Abstract
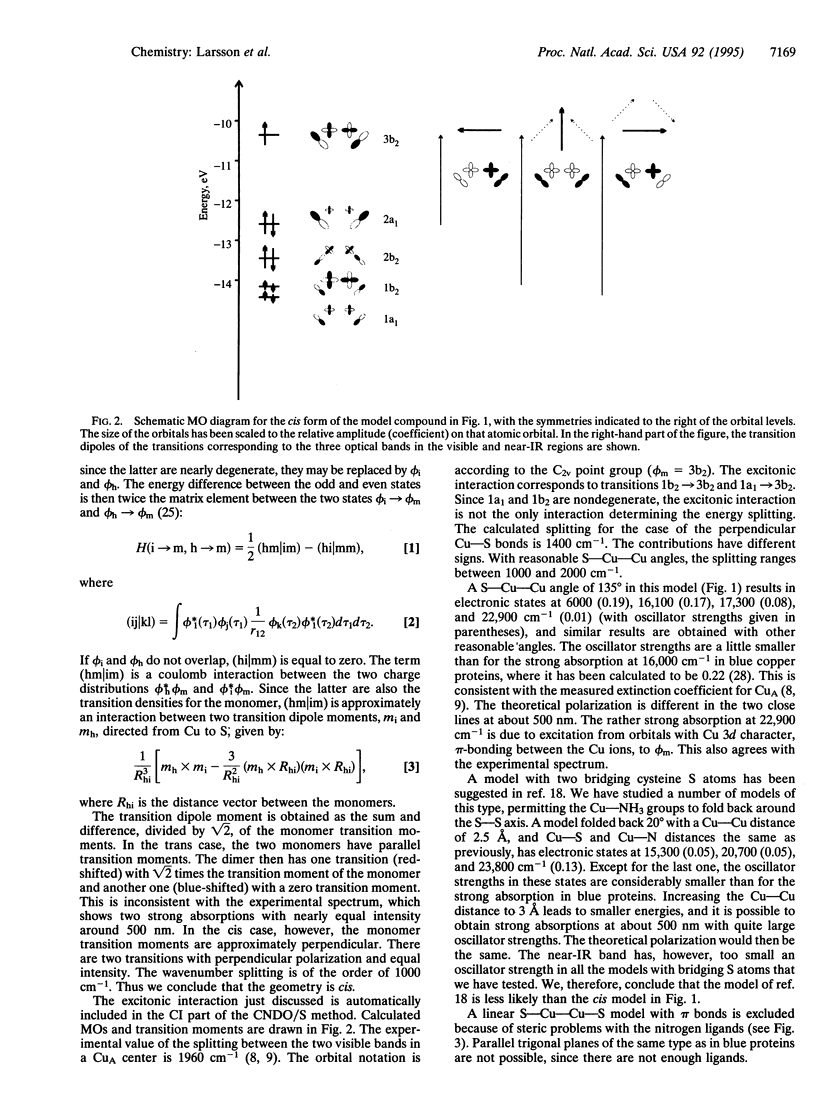
The electronic structure and spectrum of several models of the binuclear metal site in soluble CuA domains of cytochrome-c oxidase have been calculated by the use of an extended version of the complete neglect of differential overlap/spectroscopic method. The experimental spectra have two strong transitions of nearly equal intensity around 500 nm and a near-IR transition close to 800 nm. The model that best reproduces these features consists of a dimer of two blue (type 1) copper centers, in which each Cu atom replaces the missing imidazole on the other Cu atom. Thus, both Cu atoms have one cysteine sulfur atom and one imidazole nitrogen atom as ligands, and there are no bridging ligands but a direct Cu-Cu bond. According to the calculations, the two strong bands in the visible region originate from exciton coupling of the dipoles of the two copper monomers, and the near-IR band is a charge-transfer transition between the two Cu atoms. The known amino acid sequence has been used to construct a molecular model of the CuA site by the use of a template and energy minimization. In this model, the two ligand cysteine residues are in one turn of an alpha-helix, whereas one ligand histidine is in a loop following this helix and the other one is in a beta-strand.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antholine W. E., Kastrau D. H., Steffens G. C., Buse G., Zumft W. G., Kroneck P. M. A comparative EPR investigation of the multicopper proteins nitrous-oxide reductase and cytochrome c oxidase. Eur J Biochem. 1992 Nov 1;209(3):875–881. doi: 10.1111/j.1432-1033.1992.tb17360.x. [DOI] [PubMed] [Google Scholar]
- Augustin M. A., Chapman S. K., Davies D. M., Sykes A. G., Speck S. H., Margoliash E. Interaction of cytochrome c with the blue copper proteins, plastocyanin and azurin. J Biol Chem. 1983 May 25;258(10):6405–6409. [PubMed] [Google Scholar]
- BEINERT H., GRIFFITHS D. E., WHARTON D. C., SANDS R. H. Properties of the copper associated with cytochrome oxidase as studied by paramagnetic resonance spectroscopy. J Biol Chem. 1962 Jul;237:2337–2346. [PubMed] [Google Scholar]
- Babcock G. T., Wikström M. Oxygen activation and the conservation of energy in cell respiration. Nature. 1992 Mar 26;356(6367):301–309. doi: 10.1038/356301a0. [DOI] [PubMed] [Google Scholar]
- Blackburn N. J., Barr M. E., Woodruff W. H., van der Oost J., de Vries S. Metal-metal bonding in biology: EXAFS evidence for a 2.5 A copper-copper bond in the CuA center of cytochrome oxidase. Biochemistry. 1994 Aug 30;33(34):10401–10407. doi: 10.1021/bi00200a022. [DOI] [PubMed] [Google Scholar]
- Brzezinski P., Sundahl M., Adelroth P., Wilson M. T., el-Agez B., Wittung P., Malmström B. G. Triplet-state quenching in complexes between Zn-cytochrome c and cytochrome oxidase or its CuA domain. Biophys Chem. 1995 Apr;54(2):191–197. doi: 10.1016/0301-4622(94)00128-7. [DOI] [PubMed] [Google Scholar]
- Hill B. C. The reaction of the electrostatic cytochrome c-cytochrome oxidase complex with oxygen. J Biol Chem. 1991 Feb 5;266(4):2219–2226. [PubMed] [Google Scholar]
- Holm L., Saraste M., Wikström M. Structural models of the redox centres in cytochrome oxidase. EMBO J. 1987 Sep;6(9):2819–2823. doi: 10.1002/j.1460-2075.1987.tb02578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M., Lappalainen P., Talbo G., Haltia T., van der Oost J., Saraste M. Two cysteines, two histidines, and one methionine are ligands of a binuclear purple copper center. J Biol Chem. 1993 Aug 5;268(22):16781–16787. [PubMed] [Google Scholar]
- Kroneck P. M., Antholine W. A., Riester J., Zumft W. G. The cupric site in nitrous oxide reductase contains a mixed-valence [Cu(II),Cu(I)] binuclear center: a multifrequency electron paramagnetic resonance investigation. FEBS Lett. 1988 Dec 19;242(1):70–74. doi: 10.1016/0014-5793(88)80987-6. [DOI] [PubMed] [Google Scholar]
- Lappalainen P., Aasa R., Malmström B. G., Saraste M. Soluble CuA-binding domain from the Paracoccus cytochrome c oxidase. J Biol Chem. 1993 Dec 15;268(35):26416–26421. [PubMed] [Google Scholar]
- Levitt M. Conformational preferences of amino acids in globular proteins. Biochemistry. 1978 Oct 3;17(20):4277–4285. doi: 10.1021/bi00613a026. [DOI] [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Malkin R., Malmström B. G. The state and function of copper in biological systems. Adv Enzymol Relat Areas Mol Biol. 1970;33:177–244. doi: 10.1002/9780470122785.ch4. [DOI] [PubMed] [Google Scholar]
- Malmström B. G., Aasa R. The nature of the CuA center in cytochrome c oxidase. FEBS Lett. 1993 Jun 28;325(1-2):49–52. doi: 10.1016/0014-5793(93)81411-r. [DOI] [PubMed] [Google Scholar]
- Martin C. T., Scholes C. P., Chan S. I. On the nature of cysteine coordination to CuA in cytochrome c oxidase. J Biol Chem. 1988 Jun 15;263(17):8420–8429. [PubMed] [Google Scholar]
- Mchaourab H. S., Pfenninger S., Antholine W. E., Felix C. C., Hyde J. S., Kroneck P. M. Multiquantum EPR of the mixed valence copper site in nitrous oxide reductase. Biophys J. 1993 May;64(5):1576–1579. doi: 10.1016/S0006-3495(93)81527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nar H., Messerschmidt A., Huber R., van de Kamp M., Canters G. W. Crystal structure analysis of oxidized Pseudomonas aeruginosa azurin at pH 5.5 and pH 9.0. A pH-induced conformational transition involves a peptide bond flip. J Mol Biol. 1991 Oct 5;221(3):765–772. doi: 10.1016/0022-2836(91)80173-r. [DOI] [PubMed] [Google Scholar]
- Oliveberg M., Malmström B. G. Internal electron transfer in cytochrome c oxidase: evidence for a rapid equilibrium between cytochrome a and the bimetallic site. Biochemistry. 1991 Jul 23;30(29):7053–7057. doi: 10.1021/bi00243a003. [DOI] [PubMed] [Google Scholar]
- Pan L. P., Hibdon S., Liu R. Q., Durham B., Millett F. Intracomplex electron transfer between ruthenium-cytochrome c derivatives and cytochrome c oxidase. Biochemistry. 1993 Aug 24;32(33):8492–8498. doi: 10.1021/bi00084a014. [DOI] [PubMed] [Google Scholar]
- Saraste M. Structural features of cytochrome oxidase. Q Rev Biophys. 1990 Nov;23(4):331–366. doi: 10.1017/s0033583500005588. [DOI] [PubMed] [Google Scholar]
- Van Pouderoyen G., Mazumdar S., Hunt N. I., Hill A. O., Canters G. W. The introduction of a negative charge into the hydrophobic patch of Pseudomonas aeruginosa azurin affects the electron self-exchange rate and the electrochemistry. Eur J Biochem. 1994 Jun 1;222(2):583–588. doi: 10.1111/j.1432-1033.1994.tb18900.x. [DOI] [PubMed] [Google Scholar]
- Wittung P., Källebring B., Malmström B. G. The cupredoxin fold is found in the soluble CuA and CyoA domains of two terminal oxidases. FEBS Lett. 1994 Aug 1;349(2):286–288. doi: 10.1016/0014-5793(94)00694-6. [DOI] [PubMed] [Google Scholar]
- van der Oost J., Lappalainen P., Musacchio A., Warne A., Lemieux L., Rumbley J., Gennis R. B., Aasa R., Pascher T., Malmström B. G. Restoration of a lost metal-binding site: construction of two different copper sites into a subunit of the E. coli cytochrome o quinol oxidase complex. EMBO J. 1992 Sep;11(9):3209–3217. doi: 10.1002/j.1460-2075.1992.tb05398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Wachenfeldt C., de Vries S., van der Oost J. The CuAsite of the caa3-type oxidase of Bacillus subtilis is a mixed-valence binuclear copper centre. FEBS Lett. 1994 Feb 28;340(1-2):109–113. doi: 10.1016/0014-5793(94)80182-7. [DOI] [PubMed] [Google Scholar]




