Abstract
The reversal phase couples bone resorption to bone formation by generating an osteogenic environment at remodeling sites. The coupling mechanism remains poorly understood, despite the identification of a number of ‘coupling' osteogenic molecules. A possible reason is the poor attention for the cells leading to osteogenesis during the reversal phase. This review aims at creating awareness of these cells and their activities in adult cancellous bone. It relates cell events (i) on the bone surface, (ii) in the mesenchymal envelope surrounding the bone marrow and appearing as a canopy above remodeling surfaces and (iii) in the bone marrow itself within a 50-μm distance of this canopy. When bone remodeling is initiated, osteoprogenitors at these three different levels are activated, likely as a result of a rearrangement of cell–cell and cell–matrix interactions. Notably, canopies are brought under the osteogenic influence of capillaries and osteoclasts, whereas bone surface cells become exposed to the eroded matrix and other osteoclast products. In several diverse pathophysiological situations, including osteoporosis, a decreased availability of osteoprogenitors from these local reservoirs coincides with decreased osteoblast recruitment and impaired initiation of bone formation, that is, uncoupling. Overall, this review stresses that coupling does not only depend on molecules able to activate osteogenesis, but that it also demands the presence of osteoprogenitors and ordered cell rearrangements at the remodeling site. It points to protection of local osteoprogenitors as a critical strategy to prevent bone loss.
The ultimate reduction of bone physiology and pathology to the molecular biology is unavoidable and a necessary condition for a further progress in this field, but this descent to the ultimate should be balanced by the awareness of the integrating mechanisms so obvious in the making and maintenance of the skeleton(ZFG Jaworski, Calcif Tissue Int 1984; 36:531)
A role of the reversal phase in coupling bone resorption and formation
Bone remodeling replaces existing bone matrix by new bone matrix. This process has a central role in adult bone physiology, and a malfunction of bone remodeling leads to diseases such as osteoporosis. Bone remodeling is commonly seen as a two-step process: bone resorption by osteoclasts followed by bone formation by osteoblasts. These two events have been a major research focus for many years, as reflected by the current drugs used in the clinic.1 However, the most remarkable property of bone remodeling is probably the subtle coordination between osteoclasts and osteoblasts.2,3 This coordination allows keeping bone shape and structure largely unchanged throughout life, despite the repeated resorption and formation events the bone is subjected to. It has been recognized for a long time that this coordination is made possible because of the organization of osteoclasts and osteoblasts in local bone-remodeling teams, called basic multicellular units (BMUs).4 The question why osteoblasts are recruited exactly where and when osteoclasts have removed bone matrix, has prompted a lot of research in the recent years, as indicated by the number of reviews on the coupling mechanism between osteoclast and osteoblast activities.3,5,6,7,8 A major outcome of this research is the identification of a number of osteogenic molecules likely to be released by the osteoclasts. They include growth factors stored in the bone matrix and solubilized through resorptive activity, as well as so-called clastokines that can be generated by ‘non-resorbing' osteoclasts.8,9
But what are the cells that are subjected to the osteogenic factors released by the osteoclast? A simple analysis of the BMU shows that they cannot be bone-forming osteoblasts themselves, because these osteoblasts are distant from the osteoclasts (Figure 1). Histomorphometry of iliac crest biopsies from normal individuals indicates that this distance corresponds to a time interval of several weeks.10 This intermediate period starting after the osteoclast has left and lasting until bone matrix starts to be deposited is defined as the ‘reversal phase'.11,12 It thus concerns the cell activities transforming the putative osteogenic signals of the osteoclast into bone formation, but these cell activities and the origin of the osteoprogenitors targeted by these signals are poorly investigated.11 This represents a gap in the knowledge that is required to fully understand the coupling process, especially when it comes to adult human cancellous bone and osteoporosis-relevant conditions.
Figure 1.
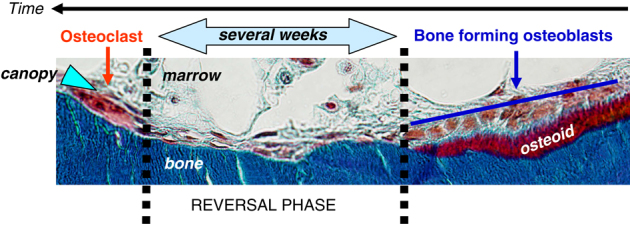
Remodeling unit in human iliac crest biopsy. Remodeling progresses to the left, as indicated by the time axis drawn relative to the bone surface. Histomorphometry indicates that a period of several weeks separates bone-resorbing osteoclasts from bone-forming osteoblasts (cells on osteoid beneath the blue line).10 This period is called reversal phase (demarcated by the dotted black lines). Mineralized matrix: blue; osteoid: red. Note the canopy of elongated cells (blue arrowhead) covering the bone-remodeling site. Image reproduced with permission.11
Overview of the putative effector cells of the reversal phase in cancellous bone
Current knowledge suggests that the coupling activity of the reversal phase starts with the release of osteogenic signals from the osteoclasts.3,6,8 These osteogenic signals will first reach the cells nearest to the osteoclast. These include both bone surface and bone marrow cells (Figure 2). The bone surface cells are the bone-lining cells of quiescent bone surfaces that have retracted to give the osteoclast access to the bone matrix,13,14,15 as well as the mononucleated cells on the eroded surface in the wake of the osteoclast.11 The latter cells are called reversal cells, and cover at least 80% of the eroded surface, known as the reversal surface (Figure 1).11 They form a cellular bridge connecting the resorbing osteoclasts and the bone-forming osteoblasts. As described earlier,11,16 reversal cells appear as elongated cells with flattened nuclei. They appear, however, less elongated than bone-lining cells, and do not show long and thin cell extensions like the latter. Reversal cells closer to bone-forming osteoblasts appear more cuboidal compared with those closer to osteoclasts. The reversal cells were also clearly identified in a rat model designed to follow the kinetics of bone remodeling, where they appear right after the osteoclasts and before the bone-forming osteoblasts.17
Figure 2.
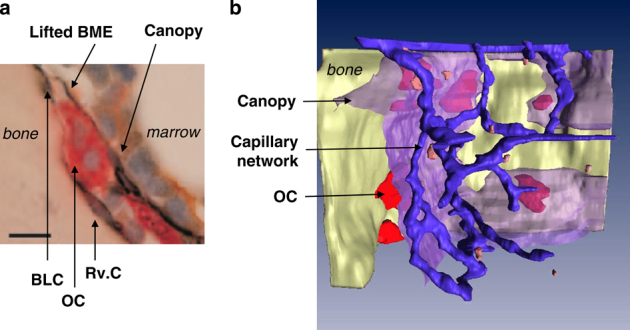
Critical components of the reversal phase. (a) Cross-section at the starting point of a remodeling unit. The bone marrow envelope is lifted over an osteoclast on initiation of bone remodeling. BME: bone marrow envelope; BLC: bone-lining cell; OC: osteoclast; Rv.C: reversal cell. (b) Three-dimensional reconstruction of a bone resorption site in human iliac crest. The bone surface is shown in yellow, the canopy in semi-transparent purple and the capillary network in blue. OC: osteoclast (red cells on the bone surface). Note the close apposition between capillaries and the canopy. Bone-lining and reversal cells are not shown. Images reproduced with permission.2
The bone marrow cells directly exposed to the osteogenic signals released by the osteoclast are the mesenchymal cells that form an envelope surrounding the red bone marrow.18,19,20 They were identified in all species investigated so far,18 and recently received new attention.21,22 This bone marrow mesenchymal envelope appears to be lifted at the level of the osteoclast (Figure 2) and forms a canopy over the whole remodeling site (Figure 1; Table 1).22 Interestingly, initiation of bone remodeling also coincides with the induction of contacts between these canopies and bone marrow capillaries (Figure 2),23 especially above osteoclasts.22 As the vasculature, perivascular cells and circulating osteoprogenitors may contribute to osteogenic events in various situations, their role during the reversal phase deserves consideration.5,24,25,26,27
Table 1. Characteristics differentiating the canopies from the rest of the bone marrow envelope22.
|
Bone marrow envelope |
||
|---|---|---|
| Canopy | ||
| Activity of neighboring bone | Quiescent | Remodeling |
| Physical separation from bone matrix | Bone-lining cells | Osteoclasts, Rv.Cs, osteoblasts and sometimes a lumen |
| Physiological status | Resting | Exposed to osteoclast and capillary osteogenic products |
| Visibility through light microscopy (in human biopsies) | Poor | Satisfying, good if immunostained |
Abbreviation: Rv.Cs, reversal cells.
The bone marrow envelope refers to a continuous layer of elongated cells lining the bone marrow. The term canopy refers to the zone of the bone marrow envelope covering bone-remodeling sites (that is, about 30% of the cancellous bone surface in human adult iliac crest22). Hypertrophy and lifting may contribute to the better visibility of the canopy. Additional discriminating characteristics are under investigation.
Finally, if the osteogenic signals released by the osteoclast cross the canopy and diffuse deeper into the bone marrow, they may reach a variety of other cells,3,28 including bone marrow osteoprogenitors.29
Thus bone-lining cells and reversal cells on the bone surface, bone marrow envelope and canopy cells, as well as capillaries are all positioned close to the remodeling site and therefore deserve special attention as potential factors in coupling bone resorption and formation during the reversal phase. This review summarizes the knowledge on how these cells may contribute in converting the osteogenic signals generated at the onset of resorption into bone formation. The osteogenic signals themselves, including osteocytic signals, have been the topic of a number of recent reviews3,5,6,7,8 and are not included herein.
Reversal surfaces
Bone-lining cells, reversal cells and osteoblast recruitment
Bone-lining cells covering quiescent bone surfaces can turn into bone-forming osteoblasts if stimulated mechanically or by intermittent parathyroid hormone (PTH).30,31,32 At resorption sites, the bone-lining cells retract making way for the osteoclast,13,14,15 but remain closely associated with the osteoclast, as shown in rat,33 mouse,16 rabbit34 and human (Figure 2) bone. In mouse bone explants, 90% of the osteoclasts were reported to show a close apposition with one or more of these cells.16 Cell extensions enwrapping demineralized collagen fibers were frequently seen, thereby raising the possibility that the eroded surface itself may exert an attraction on these cells, in addition to soluble chemoattractants.35 These observations show that the eroded surface never remains cell-free and point to the bone-lining cell as the cell colonizing it immediately after the departure of the osteoclast.16 They also show how these cells can be conditioned by both soluble and insoluble osteoclast products, in the same way as they are activated by mechanical30 or hormonal stimulation.31,32 However, as discussed elsewhere,11 the view that the cells colonizing the eroded surface are osteoblast-lineage cells, has been questioned for a long time. Some authors consider mononucleated osteoclasts or phagocytic macrophage-like cells to be involved at the beginning of the reversal phase, and pre-osteoblasts at the end.10 A recent study addressed this issue on cancellous bone of human iliac crest through immunostaining with osteoblastic and monocytic markers.11 It showed that 97% of the reversal cells were positive for the osteoblastic marker, Runx2, and were negative for monocytic markers, including osteoclast markers. Importantly, also 84% of the reversal cells immediately next to the osteoclasts were positive for Runx2, thereby indicating that the cells colonizing the eroded surface right after the departure of the osteoclasts belong to the osteoblast lineage. Furthermore, there is evidence for maturation of these reversal cells into bone-forming osteoblasts during the progress of the reversal phase, based on the inverse gradients of osterix, a later maturation marker,36 and of smooth muscle actin (SMA), a motility marker reported to decrease in maturing osteoblasts37 (Figure 3).
Figure 3.
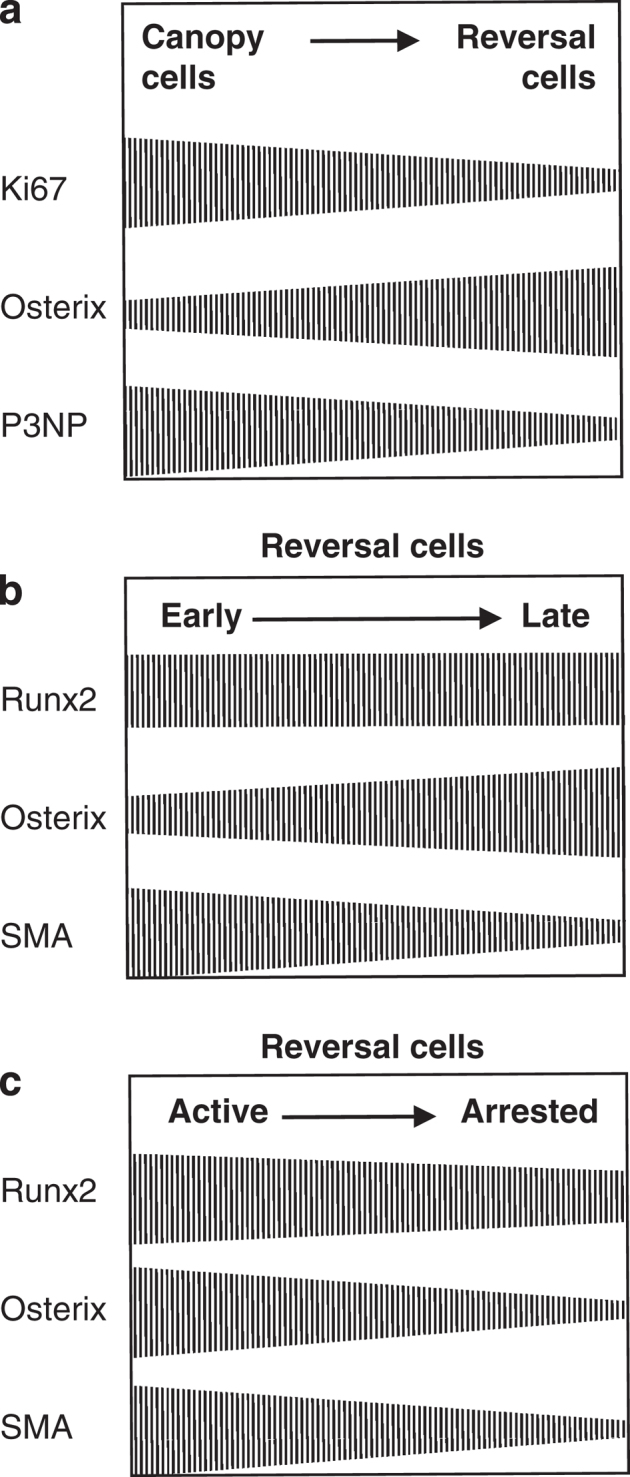
Osteoblast differentiation and proliferation at reversal sites. (a) Gradients of markers between canopy cells and reversal cells. Ki67: proliferation marker; P3NP: early differentiation marker. Data from ref. 22. (b and c) Gradients of markers between early (that is, next to the osteoclasts) and late (that is, next to osteoblasts depositing osteoid) reversal cells, and between active and arrested reversal cells. See text. Data from ref. 11.
Another important characteristic of the reversal surfaces in the context of osteoblast recruitment is that they show a higher cell density than bone-lining cells on quiescent surfaces.22 Furthermore, this enrichment was recently stressed to be obligatory for initiation of bone formation, that is, for coupling, because bone formation is detected only above a certain level of cell density,22 and because remodeling cycles abort in pathological situations where this enrichment does not occur11 (see ‘Functional evidence for a role of reversal surface events in coupling: lessons from reversal phase ‘arrest'). These recent quantitative histomorphometric studies thus demonstrate the earlier proposal that initiation of bone formation requires a sufficient number of new osteoblasts in the resorption cavity.38 An important issue is where the newly generated cells come from. Cell proliferation on the bone surface is rarely detected,38 including in human cancellous bone.22 One could consider osteocytes released by the resorbing osteoclasts39 to contribute to this colonization, as osteocytes were recently shown to be able to revert into mature osteoblasts,40 but this contribution remains to be proven during the bone-remodeling process. Thus, the contribution of reversal surface activities to osteoblastogenesis appears to be more through differentiation, and the gain in cell number on the bone surface requires recruitment from other sources (Figure 4) (see ‘Bone-remodeling compartment canopies').
Figure 4.
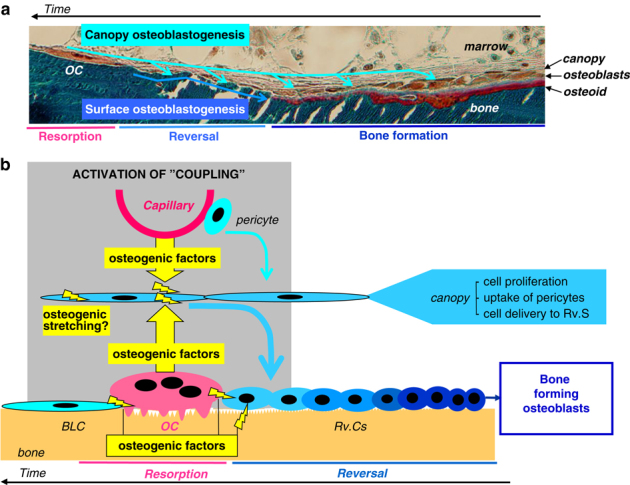
Osteoblastogenesis at bone-remodeling sites. (a) Osteoblastogenesis routes. The reversal bone surface can be seen as a recruitment platform for pre-osteoblasts. Colonization originates from bone-lining cells and canopy cells (see ‘Reversal surfaces' and ‘Bone-remodeling compartment canopies', respectively). Final differentiation occurs on the bone surface, whereas proliferation occurs mainly in the canopy. Note the time axis: canopy cells triggered by osteoclasts (and capillaries) will later cover reversal surface, and later, bone-forming osteoblasts. OC: osteoclast. (b) Scheme drawing the attention on the proximity of osteogenic triggers (yellow lightning symbol) and osteoblast-lineage cells (blue) at the starting point of the remodeling cycle. Lighter and darker blue correspond to, respectively, less- and more-differentiated osteoblastic cells. The local convergence between these triggers and osteoprogenitors activates the process coupling resorption and formation. This model provides a simple explanation of why bone is replenished at the places where osteoclasts have removed it. BLC: bone-lining cell; OC: osteoclast; Rv.Cs: reversal cells.
Reversal cells smoothing out the reversal surfaces: a role of ‘reversal matrix' in coupling?
Reversal cells also modify the surface of the eroded matrix left behind the osteoclast. Notably, this modification provides them progressively with a new matrix environment, which may be critical for the osteoblast maturation described in ‘Bone-lining cells, reversal cells and osteoblast recruitment',41 as well as for the physical connection between the new and old matrix. This modification can be seen as a smoothening process, requiring both catabolic and anabolic activities.11 Cleaning of resorption debris has been emphasized in several models, and was especially investigated in situations where excessive amounts of organic material were allowed to accumulate in the osteoclastic resorption zone.16,34,42 Interestingly, this cleaning activity corresponds with the observation that early reversal cells next to osteoclasts express collagenolytic matrix metalloproteinases (MMPs) that diffuse into the resorption area, as shown in rabbit43 and rat44 bone. In line with this, early maturation stages of osteoblasts were reported to correspond with high MMP expression.45,46 Reversal cells contribute also to the generation of cement lines.16,47,48 These are defined as basophilic material deposited on the eroded surface. Surprisingly, their composition is poorly known. They are reported to be rich in mucopolysaccharide and osteopontin. They may also contain bone sialoprotein and osteoclast products, such as TRAcP,49 which are factors able to affect osteoblast-lineage cells and bone formation.41,50
Functional evidence for a role of reversal surface events in coupling: lessons from reversal phase ‘arrest'.
A failure of a reversal surface event in a given BMU may render initiation of bone formation in this BMU completely impossible. Such failures allow to demonstrate even more convincingly the critical role of reversal cells in the coupling process. For example, blocking the cleaning of demineralized collagen in a calvaria model prevented the deposition of new matrix on the eroded surface.16 This relates perhaps also to impaired bone formation during bone development when osteoblastic collagen degradation pathways are knocked out.51 A series of other examples concern specifically bone remodeling in human adult bone. Baron et al.52,53 showed that reversal surfaces increase in biopsy specimens from patients suffering from postmenopausal or senile osteoporosis, and decrease in those of normal patients and primary hyperparathyroidism where coupling is occurring optimally. They proposed therefore that osteoporotic patients had a prolonged or even aborted reversal phase, representing uncoupling. Weinstein54 made similar conclusions in the case of long-term glucocorticoid treatment. Mosekilde55 performed a scanning electron microscopy analysis of trabeculae in vertebrae of aged patients. Connected/loaded trabeculae showed bone resorption and formation occurring at the same remodeling site. In contrast, disconnected/unloaded trabeculae showed resorption without formation, which was interpreted as due to aborted resorption cycles. Notably, this observation suggests the involvement of mechanical forces (may be osteocyte-mediated) in coupling. Makris and Saffar56 reported concomitant increase in reversal surface and decrease in formation surface, thus suggesting uncoupling, during the progress of hamster periodontitis. Parfitt57 reported that in older people, especially those with osteoporosis, a significant proportion of the eroded surface is covered by flat lining cells. He speculated that this may be due to remodeling cycles that were initiated but aborted before initiation of bone formation.
In order to definitively establish these interpretations, a systematic histomorphometry study was recently conducted on cancellous bone of human iliac crest biopsies of postmenopausal osteoporosis patients.11 The analysis of serial sections demonstrated the existence of long stretches of eroded surface, far away from bone-resorbing osteoclasts and bone-forming osteoblasts, therefore reflecting remodeling cycles that became arrested at the reversal phase. These so-called arrested reversal surfaces represented on average 30% of the total reversal surface in these postmenopausal osteoporosis biopsies, whereas the same study could hardly detect any arrested reversal surface in primary hyperparathyroidism biopsies, where coupling is occurring optimally. A direct link between reversal phase arrest and absence of initiation of bone formation was also concluded from the activation frequency of the bone formation phase determined in these biopsies.58 Furthermore, the cells on arrested reversal surface were flat and found at a twofold lower cell density compared with the cells on the so-called active reversal surface next to osteoclasts and bone-forming osteoblasts.11 They resembled thereby bone-lining cells on quiescent surfaces. They expressed also significantly less osterix, and SMA, compared with active reversal cells (Figure 3). These cell numbers and differentiation characteristics taken together with the observations of ‘Bone-lining cells, reversal cells and osteoblast recruitment' indicate that a lack of osteoblast recruitment on the reversal surface of a BMU results in the absence of initiation of bone formation.
Of note, absence of initiation of bone formation is not taken into account by classical histomorphometry, as the magnitude of bone formation is only evaluated at the sites of ongoing bone formation.11 The same holds true for important parameters such as activation frequency.11 As discussed elsewhere,11 there should thus be awareness that bone may show three concurrent types of remodeling cycles, all starting with resorption, but one where bone replenishment is complete, one where it is incomplete, and one where the replenishment process has not started. The relative frequency of each type of remodeling cycles depends on the physiological/pathlogical situation.
Bone-remodeling compartment canopies
Bone marrow envelopes, canopies and osteoblast recruitment.
The bone-lining cells coating quiescent surfaces are covered by flat (<0.1 μm) mesenchymal cells showing very long and overlapping cell extensions.22 They form an envelope surrounding the whole bone marrow.18,19,20,21 Importantly, these cells can differentiate into mature osteoblasts, as shown in culture experiments performed with cells isolated from the bone marrow envelope of human bone marrow plugs.21 The origin and role of bone marrow envelope cells in remodeling of adult human cancellous bone has recently been discussed.22 Morphologically, the bone marrow envelope cells appear much like the bone-lining cells, and have sometimes been considered as a multilayer of bone-lining cells.59 However, there are also reasons to consider them as a distinct anatomical entity, since they do not physically interact with the bone matrix itself as do the bone-lining cells, and dissociate from bone-lining cells when taking marrow plugs out of the bone,18,19,21 or as seen in images of the marrow–bone interface, where they are lifted over the osteoclast (Figure 2).22 Accordingly, they appear as a canopy covering nearly all the osteoclasts in human trabecular bone of normal individuals,2,22,58,60 and extend over the whole surface undergoing bone remodeling (Figures 1 and 4) (Table 1). Lifting of the bone marrow envelope may result from transmigration of pre-osteoclasts beneath the bone marrow envelope,61 and/or from an upward osteoclast-induced retraction when the osteoclast moves over the bone surface—similarly to the lateral induced retraction of bone-lining cells.13,15 It happens that a lumen (sometimes with erythrocytes) is seen between the canopy and the BMU cells.2,22,58,62,63 This lumen has been speculated to have a functional meaning.2,23,62,63 Ongoing research is analyzing this possibility and exploring the mechanism that may regulate fluid distribution at this level. Mechanical resistance of the canopy is supported by the presence of collagen fibers between the canopy cells.21,22 In human cancellous bone, these canopies show numerous contacts with capillaries (Figure 2),2,22,23 especially above osteoclasts, and in a rat study, intermittent PTH was also shown to induce the presence of capillaries next to remodeling sites of cancellous bone.64 Thus upon initiation of bone remodeling, canopy cells are ideally positioned to be exposed to osteogenic products provided by osteoclasts and capillaries5,24,26,27 (Figures 2 and 4), including oxygen known to promote proliferation.65 Even a third osteogenic influence may result from the stretching that occurs when they are lifted, as mechanical forces are well-known regulators of osteogenesis.66
As discussed in detail,22 several lines of evidence support that the canopies actually contribute to the enrichment of the reversal surfaces in osteoprogenitors (Figure 4). First, evidence was gained from combined measurements of cell density, proliferation and osteoblast differentiation markers. Canopy cells proved to be two to three times more proliferative and at an earlier differentiation stage, compared with reversal cells (Figure 3). Furthermore, the bone marrow region within 50 μm of the canopy was enriched in proliferative cells, putative osteoblast progenitors and capillaries surrounded by pericytes, when compared with quiescent surfaces.23 It was proposed that these osteoprogenitors might be delivered to the canopy, and from there to the bone surface23 where they might differentiate into functional osteoblasts.5 The actual influence of the capillaries on the canopies is strongly supported by the highly significant correlations between the number of capillary–canopy contacts and the density of bone-forming osteoblasts,22 taken as an index of their recruitment. Also the effect of osteoclasts is supported by the relation between the length of the osteoclast–canopy overlap and the shortness of the reversal phase in odanacatib-treated rabbits.67 More indications of the role of canopy in osteoblast recruitment come from the association between canopy deficiency and deficient bone formation in diverse pathological situations as explained in ‘Functional evidence for a role of canopies in coupling: lessons from canopy deficiency'.
The above observations focused on the reversal phase, but of note, the canopy extends over bone-forming osteoblasts, which is actually the cell layer commonly considered to consist of osteoprogenitors, and where cell proliferation was also mentioned in mouse68 and rat.31,69 This proliferation was stimulated by intermittent PTH.31 There should be awareness that these cells are part of the canopy and are the cells that were conditioned by osteoclasts and capillaries at the earlier stage of the remodeling cycle (Figure 4a). These osteoprogenitors allow continued recruitment during the bone-formation phase.
Functional evidence for a role of canopies in coupling: lessons from canopy deficiency.
The evidence for a role of canopies mentioned in ‘Bone marrow envelopes, canopies and osteoblast recruitment' is mainly based on control conditions or primary hyperparathyroidism where virtually all reversal surfaces are covered by a canopy.22,23,62 Interestingly, the extent of canopy coverage over remodeling sites has been shown to vary in several pathological situations, including multiple myeloma,2,70 glucocorticoid-induced osteoporosis60 and postmenopausal osteoporosis,58 thereby offering the possibility to analyze the consequences of diminished canopy coverage in a wide variety of disease situations. Of note, in each of these distinct pathologies, diminished canopy coverage above reversal surfaces was found to coincide with a smaller extent of bone-forming surfaces. Furthermore, deficient canopy coverage above reversal surface in postmenopausal11,58 and glucocorticoid (unpublished) osteoporosis was found to coincide with arrested reversal surfaces lined with cells at a low density, thus directly indicating deficient osteoblast recruitment with absence of initiation of bone formation. Other situations further support a role of canopies in coupling. Age, which is known to negatively affect osteoblast recruitment, also negatively affects canopy coverage over remodeling sites.22 Bisphosphonates, which are known to negatively affect bone formation, also negatively affect canopy coverage above osteoclast surfaces.67 Bone with yellow bone marrow, where bone formation is reduced, was reported not to show bone marrow envelope.71 Correlations between bone formation and canopies in these many diverse situations are unlikely to be mere coincidence, and therefore support the critical contribution of canopies in delivering osteoprogenitors to reversal surfaces, thereby allowing bone formation to be initiated, that is, coupling.
Conclusion
The reversal phase has been overlooked for a long time, although it is the remodeling step coupling bone resorption and formation.11 Recently, a lot of interest was shown for the identification of ‘coupling' molecules,3,5,6,7,8 but the search for the coupling mechanism paid little attention to the related cell activities and to their tissular context, as for instance the BMUs of adult cancellous bone. The present review creates awareness of several critical cellular aspects occurring in this specific tissue environment. (i) First of all, this review highlights previously unrecognized cellular agents of the remodeling cycle: canopies and capillaries, in addition to reversal cells recently shown to be osteoblast-lineage cells (Figure 4). (ii) It highlights previously unrecognized changes in cell–cell and cell–matrix interactions induced at the onset of bone remodeling. Contacts between bone-lining cells and bone marrow envelope are disrupted, whereas contacts are established between early reversal cells and osteoclasts, and between canopies, osteoclasts and capillaries (Figure 4). These respective interactions may relate to interruption of dormancy of the bone-lining cells and the bone marrow envelope cells, and induction of osteogenic activity in the reversal cells and in the canopy cells. Induction in the canopy may involve: paracrine interactions between canopies, osteoclasts and capillaries,5,24,26,27 mechanical signals resulting from canopy lifting and stretching;66 increased oxygen tension due to proximity of capillaries;65 and canopy–capillary contacts may also indicate transfer of pericytes23 or circulating osteoprogenitors.25 (iii) Concerning cell–matrix interactions, reversal cells are exposed to newly uncovered epitopes on the eroded surface, which are in turn modified by these cells while they differentiate into mature bone-forming osteoblasts. (iv) This review clearly shows that osteoblast recruitment is a prerequisite for initiation of bone formation, that is, for coupling. Canopies and reversal surfaces mainly contribute through, respectively, proliferation (or cell transfer) and differentiation (Figure 4). (v) Importantly, in a variety of bone diseases including osteoporosis, canopies are disrupted and cannot fulfill their obligatory role of local provider of osteoprogenitors. Destruction of local osteoprogenitors may explain why myeloma- or arthritis-induced osteolytic lesions do not heal,70,72 despite the high levels of osteoclasts and their putative release of osteogenic factors.3,5,6,7,8
Overall, this review shows that the generation of osteogenic/coupling molecules at the onset of the remodeling cycle is not sufficient for securing osteoblast recruitment and coupling. A complementary prerequisite is the availibity of local osteoprogenitors to be triggered by the coupling molecules (Figure 4). Thus, anabolic drugs will not work at the bone sites that are devoid of osteoprogenitors. Therefore, promoting survival of local osteoprogenitors deserves attention when willing to prevent bone loss in situations such as aging, glucocorticoid treatment, menopause, multiple myeloma or arthritis.
Acknowledgments
Work from the author's laboratory is supported by grants from the regional research foundation of Southern Denmark, the West Denmark research forum for health science, the Danish cancer society, the Aase and Ejnar Danielsen's foundation and Merck. The author thanks all his team members for their contribution to the observations reviewed in this manuscript.
Footnotes
The author declares no conflict of interest.
References
- Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet 2011;377:1276–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen TL, Sondergaard TE, Skorzynska KE, Dagnaes-Hansen F, Plesner TL, Hauge EM et al. A physical mechanism for coupling bone resorption and formation in adult human bone. Am J Pathol 2009;174:239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims NA, Martin TJ. Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. Bonekey Rep 2014;3:481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost HM. Dynamics of bone remodeling. In: Frost HM (ed) Bone Biodynamics Little, Brown and Co: Boston, MA, USA, 1964; p315–333. [Google Scholar]
- Dirckx N, Van HM, Maes C. Osteoblast recruitment to sites of bone formation in skeletal development, homeostasis, and regeneration. Birth Defects Res C Embryo Today 2013;99:170–191. [DOI] [PubMed] [Google Scholar]
- Henriksen K, Karsdal MA, Martin TJ. Osteoclast-derived coupling factors in bone remodeling. Calcif Tissue Int 2014;94:88–97. [DOI] [PubMed] [Google Scholar]
- Sims NA, Civitelli R. Cell-cell signaling: broadening our view of the basic multicellular unit. Calcif Tissue Int 2014;94:2–3. [DOI] [PubMed] [Google Scholar]
- Teti A. Mechanisms of osteoclast-dependent bone formation. Bonekey Rep 2013;2:449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone 2011;48:677–692. [DOI] [PubMed] [Google Scholar]
- Eriksen EF, Gundersen HJ, Melsen F, Mosekilde L. Reconstruction of the formative site in iliac trabecular bone in 20 normal individuals employing a kinetic model for matrix and mineral apposition. Metab Bone Dis Relat Res 1984;5:243–252. [DOI] [PubMed] [Google Scholar]
- Andersen TL, Abdelgawad ME, Kristensen HB, Hauge EM, Rolighed L, Bollerslev J et al. Understanding coupling between bone resorption and formation: are reversal cells the missing link? Am J Pathol 2013;183:235–246. [DOI] [PubMed] [Google Scholar]
- Baron R. Importance of the intermediate phases between resorption and formation in the measurement and understanding of the bone remodeling sequence. In: Meunier PJ (ed) Bone Histomorphometry: Second International Workshop Lyon Armour Montagu: Toulouse, France, 1977; p179–183. [Google Scholar]
- Ferrier J, Xia SL, Lagan E, Aubin JE, Heersche JNM. Displacement and translocation of osteoblast-like cells by osteoclasts. J Bone Miner Res 1994;9:1397–1405. [DOI] [PubMed] [Google Scholar]
- Karsdal MA, Fjording MS, Foged NT, Delaisse J-M, Lochter A. Transforming growth factor-B-induced osteoblast elongation regulates osteoclastic bone resorption through a p38 mitogen-activated protein kinase- and matrix metalloproteinase-dependent pathway. J Biol Chem 2001;276:39350–39358. [DOI] [PubMed] [Google Scholar]
- Perez-Amodio S, Beertsen W, Everts V. (Pre-)osteoclasts induce retraction of osteoblasts before their fusion to osteoclasts. J Bone Miner Res 2004;19:1722–1731. [DOI] [PubMed] [Google Scholar]
- Everts V, Delaissé J-M, Korper W, Jansen DC, Tigchelaar-Gutter W, Saftig P et al. The bone lining cell: its role in cleaning howship's lacunae and initiating bone formation. J Bone Miner Res 2002;17:77–90. [DOI] [PubMed] [Google Scholar]
- Tran Van PT, Vignery A, Baron R. Cellular kinetics of the bone remodeling sequence in the rat. Anat Rec 1982;202:445–451. [DOI] [PubMed] [Google Scholar]
- Bi LX, Simmons DJ, Hawkins HK, Cox RA, Mainous EG. Comparative morphology of the marrow sac. Anat Rec 2000;260:410–415. [DOI] [PubMed] [Google Scholar]
- Menton DN, Simmons DJ, Orr BY, Plurad SB. A cellular investment of bone marrow. Anat Rec 1982;203:157–164. [DOI] [PubMed] [Google Scholar]
- Simmons D. The in vivo role of bone marrow fibroblast-like stromal cells. Calcif Tissue Int 1996;58:129–132. [DOI] [PubMed] [Google Scholar]
- Bi LX, Mainous EG, Yngve DA, Buford WL. Cellular isolation, culture and characterization of the marrow sac cells in human tubular bone. J Musculoskelet Neuronal Interact 2008;8:43–49. [PubMed] [Google Scholar]
- Kristensen HB, Andersen TL, Marcussen N, Rolighed L, Delaisse JM. Osteoblast recruitment routes in human cancellous bone remodeling. Am J Pathol 2014;184:778–789. [DOI] [PubMed] [Google Scholar]
- Kristensen HB, Andersen TL, Marcussen N, Rolighed L, Delaisse JM. Increased presence of capillaries next to remodeling sites in adult human cancellous bone. J Bone Miner Res 2013;28:574–585. [DOI] [PubMed] [Google Scholar]
- Brandi ML, Collin-Osdoby P. Vascular biology and the skeleton. J Bone Miner Res 2006;21:183–192. [DOI] [PubMed] [Google Scholar]
- Eghbali-Fatourechi GZ, Lamsam J, Fraser D, Nagel D, Riggs BL, Khosla S. Circulating osteoblast-lineage cells in humans. N Engl J Med 2005;352:1959–1966. [DOI] [PubMed] [Google Scholar]
- Schipani E, Maes C, Carmeliet G, Semenza GL. Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. J Bone Miner Res 2009;24:1347–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler DA. The osteogenic-angiogenic interface: novel insights into the biology of bone formation and fracture repair. Curr Osteoporos Rep 2008;6:67–71. [DOI] [PubMed] [Google Scholar]
- Pettit AR, Chang MK, Hume DA, Raggatt LJ. Osteal macrophages: a new twist on coupling during bone dynamics. Bone 2008;43:976–982. [DOI] [PubMed] [Google Scholar]
- Bianco P, Sacchetti B, Riminucci M. Stem cells in skeletal physiology and endocrine diseases of bone. Endocr Dev 2011;21:91–101. [DOI] [PubMed] [Google Scholar]
- Chow JWM, Wilson AJ, Chambers TJ, Fox SW. Mechanical loading stimulates bone formation by reactivation of bone lining cells in 13-week-old rats. J Bone Miner Res 1998;13:1760–1767. [DOI] [PubMed] [Google Scholar]
- Dobnig H, Turner RT. Evidence that intermittent treatment with parathyroid hormone increases bone formation in adult rats by activation of bone lining cells. Endocrinology 1995;136:3632–3638. [DOI] [PubMed] [Google Scholar]
- Kim SW, Pajevic PD, Selig M, Barry KJ, Yang JY, Shin CS et al. Intermittent parathyroid hormone administration converts quiescent lining cells to active osteoblasts. J Bone Miner Res 2012;27:2075–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domon T, Suzuki R, Takata K, Yamazaki Y, Takahashi S, Yamamoto T et al. The nature and function of mononuclear cells on the resorbed surfaces of bone in the reversal phase during remodeling. Ann Anat 2001;183:103–110. [DOI] [PubMed] [Google Scholar]
- Jensen PR, Andersen TL, Pennypacker BL, Duong lT, Delaisse JM. The bone resorption inhibitors odanacatib and alendronate affect post-osteoclastic events differently in ovariectomized rabbits. Calcif Tissue Int 2014;94:212–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault MM, Hoemann CD, Buschmann MD. Fibronectin, vitronectin, and collagen I induce chemotaxis and haptotaxis of human and rabbit mesenchymal stem cells in a standardized transmembrane assay. Stem Cells Dev 2007;16:489–502. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002;108:17–29. [DOI] [PubMed] [Google Scholar]
- Ichida M, Yui Y, Yoshioka K, Tanaka T, Wakamatsu T, Yoshikawa H et al. Changes in cell migration of mesenchymal cells during osteogenic differentiation. FEBS Lett 2011;585:4018–4024. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. The cellular basis of bone remodeling: the quantum concept reexamined in light of recent advances in the cell biology of bone. Calcif Tissue Int 1984;36(Suppl 1): S37–S45. [DOI] [PubMed] [Google Scholar]
- Oguro I, Ozawa H. Cytochemical studies of the cellular events sequence in bone remodeling: cytological evidence for a coupling mechanism. J Bone Miner Metab 1989;7:30–36. [Google Scholar]
- Torreggiani E, Matthews BG, Pejda S, Matic I, Horowitz MC, Grcevic D et al. Preosteocytes/osteocytes have the potential to dedifferentiate becoming a source of osteoblasts. PLoS ONE 2013;8:e75204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner M, Jurdic P, Tuckerman JP, Block MR, Bouvard D. New insights into adhesion signaling in bone formation. Int Rev Cell Mol Biol 2013;305:1–68. [DOI] [PubMed] [Google Scholar]
- Delaisse JM, Andersen TL, Engsig MT, Henriksen K, Troen T, Blavier L. Matrix metalloproteinases (MMP) and cathepsin K contribute differently to osteoclastic activities. Microsc Res Tech 2003;61:504–513. [DOI] [PubMed] [Google Scholar]
- Andersen TL, del Carmen OM, Kirkegaard T, Lenhard T, Foged NT, Delaisse JM. A scrutiny of matrix metalloproteinases in osteoclasts: evidence for heterogeneity and for the presence of MMPs synthesized by other cells. Bone 2004;35:1107–1119. [DOI] [PubMed] [Google Scholar]
- Fuller K, Chambers TJ. Localisation of mRNA for collagenase in osteocytic, bone surface and chondrocytic cells but not osteoclasts. J Cell Sci 1995;108:2221–2230. [DOI] [PubMed] [Google Scholar]
- Rifas L, Fausto A, Scott MJ, Avioli LV, Welgus HG. Expression of metalloproteinases and tissue inhibitors of metalloproteinases in human osteoblast-like cells: differentiation is associated with repression of metalloproteinase biosynthesis. Endocrinology 1994;134:213–221. [DOI] [PubMed] [Google Scholar]
- Tuckermann JP, Pittois K, Partridge NC, Merregaert J, Angel P. Collagenase-3 (MMP-13) and integral membrane protein 2a (Itm2a) are marker genes of chondrogenic/osteoblastic cells in bone formation: sequential temporal, and spatial expression of Itm2a, alkaline phosphatase, MMP-13, and osteocalcin in the mouse. J Bone Miner Res 2000;15:1257–1265. [DOI] [PubMed] [Google Scholar]
- Zhou H, Chernecky R, Davies JE. Deposition of cement at reversal lines in rat femoral bone. J Bone Miner Res 1994;9:367–374. [DOI] [PubMed] [Google Scholar]
- McKee MD, Nanci A. Osteopontin at mineralized tissue interfaces in bone, teeth, and osseointegrated implants: ultrastructural distribution and implications for mineralized tissue formation, turnover, and repair. Microsc Res Tech 1996;33:141–164. [DOI] [PubMed] [Google Scholar]
- Sheu TJ, Schwarz EM, O'Keefe RJ, Rosier RN, Puzas JE. Use of a phage display technique to identify potential osteoblast binding sites within osteoclast lacunae. J Bone Miner Res 2002;17:915–922. [DOI] [PubMed] [Google Scholar]
- Sheu TJ, Schwarz EM, Martinez DA, O'Keefe RJ, Rosier RN, Zuscik MJ et al. A phage display technique identifies a novel regulator of cell differentiation. J Biol Chem 2003;278:438–443. [DOI] [PubMed] [Google Scholar]
- Wagenaar-Miller RA, Engelholm LH, Gavard J, Yamada SS, Gutkind JS, Behrendt N et al. Complementary roles of intracellular and pericellular collagen degradation pathways in vivo. Mol Cell Biol 2007;27:6309–6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R, Vignery A, Lang R. Reversal phase and osteopenia: defective coupling of resorption to formation in the pathogenesis of osteoporosis. In: Deluca HF, Frost HM, Jee WSS, Johnston CC, Parfitt AM (eds) Osteoporosis: Recent Advances in Pathogenesis and Treatment University Park Press: Baltimore, MD, USA, 1980; p 311–320. [Google Scholar]
- Baron R, Magee S, Silverglate A, Broadus A, Lang R. Estimation of trabecular bone resorption by histomorphometry: evidence for a prolonged reversal phase with normal resorption in post-menopausal osteoporosis and coupled increase in primary hyperparathyroidism. In: Frame B, Potts JT (eds). Clinical Disorders of Bone and Mineral Metabolism (4th edn.). Excerpta Medica: Amsterdam,, 1983; p 191–195. [Google Scholar]
- Weinstein RS. Glucocorticoid-induced osteoporosis. Rev Endocr Metab Disord 2001;2:65–73. [DOI] [PubMed] [Google Scholar]
- Mosekilde L. Consequences of the remodelling process for vertebral trabecular bone structure: a scanning electron microscopy study (uncoupling of unloaded structures). Bone Miner 1990;10:13–35. [DOI] [PubMed] [Google Scholar]
- Makris GP, Saffar JL. Disturbances in bone remodeling during the progress of hamster periodontitis. A morphological and quantitative study. J Periodontal Res 1985;20:411–420. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. The physiologic and clinical significance of bone histomorphometric data. In: Recker RR (ed) Bone Histomorphometry: Techniques and Interpretation CRC Press: Boca Raton, FL, USA, 1983; p143–223. [Google Scholar]
- Andersen TL, Hauge EM, Rolighed L, Bollerslev J, Kjaersgaard-Andersen P, Delaisse JM. Correlation between absence of bone remodeling compartment canopies, reversal phase arrest, and deficient bone formation in post-menopausal osteoporosis. Am J Pathol 2014;184:1142–1151. [DOI] [PubMed] [Google Scholar]
- Deldar A, Lewis H, Weiss L. Bone lining cells and hematopoiesis: an electron microscopic study of canine bone marrow. Anat Rec 1985;213:187–201. [DOI] [PubMed] [Google Scholar]
- Jensen PR, Andersen TL, Soe K, Hauge EM, Bollerslev J, Amling M et al. Premature loss of bone remodeling compartment canopies is associated with deficient bone formation: a study of healthy individuals and patients with Cushing's syndrome. J Bone Miner Res 2012;27:770–780. [DOI] [PubMed] [Google Scholar]
- Saltel F, Chabadel A, Zhao Y, Lafage-Proust MH, Clezardin P, Jurdic P et al. Transmigration: a new property of mature multinucleated osteoclasts. J Bone Miner Res 2006;21:1913–1923. [DOI] [PubMed] [Google Scholar]
- Hauge EM, Qvesel D, Eriksen EF, Mosekilde L, Melsen F. Cancellous bone remodeling occurs in specialized compartments lined by cells expressing osteoblastic markers. J Bone Miner Res 2001;16:1575–1582. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. The bone remodeling compartment: a circulatory function for bone lining cells. J Bone Miner Res 2001;16:1583–1585. [DOI] [PubMed] [Google Scholar]
- Prisby R, Guignandon A, Vanden-Bossche A, Mac-Way F, Linossier M-T, Thomas M et al. Intermittent PTH(1-84) is osteoanabolic but not osteoangiogenic and relocates bone marrow blood vessels closer to bone-forming sites. J Bone Miner Res 2011;26:2583–2596. [DOI] [PubMed] [Google Scholar]
- Arnett TR. Acidosis, hypoxia and bone. Arch Biochem Biophys 2010;503:103–109. [DOI] [PubMed] [Google Scholar]
- Thompson WR, Rubin CT, Rubin J. Mechanical regulation of signaling pathways in bone. Gene 2012;503:179–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PR, Andersen TL, Pennypacker BL, Duong lT, Engelholm LH, Delaisse JM. A supra-cellular model for coupling of bone resorption to formation during remodeling: lessons from two bone resorption inhibitors affecting bone formation differently. Biochem Biophys Res Commun 2014;443:694–699. [DOI] [PubMed] [Google Scholar]
- Narimatsu K, Li M, de Freitas PHL, Sultana S, Ubaidus S, Kojima T et al. Ultrastructural observation on cells meeting the histological criteria for preosteoblasts—a study in the mouse tibial metaphysis. J Electron Microscop 2010;59:427–436. [DOI] [PubMed] [Google Scholar]
- Barou O, Laroche N, Palle S, Alexandre C, Lafage-Proust M-H. Pre-osteoblastic proliferation assessed with BrdU in undecalcified, epon-embedded adult rat trabecular bone. J Histochem Cytochem 1997;45:1189–1195. [DOI] [PubMed] [Google Scholar]
- Andersen TL, Soe K, Sondergaard TE, Plesner T, Delaisse JM. Myeloma cell-induced disruption of bone remodelling compartments leads to osteolytic lesions and generation of osteoclast-myeloma hybrid cells. Br J Haematol 2010;148:551–561. [DOI] [PubMed] [Google Scholar]
- Miller SC, Bowman BM, Smith JM, Jee WS. Characterization of endosteal bone-lining cells from fatty marrow bone sites in adult beagles. Anat Rec 1980;198:163–173. [DOI] [PubMed] [Google Scholar]
- Keller KK, Thomsen JS, Stengaard-Pedersen K, Hauge EM. Systemic but no local effects of combined zoledronate and parathyroid hormone treatment in experimental autoimmune arthritis. PLoS ONE 2014;9:e92359. [DOI] [PMC free article] [PubMed] [Google Scholar]


