Abstract
Saliva has long been known for its diagnostic value in several diseases. It also has a potential to be used in forensic science.
Objective:
The objective of this study is to compare the quantity and quality of DNA samples extracted from saliva with those extracted from blood in order to assess the feasibility of extracting sufficient DNA from saliva for its possible use in forensic identification.
Materials and Methods:
Blood and saliva samples were collected from 20 volunteers and DNA extraction was performed through Phenol Chloroform technique. The quantity and quality of isolated DNA was analyzed by spectrophotometery and the samples were then used to amplify short tandem repeat (STR) F13 using the polymerase chain reaction.
Results:
Mean quantity of DNA obtained in saliva was 48.4 ± 8.2 μg/ml and in blood was 142.5 ± 45.9 μg/ml. Purity of DNA obtained as assessed by the ratio of optical density 260/280, was found to be optimal in 45% salivary samples while remaining showed minor contamination. Despite this positive F13 STR amplification was achieved in 75% of salivary DNA samples.
Conclusion:
Results of this study showed that saliva may prove to be a useful source of DNA for forensic purpose.
Keywords: Deoxyribo nucleic acid, F13, forensic identification, saliva
Introduction
Saliva is a unique fluid and interest in it as a diagnostic medium has advanced exponentially in the last 10 years. The major advantages of saliva over blood when used for diagnostic purposes include easy access, non-invasive collection, and better patient/subject compliance.[1]
Apart from its use in diagnosis of various diseases, saliva has immense potential to be used in forensic science. Saliva can be deposited on human skin through biting, sucking, licking, and kissing, etc.[2] It is found on victims of several violent crimes and has been shown to be potentially recovered and typed from bite marks, cigarette butts, postage stamps, envelopes, edibles, and other objects.[3]
Saliva is very good source of human DNA, as the mean number of epithelial cells per 1 mL of saliva is about 4.3 × 105. Moreover, the turnover of epithelial cells is quite extensive in the mouth as the surface layer of epithelial cells is replaced, on average, every 2.7 h, suggesting that there is likely to be intact genomic DNA in saliva samples. Indeed, recent studies have shown that human genomic DNA can be reliably obtained from saliva.[4] Saliva can be used even when it is stored in the most different conditions.[5]
This study was designed to assess the quantity and quality of DNA extracted from saliva, the possibility of using it for gene amplification for individualization, and its comparison with DNA extracted from blood.
Materials and Methods
This study was conducted at the Department of Oral and Maxillofacial Pathology, Saraswati Dental College and Hospital, Lucknow in collaboration with Department of Genetics, Sanjay Gandhi Postgraduate Institute of Medical Research Lucknow.
Sample collection
Blood and Saliva samples were collected from 20 non-related volunteers after informed consent and approval from Hospital Ethics Committee of Saraswati Dental College and Hospital, Lucknow. A total of 5 ml of blood was collected using sterile syringes and stored in sterile Ethylenediamine tetraacetic acid (EDTA) vials till further use. Saliva was collected by asking the subjects to spit in a sterile disposable Petri dish, 2 ml of this saliva was transferred from Petri dish to sterile vials, using a sterile pipette, and stored at − 20°C until further use. DNA extraction from blood and saliva was carried out by salting out method using the phenol-chloroform as described by Coomey et al.[6]
Quantity and quality analysis
Since, the sample size was limited analysis of quantity and the quality was performed with the help of spectrophotometer.
Concentration of deoxyribo nucleic acid
DNA was quantified by measuring the optical density (OD) at 260 nm. 5 μl of stock genomic DNA was taken and 995 μl of water was added (Dilution factor = 200), mixed well and OD was taken at 260 nm in a spectrophotometer (Hitachi). DNA concentration of the sample was calculated as follows

Determination of DNA purity
Purity of DNA was determined by taking the OD of the sample at 280 nm for protein concentration and at 260 nm for DNA concentration. The ratio OD260 /OD280 was calculated and DNA sample within the range of 1.6-2 was considered as pure. Samples above this range were considered contaminated with protein and those below by RNA.[7]
Gene amplification
Short tandem repeat (STR) marker F13 was selected for gene amplification from blood and salivary DNA as it has been shown to be one of the most polymorphic marker and can be used for individualization.[8,9]
Polymerase chain reaction
All the DNA samples of blood and saliva were subjected to PCR in automated DNA thermocycler (PTC-100, M.J Research. Inc.). The sequences of forward and reverse F13 primers are 5' GAGGTTGCACTCCAGCCTTT 3' and 5' ATGCCATGCAGATTAGAAA 3'.[10]
Results
The results of our study showed that the DNA extracted from blood samples of 20 subjects ranged from 37.5 μg/ml to 200 μg/ml with mean yield of 142.5 μg/ml. Saliva samples of same subjects showed salivary DNA yield ranged from 28.5 μg/ml to 61.5 μg/ml with the mean of 48.4 μg/ml [Table 1]. On purity assessment of blood we found that all twenty samples were within the optimal range of 1.6-2.0. While only nine out of twenty (45%) salivary DNA samples fulfilled these criteria [Table 2 and Figure 1]. The remaining eleven samples of saliva (55%) were found to have OD26o /OD280 ratio lower than 1.6, suggesting contamination with protein or phenol [Table 2]. On amplification of STR F13 it was found that all 20 (100%) blood samples [Figure 2] and 15 out of 20 (75%) saliva samples gave a positive result [Figure 3]. Further on matching amplified blood samples with saliva samples 100% matching was found. Overall success rate of 15/20 salivary samples (75%) was found [Figure 4].
Table 1.
Quantity of DNA obtained from 20 blood and saliva samples in μ/ml
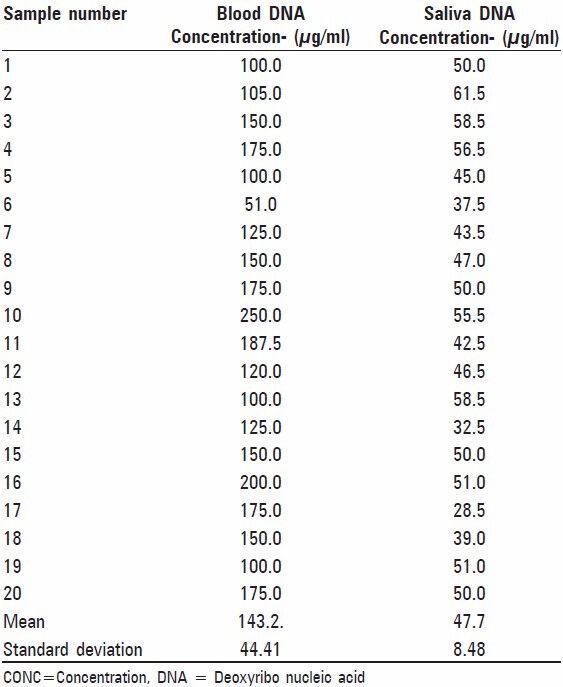
Table 2.
Quality of DNA obtained from blood and saliva samples at OD 260/280
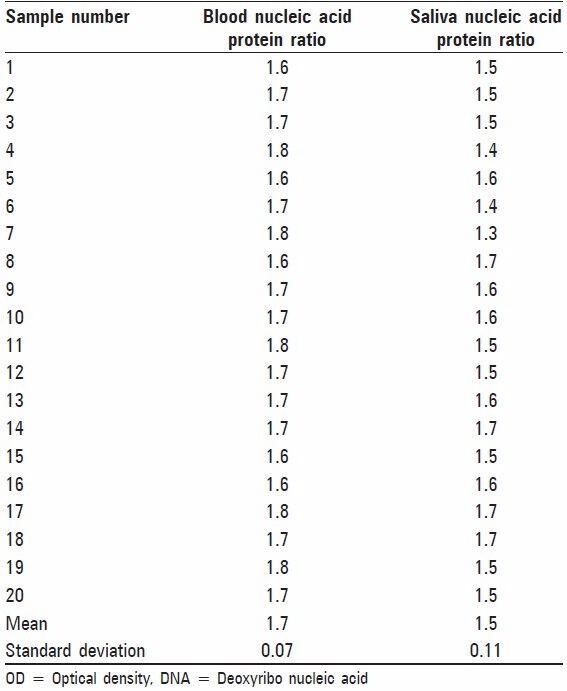
Figure 1.
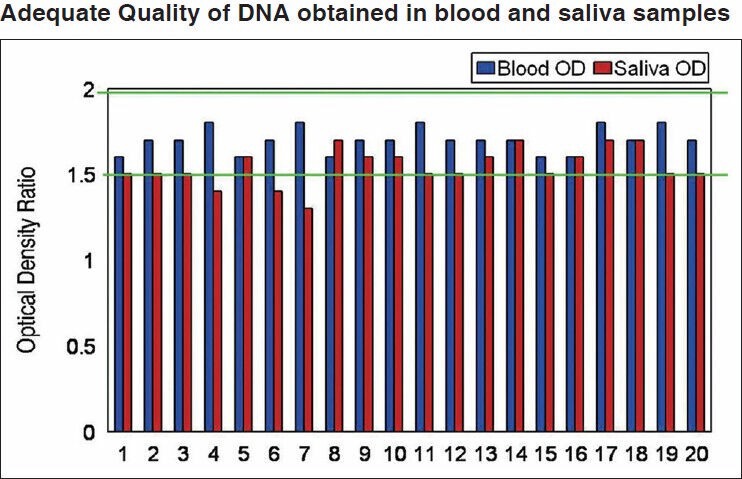
Adequate quality of DNA obtained in blood and saliva samples. It is seen that all 20 samples of blood were in optimal range of 1.6 to 2, giving a 100% result; hence all blood samples were in adequate limit. For saliva, out of 20 samples only 11 samples were in optimal range of 1.6 to 2 thus giving 45% result
Figure 2.
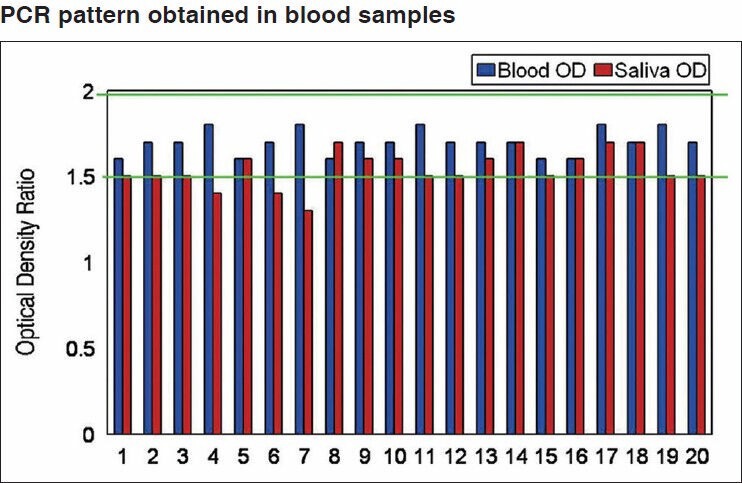
PCR pattern obtained in blood samples with F13. 0.8% agarose gel stained with ethidium bromide. Molecular weight marker for 10 bp between column 4 and 5. In columns 1-4 and 5, blood DNA samples (samples: 1,2,3,4,5)
Figure 3.
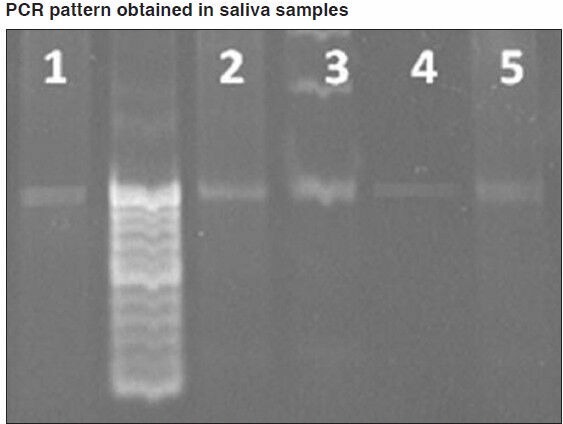
PCR pattern obtained in saliva samples with F13. 0.8% agarose gel stained with ethidium bromide. Molecular weight marker for 10 bp between column 1 and 2. In columns 1 and 3-5, saliva DNA samples (samples: 1,2,3,4,5)
Figure 4.
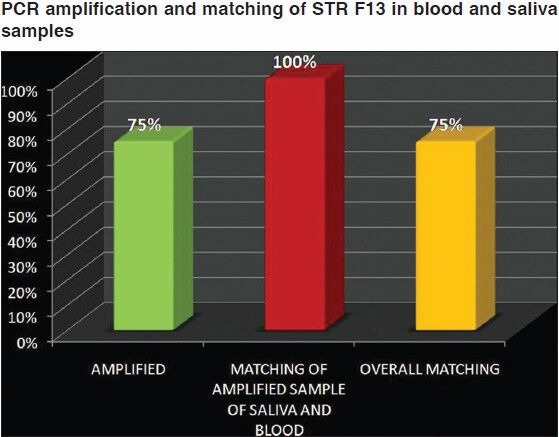
Polymerase chain reaction amplification and matching of short tandem repeat F13 in blood and saliva samples
Discussion
The use of saliva as a source of DNA allows certain technical advantages over the use of blood. Collection is easier and painless, especially considering that it can be carried out on babies, children and elderly subjects, and does not have the religious implications of using the blood. Furthermore, there are higher potential risks of disease transmission when using the blood, especially from hepatitis and AIDS patients, due to the use of sharp objects such as needles.[11] Exfoliated buccal epithelial cells and other cells found in saliva are a very promising alternative source of DNA.[12] Saliva contains cellular material and thus can be typed by DNA analysis. Swabs of the oral cavity, especially swabbing of the cheek area inside the mouth, give more than enough cells and DNA for the serologist to perform DNA typing. This can be an alternative to gathering controls by venipuncture. PCR is extremely sensitive, and from 1 ng to 5 ng of DNA can be successfully typed using the process.[13]
The purpose of this study was to find out whether sufficient quantity and quality of DNA can be extracted from saliva, to be possibly used as DNA evidence in forensics. We used the PCR technique to amplify the STR F13 in order to assess the reliability of salivary DNA. F13 STR is a polymorphic repeat that begins at base pair 248 in intron A of the human coagulation factor XIII A subunit gene on chromosome 6p24.2-p23 (F13A1).[14] Its applications in forensic analysis, paternity determination, genetic disorders, cancers, monitoring of bone marrow transplants, and human cell line identification have been previously reported.[15]
Results of our study show that DNA could be successfully extracted from saliva. Although the yield of DNA obtained was variable ranging from 28.5 μg/ml to 61.5 μg/ml, this finding is in concordance with other workers who reported yields ranging from 1.0 μg/ml to 58.9 μg/ml.[2,3,16,17]
We found mean salivary DNA yield (48.4 μg/ml) 3 times lower than mean blood DNA yield (142.5 μg/ml). Sweet has reported that it is possible to discriminate one individual from all others with a high level of confidence by starting with only 1 ng DNA,[18] and found that DNA typing strength of 1 μL of saliva is equal to 10 μL of blood.[19] DNA obtained from salivary samples in our study was sufficient enough to be used as starting material for gene amplification by PCR.
Another consideration to be kept in mind while assessing DNA samples isolated from any source is the purity of the isolate. Apart from contamination by non-cellular products the most common contaminants in an isolated DNA sample are cellular components such as protein and RNA. Furthermore, phenol used in extraction method, may be left behind in small amounts. In our study, spectrophotometry was employed to assess the purity of DNA. Nucleic acids absorb light at 260 nm through the adenine residues. Proteins absorb light at 280 nm through the tryptophane residues while phenol absorbs ultraviolet light at 270-275 nm. The absorbance of the nucleic acid at 260 nm is 1.6-2.00 times more than the absorbance at 280 nm and hence the ratio of OD readings at 260 nm to 280 nm should fall within the range of 1.6-2.0 for the isolate to be of optimal purity. Solutions having this ratio below 1.6 are thought to be contaminated either by protein or phenol. On the other hand, RNA affords a somewhat higher 260 nm/280 nm ratio, 2.0-2.3. A DNA preparation with a ratio higher than 2.0 may be contaminated with RNA.[7] In our study, we found that OD 260/280 ratio to fall within this optimal limit for all blood samples while some salivary samples were below this optimal range. Possible explanations for this could be either due to the presence of impurities such as carbohydrate, peptides, phenols, buffer salts, and other aromatic compounds or due to the presence of extracellular proteins like mucin in saliva that may contaminate the solution and may compromise the quality. Another possible reason may be that the amount of DNA present in saliva is lesser as compared to blood, and hence multiple purification steps could not be performed as it would have led to compromise in the quantity of DNA obtained.
In the final part of our study, we used the DNA isolated from our samples as a template for amplification of STR of F13 gene by PCR in order to analyze the practical utility of salivary DNA. STR F13 has been found to be useful in parentage testing, and forensic identification.[20] We found positive amplification in 75% of salivary DNA isolates and all these gave positive matching with blood DNA. In past, F13 STR has been shown to be competent enough to be amplified and successfully used for forensic identification from the small amount of DNA obtained from saliva deposited on the skin.[21]
This study was a pilot project using a small sample size in order to assess the feasibility of utilizing salivary samples for DNA based genetic analysis, the results show that DNA obtained from salivary samples could be successfully utilized for gene amplification. Hence, saliva probably has a huge potential as a source of DNA evidence in forensics, though improvements in handling technique and isolation methods are required to increase the yield and quality of DNA from salivary samples. Further studies on STRs of other genes, using a larger sample size, will help in evaluating their feasibility in the forensic application. Currently, we are working with a larger panel of genes in order to assess the possibility of their amplification from salivary DNA.
Conclusion
DNA purity and concentration from blood were found to be in the optimal range while DNA obtained from saliva was near the optimal range and could be utilized for gene amplification. Hence, we conclude that saliva may prove to be a good source of DNA and may be successfully used for forensic purposes.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Streckfus CF, Bigler LR. Saliva as a diagnostic fluid. Oral Dis. 2002;8:69–76. doi: 10.1034/j.1601-0825.2002.1o834.x. [DOI] [PubMed] [Google Scholar]
- 2.Sweet D, Lorente M, Valenzuela A, Lorente JA, Alvarez JC. Increasing DNA extraction yield from saliva stains with a modified Chelex method. Forensic Sci Int. 1996;83:167–77. doi: 10.1016/s0379-0738(96)02034-8. [DOI] [PubMed] [Google Scholar]
- 3.Anzai-Kanto E, Hirata MH, Hirata RD, Nunes FD, Melani RF, Oliveira RN. DNA extraction from human saliva deposited on skin and its use in forensic identification procedures. Braz Oral Res. 2005;19:216–22. doi: 10.1590/s1806-83242005000300011. [DOI] [PubMed] [Google Scholar]
- 4.Quinque D, Kittler R, Kayser M, Stoneking M, Nasidze I. Evaluation of saliva as a source of human DNA for population and association studies. Anal Biochem. 2006;353:272–7. doi: 10.1016/j.ab.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 5.da Silva RH, Sales-Peres A, de Oliveira RN, de Oliveira FT, Sales-Peres SH. Use of DNA technology in forensic dentistry. J Appl Oral Sci. 2007;15:156–61. doi: 10.1590/S1678-77572007000300002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comey CT, Koons BW, Kathryn MS, Presley W, Smerick JB, Sobieralski CA, et al. DNA Extraction Strategies for Amplified Fragment Length Polymorphism Analysis. Journal of Forensic Sciences. 1994;39:1254–1269. [Google Scholar]
- 7.Aggarwal S. Techniques in Molecular Biology. Lucknow: International Book Distributing CO; 2008. Short tandem repeat genotyping; pp. 127–34. [Google Scholar]
- 8.Pérez-Lezaun A, Calafell F, Mateu E, Comas D, Bosch E, Bertranpetit J. Allele frequencies for 20 microsatellites in a worldwide population survey. Hum Hered. 1997;47:189–96. doi: 10.1159/000154412. [DOI] [PubMed] [Google Scholar]
- 9.Destro-Bisol G, Boschi I, Caglià A, Tofanelli S, Pascali V, Paoli G, et al. Microsatellite variation in Central Africa: An analysis of intrapopulational and interpopulational genetic diversity. Am J Phys Anthropol. 2000;112:319–37. doi: 10.1002/1096-8644(200007)112:3<319::AID-AJPA4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 10.Short Tandem Repeat Internet Database (STR Base) with details on STR loci. [Last accessed on 2013 Dec 09]. Available from: http://www.cstl.nist.gov/biotech/strbase . http://www.cstl.nist.gov/strbase .
- 11.Carvalho SP, Sales-Peres A, Ribeiro-Bicudo LA, Silva RH. Quality evaluation of DNA obtained from stored human saliva and its applicability to identification in forensic dentistry. Rev odonto ciênc. 2010;25:48–53. [Google Scholar]
- 12.García-Closas M, Egan KM, Abruzzo J, Newcomb PA, Titus-Ernstoff L, Franklin T, et al. Collection of genomic DNA from adults in epidemiological studies by buccal cytobrush and mouthwash. Cancer Epidemiol Biomarkers Prev. 2001;10:687–96. [PubMed] [Google Scholar]
- 13.Dunncan GT, Tracey ML. Serology and DNA Typing. In: William G, Eckert, editors. Introduction to Forensic Sciences. 2nd ed. New York: Elsevier; 1992. pp. 242–304. [Google Scholar]
- 14.Polymeropoulos MH, Rath DS, Xiao H, Merril CR. Tetranucleotide repeat polymorphism at the human coagulation factor XIII A subunit gene (F13A1) Nucleic Acids Research. 1991;19:4306. [PMC free article] [PubMed] [Google Scholar]
- 15.Sprecher CJ, Puers C, Lins AM, Schumm JW. General approach to analysis of polymorphic short tandem repeat loci. Biotechniques. 1996;20:266–76. doi: 10.2144/96202rr04. [DOI] [PubMed] [Google Scholar]
- 16.Butler JM. Forensic DNA Typing Biology, Technology, and Genetics of STR Markers. 2nd ed. USA: Elsevier; 2005. Sample collection, DNA biology review; p. 23. [Google Scholar]
- 17.Buckingham L. In: Nucleic Acid Extraction Methods in Molecular Diagnostics. Davis FA, editor. Philadelphia, PA 19103: Company United States of America; 1910. pp. 65–79. [Google Scholar]
- 18.Sweet D. Why a dentist for identification? Dent Clin North Am. 2001;45:237–51. [PubMed] [Google Scholar]
- 19.Sweet D. Bite marks as biological evidence. In: Dorion RB, editor. Bitemark Evidence. USA: Routledge; 2005. pp. 183–201. [Google Scholar]
- 20.Hammond HA, Jin L, Zhong Y, Caskey CT, Chakraborty R. Evaluation of 13 short tandem repeat loci for use in personal identification applications. Am J Hum Genet. 1994;55:175–89. [PMC free article] [PubMed] [Google Scholar]
- 21.Sweet D, Shutler GG. Analysis of salivary DNA evidence from a bite mark on a body submerged in water. J Forensic Sci. 1999;44:1069–72. [PubMed] [Google Scholar]


