weighed at the time points indicated (C). The data represents means ± standard deviations. An asterisk indicates significance difference (p<0.05) between the means of treated group versus control.
Table 2.
Mortality Rate and Liver Function of Mice Treated with Dox
| WT | WT Dox | p21 KO | p21 KO Dox | |
|---|---|---|---|---|
| Procedural Mortality | 16% (n= 18) | *52% (n=25) | 7% (n=14) | 19% (n=16) |
| heart/body weight ratio | 5.22± 0.63 (n=15) | *5.93±1.04 (n=12) | 5.50±1.12 (n=13) | 5.77±1.12 (n=13) |
| Heart weight (mg) | 170.83± 30.40 | 157.68±34.78 | 152.93±16.21 | 142.55±23.36 |
| Body weight (g) | 32.59±3.57 | 27.40±5.68 | 28.85±6.18 | 24.99±2.87 |
| ALT (U/L) | 8.5 + 3.4 | 6.5 + 1.1 | 11.0 + 3.0 | 9.7 + 3.2 |
| AST (U/L) | 30.9 + 12.2 | 37.3 + 7.4 | 41.7 + 13.9 | 39.9 + 11 |
| Glucose (mg/dL) | 199.0 + 50.4 | 211.4 + 24.2 | 196.7 + 59.2 | 211.8 + 34.6 |
WT or p21KO mice were treated with saline or Dox. The mortality rate was recorded as a result of anesthesia or catheterization, since Dox treatment alone did not cause loss of mortality. The blood was collected for measurements of alanine transaminase (ALT), aspartate aminotransferase (AST) or glucose at the end of 10 weeks Dox treatment by the Pathological Service Core at University of Arizona animal care facility. The data are presented as means ± SD (n=3 unless indicated). An asterisk indicates significance difference (p<0.05) between the means of treated group versus control using Student's t test.
Figure 2 shows the results from histological and ultrastructural analyses of the left ventricles of WT or p21KO mice. Cardiomyocytes from Dox treated WT animals exhibited elongated nuclei, indicating cell size enlargement (Fig 2A). Electron microscopy images showed mitochondrial swelling, disarray of myofilaments and vacuolar structures in cardiomyocytes of WT animals treated with Dox (Fig 2B). In contrast, the p21KO animals did not show these morphological changes (Fig 2A&B).
Figure 2. Histology of Dox Induced Dilated Cardiomyopathy in Wild Type Mice.
Wild type (WT) or p21KO mice were treated with Dox over a course of 10 weeks as described in the Methods. The longitudinal sections from the left ventricles were used for histology with H&E staining (A) and electron microscopy analyses (B). Images of the tissue section of left ventricles were shown under a microscope with 4x lens (A). Arrows indicate elongated nuclei (A). Electron microscopy images of tissue blocks from left ventricles were obtained at x3500 fold magnification (B).
Hemodynamic parameters provide quantitative measurements of cardiomyopathy. Dox treated WT mice presented an impaired contractile function indicated by a decrease in the maximum rate of change in systolic pressure over time (dP/dt max), and reduced cardiac output or ejection fraction (Table 3). In contrast, p21KO mice did not show significant alterations in cardiac contractility and efficiency due to Dox administration (Table 3). Figure 3A shows representative pressure-volume loops of one animal in each group. Left ventricular performance parameters were evaluated by occlusion of the Inferior Vena Cava. The preload recruitable stroke work (PRSW), obtained by regression plot of the stroke work versus end diastolic volume, indicated a decrease in left ventricular contractility for WT but not p21KO animals after Dox treatment (Fig 3B). The end diastolic pressure volume relationship (EDPVR) was significantly altered with Dox treatment in WT but not p21KO mice (Fig 3C). A decrease in the end systolic pressure-volume relationship (ESPVR) was noticeable in the WT but not p21KO animals following Dox treatment (Fig 3D).
Table 3.
Hemodynamic Parameters of Wild Type or p21KO Mice Following Dox Treatment
| Parameters | WT | WT Dox | p21KO | p21KO Dox |
|---|---|---|---|---|
| Heart rate (bpm) | 507.59±43.25 | 544.41±39.44 | 486.17±23.63 | 503.89±35.12 |
| Maximum Volume (μL) | 15.71±2.01 | 13.29±5.10 | 13.03±5.32 | 17.49±6.13 |
| Minimum Volume (μL) | 5.14±1.47 | 5.37±3.03 | 3.96±2.46 | 5.3±2.01 |
| End-systolic Volume (μL) | 5.51±1.59 | 5.72±3.01 | 4.8±3.14 | 5.65±2.06 |
| End-diastolic Volume (μL) | 14.48±2.19 | 12.35±5.24 | 11.87±4.87 | 15.90±6.38 |
| Maximum Pressure (mmHg) | 98.18±14.74 | 86.42±28.51 | 98.54±19.54 | 92.05±15.19 |
| Minimum Pressure (mmHg) | 1.46±1.21 | 4.38±2.53 ** | 3.00±1.23 | 2.12±1.27 |
| End-systolic Pressure (mmHg) | 86.72±11.80 | 82.60±27.50 | 87.94±19.98 | 82.25±14.41 |
| End-diastolic Pressure (mmHg) | 4.64±1.59 | 8.64±6.88 | 5.94±1.33 | 5.32±0.97 |
| Stroke Volume (μL) | 10.57±1.17 | 7.99±3.59* | 9.07±4.45 | 12.19±4.91 |
| Ejection Fraction (%) | 67.66±6.33 | 52.48±10.41** | 64.38±10.27 | 68.67±8.32 |
| Cardiac Output (μL/min) | 5481.59±966.24 | 3920.31±1730.19* | 5153.52±1470.19 | 6305.82±2348.45 |
| Stroke Work (mmHg*μL) | 829.20±164.19 | 525.93±321.18* | 675.80±294.33 | 912.40±548.12 |
| Arterial Elastance (Ea) | 8.33±1.64 | 7.04±2.32 | 12.75±9.45 | 7.26±2.57 |
| dP/dt max (mmHg/sec) | 10423.97±2431.07 | 7176.26±2777.26 * | 6944.75±1141.39 | 9069.41±2718.05 |
| dP/dt min (mmHg/sec) | −6497.46±1665.83 | −5266.88±1661.75 | −5669.31±690.31 | −6878.06±2343.20 |
| dV/dt max (μL/sec) | 401.43±123.86 | 337.12±143.20 | 403.80±301.14 | 483.33±195.02 |
| dV/dt min (μL/sec) | −427.34±146.91 | −310.40±124.49 * | −333.69±98.89 | −447.31±158.41 |
| P@dVdt max (mmHg) | 10.62±19.43 | 8.94±13.84 | 16.09±12.19 | 15.44±15.43 |
| P@dPdt max (mmHg) | 49.13±10.14 | 44.57±17.99 | 39.05±6.25 | 43.02±10.4 |
| V@dPdt max (μL) | 14.87±2.03 | 13.93±6.52 | 12.69±5.11 | 15.55±5.91 |
| V@dPdt min (μL) | 5.51±1.61 | 5.74±2.30 | 4.55±2.68 | 5.80±1.27 |
| Tau_w (msec) | 6.40±1.27 | 8.22±1.41** | 7.48±0.78 | 6.45±1.52 |
| Maximal Power (mWatts) | 5.47±1.93 | 3.46±2.14* | 4.21±1.14 | 5.13±2.54 |
Hemodynamics parameters were recorded from Wild Type mice treated with Saline (WT, n=9) or with Dox (WT Dox, n=10) and p21KO mice treated with saline (p21KO, n=8) or with Dox (p21KO Dox, n=9) by Millar catheters. P=pressure, V=volume. dP/dt reflects maximum or minimum pressure change in the left ventricle, whereas dV/dt indicates maximum or minimum volume change of the left ventricle. Tau is isovolumic relaxation constant. The numbers represents means ± SD with
indicates p< 0.05
indicates p< 0.01 when treated group was compared to control group by Student's t test.
Figure 3. Hemodynamic Function Measurements Indicate p21KO Mice Were Resistant to Dox.
Mice at the end of 10 weeks of Dox treatment were used to measure hemodynamic function using a Millar 1.4 Fr catheter system. Representative pressure-volume loops were shown for one animal from each group (A). Preload Recruitable Stroke Work (PRSW, B), End Diastolic Pressure-Volume Relationship (EDPVR, C) and End Systolic Pressure-Volume Relationship (ESPVR, D) were obtained by decreasing the preload during transient Inferior Vena Cava occlusion from WT control (n=6), WT treated with Dox (n=6), p21KO control (n=6), and p21KO treated with Dox (n=6). An asterisk indicates significance difference (p<0.05) between the means of treated group versus control.
Increased expression of ANF in the ventricles serves as a biomarker of heart failure. An increase of α-smooth muscle actin (α-SMA) positive cells also correlates with heart failure. Since ANF is a secreted protein, its expression was measured by levels of mRNA. Measurements of ANF mRNA or α-SMA protein showed elevations in the myocardium of WT but not p21KO animals treated with Dox (Fig 4A&B). These findings were consistent with the histological and hemodynamic data showing p21KO animals were resistant against Dox induced cardiomyopathy.
Figure 4. Measurements of Heart Failure Biomarkers Indicate p21KO Mice Were Resistant to Dox.
The myocardial tissues from WT or p21KO mice following saline or Dox treatment were collected at the end of 10 weeks and were analyzed for ANF by RT-PCR (A,B) or α-smooth muscle actin (α-SMA) by Western blot (C). GAPDH (A, B) or actin (C) was included as a loading control. The intensities of the bands were quantified by NIH Image J program and were presented as means ± standard deviations (B). An asterisk indicates significance difference (p<0.05) between the means of treated group versus control.
Investigating the Mechanism of Resistance in p21 Knockout Mice
Since it is commonly believed that Dox induces cardiomyopathy via oxidative stress, we questioned whether p21KO animals had an elevated antioxidant reservoir. Glutathione Peroxidase-1 (GPx1) and catalase are protective against Dox toxicity and inducible upon oxidative stress (Kang et al., 1996; Gao et al., 2008). With Dox treatment, WT animals showed elevation of mRNA or activities of both GPx and catalase mRNA (Fig 5A-B). SOD activity from myocardial tissue was not significantly altered by Dox treatment in WT or p21KO mice (data not shown).
Figure 5. p21KO Mice Did Not Contain Higher Levels of Antioxidant Enzymes.
The myocardial tissues were collected at the end of the 10 weeks of Dox treatment from WT or P21KO mice for measurements of glutathione peroxidase (GPX1) mRNA or total GPX activity (A), Catalase mRNA or catalase activity (B). The data represent means ± standard deviations from measurements of 3- animals or as indicated. An asterisk indicates significance difference (p<0.05) between the means of treated group versus control.
Dox and quinones can be metabolized by NAD(P)H: quinone oxidoreductase (NQO1). A higher basal level of NQO1 in p21KO animals would indicate faster detoxification rate (Gutierrez, 2000). In contrast, p21KO animals expressed lower basal levels of NQO1 gene (data not shown). Nrf2 is a transcription factor that controls the expression of a number of antioxidant and detoxification genes. p21KO mice did not show elevated level of Nrf2 protein in the myocardium (data not shown). All these negative data lead us to direct our focus on pathways outside conventional antioxidant enzymes in an effort to understand the resistance of p21KO mice against Dox induced cardiomyopathy.
To explore the different between WT and p21KO mice with or without Dox treatment, we turned to Affymetrix microarray technique and found that Dox increased expression of 21 genes related to immune response in the myocardium of WT mice but only 4 genes in this category of p21KO mice. To test that p21 participated in an inflammatory reaction leading to heart failure, we used Multiplex Mouse Inflammation Kit to measure levels of 6 cytokines in one experiment. Dox treatment caused significant elevation of IL-6, IL-12, IFNγ and TNFα in the blood of WT animals (Fig 6). In contrast, p21KO mice did not show such changes (Fig 6). When mRNA levels of these cytokines were measured using myocardial tissues, among those detected, i.e., IL-6, IL-10, IFNγ and TNFα only IL-6 showed significant elevation in Dox treated WT but not p21KO animals (Fig 7).
Figure 6. Evaluation of Circulating Cytokines Following Dox Treatment.
Sera were collected from WT or p21KO mice at the end of 10 weeks treatment of Dox for measurements of 6 cytokines simultaneously using Mouse Inflammation Kit (BD, Bioscience) and flow cytometry. The profile of 6 cytokines from one mouse in each group was shown (A). The results from WT (n=6), WT treated with Dox (n=8), p21KO (n=6) or p21KO treated with Dox (n=7) were presented as means ± standard deviations. An asterisk indicates significance difference (p<0.05) between the means of treated group versus control.
Figure 7. Expression of Inflammatory Cytokines in the Myocardium.
The myocardial tissues were collected at the end of 10 weeks of Dox treatment from WT or P21KO mice for isolation of total RNA, and RT-PCR using primers as indicated in Table 1. The intensities of the bands were quantified by NIH Image J program and were presented as means ± standard deviations (B). An asterisk indicates significance difference (p<0.05) between the means of treated group versus control.
Discussion
This study indicates that p21KO mice were resistant to Dox induced cardiomyopathy. In WT animals, induction of p21 gene by Dox treatment correlated with morphological, hemodynamic and biochemical evidence of cardiomyopathy. In p21KO mice, most of the parameters measured were not significantly altered by Dox treatment. In addition, WT animals treated with Dox had a significantly higher mortality rate during various measurement procedures compared to p21KO mice. Although we did not observe elevations of SOD, GPx and catalase in p21KO mice, cells derived from p21KO mouse hearts have been shown to be less vulnerable to ROS generation (Roy et al., 2003). Therefore p21KO mice may have non-conventional manners of reduction of oxidative stress.
Inhibition of inflammatory response may contribute to the observed resistance of p21KO mice to Dox cardiotoxicity. Measurements of 6 cytokines in the blood confirmed the absence of systematic immune response in p21KO mice (Fig 6). In humans, inflammation is a well known risk factor for cardiovascular disease. Inflammatory response is often detected in failing hearts (Mehta and Li, 1999; Yndestad et al., 2006; Heymans et al., 2009). Heart failure patients show elevated levels of circulating IL-6 and TNFα (MacGowan et al., 1997; Plenz et al., 2001; Bradham et al., 2002; Boffa et al., 2009). Our observation of increased serum levels of IL-6 and TNFα in WT mice dosed with Dox is consistent with the literature regarding heart failure.
A few reports indicate a role of p21 in the inflammatory response. The p21 gene contributes to differentiation of monocytes to macrophage and inflammatory response in the liver, lung, vasculature and central nervous system (Wagayama et al., 2002; Ring et al., 2003; Merched and Chan, 2004). Similar to what we have observed in the myocardium, lack of p21 appears to be protective against atherosclerosis in apoE(-/-) mice or lung injury induced by cigarette smoke in correlation with reduced inflammatory response (Merched and Chan, 2004; Yao et al., 2008). Inflammation often contributes to fibrosis, and interstitial fibrosis has been observed in the myocardium in association with Dox induced cardiomyopathy (Chatterjee et al., 2010). Our observed elevation of α-SMA indicates fibrosis and proliferation of interstitial cells in the myocardium of Dox treated WT animals. Such fibrotic change was absent in p21KO mice, again supporting that lack of inflammatory response may contribute to the observed resistance of p21KO mice against Dox induced cardiomyopathy.
P21KO mice may exhibit enhanced capacity of repair and regeneration. Ray, et al. reported that p21 deficiency resulted in augmented conversion of cardiac fibroblasts to myofibroblasts, which exhibit characteristics of smooth muscle cells and are capable of contracting thus contributing to tissue repair (Roy et al., 2007; van den Borne et al., 2010). An increasing number of reports suggest that cell cycle proteins play a role in repair and regeneration of the myocardium (Ahuja et al., 2007). Down regulation of cell cycle kinases occurs during the process of human heart failure (Qiu et al., 2008), whereas stimulating cell cycle reentry such as by elevating cyclin D2 in transgenic mice improves cardiac function following cardiac injury (Hassink et al., 2008). Additional repair mechanisms involve cardiac progenitor cells, which can migrate to damaged areas of the heart, proliferate and differentiate into fully functional cardiomyocytes (Orlic et al., 2001; Anversa et al., 2006a; Anversa et al., 2006b; Kajstura et al., 2008). We have preliminary evidence that p21KO mice have a higher reservoir of cardiac progenitor cells compared to WT mice, supporting an enhanced capacity of repair and regeneration.
Clinical manifestation of cardiac injury varies tremendously among individuals. For patients treated with Dox during chemotherapy, some developed cardiomyopathy months or years later, whereas others did not experience cardiotoxicity. Currently there is no method to predict individual susceptibility to cardiotoxicity of Dox. Polymorphisms of p21 gene exist in humans (www.ncbi.nlm.nih.gov). Polymorphisms of R149G, S31R, and G215A of p21 have been found in association with an increased risk of oral, esophageal, or advanced breast cancer (Bahl et al., 2000; Ralhan et al., 2000; Staalesen et al., 2006). There is evidence that certain p21 polymorphisms affect the efficiency of p21 function and the outcome of human myocardial infarction (Rodriguez et al., 2007). In addition to polymorphism, levels of p21 protein differ considerably between individuals (Xie et al., 2004). If p21 is a central controller of the heart to chemical stress, polymorphism and differences in the expression level may contribute to individual variations in cardiotoxicity of Dox.
Highlights.
>We addressed the functional significance of p21 elevation by doxorubicin in the myocardium.
>Doxorubicin causes dilated cardiomyopathy in wild type mice.
>Knocking out p21 gene results in resistance against doxorubicin induced cardiomyopathy.
>Lack of inflammatory response correlates with the resistance in p21 knockout mice.
ACKNOWLEDGEMENTS
Work from our laboratory has been supported by NIH R01 ES 10826, R01 HL 076530, T32 ES007091, Arizona Disease Control Research Commission (QMC), and Mark and Mary Anne Fay Investigator Awards from the Sarver Heart Center at the University of Arizona (BX and SM). Histology and electron microscopy were performed by the Southwest Environmental Health Sciences Center supported by NIEHS P30 ES006694.
Abbreviation
- ANF
Atrial Natriuretic Factor
- α-SMA
alpha smooth muscle actin
- Dox
Doxorubicin
- EDPVR
end diastolic pressure volume relationship
- ESPVR
end systolic pressure-volume relationship
- GPx
glutathione peroxidase
- IL
interleukin
- IFN
Interferon
- NQO1
NAD(P)H: quinone oxidoreductase
- p21 KO
p21 knockout
- PBS
Phosphate Buffered Saline
- PCR
Polymerase Chain Reaction
- PRSW
preload recruitable stroke work
- PVDF
Polyvinylidene Fluoride
- ROS
reactive oxygen species
- SOD
Superoxide Dismutase
- RT
reverse transcription
- TNFα
Tumor Necrosis Factor alpha
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahuja P, Sdek P, MacLellan WR. Cardiac myocyte cell cycle control in development, disease, and regeneration. Physiol Rev. 2007;87:521–544. doi: 10.1152/physrev.00032.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anversa P, Kajstura J, Leri A, Bolli R. Life and death of cardiac stem cells: a paradigm shift in cardiac biology. Circulation. 2006a;113:1451–1463. doi: 10.1161/CIRCULATIONAHA.105.595181. [DOI] [PubMed] [Google Scholar]
- Anversa P, Leri A, Kajstura J. Cardiac regeneration. J Am Coll Cardiol. 2006b;47:1769–1776. doi: 10.1016/j.jacc.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Bahl R, Arora S, Nath N, Mathur M, Shukla NK, Ralhan R. Novel polymorphism in p21(waf1/cip1) cyclin dependent kinase inhibitor gene: association with human esophageal cancer. Oncogene. 2000;19:323–328. doi: 10.1038/sj.onc.1203325. [DOI] [PubMed] [Google Scholar]
- Barth E, Stammler G, Speiser B, Schaper J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J Mol Cell Cardiol. 1992;24:669–681. doi: 10.1016/0022-2828(92)93381-s. [DOI] [PubMed] [Google Scholar]
- Boffa GM, Zaninotto M, Sartor R, Mion M, Berton A, Pasqualetto C, Razzolini R, Plebani M. Interleukin-6 and tumor necrosis factor-alpha as biochemical markers of heart failure: a head-to-head clinical comparison with B-type natriuretic peptide. J Cardiovasc Med (Hagerstown) 2009;10:758–764. doi: 10.2459/JCM.0b013e32832ce8e2. [DOI] [PubMed] [Google Scholar]
- Bradham WS, Bozkurt B, Gunasinghe H, Mann D, Spinale FG. Tumor necrosis factor-alpha and myocardial remodeling in progression of heart failure: a current perspective. Cardiovasc Res. 2002;53:822–830. doi: 10.1016/s0008-6363(01)00503-x. [DOI] [PubMed] [Google Scholar]
- Bristow MR, Thompson PD, Martin RP, Mason JW, Billingham ME, Harrison DC. Early anthracycline cardiotoxicity. Am J Med. 1978;65:823–832. doi: 10.1016/0002-9343(78)90802-1. [DOI] [PubMed] [Google Scholar]
- Chatterjee K, Zhang J, Honbo N, Karliner JS. Doxorubicin cardiomyopathy. Cardiology. 2010;115:155–162. doi: 10.1159/000265166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Alexander D, Sun H, Xie L, Lin Y, Terrand J, Morrissy S, Purdom S. Corticosteroids Inhibit Cell Death Induced by Doxorubicin in Cardiomyocytes: Induction of Anti-apoptosis, Antioxidant and Detoxification Genes. Mol Pharm. 2005;67:1861–1873. doi: 10.1124/mol.104.003814. [DOI] [PubMed] [Google Scholar]
- Doroshow JH, Davies KJ. Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J Biol Chem. 1986;261:3068–3074. [PubMed] [Google Scholar]
- Friedman MA, Bozdech MJ, Billingham ME, Rider AK. Doxorubicin cardiotoxicity. Serial endomyocardial biopsies and systolic time intervals. JAMA. 1978;240:1603–1606. doi: 10.1001/jama.240.15.1603. [DOI] [PubMed] [Google Scholar]
- Fujihira S, Yamamoto T, Matsumoto M, Yoshizawa K, Oishi Y, Fujii T, Noguchi H, Mori H. The high incidence of atrial thrombosis in mice given doxorubicin. Toxicol Pathol. 1993;21:362–368. doi: 10.1177/019262339302100403. [DOI] [PubMed] [Google Scholar]
- Gao J, Xiong Y, Ho YS, Liu X, Chua CC, Xu X, Wang H, Hamdy R, Chua BH. Glutathione peroxidase 1-deficient mice are more susceptible to doxorubicin-induced cardiotoxicity. Biochim Biophys Acta. 2008;1783:2020–2029. doi: 10.1016/j.bbamcr.2008.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudin PB, Hruban RH, Beschorner WE, Kasper EK, Olson JL, Baughman KL, Hutchins GM. Myocarditis associated with doxorubicin cardiotoxicity. Am J Clin Pathol. 1993;100:158–163. doi: 10.1093/ajcp/100.2.158. [DOI] [PubMed] [Google Scholar]
- Gutierrez PL. The role of NAD(P)H oxidoreductase (DT-Diaphorase) in the bioactivation of quinone-containing antitumor agents: a review. Free Radic Biol Med. 2000;29:263–275. doi: 10.1016/s0891-5849(00)00314-2. [DOI] [PubMed] [Google Scholar]
- Hassink RJ, Pasumarthi KB, Nakajima H, Rubart M, Soonpaa MH, de la Riviere AB, Doevendans PA, Field LJ. Cardiomyocyte cell cycle activation improves cardiac function after myocardial infarction. Cardiovasc Res. 2008;78:18–25. doi: 10.1093/cvr/cvm101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymans S, Hirsch E, Anker SD, Aukrust P, Balligand JL, Cohen-Tervaert JW, Drexler H, Filippatos G, Felix SB, Gullestad L, Hilfiker-Kleiner D, Janssens S, Latini R, Neubauer G, Paulus WJ, Pieske B, Ponikowski P, Schroen B, Schultheiss HP, Tschope C, Van Bilsen M, Zannad F, McMurray J, Shah AM. Inflammation as a therapeutic target in heart failure? A scientific statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2009;11:119–129. doi: 10.1093/eurjhf/hfn043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajstura J, Urbanek K, Rota M, Bearzi C, Hosoda T, Bolli R, Anversa P, Leri A. Cardiac stem cells and myocardial disease. J Mol Cell Cardiol. 2008;45:505–513. doi: 10.1016/j.yjmcc.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Kang YJ, Chen Y, Epstein PN. Suppression of doxorubicin cardiotoxicity by overexpression of catalase in the heart of transgenic mice. J Biol Chem. 1996;271:12610–12616. doi: 10.1074/jbc.271.21.12610. [DOI] [PubMed] [Google Scholar]
- Kotamraju S, Konorev EA, Joseph J, Kalyanaraman B. Doxorubicin-induced apoptosis in endothelial cells and cardiomyocytes is ameliorated by nitrone spin traps and ebselen. Role of reactive oxygen and nitrogen species. J Biol Chem. 2000;275:33585–33592. doi: 10.1074/jbc.M003890200. [DOI] [PubMed] [Google Scholar]
- Kuhn DE, Roy S, Radtke J, Khanna S, Sen CK. Laser microdissection and capture of pure cardiomyocytes and fibroblasts from infarcted heart regions: perceived hyperoxia induces p21 in peri-infarct myocytes. Am J Physiol Heart Circ Physiol. 2007;292:H1245–1253. doi: 10.1152/ajpheart.01069.2006. [DOI] [PubMed] [Google Scholar]
- Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer. 1973;32:302–314. doi: 10.1002/1097-0142(197308)32:2<302::aid-cncr2820320205>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324:808–815. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- Ludke AR, Al-Shudiefat AA, Dhingra S, Jassal DS, Singal PK. A concise description of cardioprotective strategies in doxorubicin-induced cardiotoxicity. Can J Physiol Pharm. 2009;87:756–763. doi: 10.1139/Y09-059. [DOI] [PubMed] [Google Scholar]
- MacGowan GA, Mann DL, Kormos RL, Feldman AM, Murali S. Circulating interleukin-6 in severe heart failure. Am J Cardiol. 1997;79:1128–1131. doi: 10.1016/s0002-9149(96)00063-x. [DOI] [PubMed] [Google Scholar]
- Mehta JL, Li DY. Inflammation in ischemic heart disease: response to tissue injury or a pathogenetic villain? Cardiovasc Res. 1999;43:291–299. doi: 10.1016/s0008-6363(99)00132-7. [DOI] [PubMed] [Google Scholar]
- Merched AJ, Chan L. Absence of p21Waf1/Cip1/Sdi1 modulates macrophage differentiation and inflammatory response and protects against atherosclerosis. Circulation. 2004;110:3830–3841. doi: 10.1161/01.CIR.0000148681.01282.89. [DOI] [PubMed] [Google Scholar]
- Myers CE, McGuire WP, Liss RH, Ifrim I, Grotzinger K, Young RC. Adriamycin: the role of lipid peroxidation in cardiac toxicity and tumor response. Science. 1977;197:165–167. doi: 10.1126/science.877547. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Defreitas G, Carabello B. Contemporary topics. American Association for Laboratory Animal Surgery; 2003. Cardiac catheterization technique in a closed-chest murine model. pp. 34–38. [PubMed] [Google Scholar]
- Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- Plenz G, Song ZF, Tjan TD, Koenig C, Baba HA, Erren M, Flesch M, Wichter T, Scheld HH, Deng MC. Activation of the cardiac interleukin-6 system in advanced heart failure. Eur J Heart Fail. 2001;3:415–421. doi: 10.1016/s1388-9842(01)00137-4. [DOI] [PubMed] [Google Scholar]
- Qiu H, Dai H, Jain K, Shah R, Hong C, Pain J, Tian B, Vatner DE, Vatner SF, Depre C. Characterization of a novel cardiac isoform of the cell cycle-related kinase that is regulated during heart failure. J Biol Chem. 2008;283:22157–22165. doi: 10.1074/jbc.M710459200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralhan R, Agarwal S, Mathur M, Wasylyk B, Srivastava A. Association between polymorphism in p21(Waf1/Cip1) cyclin-dependent kinase inhibitor gene and human oral cancer. Clin Cancer Res. 2000;6:2440–2447. [PubMed] [Google Scholar]
- Ring RH, Valo Z, Gao C, Barish ME, Singer-Sam J. The Cdkn1a gene (p21Waf1/Cip1) is an inflammatory response gene in the mouse central nervous system. Neurosci Lett. 2003;350:73–76. doi: 10.1016/s0304-3940(03)00883-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez I, Coto E, Reguero JR, Gonzalez P, Andres V, Lozano I, Martin M, Alvarez V, Moris C. Role of the CDKN1A/p21, CDKN1C/p57, and CDKN2A/p16 genes in the risk of atherosclerosis and myocardial infarction. Cell Cycle. 2007;6:620–625. doi: 10.4161/cc.6.5.3927. [DOI] [PubMed] [Google Scholar]
- Roy S, Khanna S, Bickerstaff AA, Subramanian SV, Atalay M, Bierl M, Pendyala S, Levy D, Sharma N, Venojarvi M, Strauch A, Orosz CG, Sen CK. Oxygen sensing by primary cardiac fibroblasts: a key role of p21(Waf1/Cip1/Sdi1). Circ Res. 2003;92:264–271. doi: 10.1161/01.res.0000056770.30922.e6. [DOI] [PubMed] [Google Scholar]
- Roy S, Khanna S, Rink T, Radtke J, Williams WT, Biswas S, Schnitt R, Strauch AR, Sen CK. P21waf1/cip1/sdi1 as a central regulator of inducible smooth muscle actin expression and differentiation of cardiac fibroblasts to myofibroblasts. Mol Biol Cell. 2007;18:4837–4846. doi: 10.1091/mbc.E07-03-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staalesen V, Knappskog S, Chrisanthar R, Nordgard SH, Lokkevik E, Anker G, Ostenstad B, Lundgren S, Risberg T, Mjaaland I, Gram IT, Kristensen VN, Borresen-Dale AL, Lillehaug JR, Lonning PE. The novel p21 polymorphism p21G251A is associated with locally advanced breast cancer. Clin Cancer Res. 2006;12:6000–6004. doi: 10.1158/1078-0432.CCR-05-2822. [DOI] [PubMed] [Google Scholar]
- Suleiman M, Khatib R, Agmon Y, Mahamid R, Boulos M, Kapeliovich M, Levy Y, Beyar R, Markiewicz W, Hammerman H, Aronson D. Early inflammation and risk of long-term development of heart failure and mortality in survivors of acute myocardial infarction predictive role of C-reactive protein. J Am Coll Cardiol. 2006;47:962–968. doi: 10.1016/j.jacc.2005.10.055. [DOI] [PubMed] [Google Scholar]
- Sun X, Zhou Z, Kang YJ. Attenuation of doxorubicin chronic toxicity in metallothionein-overexpressing transgenic mouse heart. Cancer Research. 2001;61:3382–3387. [PubMed] [Google Scholar]
- van den Borne SW, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: the role of myofibroblasts. Nat Rev Cardiol. 2010;7:30–37. doi: 10.1038/nrcardio.2009.199. [DOI] [PubMed] [Google Scholar]
- Wagayama H, Shiraki K, Sugimoto K, Ito T, Fujikawa K, Yamanaka T, Takase K, Nakano T. High expression of p21WAF1/CIP1 is correlated with human hepatocellular carcinoma in patients with hepatitis C virus-associated chronic liver diseases. Hum Pathol. 2002;33:429–434. doi: 10.1053/hupa.2002.124724. [DOI] [PubMed] [Google Scholar]
- Xie HL, Su Q, He XS, Liang XQ, Zhou JG, Song Y, Li YQ. Expression of p21(WAF1) and p53 and polymorphism of p21(WAF1) gene in gastric carcinoma. World J Gastroenterol. 2004;10:1125–1131. doi: 10.3748/wjg.v10.i8.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Larson DF, Watson R. Age-related left ventricular function in the mouse: analysis based on in vivo pressure-volume relationships. Am J Physiol. 1999;277:H1906–1913. doi: 10.1152/ajpheart.1999.277.5.H1906. [DOI] [PubMed] [Google Scholar]
- Yao H, Yang SR, Edirisinghe I, Rajendrasozhan S, Caito S, Adenuga D, O'Reilly MA, Rahman I. Disruption of p21 attenuates lung inflammation induced by cigarette smoke, LPS, and fMLP in mice. Am J Resp Cell Mol Biol. 2008;39:7–18. doi: 10.1165/rcmb.2007-0342OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yndestad A, Damas JK, Oie E, Ueland T, Gullestad L, Aukrust P. Systemic inflammation in heart failure--the whys and wherefores. Heart Fail Rev. 2006;11:83–92. doi: 10.1007/s10741-006-9196-2. [DOI] [PubMed] [Google Scholar]