Abstract
The binding and ingestion of Mycobacterium avium subsp. paratuberculosis (MAP) by host cells are fibronectin (FN) dependent. In several species of mycobacteria, a specific family of proteins allows the attachment and internalization of these bacteria by epithelial cells through interaction with FN. Thus, the identification of adhesion molecules is essential to understand the pathogenesis of MAP. The aim of this study was to identify and characterize FN binding cell wall proteins of MAP. We searched for conserved adhesins within a large panel of surface immunogenic proteins of MAP and investigated a possible interaction with FN. For this purpose, a cell wall protein fraction was obtained and resolved by 2D electrophoresis. The immunoreactive spots were identified by MALDI-TOF MS and a homology search was performed. We selected elongation factor Tu (EF-Tu) as candidate for further studies. We demonstrated the FN-binding capability of EF-Tu using a ligand blot assay and also confirmed the interaction with FN in a dose-dependent manner by ELISA. The dissociation constant of EF-Tu was determined by surface plasmon resonance and displayed values within the μM range. These data support the hypothesis that this protein could be involved in the interaction of MAP with epithelial cells through FN binding.
1. Introduction
Paratuberculosis (PTB) is a chronic granulomatous enteritis of domestic and wild ruminants. This disease involves extensive mycobacterial shedding, which accounts for the high contagiousness, and ends with fatal enteritis. Decreases in weight, milk production, and fertility produce severe economic loss [1]. The etiological agent of PTB is Mycobacterium avium subsp. paratuberculosis (MAP). MAP enters the intestinal tissue through M cells present in the dome epithelium covering the continuous Peyer's patches in the distal ileum [2, 3]. Initially, the pathogen interacts with proteins of the extracellular matrix (ECM), which function as ligands for bacterial adhesion. Fibronectin (FN) binding is required for attachment and internalization of MAP by the epithelial cells and β1 integrins have been identified as the host cell receptors for FN-opsonized mycobacteria in vitro and in vivo [4]. M cells have the distinctive characteristic of displaying β1 integrins on their luminal face at high density; therefore, the presence of these integrins on M cells may explain why these cells are the entry of the bacteria. The interaction between MAP and FN is explained by the presence of the FN-binding proteins called adhesins. Different adhesins from several pathogens were identified as virulence factors [5–9]. Adhesins may also induce strong protective immunity in the host and, thus, remain attractive vaccine targets. For instance a 27-kDa outer membrane protein from Salmonella typhi binds to laminin and induces a strong protective antibody response in animal models and humans [10]. In addition, the antigen 85 complex (Ag85) was the first family of mycobacterial proteins to be identified as having FN-binding capability. Members of the Ag85 complex were described as a mycobacterial adhesins firstly in M. tuberculosis [11] and then in several mycobacterial species [12–15]. The members of this complex are found within the outer envelope and culture supernatants of mycobacteria and are immunodominant antigens [16, 17]. Furthermore, these proteins possess mycolyltransferase activity and catalyze the synthesis of the most abundant glycolipid of the mycobacterial cell wall, trehalose 6,6-dimycolate (TDM) [18]. Another important adhesin described in mycobacteria is the fibronectin attachment protein (FAP). FAP is a member of a family of FN-binding proteins present in several species of mycobacteria that mediate the attachment and internalization of these bacteria by epithelial cells in vitro [19–23]. This protein is also called APA (for alanine-proline-rich antigen) and is encoded by a gene annotated as MAP1569 in the MAP K-10 strain. Although the MAP-APA is not an immunodominant antigen, it activates dendritic cells and induces a Th1 polarization [24]. Furthermore, MAP-infected cattle showed a strong humoral response to recombinant APA assayed by Western blot and ELISA [7]. In a previous study conducted by our group, APA was detected mainly in the culture supernatant filtrates, demonstrating that this protein is predominantly secreted [7]. Other cell wall proteins thus could interact with FN to facilitate complex formation and, in this way, allow adherence to epithelial cells.
With all this in mind, we hypothesized that molecules with similar structure, even those from nonrelated microorganisms, could have conserved adhesin functions. In the present study, we searched for conserved adhesins within a large panel of surface immunogenic proteins of MAP and investigated a possible interaction with FN. By using the ligand blot assay (LBA), we confirmed the binding properties of a protein previously described in other bacteria and identified a novel surface component with FN-binding activity in MAP. The protein-protein interactions revealed by LBA were confirmed by ELISA binding assays and surface plasmon resonance (SPR) in order to determine the dissociation constant (KD).
2. Materials and Methods
2.1. Bacterial Strains and Culture Media
All cloning steps were performed in Escherichia coli DH5α. E. coli BL21(kDE3) was used for recombinant protein production. E. coli was grown in Luria Bertani (LB) broth or on LB agar. When necessary, ampicillin was added to the medium at a concentration of 100 μg/mL. MAP was grown in Middlebrook 7H9 medium (Difco Laboratories, USA), 0.05% Tween 80, 0.5% glycerol, AD (0.5% bovine serum albumin, 0.2% glucose), and mycobactin (2 μg/mL).
2.2. Preparation of Cell Wall Protein Fraction of MAP
MAP cultures were harvested at midlog phase, centrifuged at 14,000 ×g for 20 min at room temperature and washed twice with phosphate buffered saline (PBS). Cell pellets were resuspended in lysis buffer (PBS, 1 mM EDTA) with 1 mM phenylmethanesulfonyl fluoride (PMSF), an inhibitor of serine proteases, and then this suspension was probe sonicated in an ice bath for 15 min with pulses of 1 min on, 1 min off in a Branson Sonifier S250. Cell wall proteins were obtained as previously described by Hirschfield and collaborators [25]. Briefly, the sonic extract was centrifuged at 27,000 ×g for 20 min, and the resulting cell wall containing pellet was subjected to 2% sodium dodecyl sulfate (SDS) extraction for 2 h at 50°C. The SDS extraction was repeated twice. The protein concentration in the cell wall (CW) fraction was evaluated with Kit 2D Quant (GE Healthcare).
2.3. Two-Dimensional-SDS Polyacrylamide Gel Electrophoresis (2D-SDS-PAGE)
For 2D analysis, CW fractions were first desalted performing a gel filtration step (Sephadex G25 column). Proteins were precipitated with cold acetone and resuspended in a reswelling buffer (8 M urea, 2% CHAPS, 0.5% IPG buffer pH 4–7, 20 mM DTT, and 0.004% bromophenol blue). 2D-SDS-PAGE was performed as described by Xolalpa and collaborators [26]. The gels were transferred onto a nitrocellulose membrane (Hybond-ECL GE Healthcare) and the membranes were subjected to Western blot. The sera from 5 positive animals were pooled and diluted to 1 : 100 to detect immunogenic proteins from the CW fraction. The immunoreactive spots were manually excised from a replicate Coomassie blue stained gel and sent to the Mass Spectrometry Center for Biological and Chemical Analysis (CEQUIBIEM) at the School of Exact and Natural Sciences, University of Buenos Aires. The mass spectrometry platform used is made up of UV-MALDI-TOF/TOF Ultraflex II (Bruker Daltonics) and the software Mascot was used to identify proteins from peptide sequence databases. The protein score was calculated as −10∗Log(P), where P is the probability in which the observed match was a random event. Protein scores greater than 69 are significant (P < 0.05).
The proteins identified by MALDI-TOF MS were subjected to bioinformatic analysis including similarity searches with proteins with FN-binding domains. Sequence similarity searches were performed by BlastP (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
2.4. Recombinant MAP-EF-Tu: Cloning and Expression Assays
DNA from MAP was purified by the CTAB method as described previously by van Embden and collaborators [27]. PCR amplification was performed to amplify the complete open reading frame of EF-Tu using the forward primer eftu-fw ggatccgcgaaggcgaagttcgag (BamHI site) and the reverse primer eftu-rev aagcttctacttgatgatcttgac (HindIII site). The amplified 1,190 bp fragment was cloned into pGEM-T vector (Promega) and directionally subcloned into pRSET-A (Invitrogen). Protein expression was induced with 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG). The protein was expressed as insoluble inclusion bodies and therefore purified using a ProBond Ni-NTA Resin column (Invitrogen) under denaturing conditions with 8 M urea. After purification, EF-Tu was refolded by gradually removing the urea using a refolding buffer (Tris 50 mM pH 8, Arg-HCl, EDTA, GSH 3 mM, GSSG 0.3 mM, and PMSF 1 mM). Protein quantification was performed using BCA protein assay (Pierce) following the manufacturer's recommendations.
2.5. Western Blot
Proteins were fractionated on 12% SDS-PAGE, according to Laemmli procedure [28], and then stained with 0.25% Coomassie Brilliant Blue R250 (Sigma) or transferred onto nitrocellulose membranes (Hybond-ECL GE Healthcare). EF-Tu was assayed by Western blotting using 1 : 3,000 dilution anti-His (GE Healthcare) as primary antibody and an alkaline phosphatase-conjugated anti-mouse IgG (Sigma) as secondary antibody (1 : 3,000 dilution). A colorimetric detection was performed using BCIP/NBT (5-bromo-4-chloro-3-indolylphosphate/nitroblue tetrazolium) Color Development (Promega), according to the manufacturer's instructions.
2.6. Ligand Blot Assay (LBA)
Five μg of the purified recombinant proteins was electrophoresed in 12% SDS-PAGE gels and transferred onto nitrocellulose membranes (Hybond-ECL, GE Healthcare). Ag85 [15] and AhpC were used as positive and negative controls, respectively. The membranes were blocked with 5% BSA in PBS buffer for 1 h at room temperature and incubated with 20 μg/mL of FN for 24 hs at 4°C. The membranes were then washed three times with PBS and incubated with anti-FN in PBS-BSA 5% (1 : 100) for 2 h at room temperature, followed by a final incubation with anti-Mouse IgG alkaline phosphatase antibody (1 : 30,000) for 1 h at room temperature. The membranes were washed and the colorimetric detection of the bound bait protein was performed.
2.7. Dose-Response Curves
The 96-well plates (Polysorp Nunc) were coated with 1 μg of EF-Tu in 200 μL carbonate buffer pH 9.5 at 4°C and incubated overnight. AhpC was included as a negative control. Plates were then blocked and increasing concentrations of FN (0, 1, 10, 20, 50, and 100 μg/mL) were added in a final volume of 200 μL. Protein binding was assessed with hyperimmune anti-FN serum at the dilution of 1 : 100 followed by incubation with HRP-conjugated anti-mouse IgG (Sigma) (1 : 500). Both incubations were performed at 37°C for 1 h. The wells were washed three times, and the colorimetric detection was performed using 280 μL of developing solution 2,2′-azino-bis3-ethylbenzothiazoline-6-sulphonic acid (ABTS) at a concentration of 10 μg/mL (Sigma) and 12 μL of H2O2 30% in 10 mL of 0.1 M citrate phosphate buffer at pH 5. The absorbance at 405 nm was determined in a microplate reader Multiskan Spectrum (Thermo Scientific).
2.8. Surface Plasmon Resonance (SPR)
Protein-protein interactions were assessed by SPR [29], using a BIAcoreT100 system (GE Healthcare). Briefly, FN was covalently immobilized on the BIAcore carboxymethylated dextran matrix −5 sensor chip (GE Healthcare) according to the manufacturer's instructions. Protein solution of EF-Tu (0, 0.312, 0.625, 1.25, 2.5, 5.0, and 10.0 μM) in 10 mM HEPES, 150 mM NaCl, 3 mM EDTA, 0.005% surfactant P20, pH 7.4 was injected over immobilized FN at a flow rate of 30 μL/min for 1 min at 25°C. The dissociation was carried out with PBS-Tween 0.05% or HBS-P20. Surfaces were regenerated by applying pulses of 10 mM HCl. The KD was determined under equilibrium conditions using a nonlinear BIA evaluation program. The nonspecific binding control consisted of passing the analytes on a free surface that had been previously activated and blocked.
2.9. Humoral Response Evaluation by Line Print Immunoassay
The protein EF-Tu and a set of antigens, purified protein derivative of M. avium (PPDa), purified protein derivative of M. bovis (PPDb), and paratuberculosis protoplasmic antigen (PPA3), were evaluated with different sera. Sera were obtained from 10 healthy animals, from 8 animals with bovine tuberculosis (TBB experimentally infected animals, positive for delayed-type hypersensitivity—DTH—with PPDb and with lesions at the end of the experience), and from 25 PTB naturally infected animals, positive for DTH with PPDa and fecal culture positive. A total of 20 μL of each of the antigens was applied onto a nitrocellulose membrane (Amersham Hybond TM-ECL) using a semiautomatic aerosolizer (Camag Scientific Inc., Wilmington, Delaware) at a concentration of 100 μg/mL. The membranes were blocked and placed in a “mini blotter” (Isogen BioSolutions). This procedure allowed simultaneous analysis of the 45 sera, which were evaluated at dilutions of 1 : 100. After 1 h incubation, sera were aspirated and the membranes were washed. The membranes were then incubated with protein G conjugated to peroxidase (1 : 1,500), washed, and finally developed with chemiluminescence substrate (Pierce ECL Western blotting substrate, Thermo Scientific) according to the manufacturer's directions.
3. Results
3.1. Analysis of MAP-Cell Wall Proteins
As a first screening for surface-exposed immunogenic proteins, the MAP-cell wall (CW) protein fraction was obtained and resolved by 2D-SDS-PAGE and transferred onto a nitrocellulose membrane. Then, the membranes were analyzed by Western blot using a pool of sera from MAP-infected animals. The images were digitalized and visually analyzed (Figure 1). A total of 41 spots corresponding to proteins that were recognized by positive sera were excised from a replicate Coomassie blue stained gel and subsequently identified by MALDI-TOF MS. A total of 18 proteins were identified by this method (Table 1). We focused our interest in proteins that were previously characterized as adhesins in pathogenic microorganisms. Among these proteins, we selected elongation factor Tu (EF-Tu) because the homologous of Mycoplasma pneumoniae (identity 65%) and Acinetobacter baumannii (identity 72%) functions as a FN-binding protein that facilitates the interactions between bacteria and extracellular matrix [30–32].
Figure 1.
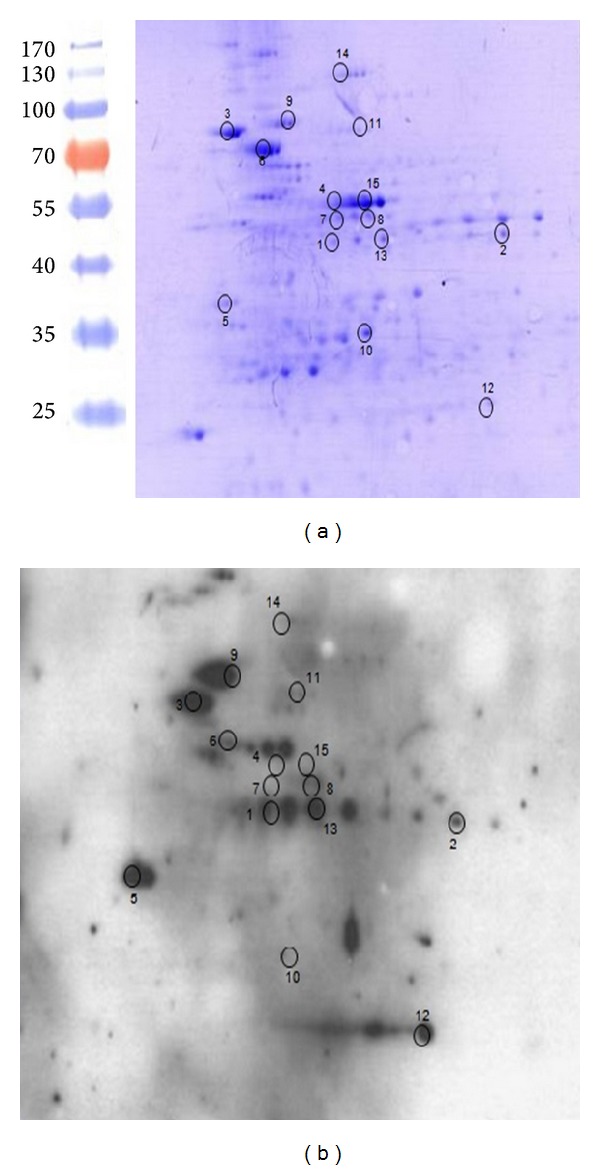
Analysis of MAP-cell wall proteins by 2D-SDS-PAGE. The cell wall protein fraction (CW) of MAP was resolved by 2D-SDS PAGE per duplicate and the resulting gels were (a) stained with Coomassie blue or (b) transferred onto a nitrocellulose membrane and subjected to Western blot. Sera from 5 positive animals were pooled and diluted to 1 : 100 to detect immunogenic proteins from the CW fraction. Molecular weight standards are shown on the left.
Table 1.
Immunogenic CW proteins of MAP identified by MALDI-TOF MS. Proteins were excised from Coomassie blue stained gels and subsequently identified by MALDI-TOF at the Mass Spectrometry Center for Biological and Chemical Analysis (CEQUIBIEM) at the School of Exact and Natural Sciences, University of Buenos Aires. ND: no data.
| Spot number | MAP Locus | Mtb Locus | Gene | Protein function/family | ORF size (bp) | Previously described as envelope protein |
|---|---|---|---|---|---|---|
| 1 | MAP1164 | Rv1436 | gapdh | Glyceraldehyde-3-phosphate dehydrogenase | 1,020 | He and de Buck, 2010 (MAP) [34] |
| 2 | MAP1205 | Rv1479 | moxR | ATPase | 1,143 | Mawuenyega et al., 2005 (Mtb) [35] |
| 3 | MAP1325 | Rv1630 | rpsA S1 | 30S ribosomal protein S1 | 1,443 | Gu et al., 2003 (Mtb) [36] |
| 4 | MAP1367 | Rv1658 | argG | Argininosuccinate synthase | 1,197 | ND |
| 5 | MAP1889c | Rv2145c | wag31 | DivIVA family protein | 783 | He and de Buck, 2010 (MAP) [34] |
| 6 | MAP1962 | Rv2220 | glnA1 | Glutamine synthetase A1 | 1,437 | Gu et al., 2003 (MAP) [36] |
| 7 | MAP1998 | Rv2245 | kasA | 3-Oxoacyl synthetase | 1,251 | He and de Buck, 2010 (MAP) [34] |
| 8 | MAP1999 | Rv2246 | kasB_1 | 3-Oxoacyl sintase 2 B | 1,323 | Mawuenyega et al., 2005 (Mtb) [35] |
| 9 | MAP2453c | Rv1308 | atpA | ATP synthase subunit alpha | 1,665 | He and de Buck, 2010 (MAP) [34] |
| 10 | MAP2855c | Rv2744c | 35kd_ag | Phage shock protein A | 828 | ND |
| 5 | MAP3152c | Rv3075c | hpcH/hpaI | Aldolase/citrate lyase | 921 | Gu et al., 2003 (MAP) [36] |
| 11 | MAP3404 | Rv3285 | accA3 | Carbamoyl-phosphate synthase subunit A | 1,824 | Mawuenyega et al., 2005 (Mtb) [35] |
| 12 | MAP3567 | Rv0148 | Hypothetical protein | Short-chain dehydrogenases/reductases | 864 | He and de Buck, 2010 (MAP) [34] |
| 13 | MAP3651c | Rv0215c | fadE3_2 | Acyl-CoA dehydrogenase FadE3 | 1,218 | He and de Buck, 2010 (MAP) [34] |
| 7 | MAP3692c | Rv0242c | fabG4 | 3-Ketoacyl reductase | 1,365 | He and de Buck, 2010 (MAP) [34] |
| 14 | MAP3853 | Rv0384c | clpB | ATP dependent protease ClpB | 2,547 | He and de Buck, 2010 (MAP) [34] |
| 3 | MAP3936 | Rv0440 | groEL2 | GroEL chaperonin | 1,626 | He and de Buck, 2010 (MAP) [34] |
| 15 | MAP4143 | Rv0685 | eftu | Elongation factor Tu | 1,191 | He and de Buck, 2010 (MAP) [34] |
The eftu gene from MAP was evaluated for orthologous in M. avium strain 104 (GenBank accession number NC_008595) and M. tuberculosis (Mtb) H37Rv (GenBank accession number NC_000962). In the MAP genome (MAP strain K-10 GenBank accession number NC_002944), this gene was annotated as MAP 4143 and shares 93% of nucleotide identity with that of Mtb and 100% with that of M. avium. The identity at the protein level is 100% between MAP and M. avium and 97% with its orthologous in Mtb.
We searched for two FN-binding regions (FBR) in the MAP-EF-Tu protein sequence, which has been previously identified in the carboxyl terminus of Mycoplasma pneumoniae EF-Tu [32, 33]. The BlastP analysis is shown in Table 2. The comparison of both regions yielded an identity of 73% for FBR1 and 69% for FBR2.
Table 2.
BlastP comparison between the two fibronectin binding regions (FBRs) of EF-Tu identified by Balasubramanian and collaborators [37] in Mycoplasma pneumoniae (MP) and the homologous regions in MAP.
| MP amino acid region | MAP amino acid region | Identity between MP and MAP (%) | |
|---|---|---|---|
| FBR1 | 192–219 | 193–220 | 73 |
| FBR2 | 340–358 | 342–360 | 69 |
3.2. Recombinant Expression and Ligand Blot Assay (LBA)
The MAP-EF-Tu protein was heterologously expressed in E. coli as a recombinant His-tagged protein and its product was purified under denaturing conditions. Purified EF-Tu protein is showed in Figure 2. The purified protein was used to perform LBA using FN as “bait.” Interactions were detected with anti-FN polyclonal antiserum and an alkaline phosphatase-conjugated antibody with further colorimetric detection of the bound bait. A strong positive signal was observed for EF-Tu and for Ag85 recombinant protein, which was assayed as a positive control. No signal was observed for the negative control, AhpC (Figure 3).
Figure 2.

Purified EF-Tu protein using a ProBond Ni-NTA Resin column (Invitrogen). (a) Detection of the protein by Western blot using an anti-Histidine Antibody (Promega). (b) Coomassie blue stained gel showing the purified protein.
Figure 3.
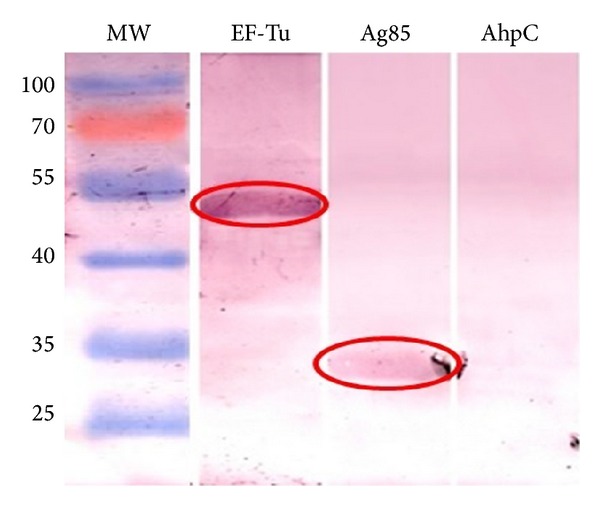
Analysis of FN-binding capability of EF-Tu through a LBA. The blot with the recombinant proteins was incubated with 20 μg/mL FN. Colorimetric detection of the bound bait protein was performed. We observed positive signal indicating the FN-binding capability of EF-Tu and the positive control Ag85 (red circles). AhpC was used as a negative control.
3.3. Dose-Response Curves
We further confirmed FN-EF-Tu interactions using an ELISA assay with some modifications. Plates were coated with 1 μg of EF-Tu or AhpC used as a negative control and then incubated with increasing concentrations of FN. After incubation with the hyperimmune anti-FN serum followed by HRP-conjugated anti-mouse IgG and the corresponding substrate, the absorbance was determined in a microplate reader at 492 nm. We observed a dose-dependent interaction confirming the binding of EF-Tu with FN (Figure 4).
Figure 4.
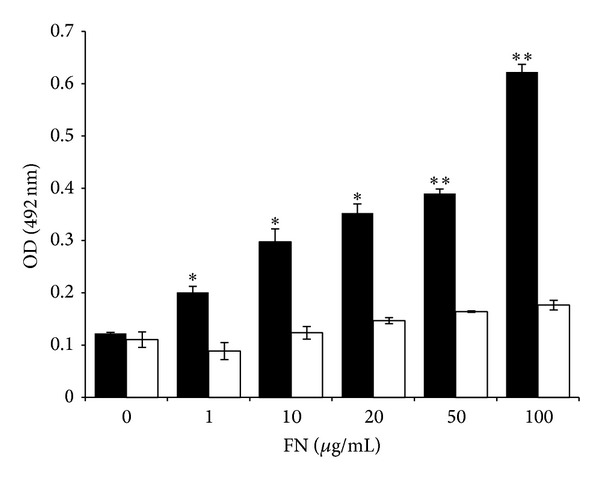
Dose-response curves assayed by ELISA. Plates were coated with EF-Tu (black bars) or AhpC (white bars) used as negative control and incubated with different concentrations of FN. The absorbance, measured at 492 nm, showed a dose-dependent interaction confirming the binding of EF-Tu with FN. Significantly different from values of the control protein AhpC **P < 0.01, *P < 0.05.
3.4. Surface Plasmon Resonance (SPR)
To finally determine the EF-Tu dissociation constant (KD), protein-protein interactions were also assessed through SPR with a BIAcoreT100 system (GE Healthcare). FN was covalently immobilized on the BIAcore CM-5 sensor chip. Solutions of EF-Tu at different concentrations were injected over immobilized FN. The obtained KD was within the μM order, (3.1 ± 0.9) 10−6 M (Figure 5). This value is similar to the values of other adhesins previously described [38]. The control using denaturized EF-Tu was not able to bind FN. Therefore, all these experiments confirmed the FN/EF-Tu binding with a moderate affinity through conformational sites.
Figure 5.
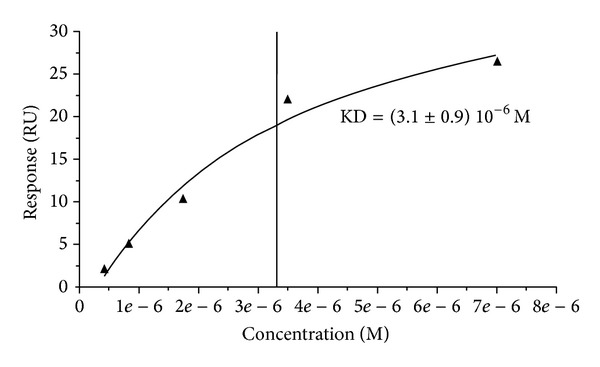
KD determination by surface plasmon resonance. Protein solutions of EF-Tu at different concentrations were injected over immobilized FN. The experiment confirmed FN-EFTu binding, with a KD of (3.1 ± 0.9) 10−6 M.
3.5. Humoral Response Evaluation by Line Print Immunoassay
FN-binding proteins could play a role in adhesion to the host and immunomodulation. With this in mind, we analyzed whether EF-Tu is able to stimulate antibody production. Using a line print assay, we evaluated a broader set of sera including TBB-infected animals and negative controls. The sera were obtained from 25 MAP-naturally infected cattle, 8 M. bovis-experimentally infected cattle, and 10 healthy bovines (negative controls). EF-Tu was recognized by 64% of the MAP positive sera. However, this protein was also recognized by sera of healthy and TBB animals, suggesting the presence of antigenic epitopes conserved among mycobacteria species, including environmental mycobacteria that sensitizes healthy cattle (Table 3).
Table 3.
Reactivity of bovine sera to the protein EF-Tu by line print immunoassay. 20 μL of antigens was applied onto a nitrocellulose membrane and simultaneously confronted to sera from 10 healthy animals, 8 animals with bovine tuberculosis (TBB), and 25 animals with paratuberculosis (PTB). Sixty four percent of MAP positive sera recognized EF-Tu.
| Number of sera with positive recognition | |||
|---|---|---|---|
| Antigen | Healthy (n = 10) | PTB infected (n = 25) | TBB infected (n = 8) |
| PPDA | 4 | 16 | 3 |
| PPA-3 | 1 | 18 | 2 |
| PPDB | 6 | 1 | 4 |
| EF-Tu | 8 | 16 | 7 |
4. Discussion
MAP invades the intestinal tissue primarily through M cells. This could occur through FN-dependent mechanisms that involve the binding of FN to proteins of the MAP CW and to integrin receptors present on the luminal surface of M cells. β1 integrins have been identified as the host cell receptors. In addition, the attachment and internalization of MAP by epithelial cells in vitro and in vivo depend on APA-FN interactions [4]. However, other MAP proteins could be involved in FN binding and they could be important for host-pathogen interactions.
In this study, we have identified immunogenic CW proteins of MAP by 2D and MALDI-TOF MS analysis. From the identified proteins, we selected one candidate based on the similarity with other proteins having the ability to bind ECM molecules in other pathogenic bacteria: elongation factor Tu (EF-Tu).
We first screened its FN-binding capability through a ligand blot assay using FN and anti-FN antibodies. This screening confirmed that, in these conditions, EF-Tu binds FN (Figure 3). Through ELISA assays, using increasing concentrations of FN, we confirmed the interaction in a dose-dependent manner (Figure 4). We finally determine the dissociation constant KD by SPR analysis, which yielded a KD within the order of μM (Figure 5) consistent with previous reports [38].
Several Mycobacterium adhesins capable to bind ECM proteins have been identified in mycobacteria, such as antigen 85 complex [11–15], APA [19–23], and GlnA1 [26]. Several reports have demonstrated that these proteins are involved in bacterial dissemination. EF-Tu is a protein responsible for critical steps in protein synthesis [39]. Moreover, this protein is a cytoplasmic protein with unusual CW location among microorganisms. For instance, when E. coli is starved for carbohydrates, nitrogen, and phosphate, this protein becomes methylated and associates to the membrane [40]. In addition, this protein was detected as a major CW protein of Mycobacterium leprae [41]. On the other hand, EF-Tu has been identified with periplasmic location in Neisseria gonorrhoeae [42] and in E. coli [43]. In Lactobacillus johnsonii and Listeria monocytogenes, EF-Tu is also associated to the membrane; in these bacteria this protein mediates binding to mucin [44] and fibrinogen [37], respectively. Recently, it has been reported that EF-Tu binds factor H and plasminogen in Pseudomonas aeruginosa [45]. Thus, EF-Tu joins the group of housekeeping enzymes, which includes enolase [46], glyceraldehyde-3-phosphate dehydrogenase [47], and pyruvate dehydrogenase [30], that exhibit unexpected biological functions in addition to their well-defined enzymatic activities. Despite the classification of EF-Tu as a cytosolic protein, Balasubramanian and collaborators [32] have shown that EF-Tu can relocate to the mycoplasma membrane surface with an exposed carboxyl terminus that facilitates FN binding. On the other hand, other reports document the presence of cytosolic proteins, including EF-Tu, on surfaces of different bacteria. Important questions regarding how these proteins translocate to the surface remain unanswered, especially since conventional secretion or anchoring signals are absent. The cytoplasmic enzymefructose-1, 6-bisphosphate aldolase (FBA) has also been found on the surface of several pathogenic bacteria. FBA is a glycolytic enzymethat, despite lacking secretion signals, translocates across the different compartments of the bacterial cell to access the surface, where it binds host molecules and exhibits nonglycolytic functions [40, 41].
The interaction of EF-Tu with the ECM could be a key mechanism during host-pathogen interactions, However, the overall contribution of this adhesin to the host cell binding remains unclear. Although there is a dose-dependent specific binding of EF-Tu to immobilized FN, the affinity of EF-Tu to FN is intermediate. In the present study, EF-Tu displayed a KD in the μM range, similar to other proteins proposed as adhesins. For instance, a surface-exposed protein of leptospira, Lsa20, binds to plasminogen and laminin with a KD in the μM range, postulated as a protein with adherence function and proteolytic activity [38]. Moreover, these adhesins can also bind other proteins of the ECM, such as elastin, laminin, and plasminogen, which contributes to adhesion, to invasion, and to the virulence of the bacteria modulating the immune response [15, 24, 45].
In our study, recombinant EF-Tu was recognized by 64% of sera from MAP-infected cattle, suggesting a putative role in the host immune response. However, sera from noninfected animals also recognize EF-Tu. This result could be due to the presence of EF-Tu in environmental mycobacteria, such as M. avium, which shares 100% identity with the MAP protein. Moreover, the suggested role of EF-Tu in the virulence of pathogenic bacteria could be related to the host-pathogen interactions.
Kuo and collaborators [15] demonstrated that reducing the expression of FN on Caco-2 cells that were transfected with FN-siRNA impaired the ability of Ag85 or Ag85-expressing MAP K-10 to bind to the Caco-2 cells. However, MAP K-10 strain binding to FN siRNA-transfected Caco-2 cells showed only a 9.7% reduction (compared with the negative siRNA transfection). This finding suggests that FN is not the only bacterial adhesion ligand contributing to the MAP ability to bind to host cells. Additionally, the MAP Ag85 interaction to FN only partially accounted for MAP's ability to bind host cells. Other surface antigens of MAP K-10 probably participate in additional ECM interactions.
In conclusion, we have identified a novel FN-binding MAP protein, EF-Tu, that could be implicated in the entrance of MAP into the epithelial cells, which is the first step of mycobacteria infection necessary for the progression of PTB.
Acknowledgments
The authors are grateful to Dr. Julia Sabio y García for critical reading of this paper. This work was funded by ANCyPT Grant PICT1170 and ANCyPT Grant FONARSEC FS Agrobiotecnología 2010. M. N. Viale and M. P. Santangelo are CONICET fellows.
Conflict of Interests
The authors declare that they have no conflict of interests regarding the publication of this paper.
Authors' Contribution
Andrea Karina Gioffré and María de la Paz Santangelo are contributed equally to this work.
References
- 1.Cocito C, Gilot P, Coene M, de Kesel M, Poupart P, Vannuffel P. Paratuberculosis. Clinical Microbiology Reviews. 1994;7(3):328–345. doi: 10.1128/cmr.7.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Momotani E, Whipple DL, Thiermann AB, Cheville NF. Role of M cells and macrophages in the entrance of Mycobacterium paratuberculosis into domes of ileal Peyer's patches in calves. Veterinary Pathology. 1988;25(2):131–137. doi: 10.1177/030098588802500205. [DOI] [PubMed] [Google Scholar]
- 3.Sigurđardóttir ÓG, Press CM, Evensen Ø. Uptake of Mycobacterium avium subsp. paratuberculosis through the distal small intestinal mucosa in goats: an ultrastructural study. Veterinary Pathology. 2001;38:184–189. doi: 10.1354/vp.38-2-184. [DOI] [PubMed] [Google Scholar]
- 4.Secott TE, Lin TL, Wu CC. Mycobacterium avium subsp. paratuberculosis fibronectin attachment protein facilitates M-cell targeting and invasion through a fibronectin bridge with host integrins. Infection and Immunity. 2004;72(7):3724–3732. doi: 10.1128/IAI.72.7.3724-3732.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joh D, Wann ER, Kreikemeyer B, Speziale P, Höök M. Role of fibronectin-binding MSCRAMMs in bacterial adherence and entry into mammalian cells. Matrix Biology. 1999;18(3):211–223. doi: 10.1016/s0945-053x(99)00025-6. [DOI] [PubMed] [Google Scholar]
- 6.Abou-Zeid C, Garbe T, Lathigra R, et al. Genetic and immunological analysis of Mycobacterium tuberculosis fibronectin-binding proteins. Infection and Immunity. 1991;59(8):2712–2718. doi: 10.1128/iai.59.8.2712-2718.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gioffré A, Echeverría-Valencia G, Arese A, et al. Characterization of the Apa antigen from M. avium subsp. paratuberculosis: a conserved Mycobacterium antigen that elicits a strong humoral response in cattle. Veterinary Immunology and Immunopathology. 2009;132(2–4):199–208. doi: 10.1016/j.vetimm.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Shin SJ, Chang CF, McDonough SP, Thompson B, Yoo HS, Chang Y. In vitro cellular immune responses to recombinant antigens of Mycobacterium avium subsp. paratuberculosis . Infection and Immunity. 2005;73(8):5074–5085. doi: 10.1128/IAI.73.8.5074-5085.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mullerad J, Michal I, Fishman Y, Hovav A, Barletta RG, Bercovier H. The immunogenicity of Mycobacterium paratuberculosis 85B antigen. Medical Microbiology and Immunology. 2002;190(4):179–187. doi: 10.1007/s00430-001-0104-z. [DOI] [PubMed] [Google Scholar]
- 10.Ghosh S, Chakraborty K, Nagaraja T, et al. An adhesion protein of Salmonella enterica serovar Typhi is required for pathogenesis and potential target for vaccine development. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(8):3348–3353. doi: 10.1073/pnas.1016180108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abou-Zeid C, Ratliff TL, Wiker HG, Harboe M, Bennedsen J, Rook GAW. Characterization of fibronectin-binding antigens released by Mycobacterium tuberculosis and Mycobacterium bovis BCG. Infection and Immunity. 1988;56(12):3046–3051. doi: 10.1128/iai.56.12.3046-3051.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thole JER, Schöningh R, Janson AAM, et al. Molecular and immunological analysis of a fibronectin-binding protein antigen secreted by Mycobacterium leprae . Molecular Microbiology. 1992;6(2):153–163. doi: 10.1111/j.1365-2958.1992.tb01996.x. [DOI] [PubMed] [Google Scholar]
- 13.Kitaura H, Ohara N, Naito M, Kobayashi K, Yamada T. Fibronectin-binding proteins secreted by Mycobacterium avium . APMIS. 2000;108(9):558–564. doi: 10.1034/j.1600-0463.2000.d01-97.x. [DOI] [PubMed] [Google Scholar]
- 14.Dheenadhayalan V, Shin K, Chang C, et al. Cloning and characterization of the genes coding for antigen 85A, 85B and 85C of Mycobacterium avium subsp. paratuberculosis . Mitochondrial DNA. 2002;13(5):287–294. doi: 10.1080/1042517021000019269. [DOI] [PubMed] [Google Scholar]
- 15.Kuo CJ, Bell H, Hsieh CL, Ptak CP, Chang YF. Novel mycobacteria antigen 85 complex binding motif on fibronectin. The Journal of Biological Chemistry. 2012;287(3):1892–1902. doi: 10.1074/jbc.M111.298687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baldwin SL, D'Souza CD, Orme IM, et al. Immunogenicity and protective efficacy of DNA vaccines encoding secreted and non-secreted forms of Mycobacterium tuberculosis Ag85A. Tubercle and Lung Disease. 1999;79(4):251–259. doi: 10.1054/tuld.1998.0196. [DOI] [PubMed] [Google Scholar]
- 17.Kamath AT, Feng CG, Macdonald M, Briscoe H, Britton WJ. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis . Infection and Immunity. 1999;67(4):1702–1707. doi: 10.1128/iai.67.4.1702-1707.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belisle JT, Vissa VD, Sievert T, Takayama K, Brennan PJ, Besra GS. Role of the major antigen of Mycobacterium tuberculosis in cell wall biogenesis. Science. 1997;276(5317):1420–1422. doi: 10.1126/science.276.5317.1420. [DOI] [PubMed] [Google Scholar]
- 19.Ratliff TL, McCarthy R, Telle WB, Brown EJ. Purification of a mycobacterial adhesin for fibronectin. Infection and Immunity. 1993;61(5):1889–1894. doi: 10.1128/iai.61.5.1889-1894.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schorey JS, Li Q, McCourt DW, et al. A Mycobacterium leprae gene encoding a fibronectin binding protein is used for efficient invasion of epithelial cells and Schwann cells. Infection and Immunity. 1995;63(7):2652–2657. doi: 10.1128/iai.63.7.2652-2657.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schorey JS, Holsti MA, Ratliff TL, Allen PM, Brown EJ. Characterization of the fibronectin-attachment protein of Mycobacterium avium reveals a fibronectin-binding motif conserved among mycobacteria. Molecular Microbiology. 1996;21(2):321–329. doi: 10.1046/j.1365-2958.1996.6381353.x. [DOI] [PubMed] [Google Scholar]
- 22.Secott TE, Lin TL, Wu CC. Fibronectin attachment protein homologue mediates fibronectin binding by Mycobacterium avium subsp. paratuberculosis . Infection and Immunity. 2001;69(4):2075–2082. doi: 10.1128/IAI.69.4.2075-2082.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Secott TE, Lin TL, Wu CC. Fibronectin attachment protein is necessary for efficient attachment and invasion of epithelial cells by Mycobacterium avium subsp. paratuberculosis . Infection and Immunity. 2002;70(5):2670–2675. doi: 10.1128/IAI.70.5.2670-2675.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jun SL, Sung JS, Collins MT, et al. Mycobacterium avium subsp. paratuberculosis fibronectin attachment protein activates dendritic cells and induces a Th1 polarization. Infection and Immunity. 2009;77(7):2979–2988. doi: 10.1128/IAI.01411-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirschfield GR, McNeil M, Brennan PJ. Peptidoglycan-associated polypeptides of Mycobacterium tuberculosis . Journal of Bacteriology. 1990;172(2):1005–1013. doi: 10.1128/jb.172.2.1005-1013.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xolalpa W, Vallecillo AJ, Lara M, et al. Identification of novel bacterial plasminogen-binding proteins in the human pathogen Mycobacterium tuberculosis . Proteomics. 2007;7(18):3332–3341. doi: 10.1002/pmic.200600876. [DOI] [PubMed] [Google Scholar]
- 27.van Embden JDA, van Soolingen D, Small PM, Hermans PWM. Genetic markers for the epidemiology of tuberculosis. Research in Microbiology. 1992;143(4):385–391. doi: 10.1016/0923-2508(92)90051-o. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Karlsson R, Michaelsson A, Mattsson L. Kinetic analysis of monoclonal antibody-antigen interactions with a new biosensor based analytical system. Journal of Immunological Methods. 1991;145(1-2):229–240. doi: 10.1016/0022-1759(91)90331-9. [DOI] [PubMed] [Google Scholar]
- 30.Dallo SF, Kannan TR, Blaylock MW, Baseman JB. Elongation factor Tu and E1 β subunit of pyruvate dehydrogenase complex act as fibronectin binding proteins in Mycoplasma pneumoniae. Molecular Microbiology. 2002;46(4):1041–1051. doi: 10.1046/j.1365-2958.2002.03207.x. [DOI] [PubMed] [Google Scholar]
- 31.Dallo SF, Zhang B, Denno J, et al. Association of Acinetobacter baumannii EF-Tu with cell surface, outer membrane vesicles, and fibronectin. The Scientific World Journal. 2012;2012:10 pages. doi: 10.1100/2012/128705.128705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Balasubramanian S, Kannan TR, Baseman JB. The surface-exposed carboxyl region of Mycoplasma pneumoniae elongation factor Tu interacts with fibronectin. Infection and Immunity. 2008;76(7):3116–3123. doi: 10.1128/IAI.00173-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balasubramanian S, Kannan TR, Hart PJ, Baseman JB. Amino acid changes in elongation factor Tu of Mycoplasma pneumoniae and Mycoplasma genitalium influence fibronectin binding. Infection and Immunity. 2009;77(9):3533–3541. doi: 10.1128/IAI.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He Z, de Buck J. Localization of proteins in the cell wall of Mycobacterium avium subsp. paratuberculosis K10 by proteomic analysis. Proteome Science. 2010;8, article 21 doi: 10.1186/1477-5956-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mawuenyega KG, Forst CV, Dobos KM, et al. Mycobacterium tuberculosis functional network analysis by global subcellular protein profiling. Molecular Biology of the Cell. 2005;16(1):396–404. doi: 10.1091/mbc.E04-04-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu S, Chen J, Dobos KM, Bradbury EM, Belisle JT, Chen X. Comprehensive proteomic profiling of the membrane constituents of a Mycobacterium tuberculosis strain. Molecular and Cellular Proteomics. 2003;2(12):1284–1296. doi: 10.1074/mcp.M300060-MCP200. [DOI] [PubMed] [Google Scholar]
- 37.Schaumburg J, Diekmann O, Hagendorff P, et al. The cell wall subproteome of Listeria monocytogenes . Proteomics. 2004;4(10):2991–3006. doi: 10.1002/pmic.200400928. [DOI] [PubMed] [Google Scholar]
- 38.Mendes RS, von Atzingen M, de Morais ZM, et al. The novel leptospiral surface adhesin Lsa20 binds laminin and human plasminogen and is probably expressed during infection. Infection and Immunity. 2011;79(11):4657–4667. doi: 10.1128/IAI.05583-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobson GR, Rosenbusch JP. Abundance and membrane association of elongation factor Tu in E. coli . Nature. 1976;261(5555):23–26. doi: 10.1038/261023a0. [DOI] [PubMed] [Google Scholar]
- 40.Young CC, Bernlohr RW. Elongation factor Tu is methylated in response to nutrient deprivation in Escherichia coli . Journal of Bacteriology. 1991;173(10):3096–3100. doi: 10.1128/jb.173.10.3096-3100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marques MAM, Chitale S, Brennan PJ, Pessolani MCV. Mapping and identification of the major cell wall-associated components of Mycobacterium leprae . Infection and Immunity. 1998;66(6):2625–2631. doi: 10.1128/iai.66.6.2625-2631.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porcella SF, Belland RJ, Judd RC. Identification of an EF-Tu protein that is periplasm-associated and processed in Neisseria gonorrhoeae . Microbiology. 1996;142(9):2481–2489. doi: 10.1099/00221287-142-9-2481. [DOI] [PubMed] [Google Scholar]
- 43.Beck BD, Arscott PG, Jacobson A. Novel properties of bacterial elongation factor Tu. Proceedings of the National Academy of Sciences of the United States of America. 1978;75(3):1250–1254. doi: 10.1073/pnas.75.3.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Granato D, Bergonzelli GE, Pridmore RD, Marvin L, Rouvet M, Corthésy-Theulaz IE. Cell surface-associated elongation factor tu mediates the attachment of lactobacillus johnsonii NCC533 (La1) to human intestinal cells and mucins. Infection and Immunity. 2004;72(4):2160–2169. doi: 10.1128/IAI.72.4.2160-2169.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kunert A, Losse J, Gruszin C, et al. Immune evasion of the human pathogen Pseudomonas aeruginosa: elongation factor Tuf is a factor H and plasminogen binding protein. Journal of Immunology. 2007;179(5):2979–2988. doi: 10.4049/jimmunol.179.5.2979. [DOI] [PubMed] [Google Scholar]
- 46.Bergmann S, Rohde M, Chhatwal GS, Hammerschmidt S. α-Enolase of Streptococcus pneumoniae is a plasmin(ogen)-binding protein displayed on the bacterial cell surface. Molecular Microbiology. 2001;40(6):1273–1287. doi: 10.1046/j.1365-2958.2001.02448.x. [DOI] [PubMed] [Google Scholar]
- 47.Pancholi V, Fischetti VA. A major surface protein on group a streptococci is a glyceraldehyde-3-phosphate-dehydrogenase with multiple binding activity. Journal of Experimental Medicine. 1992;176(2):415–426. doi: 10.1084/jem.176.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]


