Abstract
Background: This study investigates factors that are associated with nonadherence to mammography screening guidelines in Utah, a state where mammography screening rates have remained consistently lower than national averages.
Methods: We examined data on reported mammography use among women aged 40–74 years from the 2008 and 2010 Utah Behavioral Risk Factor Surveillance System (n=5,197, weighted n=417,064). Logistic regression models were used to estimate the effects of individual-level and geographic (travel time to nearest mammography facility, geographic accessibility, and rural/urban residence) factors on the odds of a woman not reporting receiving a mammogram in the last 2 years.
Results: In 2008 and 2010, a disproportionate number of women aged 40–49 (43.1%, 95% confidence interval [CI] 39.9%–46.3%) reported not receiving a mammogram within the last 2 years compared to women 50–74 (26.8%, 95% CI 24.9%–28.7%). None of the geographic factors were significant predictors of screening adherence. Based on covariate adjusted models, statistically significant (p<0.05) factors associated with increased odds of not receiving mammogram within the last 2 years included not having a regular physician, no health insurance, being aged 40–49, income less than $25,000, and the presence of three or more children in the home.
Conclusion: Mammography screening efforts in Utah should focus on improving access to insurance or a regular source of health care. Future research should also consider how best to address extreme time demands and competing priorities that present potential barriers for women with large families, resulting in lower screening levels among these women.
Introduction
Breast cancer is the most common invasive cancer diagnosed among women in the United States. In 2010 alone, 206,966 U.S. women were diagnosed with breast cancer, and 40,996 women died from the disease.1 Mammography screening remains the most effective method for the early detection of breast cancer and can detect cancer when women are asymptomatic—before the cancer becomes invasive. Despite the advantages of mammography screening for reducing mortality,2 not all women receive screening according to recommended guidelines, and screening rates remain low among certain subgroups of women.
A woman's adherence to mammography screening recommendations is driven by many factors that span a broad range of individual demographic, physical, psychological, behavioral, social/familial, and financial/economic attributes. Studies have consistently shown that mammography screening utilization is associated with individual factors, including socioeconomic status; health insurance and type of coverage (i.e., Medicaid vs. private); level of educational attainment3–5; access to a regular health care provider6; and race/ethnicity.7 Screening utilization has also been shown to be influenced by system-level factors, including the presence of specialists, types of screening,5 and whether screening had been recommended by a doctor.8 Some researchers have suggested that capacity (i.e., waiting times) and the number of mammography machines available9,10 may also affect rates of mammography screening. Elkin et al. (2010) analyzed mammography capacity, using the number of machines available at mammography facilities, and found that in areas with an inadequate supply (<1.2 machines per 10,000 women 40+), women were less likely to adhere to screening guidelines.9
The impact of geographic factors, however, such as urban-rural residence, mammography facility density, and geographic proximity, on screening adherence have not been widely studied. Such geographic measures are typically conceptualized as important enabling factors, which can facilitate or inhibit the use of health services. For example, a deficit in service distribution and/or women traveling long distances to mammography facilities can result in barriers to accessing these services and to possible geographic disparities in mammography screening rates. Studies examining the relationship between geographic factors and mammography screening have generally produced conflicting results.11–14 In the context of measuring disparities between rural and nonrural patients, Meilleur and colleagues explain that the lack of consistent findings may result from inconsistencies in how rural is defined.15 And for measures of geographic access (e.g., ratio of mammography facilities to women), the discrepancies between studies may be a result of how access is defined or measurement bias. For example, most geographic studies to date used measures of access at the county level, which can mask the heterogeneity of travel times to mammography facilities within the county, and many did not account for the use of mammography facilities outside the respondents' counties or across state boundaries. It is also possible that the inconsistent results between studies could in fact be due to the unique geographic characteristics of the different states and regions examined.
Several studies have indicated that mammography screening adherence rates, measured as the proportion of women aged 40 and over who had mammograms in the last 2 years, vary by region in the United States.16–18 In 2010, the five lowest-ranking states for mammography screening adherence were found in the Mountain West states—Idaho, Utah, Nevada, Wyoming, and Montana.19 Utah's mammography adherence rate has been persistently lower than national averages for the last 20 years,20 and the most recent screening data from 2012 indicate that Utah's screening rates were still among the lowest in the United States. To our knowledge, no studies have been published to assess the low mammography screening rates in Utah, and it remains unknown what factors contribute to these markedly low mammography screening rates among Utah women.
The purpose of this study is to investigate possible predisposing and enabling factors associated with nonadherence to screening guidelines among Utah women 40 years and older using survey data from the Utah Behavioral Risk Factor Surveillance System (BRFSS). For this study, adherence to recent screening is considered having a mammogram in the past 2 years, consistent with the U.S. Preventive Services Task Force (USPSTF) recommendations prior to 2010.21 We specifically take advantage of having data on the survey participants' ZIP codes, which allows us to estimate a unique measure of geographic access that accounts for both drive times to mammography facilities and facility capacity. Previous studies utilizing BRFSS data to examine associations between mammography screening adherence and geographic access to mammography facilities all used less precise, county-level data.
Methods
Study population
This study utilized 2008 and 2010 breast cancer screening survey data from the Utah BRFSS, which is coordinated by the Utah State Department of Health in collaboration with the Centers for Disease Control and Prevention (CDC). BRFSS is a telephone survey conducted in the noninstitutionalized population aged 18 years and older. The Utah BRFSS uses a disproportionate, stratified sampling design that oversamples rural districts to ensure a representative sample of both urban and rural populations.22 BRFSS includes specific questions about breast cancer screening history that are asked every other year. Women aged 40 and older were asked a series of questions to determine the timing of their most recent mammogram. They were first asked, “Have you ever had a mammogram?” Women who responded yes were then asked, “How long has it been since you had your last mammogram?” In this study, for survey years 2008 (n=1,470) and 2010 (n=3,727), we examined adherence to recommendations for mammography: “received mammogram within the past two years.”
USPSTF mammography screening recommendations for the BRFSS survey years included in this study differed from current recommendations for women 40–49 years old. In 2008, the USPSTF recommended screening mammography every 1–2 years for women aged 40 and over. However, at the time of the 2010 BRFSS survey, the USPSTF had just released changes to mammography screening guidelines the year before, in November 2009. They recommended against routine screening of women in their 40s and recommended instead that women 40–49 years old make individual decisions about screening based on their risk of breast cancer and the potential benefits and harms of screening. In addition to the USPSTF's mammography screening guidelines, the American Cancer Society recommends that women receive an annual mammogram beginning at age 40.23
To assess the appropriateness of pooling survey years due to changes in the USPSTF mammography screening guidelines, we examined the screening rates separately for 2008 and 2010 and found no significant change in rates between survey years for women 40–49 years old (chi-square test p=0.4294) or 50 years and older (chi-square test p=0.9583). It is not surprising that we did not find significant changes in screening rates so soon after the new recommendations in Utah, as two recent national studies also found that, as of 2011, the mammography rates did not decrease among women aged 40 years and older after publication of the USPSTF changes.24,25 Therefore, we combined data from the 2008 and 2010 surveys, resulting in a total of 5,197 women aged 40–74 included in the study (weighted population of n=416,953).
Indepedendent variables
Following Andersen's model of health care services utilization, this study examined which individual-level predisposing and enabling factors and which geographic-based enabling factors affect adherence to mammography screening guidelines. The Andersen model suggests that utilization of health care services or health-seeking behavior is a function of the predisposition of individuals to use services, factors that enable or impede use, and the need for care.26 Andersen referred to the utilization of health services as “realized access” and to the predisposing and enabling factors that facilitate the use of services as “potential access.”26 The availability of more enabling resources should increase the likelihood that a preventative health service will be used.27
The predisposing factors included in this study were age (40–74), race/ethnicity (non-Hispanic white, other/unknown), education level (<high school, high school, some college, college), marital status (single, married/partner), self-rated health (fair or poor, good to excellent), body mass index (BMI) (normal, overweight, obese), and smoking status (yes, no). The individual-level enabling factors included health insurance (yes, no), income (<25,000, 25,000–50,000, 50,000–75,000, >75,000), having a regular physician (yes, no), and number of dependent children (<18 years old) in the home (none, 1–2, 3+). Number of children in the home was conceptuzalied as a measure of time demand.28
Geographic enabling factors typically include measures of geographic access to health facilities. Geographic access refers to the “relative ease”29 by which a population can reach and use health services, when and where the services are needed.30,31 Geographic measures of access often include empirical measures that refer to proximity to a facility such as travel time or distance. Rural or urban residence is often used a surrogate measure of geographic access when more precise measures are not available. In recent years more sophisticated measures of geographic access have been developed.29 These measures specifically describe the relationship between the availability of services (supply), the number of people needing a service (demand), and the travel time (or distance) between supply and demand.29 A deficit or maldistribution of supply and/or populations traveling long distances to health services can form barriers to accessing services, which can result in lower utilization rates for specific populations.
For this study we included three geographic enabling factors: (1) rural/urban residence, (2) proximity to the nearest mammography facility, and (3) a geographic access measure that combines proximity to mammography facilities, capacity of the facility, and demand for services (e.g., women 40 years and older). Geographic measures were assigned to the survey participants' residential ZIP codes, which was the smallest geographic unit available from the Utah BRFSS. The geographic measures are described in detail in the following section.
Geographic measures
Mammography faciltiy locations
The 2008 and 2010 FDA-certified mammography facilities in Utah and surrounding states were geocoded using ArcGIS 10 software (ESRI, Redlands, CA). Capacity was calculated as the number of machines per facility, and these data were obtained through an FDA Freedom of Information Act request. To verify the number of mammography screening machines at each facility, we contacted all facilities by phone. The three mobile screening machines in Utah certfied by the FDA in both 2008 and 2010 were included in this study because they were all located at hospitals and also used as standalone screening facilities.
Rural and urban classification
Measures of urban and rural status for each survey participant's ZIP code were based on the Rural Urban Commuting Area Codes (RUCA). RUCA codes are designed to provide a definition of rural and urban based on the U.S. Census Bureau's definitions of urbanized areas and urban clusters, which are based on criteria including population density and population work commuting patterns.32 For this study, the 33 RUCA categories were aggregated into either an urban group (codes 1.0, 1.1, 2.0, 2.1, 3.0, 4.1, 5.1, 7.1, 8.1, and 10.1) or a rural group (all other codes).33
Travel time
To estimate travel time by ZIP code, we first created 1- mile2 grid cells for the entire state. Based on 2010 census block population data, populations of women aged 40+ were assigned to each of the grid cells. Drive times from each populated grid cell to each mammography facility within 60 minutes were estimated. The grid cells from each ZIP code were then used to estimate a population-weighted drive time to the nearest facility and to estimate a geographic access measure, which was later population-weighted and summarized by ZIP code. Drive times were estimated using the North American Association of Central Cancer Registries Shortest Path Tool.34 Based on the overall distribution of travel times to mammography facilities in the study population, travel times were initially grouped into five categories: >5 minutes; 5 to <10 minutes; 10 to <20 minutes; 20 to <30 minutes; and >30 minutes. Smaller intervals of time were used in the shorter categories because the majority of the study population lived less than 10 minutes from a mammography facility. Because the travel times were skewed toward shorter distances, we also considered alternative groupings to ensure an adequate number of participants per category for statistical analysis including <20 minutes and ≥20 minutes.
Geographic access (supply-demand)
Estimates of geographic access to mammography facilities were calculated for each populated grid cell using the Enhanced 2-Step Floating Catchment Area (E2SFCA) method proposed by Luo and Qi (2009).35 The E2SFCA method was used to estimate geographic access to mammography as the ratio of number of machines per mammography facility (supply) to women 40 years and older (demand). The E2SFCA method improves on prior methods by incorporating a flexible distance decay function (Wr) that allows for consideration of the increased effort, time, and/or cost associated with longer travel times in order to down weight supply-to-demand ratios (Rj). The E2SFCA was calculated in two general steps and provided a measure of the supply-to-demand ratio for each 1-mile2 grid cell.
- 1. Supply-to-demand ratios (Rj) were calculated for travel zones from each grid cell (j) as follows:

Here, Sj represented the number of mammography machines at location j, and Pk was the population of all grid cells (k) within travel zones of catchment areas j(dkjϵDr). The travel time between k and j was represented as dkj; Dr was the rth travel time zone within each catchment area, and Wr was the distance decay of access to mammography machine j. Calculations for Wr were based on a drive time of 60 minutes.
- 2. Geographic accessibility was calculated by summing supply-to-demand ratios as follows:

For distance decay (Wr), the following decay functions, based on travel time, were used: <10 minutes (1.00), >10 minutes (0.96), and at 10-minute intervals up to 60 minutes (0.82, 0.45, 0.22, 0.10). The final grid-cell measures of geographic access were population weighted and summarized by ZIP code.
Statistical analyses
Descriptive statistics were used to examine the weighted distribution of women included in the study by demographic, socioeconomic, health, household, and geographic characteristics. The primary outcome was nonadherence to mammography screening guidelines, defined as not receiving a mammogram within the last 2 years (those who never had a mammogram and those who reported having a mammogram more than 2 years ago). Bivariate analysis was used to assess the association between the weighted proportion of women who were not adherent to mammography screening guidelines and both individual-level variables and geographic measures. Due to differences in screening recommendations by age, stratified bivariate analyses were used to examine potential differences in effect sizes by age group (40–49 and 50–74 years). Bivariate associations were assessed with Wald chi-square tests. Multivariable logistic regression models were generated to identify factors associated with no adherence among all women aged 40–74 in addition to age-stratified models (40–49 and 50–74) to further delineate potential effect modification by age. All analyses were weighted to account for the disproportionate stratified sampling design used in the BRFSS. All analyses were conducted using SAS 9.3(SAS Institute) and significance determined at p<0.05.
Results
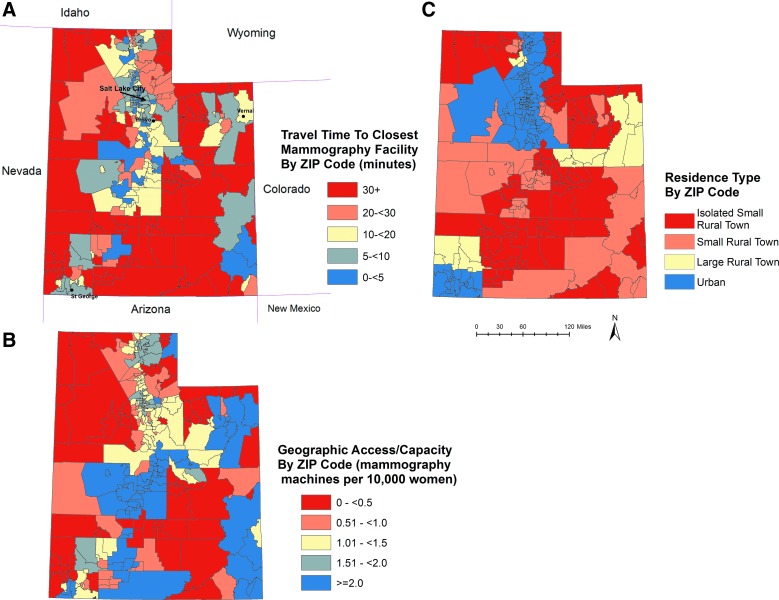
Characteristics of the study population are shown in Table 1, and Fig. 1 illustrates the distribution of the three geographic access measures in Utah. Unadjusted tests of association revealed no significant associations between mammography screening nonadherence and race/ethnicity and geographic measures (Table 1). Income and education were inversely associated with mammography screening adherence, as lower income and education groups had higher proportions of women who did not have a mammogram in the last 2 years. Single women, women without a regular physician, and women without health insurance coverage also had larger proportions of nonadherence. These findings were consistent for women in both age groups (40–49 and 50– years74). Smoking status, self-rated health, BMI, and having children in the home were not associated with mammography adherence among women aged 40–49 in bivariate analysis. However, these variables were significantly associated with nonadherence among the older age group (50–74), with smokers, women in fair or poor health, obese women, and women with 3+ children in the home reporting larger proportions of nonadherence.
Table 1.
Characteristics of Study Population and Mammography Screening Nonadhenrence Among Utah Women, Utah Behavioral Risk Factor Surveillance System 2008 and 2010
| Women aged 40–74 yearsa | Women aged 40–49 yearsa | Women aged 50–74 yearsa | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Unweighted n Weighted n | 5197 416953 | No mammogram past 2 years | 1470 153435 | No mammogram past 2 years | 3727 263517 | No mammogram past 2 years | |||
| Characteristics | %b | %b(95% CI) | p-valuec | %b | %b(95% CI) | p-valuec | %b | %b(95% CI) | p-valuec |
| Total | 32.8 (31.1–34.5) | 43.1 (39.9–46.3) | 26.8 (24.9–28.7) | ||||||
| Age (years) | |||||||||
| 40–49 | 36.8 | 43.1 (39.9–46.3) | <0.0001 | ||||||
| 50–74 | 63.2 | 26.8 (24.9–28.7) | |||||||
| Race/ethnicity | |||||||||
| Non-Hispanic White | 89.9 | 32.5 (30.7–34.3) | 0.355 | 86.0 | 42.9 (39.5–46.2) | 0.755 | 92.2 | 26.8 (24.8–28.8) | 0.816 |
| Other/unknown | 10.1 | 35.4 (29.3–41.5) | 14.0 | 44.5 (34.9–54.1) | 7.8 | 26.0 (19.1–32.9) | |||
| Income | |||||||||
| <$25,000 | 15.1 | 45.8 (41.2–50.3) | <0.0001 | 13.1 | 60.8 (51.7–69.9) | <0.0001 | 16.3 | 38.8 (34.1–43.5) | <0.0001 |
| $25,000 to $50,000 | 21.2 | 36.1 (32.4–39.9) | 16.8 | 52.4 (44.6–60.3) | 23.8 | 29.4 (25.4–33.5) | |||
| $50,000 to $75,000 | 19.5 | 32.0 (27.9–36.1) | 22.8 | 43.3 (36.3–50.2) | 17.6 | 23.5 (18.6–28.5) | |||
| >$75,000 | 32.5 | 26.0 (23.2–28.8) | 38.6 | 34.2 (29.5–38.8) | 28.9 | 19.6 (16.2–22.9) | |||
| Refused/unknown | 11.7 | 30.1 (25.3–34.8) | 8.7 | 37.5 (26.4–48.6) | 13.4 | 27.3 (22.4–32.2) | |||
| Educational attainment | |||||||||
| <High school | 4.5 | 48.6 (39.54–57.58) | <0.0001 | 4.9 | 57.4 (41.5–73.3) | 0.0377 | 4.3 | 42.8 (32.3–53.21) | <0.0001 |
| High school | 26.7 | 33.5 (30.2–36.8) | 23.2 | 43.6 (37.0–50.3) | 28.7 | 28.7 (25.0–32.4) | |||
| Some college | 36.3 | 34.7 (31.7–37.6) | 36.3 | 46.1 (40.5–51.6) | 36.2 | 28.0 (24.7–31.2) | |||
| College | 32.5 | 27.9 (25.1–30.7) | 35.6 | 37.7 (32.7–42.7) | 30.8 | 21.3 (18.2–24.4) | |||
| Smoking status | |||||||||
| Yes | 7.8 | 49.1 (43.06–55.24) | <0.0001 | 8.2 | 50.4 (39.9–60.8) | 0.1511 | 7.6 | 48.4 (40.9–55.8) | <0.0001 |
| No | 92.2 | 31.4(29.6–33.2) | 91.8 | 42.4 (39.1–45.7) | 92.4 | 25.1 (23.0–27.0) | |||
| Marital status | |||||||||
| Single | 21.6 | 38.5 (35.1–41.9) | 0.0002 | 17.2 | 52.5 (45.0–59.9) | 0.006 | 24.1 | 32.7 (29.3–36.2) | 0.0002 |
| Married/partner | 78.4 | 31.2 (29.2–33.2) | 82.8 | 41.1 (37.6–44.6) | 75.9 | 24.9 (22.6–27.2) | |||
| Regular physician | |||||||||
| No | 10.9 | 55.6 (50.2–61.0) | <0.0001 | 12.3 | 60.6 (51.8–69.4) | <0.0001 | 10.2 | 52.1 (45.2–58.9) | <0.0001 |
| Yes | 89.1 | 30.0 (28.2–31.85) | 87.8 | 40.6 (37.2–44.0) | 89.8 | 23.9 (22.0–25.9) | |||
| Any health insurance coverage | |||||||||
| No | 9.6 | 59.0 (53.2–64.8) | <0.0001 | 11.7 | 69.2 (60.9–77.6) | <0.0001 | 8.4 | 50.9 (43.2–58.3) | <0.0001 |
| Yes | 90.4 | 30.0 (28.2–31.8) | 88.3 | 39.6 (36.3–43.0) | 91.6 | 24.6 (22.6–26.5) | |||
| Self-rated health general health status | |||||||||
| Fair or poor | 14.8 | 31.2 (29.48–33.1) | <0.0001 | 12.2 | 52.1 (42.0–62.2) | 0.0553 | 16.3 | 37.2 (32.4–42.1) | <0.0001 |
| Good to excellent | 85.2 | 41.8 (37.1–46.4) | 87.8 | 41.83 (38.5–45.2) | 83.7 | 24.7 (22.7–26.8) | |||
| BMI | |||||||||
| Normal | 38.7 | 32.6 (29.8–35.4) | 0.015 | 45.8 | 49.3(36.2–62.4) | 0.16 | 34.6 | 25.7 (22.6–28.7) | 0.046 |
| Overweight | 29.8 | 29.2 (26.2–32.3) | 29.8 | 41.5(36.9–46.2) | 31.4 | 24.1 (20.8–27.3) | |||
| Obese | 23.9 | 35.8 (32.2–39.5) | 20.2 | 39.6(33.4–45.7) | 26.0 | 29.8 (25.7–34.0) | |||
| no data | 7.6 | 38.1 (31.5–44.7) | 5.0 | 49.08(41.8–56.3) | 8.0 | 32.3 (25.3–39.3) | |||
| No. children <18 years in home | |||||||||
| 3+ Children | 11.9 | 49.5 (43.7–55.3) | <0.0001 | 28.0 | 49.1 (42.9–55.2) | 0.0662 | 2.5 | 52.3 (35.7–68.8) | <0.0001 |
| 1–2 Children | 26.5 | 38.0 (34.4–41.7) | 46.0 | 40.0 (35.3–44.7) | 15.2 | 34.4 (28.4–40.5) | |||
| 0 Children | 61.6 | 27.3 (25.5–29.3) | 26.0 | 42.1 (35.8–48.4) | 82.4 | 24.6 (22.6–26.6) | |||
| Median travel time to nearest mammography (minutes) | |||||||||
| ≥30 | 2.2 | 37.8 (28.6–47.0) | 0.608 | 1.7 | 49.4 (29.6–69.2) | 0.7799 | 2.5 | 33.3 (23.4–43.2) | 0.6014 |
| 20 to <30 | 3.2 | 38.6 (30.3–46.8) | 3.11 | 52.0 (35.7–68.2) | 3.2 | 30.9 (22.2–39.6) | |||
| 10 to <20 | 13.9 | 33.4 (28.8–38.1) | 14.67 | 39.9 (31.4–48.3) | 13.5 | 29.4 (24.0–34.8) | |||
| 5 to <10 | 47.5 | 32.6 (30.1–35.1) | 47.77 | 44.2 (39.5–48.9) | 47.4 | 25.8 (22.9–28.6) | |||
| <5 | 31.1 | 31.8 (28.8–34.9) | 30.2 | 41.7 (35.8–47.6) | 31.6 | 26.4 (23.0–29.7) | |||
| Unknown | 2.2 | 32.7 (20.9–44.6) | 2.6 | 41.5 (21.9–61.1) | 1.8 | 25.9 (11.1–40.6) | |||
| Geographic accessibility | |||||||||
| 0 to <0.50 (Low) | 3.3 | 32.7 (20.9–44.6) | 0.7482 | 3.1 | 48.2 (35.1–61.2) | 0.8194 | 3.5 | 28.4 (21.5–35.2) | 0.9379 |
| 0.51 to <1.0 | 12.2 | 30.2 (25.4–35.0) | 12.5 | 37.8 (28.5–47.0) | 12.0 | 25.6 (20.3–30.8) | |||
| 1.0 to <1.5 | 43.9 | 32.9 (30.2–35.7) | 47.1 | 43.2 (38.4–48.0) | 42.1 | 26.3 (23.1–29.4) | |||
| 1.5 to <2.0 | 28.5 | 34.0 (30.7–37.3) | 27.3 | 44.57 (38.3–50.8) | 29.1 | 28.2 (24.6–31.8) | |||
| ≥2.0 (High) | 9.9 | 31.0 (26.7–35.3) | 7.5 | 44.0 (34.7–53.4) | 11.4 | 26.0 (21.2–30.8) | |||
| Unknown | 2.2 | 32.7 (20.9–44.6) | 2.5 | 41.5 (21.9–61.1) | 1.9 | 25.9 (11.1–40.5) | |||
| Area of residence | |||||||||
| Urban | 85.3 | 32.5 (30.6–34.4) | 0.4505 | 86.9 | 42.5 (38.9–46.0) | 0.4063 | 84.3 | 26.5 (24.3–28.7) | 0.3355 |
| Rural | 12.7 | 35.2 (31.6–38.8) | 10.5 | 48.8 (41.4–56.3) | 13.9 | 29.2 (25.4–33.0) | |||
| Unknown | 2.1 | 30.2 (19.0–41.1) | 1.5 | 41.1 (21.4–60.8) | 1.8 | 21.0 (9.0–33.0) | |||
Utah 2008 and 2010 Behavioral Risk Factor Surveillance System (BRFSS) respondents with no mammogram within the past 2 years.
Weighted percentage.
Wald chi-square p-value.
CI, confidence interval.
FIG. 1.
ZIP code level geographic measures: (A) Travel times to nearest mammography facility; (B) geographic access, including facility capacity; and (C) residence type (urban/rural). Color images available online at www.liebertpub.com/jwh
Table 2 summarizes the results of the multivariable analyses. In the unstratified model, women aged 40–49 were more likely to be nonadherent to mammography screening guidelines compared with women aged 50–74 (Model 1 odds artio [OR] 1.71, 95% confidence interval [CI] 1.36–2.14). The geographic variables (i.e., geographic accessibility, rural/urban residence, and travel time) remained nonsignificant even after controlling for individual-level factors. In age-stratified models, race/ethnicity was not significant among women aged 40–49, but non-Hispanic white women aged 50–74 were 1.85 times more likely to be nonadherent as compared with women of other race/ethnicities in the same age group, ceteris paribus.
Table 2.
Multivariate Analysis of Factors Related to Mammography Screening Nonadherence Among Utah Women
| Nonadherence: No mammogram within past two years | |||
|---|---|---|---|
| Age 40–74 | Age 40–49 | Age 50–74 | |
| Adjusted OR (95% CI)a | Adjusted OR (95% CI)a | Adjusted OR (95% CI)a | |
| Age (years) | |||
| 40–49 | 1.71 (1.36–2.14) | ||
| 50–74 | Ref | ||
| Race/ethnicity | |||
| Non-Hispanic white | 1.62 (1.16–2.27) | 1.43 (0.88–2.33) | 1.85 (1.21–2.82) |
| Other/unknown | Ref | Ref | Ref |
| Income | |||
| Less than $25,000 | 1.85 (1.35–2.54) | 2.18 (1.21–3.95) | 1.74 (1.2–2.54) |
| $25,000 to $50,000 | 1.56 (1.21–2.01) | 1.82 (1.2–2.77) | 1.44 (1.04–2) |
| $50,000 to $75,000 | 1.27 (0.98–1.65) | 1.33 (0.92–1.91) | 1.23 (0.84–1.79) |
| More than $75,000 | Ref | Ref | Ref |
| Refused/unknown | 1.28 (0.95–1.73) | 1.01 (0.59–1.74) | 1.41 (0.98–2.04) |
| Educational attainment | |||
| <High school | 1.39 (0.85–2.26) | 1.04 (0.44–2.46) | 1.83 (1.05–3.22) |
| High school | 1.13 (0.89–1.44) | 1.03 (0.7–1.52) | 1.18 (0.87–1.62) |
| Some college | 1.24 (1.01–1.52) | 1.3 (0.94–1.8) | 1.18 (0.99–1.55) |
| College | Ref | Ref | Ref |
| Smoking status | |||
| Yes | 1.70 (1.28–2.24) | 1.19(0.73–1.94) | 2.03 (1.45–2.86) |
| No | Ref | Ref | Ref |
| Marital status | |||
| Single | 1.07 (0.87–1.32) | 1.01 (0.68–1.50) | 1.06 (0.83–1.35) |
| Married/partner | Ref | Ref | Ref |
| Regular physician | |||
| No | 2.57 (1.98–3.34) | 1.92 (1.24–2.97) | 3.06 (2.19–4.27) |
| Yes | Ref | Ref | Ref |
| Any health insurance coverage | |||
| No | 2.17 (1.64–2.86) | 2.46 (1.53–3.96) | 2.0 (1.42–2.82) |
| Yes | Ref | Ref | Ref |
| Self-rated health | |||
| Fair or poor | 1.5 (1.18–1.9) | 1.31 (0.81–2.12) | 1.58 (1.21–2.05) |
| Good to excellent | Ref | Ref | Ref |
| Children <18 years in home | |||
| 3+ Children | 2.16 (1.57–2.98) | 1.65 (1.12–2.44) | 3.11 (1.42–6.83) |
| 1–2 Children | 1.43 (1.13–1.81) | 1.11 (0.78–1.57) | 1.64 (1.2–2.24) |
| 0 Children | Ref | Ref | Ref |
| Geographic accessibility | |||
| 0–0.50 (Low) | 1.04 (0.66–1.63) | 1.06 (0.46–2.46) | 1.04 (0.61–1.78) |
| 0.51–1.0 | 0.95 (0.66–1.38) | 0.87 (0.43–1.73) | 1 (0.64–1.56) |
| 1.01–1.50 | 1.17 (0.86–1.57) | 1.13 (0.64–2.00) | 1.18 (0.83–1.67) |
| 1.51–2.0 | 1.28 (0.94–1.74) | 1.27 (0.71–2.27) | 1.28 (0.89–1.83) |
| >2.0 (High) | Ref | Ref | Ref |
| Area of residence | |||
| Rural | 1.14 (0.88–1.48) | 1.19 (0.75–1.89) | 1.12 (0.83–1.53) |
| Urban | Ref | Ref | Ref |
| Median travel time to nearest mammography (minutes) | |||
| ≥20 | 1.29 (0.91–1.82) | 1.2 (0.64–2.26) | 1.34 (0.89–2.03) |
| <20 | Ref | Ref | Ref |
Adjusted for all variables in the model.
OR, odds ratio; Ref, reference.
Effect modification by age group (40–49 and 50–74 years) was evident in stratified analyses for income, having a regular physician, having health insurance, self-rated health, and having children in the home. Among women aged 40–49, incomes less than $25,000 and between $25,000 and $50,000 were associated with 2.18 (95% CI 1.21–3.95) and 1.82 (95% CI 1.20–2.77) greater odds, respectively, of being nonadherent compared with women with incomes greater than $75,000 in the same age group. The association of income among the older age group (50–74), although significant, was not as pronounced with OR=1.74 (95% CI 1.20–2.54) and OR=1.44 (95% CI 1.04–2.00), respectively. As compared with women with health insurance, women aged 40–49 without health insurance have 2.46 times the odds of being nonadherent (95% CI 1.53–3.96). Among women aged 50–74, the OR was slightly lower, 2.0 (95% CI 1.42–2.82). Women aged 50–74 who reported not having a regular physician had more than three times the odds of being nonadherent as compared with women in the same age group with a regular physician. Comparatively, not having a regular physician only resulted in a 92% increased risk of nonadherence among women aged 40–49.
Having children under the age of 18 in the home was associated with nonadherence, but to varying degrees, for women aged 40–49 and 50–74. Among women aged 40–49, there were no significant differences in the odds of having a mammogram within the last 2 years between women with 1–2 children in the home compared with women with no children in the home. However, in this age group, having 3 or more children in the home was associated with a 65% greater risk of nonadherence as compared with women with no children in the home. Having any children in the home had a significant effect on the odds of mammography screening nonadherence among women in the older age group 50–74 (1–2 children: OR 1.64, 95% CI 1.2–2.24; 3+ children OR 3.11, 95% CI 1.42–6.83). Women aged 50–74 with less than a high school education (OR 1.83, 95% 1.05–3.22), being a current smoker (OR 2.03, 95% CI 1.45–2.86), and reporting fair or poor health (OR 1.58, 95% CI 1.21–2.05) were more likely to be nonadherent compared with women with a college education, being a non-smoker, and in good to excellent health in the same age group, respectively. These associations were not significant among women aged 40–49.
Discussion
This study is the first to attempt to identify factors affecting adherence to mammography screening guidelines among Utah women and the first to utilize ZIP code data from BRFSS to assess geographic access to mammography. Overall, only 57% of women aged 40–49 and 67.2% of women aged 40–74 in the study population were adherent to mammography screening guidelines. Both rates were below the national screening rate in 2010 (75%), failed to meet the national Healthy People 2010 goal of 70% for screening mammography,36 and were even more disparate from the national Healthy People goal set for 2020 (81.1%).37 Using multivariable analysis, we identified several predisposing factors associated with nonadherence that were similar to findings from previous studies, including younger age (40–49), less than high school education, and poor to fair self-rated health.3,5,6,8
The difference in screening rates between women 40–49 and 50–74 years of age has been attributed to lack of consensus about the efficacy of screening among providers, differences in perceived cancer risk by both patients and providers, and, for older women, access to Medicare insurance and more accumulated health factors that increase cancer screening among older women.24,38,39 Utah women in the younger age group have had especially low screening rates compared with the national average for nearly the last 20 years.20 The lower rates of screening among Utah women with less than a high school education is likely due to low literacy among those with low education. Low literacy can affect patient–physician communication regarding screening or understanding of cancer screening concepts that are presented in educational materials. Furthermore, because low education is often correlated with low income, these same women might have difficulties seeking access to free screening services. Cancer screening and early detection efforts should, therefore, enhance outreach to these at-risk women with more effective evidence-based public health interventions to address low-income and low-literacy populations.
We also identified several individual barriers that were associated with nonadherence, including insurance and income.3,5,6,8 Results from this study support previous research that low-income women and women without health insurance received fewer recommended cancer screening tests and preventative services compared with women with higher income or insurance, respectively.40,41 Mammography screening for low-income, uninsured, or underinsured women was available in 2008 and 2010 for women aged 40–74 through Utah's Cancer Control Program in partnership with the CDC's National Breast and Cervical Cancer Early Detection Program (NBCCEDP).42 However, gaps in financial coverage for mammography screening remained during the study period. Thirty-three percent of women aged 40–74 and 59% of uninsured women did not receiving mammography screening services in Utah in the study. In our study, we found greater disparities among younger women aged 40–49 as nearly 70% of the uninsured ‘did not’ have a mammogram in the last 2 years as compared to only 40% of insured women in the same age group. Furthermore, after including women aged 50–74 who fall within the age range of current mammography screening guidelines, the percent of women with insurance who did not have a mammogram in the last 2 years was 25% compared to 51% for women with no insurance.
Proponents of the U.S. Affordable Care Act (ACA) anticipate reductions in mammography screening disparities from providing greater access to affordable health insurance offering coverage for mammography screening with no out-of-pocket expenses. However, according to the American Cancer Society Cancer Action Network, “Since Utah will not be participating in the Medicaid expansion, more than 31,000 women will not gain access to any affordable health care coverage in 2014.” 43 Given this potential gap, continued monitoring of mammography screening rates in the context of the implementation of the ACA is imperative to not only assess the effectiveness of current cancer control efforts, but to consider future needs and resource allocations that will ensure that all women have access to screening services. Furthermore, even if financial barriers to accessing screening are removed for the majority of women of screening age, state cancer control programs—including NBCCEDP—will need to expand their focus to address predisposing factors and other enabling factors, since health insurance is only one barrier women face in accessing preventative services.
In addition to insurance and income, we also considered time demands as a potential barrier. Considering our study population, we hypothesized that family size presented time demands and competing priorities that outweighed the perceived need for breast cancer screening. Utah has the highest percentage of households with children (43.3%) and the most people per household (3.1 per household) compared with other U.S. states. National averages are substantially lower, at only 33.4% and 2.58 respectively.44 Using the number of children in the home as proxy for demands on women's time, we found that having children in the home was associated with as much as a 3-fold increase in the odds of not having a mammogram within the last 2 years. This finding was consistent with Brown et al. who found lower mammography screening rates among women with one or more children in the home.28 We found additional support for this hypothesis in post-hoc analysis, where we reviewed responses from a 2010 Utah BRFSS question which asked women who had not had a mammogram in the last 3–5 years: “What is the most important reason you have not had a mammogram in the last 2 years?” Of the 306 respondents, 18% cited “no time” as the most important reason for not having had a mammogram in the last 2 years, second only to “cost/not covered by insurance,” which 20% of respondents cited. Future research is necessary to better understand how time demands related to work and family impact adherence to mammography screening guidelines in Utah and elsewhere.
Other systemic or cultural factors that have persisted over time and are highly prevalent in the Utah population may be driving these trends. It is possible that Utah women perceive their breast cancer risk as lower because risk declines with increases in parity.45–47 To better understand barriers to screening among women in Utah, future work should investigate other social and behavioral factors that may explain these trends, including cultural, religious, risk perceptions, family resources, and time demands. Future work might also compare and contrast our Utah findings with other states in the greater Mountain West region that also have screening rates lower than the national average of 75%. In 2010, the five lowest-ranking states in 2010 for mammography screening rates were found in the Mountain West states of Idaho (64%), Utah, Nevada, Wyoming, and Montana (all 67%).47
We did not find any indication that Utah-specific geographic factors, including median travel time, geographic access based on facility capacity, and rural/urban residence, were significantly associated with receiving a mammogram within the last 2 years for women aged 40–74. This is inconsistent with several previous studies in other states that found that poorer access (defined as long drive times to mammography facilities), rural residence, and/or low capacity decreased mammography screening adherence.9,11–14,17 The inconsistencies are unsurprising considering that the majority of these studies used county-based geographic access measures, were confined to small geographic areas, or included only women 64 years and older; therefore, it is difficult to make direct comparisons. County measures of geographic access are particularly problematic because they do not account for county border crossings; they provide no information about spatial variation within a county, and their measures can vary significantly depending on the size, number, and configuration of the study zones. And although we report null findings, we avoided some of these measurement challenges by utilizing population-weighted ZIP code measures based on small-area estimates of drive times and population demand.
There are a few possible reasons we did not find geographic factors significant. First, the overall drive times for Utah women are lower compared to those in many other U.S. areas. Whereas 63% of U.S. women travel only 10 or less minutes to the nearest mammography facility,48 over 86% of Utah women travel that long to their nearest mammography facility. Second, despite Utah's large land area, only 15% of the population lives in rural areas. Finally, in spite of a nationwide trend toward consolidation of mammography facilities.9,49 Utah appears to have adequate coverage, even in rural parts of the state. This is partly the result of several new facilities opening in rural areas between 2004 and 2007.50
A few study limitations should be noted. First, the Utah BRFSS survey data were collected using only a landline-based phone survey; therefore, respondents were limited to women who have a phone in their homes. Second, the data used in this study are based on self-reported responses to the survey questions, which can be subject to recall bias. Third, our measures of geographic access and travel times were based on travel through the street network and assumed access to a household vehicle and the same mode of travel. This methodology fails to recognize other methods of transport, such as public transportation and walking, and women without access to a private vehicle may face a greater travel burden in getting to mammography facilities. Fourth, the drive times and geographic access measures were based on population-weighted estimates of these measures because ZIP codes were the only geographic variable available from BRFSS. While the use of population-weighted estimates is a better approach than simply using ZIP code centroids, which can fall in areas far from populated places, measurement error is still possible. However, it should be noted that ZIP codes in many rural areas in Utah provide better geographic coverage than census tracts because they tend to be more compact in size and more directly surround towns and cities than do census tracts. A final limitation is that distance to mammography services were calculated for stationary facilities only. The impact of mobile mammography coverage in Utah is unclear. During the study period, three mobile facilitates were located in rural parts of the state, based out of hospitals where they are also used for screening onsite. The hospital location was used for estimating distance to screening, and therefore, our measures of capacity and access are likely underestimated, so accessibility is likely better than we measured. There are current ongoing efforts to increase mammography rates with new mobile units.51 However, these enterprises alone may not increase mammography rates if individual-level factors are more important than geographic access.
Conclusions
Although one of the results from this study suggests that the poor mammography screening rates in Utah may not be attributable to geographic accessibility, it is important to understand whether the lack of significance is a regional factor and may in fact speak to the success of current cancer control screening efforts. Access to healthcare through health insurance coverage is clearly a primary intervention point for increasing mammography screening. However, since Utah will not be participating in the Medicaid expansion, some women will not gain access to any affordable healthcare coverage. A policy focusing on ensuring that there are no women without insurance coverage may be an effective first step toward increasing mammography screening in Utah.
Other results offer potential targets for future breast cancer screening interventions among Utah women. Cancer control efforts should use evidenced-based public health interventions to lower potential mammography screening barriers associated with obtaining a usual source of care or physician, even among the insured; low literacy rates, which may be a driving force for nonadherence among the poorly educated and low income; and competing time demands, which present unique challenges for women with young children who may also work outside the home and generally have busy schedules. This final barrier may be the most challenging to address, but likely interventions could include implementing or enhancing existing employer-based preventative health programs (e.g., mobility units, paid leave time for cancer screening during work hours), extending cancer screening hours to include evening and weekend appointments, and providing onsite childcare for women with small children.
Author Disclosure Statement
The authors declare no competing financial interests exist.
References
- 1.U.S. Cancer Statistics Working Group. United states cancer statistics: 1999–2009 Incidence and mortality web-based report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; 2013. Available at: www.cdc.gov/uscs [Google Scholar]
- 2.Hendrick RE, Helvie MA. United States preventive services task force screening mammography recommendations: science ignored. AJR Am J Roentgenol 2011;196:W112–W116 [DOI] [PubMed] [Google Scholar]
- 3.Kim J, Jang SN. Socioeconomic disparities in breast cancer screening among U.S. women: Trends from 2000 to 2005. J Prev Med Public Health. 2008;41:186–194 [DOI] [PubMed] [Google Scholar]
- 4.Rattanawatkul K, Carter-Pokras O. Decline and disparities in mammography use trends by socioeconomic status and race/ethnicity. U Maryland McNair Scholars Undergrad Res J 2011;3:10 [Google Scholar]
- 5.Rauscher GH, Allgood KL, Whitman S, Conant E. Disparities in screening mammography services by race/ethnicity and health insurance. J Womens Health (Larchmt) 2012;21:154–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kempe KL, Larson RS, Shetterley S, Wilkinson A. Breast cancer screening in an insured population: Whom are we missing? The Permanente Journal. 2013;17:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wells KJ, Roetzheim RG. Health disparities in receipt of screening mammography in Latinas: a critical review of recent literature. Cancer Control 2007;14:369–379 [DOI] [PubMed] [Google Scholar]
- 8.Finney Rutten LJ, Nelson DE, Meissner HI. Examination of population-wide trends in barriers to cancer screening from a diffusion of innovation perspective (1987–2000). Prev Med 2004;38:258–268 [DOI] [PubMed] [Google Scholar]
- 9.Elkin EB, Ishill NM, Snow JG, et al. Geographic access and the use of screening mammography. Med Care 2010;48:349–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elkin EB, Snow JG, Leoce NM, Atoria CL, Schrag D. Mammography capacity and appointment wait times: Barriers to breast cancer screening. Cancer Causes Control CCC. 2012;23:45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elting LS, Cooksley CD, Bekele BN, et al. Mammography capacity impact on screening rates and breast cancer stage at diagnosis. Am J Preventive Med 2009;37:102–108 [DOI] [PubMed] [Google Scholar]
- 12.Khan N, Kaestner R, Salmon JW, Gutierrez B. Does supply influence mammography screening? Am J Health Behav 2010;34:465–475 [DOI] [PubMed] [Google Scholar]
- 13.Meersman SC, Breen N, Pickle LW, Meissner HI, Simon P. Access to mammography screening in a large urban population: A multi-level analysis. Cancer Causes Control 2009;20:1469–1482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coughlin SS, Thompson TD, Hall HI, Logan P, Uhler RJ. Breast and cervical carcinoma screening practices among women in rural and nonrural areas of the United States, 1998–1999. Cancer 2002;94:2801–2812 [DOI] [PubMed] [Google Scholar]
- 15.Meilleur A, Subramanian SV, Plascak JJ, Fisher JL, Paskett ED, Lamont EB. Rural residence and cancer outcomes in the United States: Issues and challenges. Cancer Epidemiol Biomarkers Prev 2013;22:1657–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller JW, King JB, Ryerson AB, Eheman CR, White MC: Mammography use from 2000 to 2006: state-level trends with corresponding breast cancer incidence rates. AJR Am J Roentgenol 2009;192:352–360 [DOI] [PubMed] [Google Scholar]
- 17.Mobley LR, Kuo TM, Driscoll D, Clayton L, Anselin L. Heterogeneity in mammography use across the nation: separating evidence of disparities from the disproportionate effects of geography. Int J Health Geogr 2008;7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabatino SA, Coates RJ, Uhler RJ, Breen N, Tangka F, Shaw KM. Disparities in mammography use among US women aged 40–64 years, by race, ethnicity, income, and health insurance status, 1993 and 2005. Med Care 2008;46:692–700 [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC). Behavioral Risk Factor Surveillance System Survey data. Atlanta, Georgia: U.S. Department of Health and Human Services, 2010 [Google Scholar]
- 20.Office of Public Health Assessment. Utah's Behavioral Risk Factor Surveillance System trend report 1989–1999. Salt Lake City, UT: Utah Department of Health, 2001 [Google Scholar]
- 21.Force USPST. Screening for breast cancer: recommendations and rationale. Ann Intern Med 2002;137:344–346 [DOI] [PubMed] [Google Scholar]
- 22.Behavioral Risk Factor Surveillance System (BRFSS). Operational and user's guide,version 3.0. U.S. Department of Health and Human Services, 2006:1–112 Available at: http://ftp.cdc.gov/pub/Data/Brfss/userguide.pdf Accessed December29, 2012 [Google Scholar]
- 23.American Cancer Society. Breast cancer facts and figures 2011–2012. Atlanta, GA: American Cancer Society, Inc., 2011 [Google Scholar]
- 24.Block LD, Jarlenski MP, Wu AW, Bennett WL. Mammography use among women ages 40–49 after the 2009 U.S. Preventive Services Task Force recommendation. J Gen Intern Med 2013;28:1147–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pace LE, He Y, Keating NL. Trends in mammography screening rates after publication of the 2009 US Preventive Services Task Force recommendations. Cancer 2013;119:2518–2523 [DOI] [PubMed] [Google Scholar]
- 26.Andersen RM. Revisiting the behavioral model and access to medical care: Does it matter? J Health Social Behav 1995;36:1–10 [PubMed] [Google Scholar]
- 27.Andersen RM, Davidson PL. Improving access to care in America: Individual and contextual indicators. In: Andersen R, Rice T, Kominski J, eds. Changing the U.S. health care system: Key issues in health services, policy, and management; San Francisco: Jossey-Bass Publishers, 2001:3–30 [Google Scholar]
- 28.Brown SL, Gibney TM, Tarling R. Busy lifestyles and mammography screening: Time pressure and women's reattendance likelihood. Psychol Health. 2013:1–11 [DOI] [PubMed] [Google Scholar]
- 29.Wang F. Measurement, optimization, and impact of health care accessibility: A methodological review. Ann Assoc Am Geogr 2012;102:1104–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aday LA, Andersen RM. Equity of access to medical care: a conceptual and empirical overview. Med Care 1981;19:4–27 [PubMed] [Google Scholar]
- 31.Cromley EK, McLafferty S. GIS and public health. 2nd ed. New York: Guilford Press, 2012:304–337 [Google Scholar]
- 32.U.S. Department of Agriculture (USDA). 2000 Rural-urban commuting area (ruca) codes: Documentation. 2013. Available at: www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/documentation.aspx#.UfQPF421HX5 Accessed December1, 2012
- 33.University of Washington Rural Health Research Center. Rural urban commuting areas (RUCAs). 2007. Available at: http://depts.washington.edu/uwruca/index.php Accessed December1, 2012
- 34.University of Southern California GIS Research Laboratory. North American Association of Central Cancer Registries (NAACCR) Shortest Path Finder Tool, NAACCR Inc, Springfield, IL: Available at: www.naaccr.org/Research/ShortestPathFinder.aspx Accessed March1, 2013 [Google Scholar]
- 35.Luo W, Qi Y. An enhanced two-step floating catchment area (E2SFCA) method for measuring spatial accessibility to primary care physicians. Health Place 2009;15:1100–1107 [DOI] [PubMed] [Google Scholar]
- 36.U.S. Department of Health and Human Services. Office of Disease Prevention and Health Promotion. Healthy People 2010. Washington, DC: U.S. Department of Health and Human Services; Available at: www.healthypeople.gov/2010 Accessed March5, 2013 [Google Scholar]
- 37.U.S. Department of Health and Human Services. Office of Disease Prevention and Health Promotion. Healthy People 2020. Washington, DC: U.S. Department of Health and Human Services; Available at: www.healthypeople.gov/2020/topicsobjectives2020/default.aspx Accessed March5, 2013 [Google Scholar]
- 38.Nutting PA, Baier M, Werner JJ, Cutter G, Conry C, Stewart L. Competing demands in the office visit: What influences mammography recommendations? J Am Board Fam Pract 2001;14:352–361 [PubMed] [Google Scholar]
- 39.Ryerson AB, Miller JW, Eheman CR, Leadbetter S, White MC. Recent trends in U.S. mammography use from 2000–2006: A population-based analysis. Prev Med 2008;47:477–482 [DOI] [PubMed] [Google Scholar]
- 40.Carney PA, O'Malley J, Buckley DI, et al. Influence of health insurance coverage on breast, cervical, and colorectal cancer screening in rural primary care settings. Cancer 2012;118:6217–6225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sabatino SA, Thompson TD, Richardson LC, Miller J. Health insurance and other factors associated with mammography surveillance among breast cancer survivors: Results from a national survey. Med Care 2012;50:270–276 [DOI] [PubMed] [Google Scholar]
- 42.Utah Department of Health, Utah Cancer Control Program (UCCP). Eligibility requirements. Available at: www.cancerutah.org/Screening_Services/Eligibility_Requirements.php Accessed March12, 2014
- 43.American Cancer Society. The national breast and cervical cancer early detection program saves lives, Available at: www.acscan.org/pdf/breastcancer/factsheets/state-facts/Utah.pdf Accessed August24, 2013
- 44.Lofquist D, Lugaila T, O'Connell M, Feliz S. The Census Bureau's Households and Families: 2010, U.S. Census Bureau,. Available at: www.census.gov/prod/cen2010/briefs/c2010br-14.pdf, ed2012 Accessed August7, 2011 [Google Scholar]
- 45.Lambe M, Hsieh CC, Chan HW, Ekbom A, Trichopoulos D, Adami HO. Parity, age at first and last birth, and risk of breast cancer: A population-based study in Sweden. Breast Cancer Res Treat 1996;38:305–311 [DOI] [PubMed] [Google Scholar]
- 46.Colditz G, Baer H, Tamimi R. Breast cancer. In: Schottenfeld D, Fraumeni JF, eds. Cancer epidemiology and prevention. New York: Oxford University Press, 2006:995–1012 [Google Scholar]
- 47.Centers for Disease Control and Prevention (CDC). Behavioral Risk Factor Surveillance System Survey data. Centers for Disease Control and Prevention, 2011. Available at: http://apps.nccd.cdc.gov/brfss Accessed August7, 2011
- 48.Henry KA, Sherman R, Farber S, Cockburn M, Goldberg DW, Stroup AM. The joint effects of census tract poverty and geographic access on late-stage breast cancer diagnosis in 10 US States. Health Place. 2013;21:110–121 [DOI] [PubMed] [Google Scholar]
- 49.U.S. Government Accountability Office (GAO). Report to Congressional requesters: Mammography: Current nationwide capacity is adequate, but access problems may exist in certain locations. In: Report to Congressional Requesters, GAO-06-724. Washington, DC: US G A O, 2006 [Google Scholar]
- 50.FDA Certified Mammography Facilities 2008–2010 (Raw Data on CD-ROM), Rockville MD: Center for Devices and Radiological Health, 2011. http://webarchive.library.unt.edu/eot2008/20080917140511/http://www.ntis.gov/products/mammog.aspx Accessed August1, 2011 [Google Scholar]
- 51.May H. Utah doctors will soon provide mammogram on wheels. Salt Lake Tribune, July, 162012. Available at: www.sltrib.com/sltrib/news/54463626-78/women-utah-medical-babcook.html.csp Accessed August15, 2013 [Google Scholar]