Abstract
Purpose.
We determined whether photic stress differentially impairs organelle motility of RPE lipofuscin and melanin granules, whether lethal photic stress kills cells in proportion to lipofuscin abundance, and whether killing is modulated by melanosome content.
Methods.
Motility of endogenous lipofuscin and melanosome granules within the same human RPE cells in primary culture was quantified by real-time imaging during sublethal blue light irradiation. Cell death during lethal irradiation was quantified by dynamic imaging of the onset of nuclear propidium iodide fluorescence. Analyzed were individual cells containing different amounts of autofluorescent lipofuscin, or similar amounts of lipofuscin and a varying content of phagocytized porcine melanosomes, or phagocytized black latex beads (control for light absorbance).
Results.
Lipofuscin granules and melanosomes showed motility slowing with mild irradiation, but slowing was greater for lipofuscin. On lethal irradiation, cell death was earlier in cells with higher lipofuscin content, but delayed by the copresence of melanosomes. Delayed death did not occur with black beads, suggesting that melanosome protection was due to properties of the biological granule, not simple screening.
Conclusions.
Greater organelle motility slowing of the more photoreactive lipofuscin granule compared to melanosomes suggests that lipofuscin mediates mild photic injury within RPE cells. With lethal light stress endogenous lipofuscin mediates killing, but the effect is cell autonomous and modulated by coincident melanosome content. Developing methods to quantify the frequency of individual cells with combined high lipofuscin and low melanosome content may have value for predicting the photic stress susceptibility of the RPE monolayer in situ.
Keywords: lipofuscin, photic stress, melanosome, retinal pigment epithelium
Endogenous lipofuscin makes human RPE cells more susceptible to photic stress. This effect is modified by the content of melanosomes in individual cells.
Introduction
Cells of the RPE contain two types of pigment granules that are believed to have opposing effects in determining the cellular susceptibility to photic stress: lipofuscin granules and melanosomes. In the RPE, lipofuscin forms largely from the incomplete lysosomal digestion of lipids and proteins derived from phagocytized shed photoreceptor outer segment membranes.1,2 Lipofuscin, which accumulates with age and occupies an increasing cytoplasmic volume over time,3 is a complex, photoreactive material containing several fluorophores that are responsible for its characteristic broad-band, golden fluorescence when excited with blue light.4,5 Blue light irradiation of isolated lipofuscin generates superoxide anion, hydrogen peroxide, and singlet oxygen,6–8 reactive species that cause lipid peroxidation, enzyme inactivation, and protein oxidation in model systems.9 Chloroform-soluble extracts6,8,10 and chloroform-insoluble components of lipofuscin granules11 are capable of generating toxic reactive species on excitation with blue light, but the main lipofuscin chromophore(s) responsible for the granule's phototoxicity remain unknown.
The best characterized fluorophore of lipofuscin is A2E,12,13 which has been used extensively to model the photochemical and photobiological properties of RPE lipofuscin. Despite its extensive experimental use, A2E is neither a major chromophore of lipofuscin granules14 nor a major photoreactive component of the granules,15 being capable of only inefficient photogeneration of superoxide anion and singlet oxygen.16–18 Given the small content of A2E estimated in each lipofuscin granule,19 perhaps it is not surprising that a recent report found that the distribution of A2E and of lipofuscin does not correlate in human RPE cells.20 Despite that the photoreactivity of A2E is low and significantly less than RPE lipofuscin,16–18 A2E has been used widely. When added experimentally to cultured RPE cells, even in the absence of irradiation, A2E affects cholesterol metabolism,21 impairs mitochondrial function and phagocytosis of outer segments,22 and destabilizes cellular membranes.23 With short wavelength visible light irradiation, A2E-laden RPE cells exhibit altered gene expression24 and cell death via apoptosis25–27 or loss of lysosomal integrity.28
Lipofuscin granules, like A2E, also have been experimentally introduced into cultured RPE cells followed by irradiation, which produces outcomes, such as the extragranular oxidation of lipids, inactivation of lysosomal and antioxidant enzymes, loss of lysosomal integrity, and cell death.19,29 These damaging effects have led to the view that lipofuscin is phototoxic within the aging RPE, contributing to light-induced tissue dysfunction and predisposing to age-related macular degeneration (AMD).30 Despite the evidence in support of this notion, endogenous lipofuscin granules, within the RPE cells that generated them, have not been explicitly shown to mediate phototoxicity in live cells. This is a relevant issue for two reasons: although lipofuscin accumulates with age, it varies among individual RPE cells, and it coexists with another pigment granule, the melanosome, which is believed to be photoprotective.
Melanosomes are the dominant membrane-limited pigment granules in young RPE cells. They contain melanin, a complex oligomeric aggregate consisting of products of enzymatic oxidation of tyrosine and nonenzymatic transformations of melanin precursors deposited on a protein matrix.31,32 When illuminated under aerobic conditions, melanin can generate superoxide anion and hydrogen peroxide.33–35 Despite its photoreactivity, RPE melanin usually is considered photoprotective,36,37 although how melanin protects from light damage is not entirely clear. Apart from absorbing light, melanin may act as a cellular antioxidant.37,38 In a variety of model systems, melanin has been shown to scavenge reactive free radicals,39,40 quench singlet oxygen41,42 and excited states of photosensitizing molecules,43–45 and to inhibit lipid peroxidation.46–48 Melanin also can sequester redox-active metal ions, making them less likely to induce lipid peroxidation,46 and less available to act as cofactors in Fenton-type reactions that yield extremely reactive hydroxyl radicals.49,50 Despite the many properties that predict melanin would photoprotect RPE cells by acting as an antioxidant, showing this effect in a cellular system has presented an experimental challenge. Using conventional measures of antioxidant-mediated cytoprotection, we previously were unable to detect photoprotection of ARPE-19 cells by phagocytized porcine melanosomes when compared to phagocytized black beads, which were used to control for the effect of optical screening.51 Subsequent experiments, using a more sensitive dynamic imaging protocol to quantify the timing of cell injury, revealed no protection, but rather detected a slight increase in phototoxicity conferred by melanosomes compared to beads,52 an outcome consistent with observations that melanosomes generate superoxide and hydrogen peroxide on blue light irradiation.35 In the same study, we observed that untreated melanosomes conferred protection when compared to melanosomes that were photobleached, a process that changes the properties of melanin rendering it more photoreactive.52 This raised the possibility that, despite melanin's photoreactivity, melanosomes may help photoprotect RPE cells when irradiation occurs in the presence of other, more photoreactive substances or organelles, a context in which the light screening and the antioxidant properties of melanosomes could be functionally relevant. This is the typical state for RPE cells in which melanosomes coexist with the naturally-occurring and more highly photoreactive organelle, the lipofuscin granule.35
We used cultured human RPE cells containing melanosomes and lipofuscin granules to analyze biological responses to treatment with phototoxic blue light delivered at either sublethal or lethal levels. The outcomes demonstrated that endogenous lipofuscin granules sensitize living cells to sublethal irradiation more than melanosomes. This sensitization was revealed as greater impairment of organelle motility, a previously-identified consequence of mild light treatment especially for organelles with photoreactive contents.53 When irradiation was elevated to lethal levels, lipofuscin-containing cells were killed preferentially in proportion to their lipofuscin content; to our knowledge this is the first explicit demonstration of the phototoxic effect of endogenous, not phagocytized, lipofuscin within RPE cells. Further, experimental manipulation of melanosome content in cells containing endogenous lipofuscin revealed that the susceptibility of each individual RPE cell to light–induced injury was modulated by its coincident melanosome content and not solely dependent upon its lipofuscin abundance. Additionally, comparable numbers of black beads did not similarly reduce susceptibility in this model, suggesting that the mechanism of melanosome protection was not simple light absorbance, but was due to other properties of the biological granule. Overall the results in this culture system indicated that melanosomes can protect RPE cells from photic stress induced in the presence of lipofuscin. The outcomes raise the possibility that the ratio of melanin to lipofuscin in individual RPE cells, not the overall content of autofluorescent lipofuscin throughout the tissue, may be an effective predictor of the photic stress susceptibility of the RPE within eyes.
Methods
Cell Cultures
The RPE cells were isolated from the eyes of four adult human donors, aged 37, 62, 84, and 86 years, by enzymatic methods similar to those we have used previously.54 To produce the cultures, the anterior segment was excised, the vitreous and retina were removed, then the RPE monolayer lining the eyecup was dislodged by a 3-hour incubation with 0.3% trypsin in culture medium (Eagle's Minimum Essential Medium [EMEM]). Cells were plated in multiple wells of 24-well plates in EMEM supplemented with 10% fetal bovine serum (FBS). Nonadherent cells were removed after 24 hours, followed by refeeding. To maintain their content of endogenous pigment granules, the primary cultures were subjected to no or limited passage (no more than twice) and maintained at confluent density with biweekly feedings before use. For the experiments, cells were replated in the wells of Nunc Lab-Tek II 8-chamber glass slides (Thermo Fisher Scientific, Waltham, MA, USA) at the density required for each type of analysis as indicated with the Results. Outcomes for experiments did not differ with RPE donor age. For the representative results that are shown, the age of the donor providing the cells is indicated in the Figure legends.
Preparation and Phagocytosis of Melanosomes
For some experiments in which the melanosome content of individual cells was experimentally manipulated, porcine RPE melanosomes were delivered to human RPE cells for phagocytic uptake. For this purpose, melanosomes were isolated from porcine RPE as described previously.52,55 Briefly, RPE cells were scraped from eyecups and homogenized. The granules then were purified by ultracentrifugation in a sucrose gradient followed by removal of contaminating materials and membranes associated with the granule surface by incubation in Tris buffer containing 0.25% Triton X-100, 2.0% SDS, and a cocktail of protease inhibitors. Delivery of melanosomes to RPE cells for phagocytosis was by previously-described methods, with granule internalization proceeding over 24 hours.56 To control for light screening in some irradiation experiments, black latex beads (1 μm; Invitrogen, Carlsbad, CA, USA) were delivered to control cell populations for phagocytic uptake by the same methods we have used previously.56
Light Irradiation, Organelle Motility Tracking and Cell Survival Analysis
Chamber slides containing RPE cells were mounted on the stage of a Nikon Eclipse TE2000U microscope outfitted with a motorized, computer-controlled stage (Nikon Instruments, Melville, NY, USA) and a CoolSnap ES digital camera (Photometrics, Tucson, AZ, USA). The stage was equipped with a Live Cell 3 environmental chamber (Pathology Devices, Westminster, MD, USA) to control temperature, humidity, and CO2 levels. Image acquisition and data analysis were performed using Premier MetaMorph software (Molecular Devices, Sunnyvale, CA, USA).
Sublethal blue light treatment of RPE cells, and motility tracking of endogenous lipofuscin granules and melanosomes were performed as described previously56 with some modifications. Fluorescence (lEx = 490 nm, lEm = 617 nm) images were first captured to identify lipofuscin granules, then the fluorescence images were merged with bright field images of the same field to permit discrimination of the two granule types within the same cells as explained further in the Results. For motility tracking of individual granules, time lapse bright-field images were acquired at 1-second intervals over 9 minutes using the following light irradiation protocol: light OFF during the first 3 minutes (for tracking of baseline granule motility), light ON during the second 3 minutes (for motility tracking during light stress), and light OFF during the final 3 minutes (for postirradiation motility tracking). Light (400–410 nm, 100 J/cm2) was delivered using the epi-illumination port of the microscope. After treatment with blue light, images were collected for another 18 to 24 hours to confirm that the photic stress was sublethal.53 Movement of granules was quantified by assembling the time lapse images into stacks and following selected granules using the Track Objects function of MetaMorph software, as previously described.53
For lethal light irradiation followed by survival analysis, confluent cultures were used in which the cells contained endogenous lipofuscin granules, without or with varying numbers of porcine melanosomes or black latex beads introduced by phagocytosis as described above. For the experiments, cultures were irradiated continuously with blue light (490 nm, 56 mW/cm2) using the microscope's mercury lamp by methods similar to those we have used previously.53 Irradiation was in the presence of 100 μM propidium iodide (PI), a membrane impermeant red-emitting fluorescent dye (lEx = 555 nm, lEm = 617 nm) that stains the nuclei of damaged cells. Before light treatment, fluorescence images were captured to identify lipofuscin granules (lEx = 490 nm, lEm = 617 nm) or latex beads (lEx = 555 nm, lEm = 685 nm), which have a characteristic fluorescence.56 During light treatment, phase contrast and PI fluorescence images were collected at 30-second intervals for up to 15 hours. For analysis, image stacks were generated and the time of onset of thresholded nuclear PI fluorescence was recorded as a dynamic measure of cell death using published methods.56 To estimate lipofuscin content in individual cells, each cell was outlined manually and the percent of cell area occupied by thresholded lipofuscin fluorescence was determined using the Threshold Image function of MetaMorph. The same approach was used to determine the content of phagocytized black latex beads, using their fluorescence, and phagocytized melanosomes, using bright-field images. Cells were grouped according to granule content, as described further in the Results, and survival curves were plotted using GraphPad Prism software (GraphPad Software, La Jolla, CA, USA).
Results
Sublethal Irradiation Differentially Affects the Motility of Endogenous Lipofuscin Granules and Melanosomes
The motility of endogenous lipofuscin granules and melanosomes within the same individual RPE cells during treatment with sublethal blue light treatment was analyzed by published methods53 (Figs. 1, 2). Using well-spread cells in subconfluent cultures, granule types were identified by capture of sequential bright field and fluorescence images before each experiment, and confirmed by imaging again at the experiment's end. Bright field micrographs revealed both granule types (Fig. 1A); the subset of granules that was lipofuscin was identified by their autofluorescence (Figs. 1B–D), and those lacking detectable autofluorescence were identified as melanosomes (Fig. 1D). In the perinuclear region of the primary cultures, granules are abundant, closely packed, and relatively nonmotile. However, in the periphery of spread cells individual granules are more widely separated, which permits them to translocate over longer distances and allows individual granules to be discriminated in bright field-fluorescence image overlays (Fig. 1D). Granules in this subcellular location were selected for motility tracking (Fig. 2).
Figure 1.

Discrimination of endogenous lipofuscin granules and melanosomes in a human RPE cell. Bright field imaging (A) shows all granules, and fluorescence imaging (B) of the same cell identifies autofluorescent lipofuscin granules. In the cell periphery, where granules are less numerous, individual granules can be discriminated on merged images (C). A magnified region (D) illustrates an adjacent melanosome (black arrow) and lipofuscin granule (white arrow). Note that the two images of the lipofuscin granule do not align due to the granule's movement between capture of sequential bright field and fluorescence images. N, nucleus. Age of RPE donor: 84 years. Magnifications: (A–C) ×400, (D) ×600.
Figure 2.
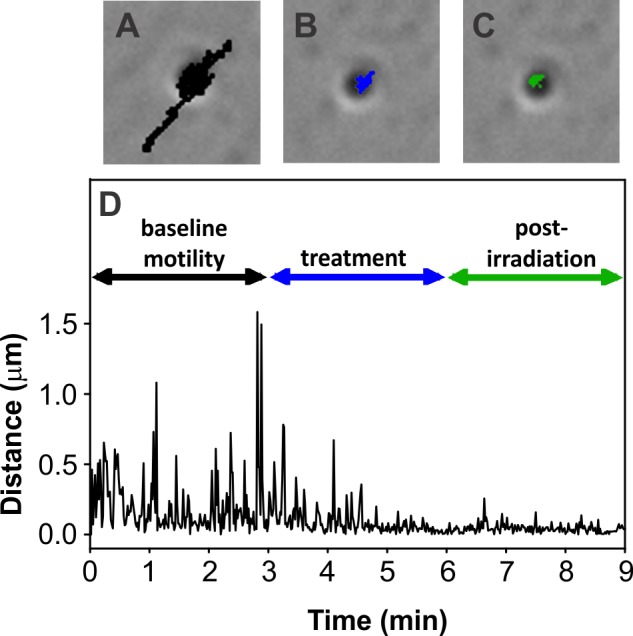
Illustration of the movement of a representative lipofuscin granule at baseline and with sublethal blue light treatment. Bright field micrographs, with overlaid movement tracks, show organelle motility over 3-minute intervals at baseline ([A], track shown in black), then during ([B], track shown in blue) and after ([C], track shown in green) blue light irradiation. (D) Graphical representation of the movement of the lipofuscin granule shown in (A–C). Age of RPE donor: 84 years. Magnification for (A–C): ×600.
In previous studies, melanosomes (endogenous and phagocytized granules) within the cytoplasm of cultured RPE cells were shown to exhibit variable constitutive baseline organelle movement that was impaired on irradiation with sublethal blue light.53 Using the same protocol, similar outcomes were obtained here for endogenous lipofuscin granules (Figs. 2A–D). Baseline lipofuscin granule motility consisted of short, oscillatory movements and longer distance translocations (Fig. 2D). During 3 minutes of sublethal blue light treatment, lipofuscin motility slowed and remained impaired during a 3-minute posttreatment interval (Figs. 2B–D).
When cells containing endogenous granules of both types were blue light–treated, lipofuscin granules showed greater impairment of movement than melanosomes within the same individual human RPE cells (Fig. 3). The total distance traveled by melanosomes at baseline (75 μM ± 41 SD) was slightly, but not significantly, longer than the baseline distance traveled by lipofuscin granules (60 μM ± 37 SD, t-test, P = 0.14).
Figure 3.
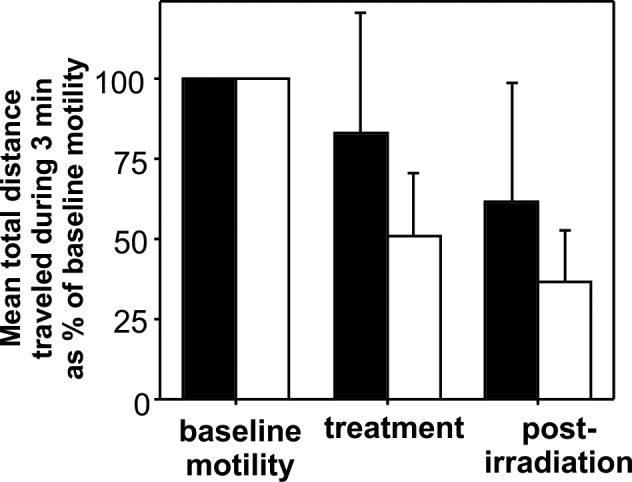
Mean total distances traveled over 3-minute intervals (by the methods illustrated in Fig. 2) by melanosomes (black bars, n = 65) and lipofuscin granules (white bars, n = 56) within the same RPE cells. Data for distance traveled during and after light treatment are expressed as a percent of baseline motility (± SD). Reductions in movement relative to baseline are significantly greater for lipofuscin granules than for melanosomes during light treatment and after irradiation (P < 0.05, t-test). Age of RPE donor: 62 years.
Individual RPE Cells Are Susceptible to Lethal Photic Stress in Proportion to Their Lipofuscin Content
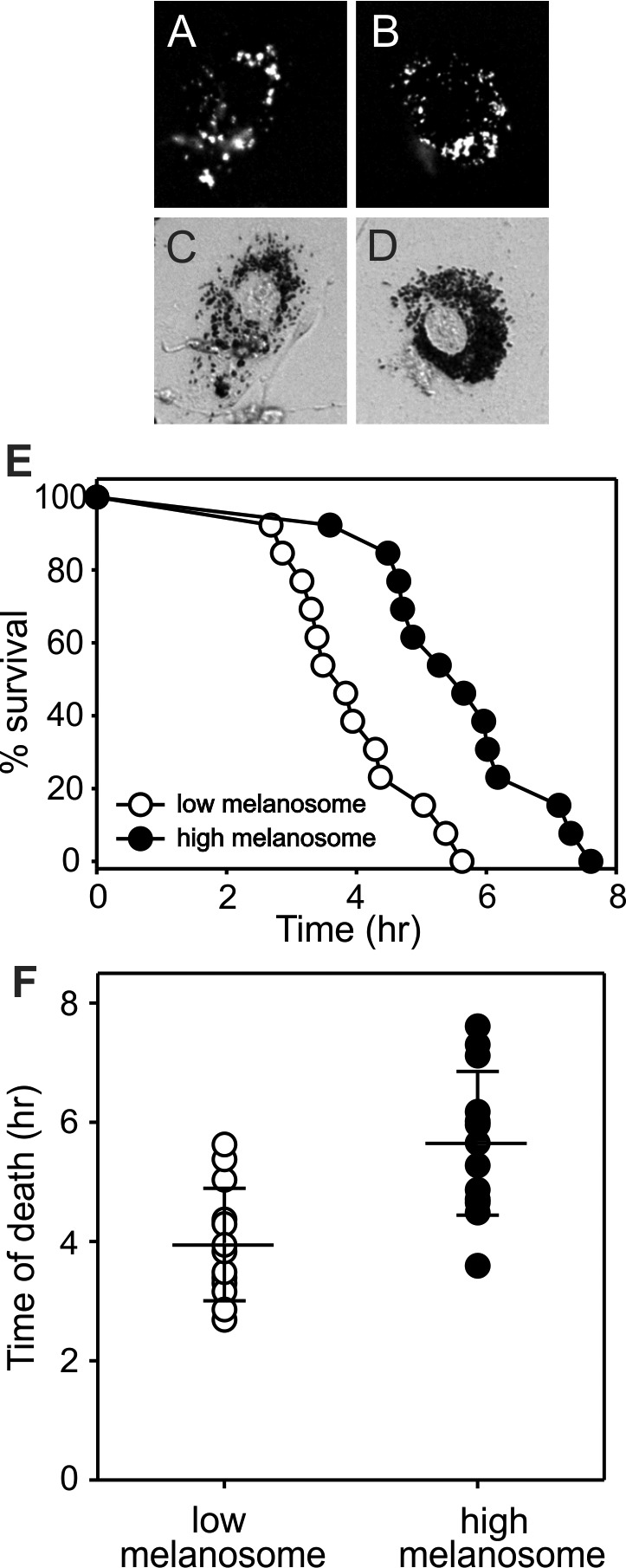
The slowing of organelle motility exhibited by lipofuscin following mild light treatment (Fig. 3) suggested that lipofuscin is a greater source of local photogeneration of damaging reactive species within cells than melanosomes,53 as long has been presumed. Given that lipofuscin content is a cell autonomous feature, we next sought to determine whether greater light stress, at lethal levels, preferentially killed cells containing lipofuscin, and, more specifically, whether the killing differed with the amount of lipofuscin in individual cells. To address this question, confluent human RPE cultures were used that consisted of cells with varying amounts of endogenous autofluorescent lipofuscin (Fig. 4A). Lipofuscin abundance was quantified in individual cells by determining the percent of cell area occupied by autofluorescent material as described in the Methods. Lethal light stress induced by continuous irradiation with blue light for 15 hours in the presence of PI produced a time-dependent increase in the fraction of cells with PI-positive nuclei as a marker of cell death (Fig. 4B). The time of cell death for individual cells with varying amounts of lipofuscin was recorded and cells were grouped by lipofuscin content for analysis. Individual cells with more abundant lipofuscin exhibited an earlier onset and a more rapid rate of cell death than cells with low lipofuscin content, in which cell death was delayed (Fig. 4C).
Figure 4.

Confluent cultures of human RPE cells containing endogenous lipofuscin and subjected to lethal blue light irradiation. (A) Fluorescence micrograph of a representative culture imaged to show lipofuscin. Three cells containing abundant lipofuscin are indicated (white arrowheads). (B) Merged phase contrast and PI fluorescence images of the same field shown in (A) illustrated before light treatment (0 hours) and at two time points during irradiation (4 and 8 hours) to show the time-dependent increase in PI-positive nuclei. The same high lipofuscin cells indicated in (A) are highlighted (white arrowheads), which became PI-positive early in the irradiation time course (by 4 hours). Three low lipofuscin cells also are indicated (white arrows) with nuclei that remain PI-negative through 8 hours. (C) Survival curves for RPE cells grouped by lipofuscin content (% cell area occupied by thresholded autofluorescence) showing the time of onset of nuclear PI fluorescence as a real-time measure of cell death during blue light irradiation over 15 hours. Each dot represents an individual cell. All survival curves for cells with different lipofuscin content differ significantly from each other (GraphPad Prism, Comparison of Survival Curves function, P < 0.0001). Age of RPE donor: 37 years.
The Susceptibility to Lethal Photic Stress of Individual RPE Cells Containing Lipofuscin Is Reduced in Proportion to Their Melanosome Content and the Reduction Is Not Due to Optical Screening
Individual cells differ in their susceptibility to lethal light stress in proportion to their lipofuscin content (Fig. 4), but lipofuscin granules are not the only granule with the potential to affect sensitivity to photic stress; melanosomes coexist with lipofuscin in RPE cells. To determine whether melanosomes modify light stress susceptibility in cells containing lipofuscin, cultures of cells containing endogenous lipofuscin were used. Since the cells contain relatively few unmodified melanosomes (not shown), cells were rendered more melanotic by phagocytic uptake of porcine melanosomes. Individual cells then were selected for analysis that had comparable lipofuscin content, determined by quantifying cell area occupied by autofluorescent material in fluorescence images (Figs. 5A, 5B), and either low (Fig. 5C) or high (Fig. 5D) numbers of melanosomes, determined by quantifying light absorbing material in paired bright field images. Time of cell death was recorded in these two cell subpopulations during irradiation of the cultures to lethality with blue light in the presence of PI. Cells with a similar lipofuscin content, but more abundant melanosomes exhibited delayed cell death compared to cells in the same culture with fewer melanin granules (Figs. 5E, 5F).
Figure 5.
Human RPE cells containing endogenous lipofuscin granules and differing numbers of phagocytized porcine melanosomes. Paired fluorescence (A, B) and bright field micrographs (C, D, respectively) illustrate cells with comparable lipofuscin and either low (C) or high (D) melanosome content. (E) Survival curves for RPE cells with low and high melanosome content during irradiation with blue light. Each dot represents an individual cell. (F) Demonstration of the mean time of cell death for cells with low and high melanosome content. Cell death was significantly later than for cells with higher melanosome numbers (P < 0.05, t-test). Wide cross bars indicate means, shorter error bars indicate SD. Age of RPE donor: 37 years.
To determine whether the protective effect of melanosomes coexisting with lipofuscin granules (shown in Fig. 5) resulted from simple optical screening by the melanin granules, similar experiments were performed using phagocytized black latex beads in place of the melanosomes. The beads are chemically inert, but have light-blocking properties similar to melanosomes, can be loaded similarly into cells by phagocytosis, and can be discriminated by their endogenous fluorescence.56 The RPE cells containing comparable endogenous lipofuscin and either low or high numbers of black latex beads were analyzed for time of death in cultures subjected to lethal blue light irradiation in the presence of PI. In contrast to cells containing varying numbers of melanosomes (Fig. 5), time of death was similar for cells containing low versus high numbers of black latex beads (Fig. 6).
Figure 6.
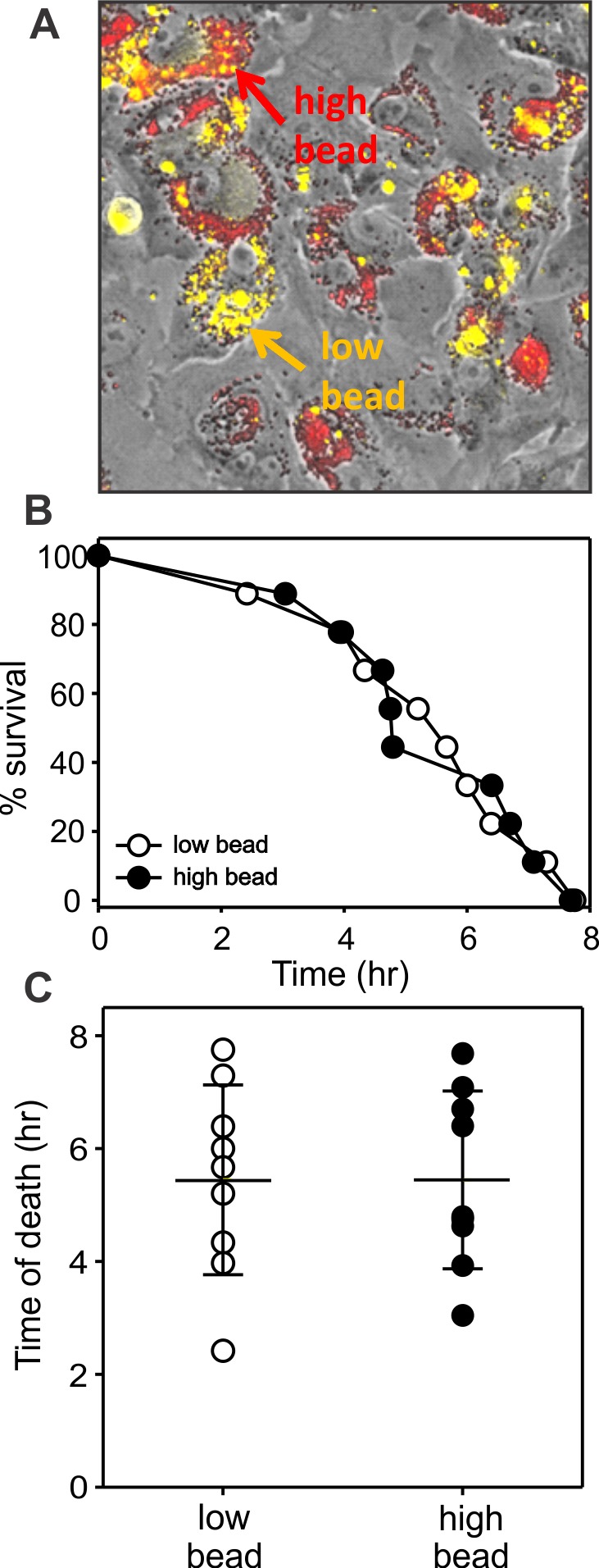
Human RPE cells containing endogenous lipofuscin granules and differing numbers of phagocytized black latex beads. (A) Merged phase-contrast, far-red fluorescence of black latex beads (shown red), and autofluorescence of lipofuscin (shown yellow) to illustrate cells with comparable lipofuscin and low (yellow arrow and label) or high bead content (red arrow and label). (B) Survival curves for RPE cells with similar lipofuscin content and low or high content of black beads during irradiation with blue light. Each dot represents an individual cell. (C) Demonstration of the mean time of cell death for cells with low and high bead content. Times of death did not differ. Wide cross bars indicate means; shorter error bars indicate SD. Age of RPE donor: 37 years.
Discussion
Previous studies showed that isolated RPE lipofuscin granules are competent to photo-generate oxidizing species 6–8,57 and to mediate light-induced cell killing when delivered by phagocytosis to cultured cells.19 Here, explicit evidence is provided to indicate that endogenous lipofuscin granules behave similarly, mediating photoxic responses in cultured human RPE cells exposed to either sublethal or lethal levels of blue light. Following mild light irradiation, the response manifests as reduced lipofuscin granule motility, and on lethal irradiation lipofuscin is shown to mediate cell killing. Significantly, the killing was cell autonomous; that is, susceptibility was determined by the lipofuscin abundance in individual cells. Further, melanosomes were shown to reduce the light susceptibility of lipofuscin-containing cultured cells by a mechanism that was cell autonomous and not due to simple light absorbance.
Sublethal photic stress was previously shown to impair the motility of melanosomes and phagosomes within cultured RPE cells.53 Photosensitizer-loaded phagosomes exhibited greater motility impairment than those lacking photosensitizer,53 which supported the interpretation that damaging species that were photo-generated locally, that is, in the subcellular domain of the organelle, slowed organelle movement in proportion to their abundance. Here endogenous RPE lipofuscin granules, compared to melanosomes in the adjacent cytoplasm of the same cells, behaved like photosensitizer-loaded phagosomes by demonstrating greater motility impairment on mild light treatment. This observation is consistent with conclusions from studies of isolated RPE granules showing that melanosomes and lipofuscin granules are photoreactive, but that the photoreactivity of lipofuscin is greater,35 and suggests that the relative photoreactive potentials of the two granule types occur within cells.
In examining the effects of lipofuscin following lethal irradiation, the focus here was not only on whether endogenous granules can be shown to increase light susceptibility, but more specifically on whether susceptibility differed among cells depending on their lipofuscin content and on the copresence of melanosomes. We took advantage of the naturally occurring cell–cell heterogeneity in lipofuscin content in RPE cells58 and on the degranulation that occurs when RPE cells are propagated in vitro59 to generate a culture system in which cells varying in lipofuscin abundance could be identified readily and selected for analysis. Using this approach, coupled with a real-time assay that quantifies the timing of cell death,52 it was possible to demonstrate a greater susceptibility to lethal light exposure of cells containing greater amounts of autofluorescent lipofuscin compared to those with few granules. This observation illustrates the importance of a feature of lipofuscin that is not often considered: phototoxicity mediated by lipofuscin is cell autonomous and related to the content in individual cells rather than producing cell death equivalently throughout the monolayer.
The cell autonomy of light susceptibility was illustrated further by demonstrating photo-protection conferred by melanosomes that coexisted with lipofuscin granules in the cytoplasm of the same cultured RPE cells. Within the aging eye, RPE pigment granules become more complex, melanosomal melanin undergoes oxidative modification60 that is believed to change the photoreactive properties of the pigment granule,35 and compound granules, like melanolipofuscin, increase.3 Therefore, melanosomes lacking detectable lipofuscin autofluorescence are infrequent in RPE cultures from adult human donors, such as those used here. To study the photophysical properties of melanosomes that have undergone limited age-related modifications, we have used previously granules isolated from the RPE of young pigs.55,61 Porcine granules were used here as well and delivered to cultures for phagocytic uptake to generate cells with measurably different ratios of melanosomes to lipofuscin, and to demonstrate decreased light susceptibility for cells with higher numbers of melanosomes. This outcome indicates that the susceptibility of RPE cells to photic injury is determined not by their lipofuscin content alone, but is modulated further by their coincident content of melanosomes. The observation that higher numbers of melanosomes conferred photoprotection may appear to conflict with our previous observation of melanosome-mediated phototoxicity.52 In the previous study, photoxicity was detected by comparing cells phagocytically-loaded with melanosomes to cells similarly loaded with black beads, conditions under which optical screening was comparable and the phototoxic properties of melanosomal melanin apparently were revealed. Here, the comparison groups differed; all cells contained highly photoreactive lipofuscin in addition to melanosomes, conditions under which the photoprotective properties of melanosomal melanin were apparently revealed. Stated differently, the melanosome has photoxic and photoprotective properties, and the net effect when irradiated cells also contain lipofuscin is melanosome-mediated photoprotection.
An additional noteworthy observation was that the photoprotective effect of melanosomes could not be attributed to simple light absorbance, since similarly absorbing black particles did not similarly protect. This outcome was not surprising given that, to permit detection of granule abundance and type in this model, the cultured cells were somewhat flattened on the substrate. Therefore, the granules were not in an optimal position to screen one another or important subcellular structures, notably nuclei. This granule position was useful for the purposes of this investigation where one goal was to detect nonscreening effects of melanosomes. We found that, despite the limited light blockade, protection by melanosomes was nonetheless demonstrable, indicating that the melanin granule exhibits other photoprotective properties aside from light absorbance. The most likely are the antioxidant properties of melanin, which have been shown many times in noncellular systems,39,46,49,50 but which have been difficult to demonstrate explicitly within living cells. We have reported previously indirect evidence for an antioxidant function of melanosomes within cultured cells in various experimental systems,56,62,63 and perhaps the protection by melanosomes of light-irradiated cells containing lipofuscin shown here is another example. We would like to suggest the possibility that melanosomes function as an antioxidant under these conditions, because the two granule types closely codistribute in the perinuclear cytoplasm. In this subcellular domain, the short-lived and locally-generated reactive species originating from irradiated lipofuscin may be scavenged efficiently by melanin in nearby melanosomes. This granule codistribution occurs in RPE cells in situ as well where, with aging, the preferential segregation of melanosomes to the apical domain declines leading to cosegregation with more basal lipofuscin granules. In this regard, the culture system used here bears more resemblance to granule location found in aged than young tissue. Optical screening is likely to be a more significant function of melanosomes in situ than in vitro due to the more cuboidal cytoarchitecture of the RPE within eyes, especially young eyes.
The observation that RPE cells exhibit toxic responses to light irradiation in response to their individual lipofuscin content, as modified by their individual melanosome content, may have ramifications for understanding in vivo phototoxicity and the role of photic stress in predisposing to AMD. There has been considerable interest in recent years in whether fundus autofluorescence might be useful for predicting AMD disease susceptibility.5,64,65 The results here raise the possibility that the frequency and/or topographical location in the monolayer of RPE cells with a high content of lipofuscin and a low melanosome number may be more relevant than total autofluorescence. Newer imaging systems with the ability to quantify noninvasively the ratio of autofluorescence to absorbance at the single RPE cell level66 may reveal useful features of the monolayer for predicting disease or tracking its progression.
Acknowledgments
Supported by Research Grants R01EY019664 (JMB) and P30EY01931 from the National Eye Institute (Bethesda, MD, USA), and an unrestricted grant from Research to Prevent Blindness, Inc. Research in Poland was supported by the National Science Center (Maestro-2013/08/A/NZ1/00194). This investigation was conducted in a facility constructed with support from National Center for Research Resources Grant C06 RR-RR016511 from the National Institutes of Health (Bethesda, MD, USA).
Disclosure: M. Zareba, None; C.M.B. Skumatz, None; T.J. Sarna, None; J.M. Burke, None
References
- 1. Boulton M, McKechnie NM, Breda J, Bayly M, Marshall J. The formation of autofluorescent granules in cultured human RPE. Invest Ophthalmol Vis Sci. 1989; 30: 82–89 [PubMed] [Google Scholar]
- 2. Feeney-Burns L, Eldred GE. The fate of the phagosome: conversion to ‘age pigment' and impact in human retinal pigment epithelium. Trans Ophthalmol Soc U K. 1983; 103 (Pt 4): 416–421 [PubMed] [Google Scholar]
- 3. Feeney-Burns L, Hilderbrand ES, Eldridge S. Aging human RPE: morphometric analysis of macular, equatorial, and peripheral cells. Invest Ophthalmol Vis Sci. 1984; 25: 195–200 [PubMed] [Google Scholar]
- 4. Sparrow JR, Gregory-Roberts E, Yamamoto K, et al. The bisretinoids of retinal pigment epithelium. Prog Retin Eye Res. 2012; 31: 121–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Delori FC, Dorey CK, Staurenghi G, Arend O, Goger DG, Weiter JJ. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci. 1995; 36: 718–729 [PubMed] [Google Scholar]
- 6. Gaillard ER, Atherton SJ, Eldred G, Dillon J. Photophysical studies on human retinal lipofuscin. Photochem Photobiol. 1995; 61: 448–453 [DOI] [PubMed] [Google Scholar]
- 7. Rozanowska M, Jarvis-Evans J, Korytowski W, Boulton ME, Burke JM, Sarna T. Blue light-induced reactivity of retinal age pigment. In vitro generation of oxygen-reactive species. J Biol Chem. 1995; 270: 18825–18830 [DOI] [PubMed] [Google Scholar]
- 8. Rozanowska M, Wessels J, Boulton M, et al. Blue light-induced singlet oxygen generation by retinal lipofuscin in non-polar media. Free Radic Biol Med. 1998; 24: 1107–1112 [DOI] [PubMed] [Google Scholar]
- 9. Wassell J, Davies S, Bardsley W, Boulton M. The photoreactivity of the retinal age pigment lipofuscin. J Biol Chem. 1999; 274: 23828–23832 [DOI] [PubMed] [Google Scholar]
- 10. Reszka K, Eldred GE, Wang RH, Chignell C, Dillon J. The photochemistry of human retinal lipofuscin as studied by EPR. Photochem Photobiol. 1995; 62: 1005–1008 [DOI] [PubMed] [Google Scholar]
- 11. Rozanowska M, Pawlak A, Rozanowski B, et al. Age-related changes in the photoreactivity of retinal lipofuscin granules: role of chloroform-insoluble components. Invest Ophthalmol Vis Sci. 2004; 45: 1052–1060 [DOI] [PubMed] [Google Scholar]
- 12. Reinboth JJ, Gautschi K, Munz K, Eldred GE, Reme CE. Lipofuscin in the retina: quantitative assay for an unprecedented autofluorescent compound (pyridinium bis-retinoid, A2-E) of ocular age pigment. Exp Eye Res. 1997; 65: 639–643 [DOI] [PubMed] [Google Scholar]
- 13. Parish CA, Hashimoto M, Nakanishi K, Dillon J, Sparrow J. Isolation and one-step preparation of A2E and iso-A2E, fluorophores from human retinal pigment epithelium. Proc Natl Acad Sci U S A. 1998; 95: 14609–14613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haralampus-Grynaviski NM, Lamb LE, Clancy CM, et al. Spectroscopic and morphological studies of human retinal lipofuscin granules. Proc Natl Acad Sci U S A. 2003; 100: 3179–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pawlak A, Rozanowska M, Zareba M, Lamb LE, Simon JD, Sarna T. Action spectra for the photoconsumption of oxygen by human ocular lipofuscin and lipofuscin extracts. Arch Biochem Biophys. 2002; 403: 59–62 [DOI] [PubMed] [Google Scholar]
- 16. Cantrell A, McGarvey DJ, Roberts J, Sarna T, Truscott TG. Photochemical studies of A2-E. J Photochem Photobiol B. 2001; 64: 162–165 [DOI] [PubMed] [Google Scholar]
- 17. Lamb LE, Ye T, Haralampus-Grynaviski NM, et al. Primary photophysical properties of A2E in solution. J Phys Chem B. 2001; 105: 11507–11512 [Google Scholar]
- 18. Pawlak A, Wrona M, Rozanowska M, et al. Comparison of the aerobic photoreactivity of A2E with its precursor retinal. Photochem Photobiol. 2003; 77: 253–258 [DOI] [PubMed] [Google Scholar]
- 19. Davies S, Elliott MH, Floor E, et al. Photocytotoxicity of lipofuscin in human retinal pigment epithelial cells. Free Radic Biol Med. 2001; 31: 256–265 [DOI] [PubMed] [Google Scholar]
- 20. Ablonczy Z, Higbee D, Anderson DM, et al. Lack of correlation between the spatial distribution of A2E and lipofuscin fluorescence in the human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2013; 54: 5535–5542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lakkaraju A, Finnemann SC, Rodriguez-Boulan E. The lipofuscin fluorophore A2E perturbs cholesterol metabolism in retinal pigment epithelial cells. Proc Natl Acad Sci U S A. 2007; 104: 11026–11031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vives-Bauza C, Anand M, Shirazi AK, et al. The age lipid A2E and mitochondrial dysfunction synergistically impair phagocytosis by retinal pigment epithelial cells. J Biol Chem. 2008; 283: 24770–24780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sparrow JR, Cai B, Jang YP, Zhou J, Nakanishi K. A2E, a fluorophore of RPE lipofuscin, can destabilize membrane. Adv Exp Med Biol. 2006; 572: 63–68 [DOI] [PubMed] [Google Scholar]
- 24. van der Burght BW, Hansen M, Olsen J, et al. Early changes in gene expression induced by blue light irradiation of A2E-laden retinal pigment epithelial cells. Acta Ophthalmol. 2013; 91: e537–e545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sparrow JR, Nakanishi K, Parish CA. The lipofuscin fluorophore A2E mediates blue light-induced damage to retinal pigmented epithelial cells. Invest Ophthalmol Vis Sci. 2000; 41: 1981–1989 [PubMed] [Google Scholar]
- 26. Sparrow JR, Cai B. Blue light-induced apoptosis of A2E-containing RPE: involvement of caspase-3 and protection by Bcl-2. Invest Ophthalmol Vis Sci. 2001; 42: 1356–1362 [PubMed] [Google Scholar]
- 27. Westlund BS, Cai B, Zhou J, Sparrow JR. Involvement of c-Abl, p53 and the MAP kinase JNK in the cell death program initiated in A2E-laden ARPE-19 cells by exposure to blue light. Apoptosis. 2009; 14: 31–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schutt F, Davies S, Kopitz J, Holz FG, Boulton ME. Photodamage to human RPE cells by A2-E, a retinoid component of lipofuscin. Invest Ophthalmol Vis Sci. 2000; 41: 2303–2308 [PubMed] [Google Scholar]
- 29. Shamsi FA, Boulton M. Inhibition of RPE lysosomal and antioxidant activity by the age pigment lipofuscin. Invest Ophthalmol Vis Sci. 2001; 42: 3041–3046 [PubMed] [Google Scholar]
- 30. Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000; 45: 115–134 [DOI] [PubMed] [Google Scholar]
- 31. Boulton M. Melanin and the retinal pigment epithelium. In: Marmor MF, Wolfensberger TJ. eds The Retinal Pigment Epithelium. New York, NY: Oxford University Press, 1998: 68–85 [Google Scholar]
- 32. Meredith P, Sarna T. The physical and chemical properties of eumelanin. Pigment Cell Res. 2006; 19: 572–594 [DOI] [PubMed] [Google Scholar]
- 33. Felix CC, Hyde JS, Sarna T, Sealy RC. Melanin photoreactions in aerated media: electron spin resonance evidence for production of superoxide and hydrogen peroxide. Biochem Biophys Res Commun. 1978; 84: 335–341 [DOI] [PubMed] [Google Scholar]
- 34. Korytowski W, Pilas B, Sarna T, Kalyanaraman B. Photoinduced generation of hydrogen peroxide and hydroxyl radicals in melanins. Photochem Photobiol. 1987; 45: 185–190 [DOI] [PubMed] [Google Scholar]
- 35. Rozanowska M, Korytowski W, Rozanowski B, et al. Photoreactivity of aged human RPE melanosomes: a comparison with lipofuscin. Invest Ophthalmol Vis Sci. 2002; 43: 2088–2096 [PubMed] [Google Scholar]
- 36. Handelman GJ, Dratz EA. The role of antioxidants in the retina and retinal pigment epithelium and the nature of prooxidant induced damage. Free Radic Biol Med. 1986; 2: 1–89 [Google Scholar]
- 37. Sarna T. Properties and function of the ocular melanin--a photobiophysical view. J Photochem Photobiol B. 1992; 12: 215–258 [DOI] [PubMed] [Google Scholar]
- 38. Ostrovsky MA, Sakina NL, Dontsov AE. An antioxidative role of ocular screening pigments. Vision Res. 1987; 27: 893–899 [DOI] [PubMed] [Google Scholar]
- 39. Rozanowska M, Sarna T, Land EJ, Truscott TG. Free radical scavenging properties of melanin interaction of eu- and pheo-melanin models with reducing and oxidising radicals. Free Radic Biol Med. 1999; 26: 518–525 [DOI] [PubMed] [Google Scholar]
- 40. Sarna T, Pilas B, Land EJ, Truscott TG. Interaction of radicals from water radiolysis with melanin. Biochim Biophys Acta. 1986; 883: 162–167 [DOI] [PubMed] [Google Scholar]
- 41. Sarna T, Menon IA, Sealy RC. Photosensitization of melanins: a comparative study. Photochem Photobiol. 1985; 42: 529–532 [DOI] [PubMed] [Google Scholar]
- 42. Sealy RC, Sarna T, Wanner EJ, Reszka K. Photosensitization of melanin: an electron spin resonance study of sensitized radical production and oxygen consumption. Photochem Photobiol. 1984; 40: 453–459 [DOI] [PubMed] [Google Scholar]
- 43. Bielec J, Pilas B, Sarna T, Truscott TG. Photochemical studies of porphyrin – melanin interaction. J Chem Soc. 1986; 82: 1469–1474 [Google Scholar]
- 44. Ito AS, Azzellini GC, Silva SC, Serra O, Szabo AG. Optical absorption and fluorescence spectroscopy studies of ground state melanin-cationic porphyrins complexes. Biophys Chem. 1992; 45: 79–89 [DOI] [PubMed] [Google Scholar]
- 45. Wrobel D, Planner A, Hanyz I, Wielgus A, Sarna T. Melanin-porphyrin interaction monitored by delayed luminescence and photoacoustics. J Photochem Photobiol B. 1997; 41: 45–52 [DOI] [PubMed] [Google Scholar]
- 46. Korytowski W, Sarna T, Zareba M. Antioxidant action of neuromelanin: the mechanism of inhibitory effect on lipid peroxidation. Arch Biochem Biophys. 1995; 319: 142–148 [DOI] [PubMed] [Google Scholar]
- 47. Porebska-Budny M, Sakina NL, Stepien KB, Dontsov AE, Wilczok T. Antioxidative activity of synthetic melanins. Cardiolipin liposome model. Biochim Biophys Acta. 1992; 1116: 11–16 [DOI] [PubMed] [Google Scholar]
- 48. Scalia M, Geremia E, Corsaro C, Santoro C, Baratta D, Sichel G. Lipid peroxidation in pigmented and unpigmented liver tissues: protective role of melanin. Pigment Cell Res. 1990; 3: 115–119 [DOI] [PubMed] [Google Scholar]
- 49. Pilas B, Sarna T, Kalyanaraman B, Swartz HM. The effect of melanin on iron associated decomposition of hydrogen peroxide. Free Radic Biol Med. 1988; 4: 285–293 [DOI] [PubMed] [Google Scholar]
- 50. Zareba M, Bober A, Korytowski W, Zecca L, Sarna T. The effect of a synthetic neuromelanin on yield of free hydroxyl radicals generated in model systems. Biochim Biophys Acta. 1995; 1271: 343–348 [DOI] [PubMed] [Google Scholar]
- 51. Zareba M, Raciti MW, Henry MM, Sarna T, Burke JM. Oxidative stress in ARPE-19 cultures: do melanosomes confer cytoprotection? Free Radic Biol Med. 2006; 40: 87–100 [DOI] [PubMed] [Google Scholar]
- 52. Zareba M, Sarna T, Szewczyk G, Burke JM. Photobleaching of melanosomes from retinal pigment epithelium: II. Effects on the response of living cells to photic stress. Photochem Photobiol. 2007; 83: 925–930 [DOI] [PubMed] [Google Scholar]
- 53. Burke JM, Zareba M. Sublethal photic stress and the motility of RPE phagosomes and melanosomes. Invest Ophthalmol Vis Sci. 2009; 50: 1940–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Burke JM, Skumatz CM, Irving PE, McKay BS. Phenotypic heterogeneity of retinal pigment epithelial cells in vitro and in situ. Exp Eye Res. 1996; 62: 63–73 [DOI] [PubMed] [Google Scholar]
- 55. Burke JM, Henry MM, Zareba M, Sarna T. Photobleaching of melanosomes from retinal pigment epithelium: I. Effects on protein oxidation. Photochem Photobiol. 2007; 83: 920–924 [DOI] [PubMed] [Google Scholar]
- 56. Burke JM, Kaczara P, Skumatz CM, Zareba M, Raciti MW, Sarna T. Dynamic analyses reveal cytoprotection by RPE melanosomes against non-photic stress. Mol Vis. 2011; 17: 2864–2877 [PMC free article] [PubMed] [Google Scholar]
- 57. Boulton M, Dontsov A, Jarvis-Evans J, Ostrovsky M, Svistunenko D. Lipofuscin is a photoinducible free radical generator. J Photochem Photobiol B. 1993; 19: 201–204 [DOI] [PubMed] [Google Scholar]
- 58. Burke JM, Hjelmeland LM. Mosaicism of the retinal pigment epithelium: seeing the small picture. Mol Interv. 2005; 5: 241–249 [DOI] [PubMed] [Google Scholar]
- 59. Burke JM, Skumatz CM. Autofluorescent inclusions in long-term postconfluent cultures of retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1998; 39: 1478–1486 [PubMed] [Google Scholar]
- 60. Ito S, Pilat A, Gerwat W, et al. Photoaging of human retinal pigment epithelium is accompanied by oxidative modifications of its eumelanin. Pigment Cell Melanoma Res. 2013; 26: 357–366 [DOI] [PubMed] [Google Scholar]
- 61. Zareba M, Szewczyk G, Sarna T, et al. Effects of photodegradation on the physical and antioxidant properties of melanosomes isolated from retinal pigment epithelium. Photochem Photobiol. 2006; 82: 1024–1029 [DOI] [PubMed] [Google Scholar]
- 62. Kaczara P, Zareba M, Herrnreiter A, et al. Melanosome-iron interactions within retinal pigment epithelium-derived cells. Pigment Cell Melanoma Res. 2012; 25: 804–814 [DOI] [PubMed] [Google Scholar]
- 63. Pilat A, Herrnreiter AM, Skumatz CM, Sarna T, Burke JM. Oxidative stress increases HO-1 expression in ARPE-19 cells, but melanosomes suppress the increase when light is the stressor. Invest Ophthalmol Vis Sci. 2013; 54: 47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bindewald A, Bird AC, Dandekar SS, et al. Classification of fundus autofluorescence patterns in early age-related macular disease. Invest Ophthalmol Vis Sci. 2005; 46: 3309–3314 [DOI] [PubMed] [Google Scholar]
- 65. Rossi EA, Rangel-Fonseca P, Parkins K, et al. In vivo imaging of retinal pigment epithelium cells in age related macular degeneration. Biomed Opt Express. 2013; 4: 2527–2539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zhang X, Zhang HF, Puliafito CA, Jiao S. Simultaneous in vivo imaging of melanin and lipofuscin in the retina with photoacoustic ophthalmoscopy and autofluorescence imaging. J Biomed Opt. 2011; 16: 080504 [DOI] [PMC free article] [PubMed] [Google Scholar]


