Abstract
Twenty publications from twelve prospective cohorts evaluated associations between flavonoid intakes and incidence or mortality from cardiovascular disease among adults in Europe and the United States (US). The most common outcome was coronary heart disease mortality, and four of eight cohort studies reported significant inverse associations for at least one flavonoid class (multivariate adjusted ptrend <.05). Three of seven cohorts reported that greater flavonoid intake was associated with lower risk of incident stroke. Comparisons were difficult because of variability in the flavonoid classes included, demographic characteristics of the populations, outcomes assessed, and length of follow up. The most common flavonoid classes examined were flavones and flavonols combined (11 studies). Only one study examined all seven flavonoid classes. The flavonol and flavone classes were most strongly associated with lower CHD mortality. Evidence for protection from other flavonoid classes and CVD outcomes was more limited. The hypothesis that flavonoid intakes are associated with lower CVD incidence and mortality requires further study.
Keywords: flavonoids, cardiovascular disease, coronary heart disease, stroke, prospective cohort studies, United States, Europe
INTRODUCTION
Flavonoids are bioactive, polyphenolic, non-caloric, non-nutrient compounds – which are ubiquitous in fruits, vegetables and other vascular plants and cannot be synthesized by humans.1 They are present in relatively high amounts in the diets of US and European populations, who have total intakes of flavonoids that vary from ~200–400 mg/day.2–8 Single cell,9–11 in vitro,12–14 animal,15–24 and clinical studies25–40 suggest that intakes of certain flavonoid classes reduce cardiovascular disease risk.2,41–53 Consequently some experts have recommended increased intakes of flavonoid rich diets for preventive purposes.54–62
This article reviews the evidence on the association between intakes of all the flavonoid classes with cardiovascular disease mortality and incidence in prospective adult cohorts in Europe and the US.63–82
METHODS
Literature search method
We used Ovid Medline and Ovid Commonwealth Agricultural Bureau (CAB) to search the literature using the following basic search terms: [Flavonoids AND (Cohort or Prospective or Nested or Cross-sectional) AND Cardiovascular diseases]. Additional search terms used were Arteriosclerosis, Cerebrovascular Disorders, Cholesterol, Coronary Disease, Heart Diseases, Hypercholesterolemia, Hypertension, Metabolic Syndrome, Myocardial infarction, Phytoestrogens, and specific flavonoid classes to enhance our ability to identify all possible relevant studies. Retrospective (case-control and cross-sectional) studies83,84 were excluded to avoid potential recall bias associated with retrospective data collection.85
We found twenty publications from twelve prospective cohorts in three European countries and the US, as described in Table 1: Finland (alpha-tocopherol beta-carotene Cancer Prevention [ATBC] Study,69,70 Kuopio Ischemic Heart Disease Risk Factor Study,78 the Finnish Mobile Clinic Health Examination Survey,72–74 and the Turku and Environs Health Survey76), the Netherlands (Dutch Prospect - European Prospective Study into Cancer and Nutrition [EPIC] Cohort,81 the Rotterdam Study,65 and the Zutphen Elderly Study63,66,67,71), Wales, United Kingdom (Caerphilly Study68), and the United States (Health Professionals Follow-up Study [HPFS],79 Iowa Women’s Health Study,64,77,82 Nurses’ Health Study [NHS],75 and the Women’s Health Study [WHS]80). Only three of these cohorts (the Finnish Mobile, Turku and Rotterdam cohorts) included both men and women. Five of the cohorts (ATBC, Kuopio, Zutphen, Caerphilly, and HPFS cohorts) included only men. One European cohort (Dutch EPIC) and three of the US cohorts (Iowa, NHS, and WHS cohorts) included only women.
Table 1.
Prospective cohorts examined
| Cohort description | Studies N |
Endpoints | Flavonoid intake mean mg/d (median mg/d) |
|---|---|---|---|
| Finland | |||
|
Finnish Mobile Clinic Health Examination Survey M & F, 1966–1972 ages 30–69 |
Knekt 199673 2,748 M & 2,385 F |
CHD mortality | Flavones & flavonols (3.4) |
| Knekt 200072 9,208 M & F |
Stroke incidence | Flavonol (quercetin) 3.68 M, 4.07 F | |
| Knekt 200274 9,131 M & F |
CHD mortality Stroke incidence |
Flavonols 4.0 Flavones <0.1 Flavanones 20.2 |
|
|
alpha-tocopherol, beta-carotene Cancer Prevention Study (ATBC) M, 1985–1988 ages 50–69 |
Hirvonen 200070 26,497 M |
Stroke incidence | Flavones & flavonols (8.0) |
| Hirvonen 200169 25,372 M |
CHD mortality CHD incidence |
Flavones & flavonols 9.9 | |
|
Turku and Environs Health Survey M & F, in 1986–1987 ages 65–99 |
Marniemi 200576 361 M & 394 F |
CHD incidence Stroke incidence |
Flavones 0.51 Flavonols 9.61 |
|
Kuopio Ischemic Heart Disease Risk Factor Study M, in 1984–1989 ages 42, 48, 54,or 60 |
Mursu 200878 1,950 M |
CVD mortality Stroke incidence |
Flavones 0.3 Flavonols 10.0 Flavanones 3.1 Flavan-3-ols 119.72 Anthocyanidins 6.2 |
| Netherlands | |||
|
Zutphen Elderly Study M, in 1985 ages 65–84 |
Hertog 199366 805 M |
CHD mortality CHD incidence |
Flavones & flavonols 25.9 |
| Keli 199671 552 M |
Stroke incidence | Flavones & flavonols 22.2 | |
| Hertog 199767 804 (mortality) 692 (incidence) |
CHD mortality CHD incidence |
Flavones & flavonols 25.93 | |
| Arts 200163 806 M |
CHD mortality CHD incidence Stroke mortality Stroke incidence |
Flavan-3-ols 72 | |
|
Rotterdam Study M & F, 1990–1993 aged ≥55 |
Geleijnse 200265 1836 M & 2971 F |
CHD mortality CHD incidence |
Flavonols 28.6 |
|
Dutch Prospect-European Prospective Study Into Cancer and Nutrition Cohort (EPIC) F, 1993–1997 ages 49–70 |
van der Schouw 200581 16,165 F |
CHD incidence Stroke incidence CVD incidence |
Isoflavones (0.4)4 |
| Wales, UK | |||
|
Caerphilly Study M, 1979–1983 ages 45–59 |
Hertog 199768 1,900 M |
CHD mortality CHD incidence |
Flavonols 26.3 |
| USA | |||
|
Health Professionals Follow-up Study (HPFS) M, in 1986 ages 40–75 |
Rimm 199679 34,789 M |
CHD mortality CHD incidence |
Flavones & flavonols 20.1 |
|
Iowa Women's Health Study F, in 1986 ages 55–69 |
Yochum 199982 34,492 F |
CHD mortality Stroke mortality |
Flavones & flavonols 13.9 |
| Arts 200164 32,857 F |
CHD mortality | Flavan-3-ols 25.4 | |
| Mink 200777 34,492 F |
CHD mortality Stroke mortality CVD mortality |
Flavones (0.4) Flavonols (8.9) Flavanones (40.4) Flavan-3-ols (20.4)5 Anthocyanidins (0.2)6 Isoflavones (0.3) Proanthocyanidins (175.2)7 |
|
|
Women's Health Study (WHS) F, in 1992 ages ≥45 |
Sesso 200380 38,445 F |
CVD mortality CHD incidence Stroke incidence CVD incidence |
Flavones & flavonols 24.6 |
|
Nurses' Health Study (NHS) F, in 1976 ages 30–55 |
Lin 200775 66,360 F |
CHD mortality CHD incidence |
Flavones & flavonols 21.2 |
M – males, F- females
Average of controls for stroke and acute myocardial infarction
Includes theaflavins and thearubigins
Not stated in article but article refers to Hertog et al66
Isoflavone data is scored, analytical values not used
Flavan-3-ols and proanthocyanidin monomers averaged to determine intake
Approximation not specifically stated
Proanthocyanidins minus proanthocyanidin monomers
Assessment of flavonoid intakes
Flavonoid intakes were assessed in eight cohorts using five different food frequency questionnaires,64,65,68–70,75,77,79–82 which varied in length from 5668 to 27669,70 items. Diet histories were used in three cohorts,63,66,67,71–74,76 a 4-day dietary record in one,78 and the last used a checklist along with a dietician interview and food frequency questionnaire.65
The data sources for the flavonoid content of foods and beverages also varied. Thirteen studies65–75,79,82 utilized flavonoid data developed by Hertog et al.86–90 Two studies63,64 utilized flavan-3-ol data developed by Arts et al.91–93 One study81 on isoflavones used data and the assessment methodology of Boker et al.94 Mink et al77 and Mursu et al78 used the USDA 2003 flavonoid database95 and Mink et al77 also used the USDA 1999 isoflavone database96 and the USDA 2004 proanthocyanidin database.97
In addition to using Hertog’s flavonoid data, four Finnish studies69,70,72,74 used data from Hakkinen et al.98 Knekt et al73 (1996) employed flavonoid data from Starke et al99 and Wildanger et al,100 and Knekt et al (2002)74 used data by Mattila et al.101 The Finnish Turku study by Marniemi et al76 did not explicitly state the source of its flavonoid data.
For three US studies (NHS,75 HPFS,79 WHS80), certain American foods were analyzed by Hertog’s laboratory at the Netherlands State Institute for Quality Control of Agricultural Products for flavonoids and included in the food frequency composition tables maintained by the Harvard School of Public Health’s Department of Nutrition.102
Flavonoid classes
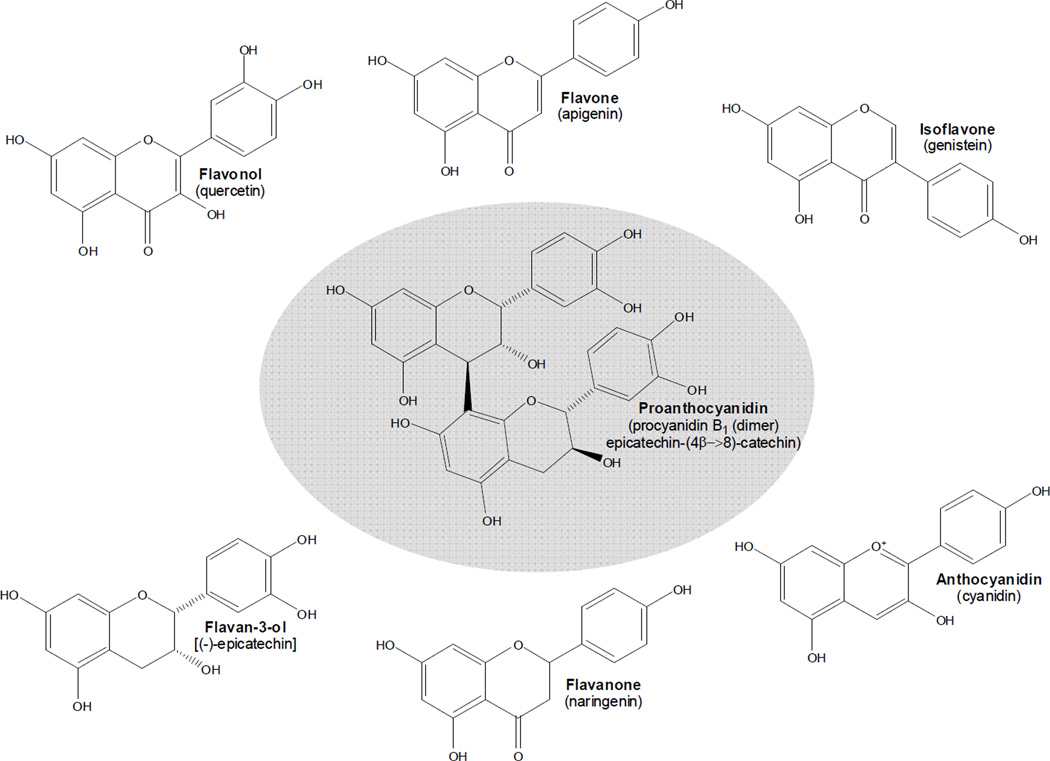
Figure 1 provides examples of common compounds in the monomeric and polymeric flavonoid classes in foods used in the flavonoid food composition tables of the USDA.97,103,104 The “monomeric” (single flavonoid structure) flavonoids studied listed here by class (and the compounds within each class) include: flavonols (isorhamnetin, kaempferol, myricetin, quercetin), flavones (apigenin, luteolin), flavanones (eriocitrin, hesperetin, naringenin), flavan-3-ols (catechin, epicatechin, epigallocatechin, epicatechin gallate, epigallocatechin gallate, gallocatechin), anthocyanidins (cyanidin, delphinidin, malvidin, pelargonidin, peonidin, petunidin), and isoflavones (daidzein, genistein). The “polymeric” (two or more connected flavan-3-ol structures) flavonoids are the proanthocyanidins (which include flavan-3-ol monomers [due to current analytic methods], dimers, trimers, 4–6mers, 7–10mers, and oligomers [usually up to twenty or twenty-five flavan-3-ol units]).
Figure 1.
Flavonoid classes commonly found in plant foods with representative examples of each. Except for flavan-3-ols and proanthocyanidins, most compounds in the other classes have sugars attached (flavonoid glycosides). Here they are all presented as aglycones (without sugars).
Total flavonoid intakes could not be considered because different classes were assessed from study to study. Only one study (Mink et al77) assessed all seven classes.
Classification of mortality outcomes
International Classification of Disease (ICD) codes shown in Table 2 were those for the time at which each study was done (8th and 9th revisions,105,106 with the 10th revision107 used only by Geleijnse et al65 and Mursu et al78); Rimm et al,79 and Sesso et al80 used World Health Organization classifications108,109 but did not explicitly state the ICD code revision used.
Table 2.
International Classification of Disease Codes (ICD) for Cardiovascular Disease
| Cardiovascular Disease Outcomes | ICD 8 | ICD 9 | ICD 10 |
|---|---|---|---|
| Mortality | |||
| Coronary Heart Disease Deaths* | 410–414 | 410–414, 429.2 | I20–I25 |
| Stroke Deaths | 430–438 | 430–438 | I60–I69 |
| Cardiovascular Disease Deaths | 410–438 | 390–459 | I20–I99 |
| Incidence | |||
| Coronary Heart Disease Incidence* | 410–414 | 410–414, 427.5 | I20–I25 |
| Stroke Incidence** | 430–438 | 430–438 | I60–I69 |
| Cardiovascular disease Incidence | 410–438 | 390–459 | I20–I99 |
Ischemic heart disease
Hemorrhagic stroke ICD 8-430-431, ICD 9-430-432, ICD 10-I60-I62
Ischemic stroke ICD 8-433-434, ICD 9-433-434, ICD 10-I63
RESULTS
We present a synopsis of the studies of the various cardiovascular disease outcomes based on the flavonoid classes most frequently assessed. Although there is some evidence that total flavonoid intakes may be relevant to some cardiovascular disease endpoints in experimental animals,15 the differences in which flavonoid classes were examined made calculation of total flavonoid intakes that were comparable from study to study impossible.
The most commonly measured flavonoids were the flavonol and flavone classes combined or flavonols alone. All studies, with the single exception of the Netherlands EPIC cohort,81 assessed the flavonols63–80,82 (two studies63,64 included flavonol intake in their articles but not in their analyses because they were examining flavan-3-ols and cardiovascular disease endpoints only). All except three cohorts (Netherlands EPIC81 and Rotterdam,65 Caerphilly, Wales68) estimated intakes of the flavones, either as an individual class or compounds74,76–78,80 or combined with the flavonols66,67,69–71,73,75,76,79,80,82 (three studies did not use flavones63,64,72 in their analyses). Only three cohorts (Iowa,64,77 Kuopio,78 and Zutphen63) assessed intakes of the flavan-3-ols. Similarly, three cohorts (Finnish Mobile,74 Iowa,77 Kuopio78) estimated flavanone intakes. Two cohorts (Netherlands EPIC81 and Iowa77) estimated isoflavone intakes, two cohorts (Iowa77 and Kuopio78) anthocyanidin intakes, and only one cohort (Iowa77) estimated intakes of the proanthocyanidins. In evaluating studies, results were declared significant if the multivariate adjusted test for trend was p<.05.
Tables 3 and 4 summarize the published studies on the relationship between flavonoid intakes and cardiovascular disease incidence or mortality in European and US prospective cohorts. The summary begins with the studies of flavonoid intakes and cardiovascular disease mortality because more publications had their primary analysis on flavonoid intakes and mortality than on incidence.
Table 3.
Associations between flavonoid intakes, incidence of coronary heart disease, stroke, and total cardiovascular disease mortality and incidence by cohort and country (multivariate adjusted ptrend <.05 in bold face)*
| Cohort | Study | Flavonoid classes studied |
Population | Follow up yrs |
Covariate measures |
CHD mortality (deaths) |
Stroke mortality (deaths) |
CVD mortality (deaths) |
CHD incidence (events) |
Stroke incidence (events) |
CVD incidence (events) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Finland | |||||||||||
| Finnish Mobile Clinic Health Examination Survey | Knekt 199673 | Flavones & flavonols | 2,748 M & 2,385 F | 26 | age, BMI, BP, chol, smoking BC, energy, FA, fiber, vit C, vit E |
Flavones & flavonols 0.67 RR (0.44, 1.00) CI ptrend 0.12 [4.8 vs 2.1 mg/d]b (n 324 men) Flavones & flavonols 0.73 RR (0.41, 1.32) CI ptrend 0.21 [5.5 vs 2.4 mg/d]b (n 149 women) |
|||||
| Knekt 200072 | Flavonol (Quercetin) | 9,208 M & F | 28 | age, BMI, BP, chol, diabetes, region, SE, smoking BC, energy, FA, fiber, quercetin, vit C, vit E, |
Flavonols 0.99 RR (0.71, 1.38) CI ptrend 0.80 [4.6 vs 2.0 mg/d]b (n 445 men) Flavonols 0.85 RR (0.60, 1.21) CI ptrend 0.62 [5.2 vs 2.3 mg/d]b (n 378 women) |
||||||
| Knekt 200274 | Flavanones, flavones, flavonols | 9,131 M & F | 28 | age, BMI, BP, chol, diabetes, region, SE, sex, smoking |
Flavonol (Quercetin) 0.79 RR (0.63, 0.99) CI ptrend 0.02 [M 3.9 vs 1.5, F 4.7 vs 1.8 mg/d]b (n 681) (Kaempferol) 0.82 RR (0.66, 1.02) CI ptrend 0.06 [M 0.8 vs 0.1, F 0.9 vs 0.2 mg/d]b (n 681) |
Flavonol (Kaempferol) 0.70 RR (0.56, 0.86) CI ptrend 0.003 [M 0.8 vs 0.1, F 0.9 vs 0.2 mg/d]b (n 806) Flavanones (Hesperetin) 0.80 RR (0.64, 0.99) CI ptrend 0.008 [M 15.4 vs 0, F 26.8 vs 3.2 mg/d]b (n 806) (Naringenin) 0.79 RR (0.64, 0.98) CI ptrend 0.006 [M 4.7 vs 0, F 7.7 vs 0.9 mg/d]b (n 806) Flavanones & flavones & flavonols 0.79 RR (0.64, 0.98) CI ptrend 0.006 [M 26.9 vs 4.3, F 39.5 vs 8.5 mg/d]b (n 806) |
|||||
|
alpha-tocopherol, beta-carotene Cancer Prevention Study (ATBC) |
Hirvonen 200070 | Flavones & flavonols | 26,497 M | 6.1 | age, BMI, BP, chol, diabetes, hist CHD, SE, smoking alcohol, suppl |
Flavones & flavonols 0.98 RR (0.80, 1.21) CI ptrend 0.81 [16.4 vs 4.2 mg/d (medians)]b (n 736) |
|||||
| Hirvonen 200169 | Flavones & flavonols | 25,372 M | 6.1 | age, BMI, BP, chol, diabetes, hist CHD, MS, PA, SE, smoking suppl, |
Flavones & flavonols 0.89 RR (0.71, 1.11) CI trend not given [17.8 vs 3.94 mg/d (medians)]c (n 815) |
Flavones & flavonols 0.77 RR (0.64, 0.93) CI trend not given [17.8 vs 3.94 mg/d (medians)]c (n 1,122, nonfatal) |
|||||
| Turku and Environs Health Survey | Marniemi 200576 | Flavones & flavonols | 361 M & 394 F elderly | 10 | age, FC, sex, smoking energy |
Flavone (Luteolin) 0.53 RR (0.33, 0.86) CI p = 0.0096a (n 130, fatal & nonfatal) |
Flavones & flavonols 0.65 RR (0.34, 1.23) CIa p not given (n 70) |
||||
| Kuopio Ischemic Heart Disease Risk Factor study | Mursu 200878 |
5 classes (flavonols, flavones, flavanones, flavan-3-ols, anthocyanidins) |
1,950 M | 15.2 | age, BMI, BP, BP meds, chol, diabetes, exam, fam CHD, O2, smoking, TAG alcohol, FA, folate, vit E |
5 classes 1.25 RR (0.74, 2.11) CI ptrend 0.73 [435 vs 9.5 mg/d]b (n 153) Flavanones 0.54 RR (0.32, 0.92) CI ptrend 0.27 [3.1 mg/d]b (n 153) |
Flavonols 0.55 RR (0.31, 0.99) CI ptrend 0.027 [10.0 mg/d]b (n 102) 5 classes 0.71 RR (0.37, 1.37) CI ptrend 0.137 [435 vs 9.5 mg/d]b (n 102) |
||||
| Netherlands | |||||||||||
| Zutphen Elderly Study | Hertog 199366 | Flavones & flavonols | 805 M | 5 | BMI, BP, chol, PA, smoking energy, FA |
Flavones & flavonols 0.32 RR (0.15, 0.71) CI ptrend 0.003 [29.9 vs 19.0 mg/d]a (n 43) |
Flavones & flavonols 0.52 RR (0.22,1.23) CI ptrend 0.15 [29.9 vs 19.0 mg/d]a (n 38, fatal & nonfatal) |
||||
| Keli 199671 | Flavones & flavonols | 552 M | 15 | age, BP, chol, smoking alcohol, energy, fish |
Flavones & flavonols 0.27 RR (0.11, 0.70) CI ptrend 0.004 [28.6 vs 18.3 mg/d]a (n 42) |
||||||
| Hertog 199767 | Flavones & flavonols | 804 (mortality) 692 (incidence) | 10 | BMI, BP, chol, PA, smoking energy, FA, |
Flavones & flavonols 0.47 RR (0.27, 0.82) CI ptrend 0.006 [29.9 vs 19.0 mg/d]a (n 90) |
Flavones & flavonols 0.62 RR (0.24,1.05) CI ptrend 0.078 [29.9 vs 19.0 mg/d]a (n 92, fatal & nonfatal) |
|||||
| Arts 200163 | Flavan-3-ols | 806 M | 15 | age, BMI, PA, smoking alcohol, BC, coffee, diet, energy, FA, fiber, fish, vit C, vit E |
Flavan-3-ols 0.49 RR (0.27, 0.88) CI ptrend 0.017 [85.9 vs 49.0 mg/d]a (n 90) |
Flavan-3-ols 0.81 RR (0.36, 1.83) CI ptrend 0.61 [85.9 vs 49.0 mg/d]a (n 47) |
Flavan-3-ols 0.70 RR (0.39, 1.26) CI ptrend 0.23 [85.9 vs 49.0 mg/d]a (n 90, fatal & nonfatal) |
Flavan-3-ols 0.921 RR (0.51, 1.68) CI ptrend 0.606 [85.9 vs 49.0 mg/d]a (n 88) |
|||
| Rotterdam Study | Geleijnse 200265 | Flavonols | 1836 M & 2971 F | 5.6 | age, BMI, SE, sex, smoking alcohol, coffee, energy, FA, fiber, vit E |
Flavonols 0.35 RR (0.13, 0.98) CI trend not given [32.9 vs 22.8 mg/d]a (n 30) |
Flavonols 0.93 RR (0.57, 1.52) CI nsd [32.9 vs 22.8 mg/d]a (n 116, nonfatal) Flavonols 0.76 RR (0.49, 1.18) CI nsd [32.9 vs 22.8 mg/d]a (n 146, fatal & nonfatal) |
||||
| Dutch Prospect-EPIC (European Prospective Study Into Cancer and Nutrition) Cohort | Van der Schouw 200581 | Isoflavones | 16,165 F | 6.25 median | age, BMI, BP, chol, diabetes, HRT, OC, PA, smoking alcohol, energy, F&V, FA, fiber, protein, |
Isoflavones 0.94 HR (0.68, 1.30) CI nsd [0.54 vs 0.26 mg/d]b,d (n 372, fatal & nonfatal) |
Isoflavones 1.05 HR (0.64, 1.70) CI nsd [0.54 vs 0.26 mg/d]b,d (n 147) |
Isoflavones 0.97 HR (0.74, 1.27) CI nsd [0.54 vs 0.26 mg/d]b,d (n 519) |
|||
| Wales, UK | |||||||||||
| Caerphilly Study | Hertog 199768 | Flavonols | 1,900 M | 10 incidence 14 mortality |
age, BMI, BP, chol, hist CHD, SE, smoking alcohol, BC, energy, FA, vit C, vit E, |
Flavonols 1.6 RR (0.9, 2.9) CI ptrend 0.119 [34 vs 19 mg/d]b (n 131) |
Flavonols 1.1 RR (0.6, 1.6) CI ptrend 1.00 [34 vs 19 mg/d]b (n 186, fatal & nonfatal) |
||||
| US | |||||||||||
|
Health Professionals Follow-up Study (HPFS) |
Rimm 199679 | Flavones & flavonols | 34,789 M | 2 or 6 | age, BMI, BP, chol, diabetes, fam CHD, SE, smoking alcohol, vit E, |
Flavones & flavonols 0.77 RR (0.45, 1.35) CI nsd [40.0 vs 7.1 mg/d (medians)]c (n 140, 2 yr FU, N 38,036) Flavones & flavonols 0.63 RR (0.33, 1.20) CI nsd [40.0 vs 7.1 mg/d (medians)]c (n 105, 2 yr FU, N 4838 CHD prevalent) |
Flavones & flavonols 1.08 RR (0.81, 1.43) CI nsd [40.0 vs 7.1 mg/d (medians)]c (n 486 6 yr FU nonfatal) Flavones & flavonols 0.94 RR (0.68 – 1.31) CI nsd [40.0 vs 7.1 mg/d (medians)]c (n 373, 2 yr FU, fatal & nonfatal) |
||||
| Iowa Women's Health Study | Yochum 199982 | Flavones & flavonols | 34,492 F post | 10 | BP, chol, diabetes, HRT, MS, PA, SE, smoking, WH alcohol, energy, FA, fiber, grains, vit E, |
Flavones & flavonols 0.62 RR (0.44, 0.87) CI ptrend 0.11 [32.3 vs 4.3 mg/d]c (n 438) |
Flavones & flavonols 1.18 RR (0.70, 2.00) CI ptrend 0.83 [18.7 vs 5.7 mg/d]c (n 131) |
||||
| Arts 200164 | Flavan-3-ols | 32,857 F post | 13 | age, BMI, BP, diabetes, HRT, MS, PA, SE, smoking, WH alcohol, BC, energy, FA, folate, grains, vit C, vit E, vit suppl, |
Flavan-3-ols 0.85 RR (0.67, 1.07) CI nsd [36.8 vs 6.3 mg/d]c (n 767) |
||||||
| Mink 200777 |
7 classes (flavonols, flavones, flavanones, flavan-3-ols, anthocyanidins, isoflavones, proanthocyanidins) |
34,492 F post | 16 | age, BMI, BP, diabetes, HRT, MS, PA, SE, smoking, WH energy |
Flavanones 0.78 RR (0.65, 0.94) CI ptrend 0.010 [72.8 vs 16.1 mg/d]c (n 1329) Anthocyanidins 0.88 RR (0.78, 0.99) CI ptrend 0.031 [0.01 vs 0 mg/d] (n 1329) |
7 classes 0.94 RR (0.69, 1.29) CI ptrend 0.80 [425.3 vs 133.2 mg/d] c (n 469) |
Flavanones 0.88 RR (0.77, 1.01) CI ptrend 0.054 [72.8 vs 16.1 mg/d]c (n 2316) Anthocyanidins 0.91 RR (0.83, 0.99) CI ptrend 0.032 [0.1 vs 0 mg/d]c (n 2316) |
||||
|
Women's Health Study (WHS) |
Sesso 200380 | Flavones & flavonols | 38,445 F | 6.9 | age, aspirin, BMI, BP, chol, diabetes, fam CHD, HRT, PA, smoking alcohol, BC, F&V, FA, fiber, folate, vit E |
Flavones & flavonols 1.05 RR (0.62, 1.78) CI nsd [47.4 vs 8.9 mg/d (medians)]c (n not given) |
Flavones & flavonols 0.82 RR (0.51, 1.30) CI nsd [47.4 vs 8.9 mg/d (medians)]c (n not given, fatal & nonfatal) |
Flavones & flavonols 0.70 RR (0.46, 1.07) CI nsd [47.4 vs 8.9 mg/d (medians)]c (n not given) |
Flavones & flavonols 0.80 RR (0.59, 1.09) CI ptrend 0.80 [47.4 vs 8.88 mg/d (medians)]c (n 519 events) |
||
|
Nurses' Health Study (NHS) |
Lin 200775 | Flavones & flavonols | 66,360 F | 12 | age, aspirin, BMI, BP, chol, diabetes, fam CHD, HRT, meno, PA, smoking alcohol, energy, vit E, vit suppl |
Flavonol (Kaempferol) 0.66 RR (0.48, 0.93) CI ptrend 0.04 [4.7 mg/d mean]c (n 324) |
Flavones & flavonols 1.05 RR (0.85, 1.29) CI ptrend 0.55 [30.9 vs 9.6 mg/d]c (n 938, nonfatal) |
Blank cells – outcome not studied
Bold font indicates the class(es) significant for inverse association with the given event at multivariate adjusted ptrend<.05 or less.
nsd – association not significant at ptrend<.05 for compounds or classes studied.
F – females, HR – Hazard ratios, M – males, Post – postmenopausal, RR – Risk ratios
General covariate measures: age; aspirin – aspirin use; BMI – body mass index; BP – blood pressure (diastolic, hypertension, systolic); BP meds – blood pressure medication; chol – cholesterol (HDL, high, LDL, total cholesterol); diabetes; exam - examination years; fam CHD – history of family CHD; FC - functional capacity; hist CHD - history of CHD; HRT – hormone replacement therapy; meno - menopausal; MS - marital status; O2 - oxygen uptake; OC - oral contraceptive; PA - physical activity; region - geographic region; SE – socioeconomic demographic (education, occupation, profession, social class); sex; smoking; TAG - triglycerides; WH - waist to hip ratio
Intake covariate measures: alcohol; BC – beta-carotene; coffee; diet - prescribed diet; energy - total energy; F&V - fruit & vegetable; FA – fat (cholesterol, fat, monounsaturated fatty acids, polyunsaturated fatty acids, saturated fatty acids); fiber; fish; folate; grains - whole grains; protein - animal protein; quercetin; suppl - supplementation group; vit C - vitamin C; vit E - vitamin E; vit suppl - multivitamin supplement
tertiles
quartiles
quintiles
Intake from FFQ but food items scored for isoflavones, analytical values were not used.
Table 4.
Summary of significant (multivariate adjusted ptrend<.05) associations between flavonoid intakes and various cardiovascular outcomes in prospective cohorts in US and European countries
Number of cohorts with significant associations/number of cohorts examined
| Mortality | Incidence | Number cohorts |
All publications |
|||||
|---|---|---|---|---|---|---|---|---|
| Flavonoid Class | CHD | Stroke | CVD | CHD | Stroke | CVD | ||
| Flavonols & flavones | 1a/6e | 0/1 | 0/1 | 0/6e | 1/4 | 0/1 | 1/8 | 3/11 |
| Flavonols | 2/5d | 0/1 | 0/2 | 0/5 | 2/3c | 0/1 | 3/9 | 3/10 |
| Flavones | 0/1a | 0/1 | 0/2 | 1/1 | 0/2 | 0/1 | 1/4 | 1/5 |
| Flavanones | 1/2 | 0/1 | 1/2 | 1/2 | 2/3 | 2/3 | ||
| Flavan-3-ols | 1/2b | 0/2 | 0/2 | 0/1 | 0/2 | 1/3 | 1/4 | |
| Anthocyanidins | 1/1 | 0/1 | 1/2 | 0/1 | 1/2 | 1/2 | ||
| Isoflavones | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | 0/2 | 0/2 |
| Proanthocyanidins | 0/1 | 0/1 | 0/1 | 0/1 | 0/1 | |||
| Number of cohorts studied | 4/8 | 0/2 | 1/3 | 1/9 | 3/7 | 0/2 | 6/12 | |
| All publications | 6/13 | 0/3 | 1/3 | 1/11 | 3/9 | 0/2 | 8/20 | |
two publications
three publications
four publications
six publications
seven publications
Mortality
Coronary Heart Disease Mortality
Coronary heart disease mortality was the most extensively studied outcome (with a total of thirteen publications from eight prospective cohorts)63,65–69,73–75,77,79,82 in four countries (Finland, the Netherlands, United States and Wales). Only one of these studies (Iowa77) assessed intakes of all seven flavonoid classes. Most of the other studies assessed intakes of the flavonols and flavones combined (six cohorts). Five cohorts assessed associations with flavonol intakes, one with flavones, and two each with flavanones and flavan-3-ols.
Flavonols and Flavones
All studies of fatal coronary heart disease examined flavonol intakes (either the flavonols separately65,68,74,75,77,82 or the flavone and flavonol classes combined66,67,69,73,75,79,82). Four66,67,73,82 out of the seven66,67,69,73,75,79,82 studies that examined the relationship of flavones and flavonols combined reported lower risk of fatal coronary heart disease with higher flavonoid consumption.
Specifically, flavonols and flavones were significantly associated with reduced coronary heart disease mortality in the Zutphen Elderly Study cohort at both five and ten years of follow-up (Hertog et al66,67). Although confidence intervals (CI) were less than 1.0 for point estimates, there was not a statistically significant trend for an inverse association over the intake range investigated in the small Finnish Mobile cohort (Knekt et al73) and the large Iowa cohort of postmenopausal women (Yochum et al82). These compounds were not associated with fatal coronary heart disease in the ATBC cohort in Finland (Hirvonen et al69), the female NHS (Lin et al75) or the male HPFS cohort (Rimm et al79).
Five studies65,68,74,75,77 examined the flavonol class or its individual compounds separately. In the Dutch Rotterdam study65 there was an inverse association between flavonol intake and risk of fatal coronary heart disease after almost six years of follow up. In the Finnish Mobile Clinic study by Knekt et al,74 after twenty-eight years of followup, the flavonols quercetin (significantly) and kaempferol (borderline) were associated with lower coronary heart disease mortality but the flavonol myricetin was not. Lin et al,75 who examined intakes of the individual flavonol compounds separately in the NHS, found that only the flavonol kaempferol was significantly associated with lower coronary heart disease mortality in US nurses after twelve years of followup. However, three other studies (Caerphilly,68 Iowa77,82) reported no association with flavonol intake.
A 2003 meta-analysis110 which included six67–69,73,79,82 of the studies reviewed here, found a statistically significant 20% lower association between flavonol intakes in the highest compared to lowest tertile (RR [risk ratio] 0.80, 95% CI 0.69, 0.93 after adjustment for known coronary heart disease risk factors and other dietary components) and coronary heart disease mortality, and subsequent studies appear to be in the same direction.65,74,75,77
Other Flavonoid Classes (Flavan-3-ols, Flavanones, Isoflavones, Anthocyanidins and Proanthocyanidins)
Far fewer studies examined the other classes (flavanones,74,77 flavan-3-ols,63,64,77 anthocyanidins,77 isoflavones,77 and proanthocyanidins77), probably because comprehensive food composition data for these five classes were lacking at the time the earlier studies were done.
Arts et al63 studied intakes of the flavan-3-ols in the Dutch Zutphen cohort and found that higher intakes (catechins and epicatechins) were significantly associated with a lower risk of coronary heart disease mortality after ten years of followup. However, these same investigators64 found no significant associations between flavan-3-ol intakes and deaths from coronary heart disease among Iowa women after thirteen years of followup, nor were flavan-3-ols associated with coronary heart disease mortality in a subsequent analysis in this same cohort (Mink et al77).
Knekt et al74 studied the associations between flavanones individually and combined with the flavonol and flavone classes and found no associations with coronary heart disease mortality in the Finish Mobile clinic cohort. In the Iowa postmenopausal cohort with fifteen years of follow up, Mink et al77 studied all seven classes of flavonoids and found significant inverse associations between intakes of anthocyanidins and flavanones and coronary heart disease mortality. However, the associations between flavonols, flavones, isoflavones, or proanthocyanidins and this outcome were not statistically significant in this study.77
In summary, six63,66,67,74,75,77 out of the thirteen studies63–69,73–75,77,79,82 (four out of eight cohorts) found significant inverse associations between at least one of the flavonoid classes and coronary heart disease mortality, and three additional studies65,73,82 found nonsignificant trends toward lower risk. Only the Caerphilly Study (Hertog et al68) suggested a higher risk of fatal coronary heart disease with greater flavonol intake; however, the confidence interval included 1.0 and the trend was not statistically significant.
Stroke mortality
Only three studies from two cohorts (Iowa and Zutphen) examined the relationship between flavonoid intakes and fatal stroke.
Flavonols and Flavones
In the Iowa cohort, neither the flavonols and flavones combined at ten years of followup (Yochum et al82) nor the flavonol and flavone classes examined separately at sixteen years followup were associated with stroke mortality (Mink et al77).
Other Flavonoid Classes (Flavan-3-ols, Flavanones, Isoflavones, Anthocyanidins and Proanthocyanidins)
The flavan-3-ols were not associated with stroke mortality in the Zutphen cohort (Arts et al63) at ten years followup. Intakes of flavan-3-ols, flavanones, anthocyanidins, isoflavones, proanthocyanidins, and total flavonoid intake were not associated with stroke mortality after sixteen years of followup in the Iowa cohort of postmenopausal women (Mink et al77).
In summary, no associations were observed between flavonoid intakes and fatal stroke in three different studies63,77,82 of two cohorts.
Total Cardiovascular Disease Mortality
Three studies in three separate cohorts (Iowa,77 Kuopio,78 WHS80) assessed flavonoid intakes and total cardiovascular disease mortality (fatal coronary heart disease and stroke combined).
Flavonols and Flavones
Sesso et al80 found no associations between intakes of flavonol plus flavone classes combined or their individual compounds and cardiovascular disease death after nearly seven years of follow up in a study of American postmenopausal women (WHS). Neither Mink et al77 in the Iowa cohort at sixteen years of followup nor Mursu et al78 in the Kuopio cohort at fifteen years of follow up found associations between the flavone or the flavonol classes examined separately and cardiovascular disease mortality.
Other Flavonoid Classes (Flavan-3-ols, Flavanones, Isoflavones, Anthocyanidins and Proanthocyanidins)
Mink et al77 also estimated intakes of the flavan-3-ols, flavanones, anthocyanidins, isoflavones, and proanthocyanidins in the Iowa cohort after sixteen years of follow up and associations with cardiovascular disease mortality. Flavanones were inversely associated with a borderline significantly lower risk and anthocyanidins with a significantly lower risk of cardiovascular disease mortality in this cohort.77 However, in the Kuopio cohort, Mursu et al78 found no associations between anthocyanidins, flavanones, and flavan-3-ols and cardiovascular disease mortality after more than fifteen years of followup.
In summary, in the three prospective cohorts in which flavone and flavonol intakes and total cardiovascular mortality were examined (Iowa,77 Kuopio,78 WHS80), there was no observed relationship between these intakes and death from cardiovascular disease. However, the flavanones and anthocyanidins were significantly associated with lower risk of cardiovascular disease mortality in a recent Iowa cohort analysis.77
Incidence
Coronary Heart Disease Incidence
The relationship between flavonoid intakes and coronary heart disease incidence (either as nonfatal or nonfatal and fatal disease combined) was investigated in eleven studies.63,65–69,75,76,79–81
Nonfatal coronary heart disease incidence
Flavonols and Flavones
Hirvonen et al69 (2001) assessed intakes of the flavonol plus flavone classes combined among male Finnish smokers in the ATBC cohort. Higher intakes were nonsignificantly associated with lower incidence of coronary heart disease. In contrast, there were no observed associations between intakes of either the flavonols individually (kaempferol, myricetin, quercetin) or the flavonol and flavone classes combined and coronary heart disease incidence in the HPFS cohort (Rimm79) of US men after six years of follow up. Similar findings were observed in US women; Lin et al75 found no association between intakes of the flavonols individually (kaempferol, myricetin, quercetin) or the flavones and flavonols combined and coronary heart disease incidence after twelve years of followup in the NHS cohort. In the Dutch Rotterdam study, Geleijnse et al65 found no significant associations between flavonols and nonfatal coronary heart disease among Rotterdam men and women after almost six years of follow up.
Other Flavonoid Classes (None)
No prospective cohort studies were available on nonfatal coronary heart disease and the other five classes of flavonoids.
In summary, three of the four of the cohorts65,69,75,79 that examined flavonoid intakes and nonfatal coronary heart disease were null for flavonols and flavones. Only one study69 reported a protective association with the flavonol plus flavones classes combined.
Nonfatal and fatal coronary heart disease incidence
Nine studies in seven cohorts63,65–68,76,79–81 examined the relationship between flavonoid intake and fatal and nonfatal coronary heart disease combined.
Flavonols and Flavones
Among elderly Dutch men in Zutphen, flavonols and flavones combined were inversely but not significantly associated with nonfatal and fatal coronary heart disease at five years66 and ten years67 of followup. Intakes of flavonols plus flavones combined were also not associated with incident coronary heart disease in the HPFS cohort (Rimm et al79). In the Caerphilly study neither quercetin (a flavonol) nor total flavonol intakes were associated with incident coronary heart disease after ten years of followup.68 In the Dutch Rotterdam study65 there were no significant associations between flavonol intakes and nonfatal and fatal coronary heart disease after almost six years of follow up.
Sesso et al80 reported no associations between intakes of flavonol plus flavone classes combined and incident coronary heart disease in the US WHS. Similarly, no significant associations for individual flavonol and flavone compounds or flavonols plus flavones combined were found among men and women in the Finnish Turku study76 after ten years of followup except for the flavone luteolin which was significantly associated with lower coronary heart disease incidence.
Other Flavonoid Classes (Flavan-3-ols, Isoflavones)
Arts et al63 found no associations between flavan-3-ol intakes and fatal and nonfatal coronary heart disease in the Zutphen cohort. In the Dutch EPIC cohort, van der Schouw et al81 found no associations between estimated median intakes of ~0.4 mg isoflavones per day and incident coronary heart disease in women. It should be noted that these isoflavone intakes were less than a fiftieth of those reported in recent Asian studies (~29 mg/day).111
In summary, only one cohort76 found protective associations between flavonoid intake (the flavone luteolin) and lower risk of fatal and non-fatal coronary heart disease.
Stroke incidence
Nine studies from seven cohorts63,70–72,74,76,78,80,81 assessed the relationship between flavonoid intakes and both fatal and nonfatal stroke.
Total stoke incidence
Flavonols and Flavones
In the Zutphen study after fifteen years of followup, Keli et al71 found significant inverse associations between flavonol plus flavone intakes combined and stroke incidence for elderly Dutch men as did Mursu et al78 for flavonols in the Kuopio cohort. In contrast, in the ATBC cohort of male Finnish smokers, Hirvonen et al70 found no significant association between flavonol plus flavone intakes and stroke incidence. Likewise, Sesso et al80 found no associations between flavonol and flavone intakes their combined and stroke incidence among American postmenopausal women from the WHS cohort with nearly seven years of follow up, and Marniemi et al76 found no association between flavonols and flavones either as individual compounds or combined intake and stroke in the Turku cohort of Finnish men and women after ten years of follow up. Similarly, in the Finnish Mobile Clinic study of men and women, Knekt et al (2000,72 200274) found no association between the intake of the flavonol quercetin72,74 or myricetin74 and stroke incidence in adults after twenty-eight years of followup. However, they did observe an association between intake of the flavonol kaempferol as well as total intakes of flavonols, flavones and flavanones combined and incident stroke.74 A meta-analysis by Hollman et al112 of six cohorts included in this review,70,71,74,77,78,80 with a total of 111,067 participants, 2,155 cases, and six to twenty-eight years of followup, found borderline significant inverse associations between greater flavonol intake and incident stroke (RR 0.80, 95% CI 0.65, 0.98), for the top vs bottom intake quartile.
Other Flavonoid Classes (Flavan-3-ols, Flavanones, Isoflavones, Anthocyanidins)
In studies of fatal and nonfatal stroke incidence, Arts et al63 observed that flavan-3-ol intake was not associated with stroke incidence in the Zutphen cohort with ten years of follow-up. The EPIC study of Dutch women81 found no association between isoflavone intakes (estimated median intakes ~0.4 mg/day) and incident stroke. In the Finnish Mobile Clinic cohort74 in addition to the flavonol kaempferol, as noted above, the flavanone hesperetin and the flavanone naringenin as well as total intakes of flavonols, flavones and flavanones combined were inversely and significantly associated with stroke incidence. Mursu et al78 found no association with intakes of anthocyanidins, flavanones, flavan-3-ols, or total intake of all five classes (flavonols, flavones, flavanones, flavan-3-ols, anthocyanidins) and stroke incidence in the Finnish Kuopio cohort.
In summary, only three71,74,78 of nine studies (from seven prospective cohorts)63,70–72,74,76,78,80,81 reported lower risk of fatal and nonfatal stroke combined and these inverse associations were confined to the flavonols, the flavones, and the flavanones.
Hemorrhagic and Ischemic stroke incidence
Three studies from two cohorts examined hemorrhagic stroke.70,72,74 Ischemic stroke was considered in two studies of one cohort.72,74
Flavonols and Flavones
In the Finnish Mobile cohort, Knekt et al (200072 200274) reported a significant inverse association between kaempferol74 and ischemic (RR 0.63, 95% CI 0.47, 0.85 ptrend 0.004), but not hemorrhagic, stroke. No association was seen between intake of the flavonols quercetin72,74 and myricetin74 and incident hemorrhagic or ischemic stroke. Hirvonen et al’s study70 of male Finnish smokers from the ATBC cohort found no significant associations between flavonol and flavone intakes combined and hemorrhagic stroke incidence.
Other Flavonoid Classes (Flavanones)
In his study of the Finnish Mobile Clinic two years after his first report,72 Knekt et al74 found significant associations between the flavanone hesperetin (RR 0.74, 95% CI 0.55, 1.00; ptrend 0.01), the flavanone naringenin (RR 0.73, 95% CI 0.54, 0.98; ptrend 0.009), and the total of flavone, flavonol and flavanone intakes combined (RR 0.73, 95% CI 0.54, 0.98; ptrend 0.004) and ischemic stroke incidence but no associations with hemorrhagic stroke incidence.
In summary, only one74 of the three studies (in two cohorts)70,72,74 that examined flavonoid intakes in relation to hemorrhagic or ischemic stroke incidence found an significant association with intakes of the flavonol kaempferol, the flavanones hesperetin and naringenin and the flavanones, flavonols, and flavones combined and lower risk of ischemic stroke.74
Cardiovascular disease incidence
Only two cohorts (WHS80 and Dutch EPIC81) examined the relationship between flavonoids and cardiovascular disease incidence, and neither cohort found any associations.
Flavonols and Flavones
Sesso et al80 studied the associations between flavonols and flavones and cardiovascular disease incidence in American women from the WHS cohort. They found no associations between total intakes of flavones and flavonols or the individual compounds (quercetin, kaempferol, myricetin, apigenin, luteolin) and cardiovascular disease incidence.80
Other Flavonoid Classes (Isoflavones)
Van der Schouw81 estimated median intakes of isoflavones in Dutch women at (~0.4 mg/day) and found no association between isoflavone intakes and cardiovascular disease incidence.
In summary, the limited data on associations between flavonoids and cardiovascular disease incidence were null.
Overall Summary
Intakes of flavonoids, particularly of the flavonol and flavone classes, were associated with lower cardiovascular disease mortality and incidence in eight of the twenty prospective studies reviewed (six out of twelve cohorts), although results were not entirely consistent for any particular flavonoid class or compound (Table 4). The associations between flavonoid consumption and non-fatal cardiovascular disease were weaker, but there were fewer studies of these outcomes.
DISCUSSION
Our review of existing cohort studies of flavonoid intake and cardiovascular disease risk in the US and Europe provides some, albeit limited, evidence that certain flavonoids are related to lower risk of mortality from coronary heart disease. What was strikingly apparent in this review was the need for greater uniformity on a number of factors before definitive conclusions could be drawn from existing studies. Comparisons among the studies included in this review of European and American cohorts were complicated by variability of the study designs including flavonoid classes assessed, dietary assessment tools used, population characteristics (such as age, sex, and health status), cardiovascular endpoints chosen, and length of followup.
We did not consider total flavonoid intake in our summary because the term “total flavonoids” was used in the individual cohorts to represent the total of different and varying numbers of flavonoid classes. In addition to the questions of the practical utility of such a term, the theoretical basis for assessing the impact of “total” flavonoid intakes on cardiovascular disease is also uncertain. Although there are many putative biological mechanisms underlying a possible cardioprotective role for flavonoids,113,114 including antioxidant,16,115,116 vasodilatory,117–123 antithrombotic23,124–127 anti-inflammatory,19,21,22,48,128 and endothelial protective23,35,48,129,130 properties of some of the compounds,131 the effects of the flavonoids appear to vary from compound to compound within each class, rather than being inherent in all compounds in each of the several classes.1,50
Because the flavonoids are very diverse in their physiochemical properties (lipophilicity, polarity, etc.) as well as very different in their bioavailability and bioactivity (such as antioxidant capacity or binding at receptor sites), the rationale for assuming that exposures to all flavonoid classes might have effects on cardiovascular disease needs more consideration. At present, the evidence that flavonoid classes or compounds have impact on cardiovascular disease relies chiefly on in vitro12–14 and animal studies.15–24 Thus, the biological rationale for suggesting that flavonoid classes grouped by their structural features have common functional effects that lower cardiovascular disease incidence or mortality remains unclear. Differences between compounds within each class may be significant.
The associations between flavonoid intakes and coronary heart disease mortality were strongest for the flavonol class. Our results support the findings of a prior meta-analysis110 of flavonol intakes and coronary heart disease mortality that covered six67–69,73,79,82 of the eight prospective cohorts67–69,73–75,77,79,82 we reviewed. In the studies we reviewed, protective associations by the flavanones and anthocyanidins against coronary heart disease mortality were somewhat stronger in a recent study77 using more complete food flavonoid composition tables. In contrast to findings for coronary heart disease mortality, only one of the eleven studies63,65–69,75,76,79–81 of coronary heart disease incidence in these nine cohorts was statistically significant, and solely for the flavone luteolin.76
Unlike the positive findings for reduced risk of coronary heart disease mortality, three studies (from two cohorts)63,77,82 of stroke mortality were null. However, three71,74,78 of the nine studies of stroke incidence63,70–72,74,76,78,80,81 reported some associations between greater consumption of flavonols, flavones and flavanones and reduced risk.
Total cardiovascular disease (coronary heart disease and stroke combined) were examined in four studies from four cohorts,77,78,80,82 but only one study77 reported significant inverse associations for flavonoids, specifically for intakes of flavanones and anthocyanidins and cardiovascular mortality.
The inconsistencies across the epidemiologic studies that were evident in this review complicated interpretation. Differences in exposure assessment were many (due to varied dietary assessment instruments and incompleteness of flavonoid food composition databases used), making comparisons between studies difficult. Errors of exposure measurement were due in large part to the lack of analytic values for specific foods and flavonoid classes. The more recent studies assessed more flavonoid classes, taking advantage of the more recent flavonoid analytic techniques and flavonoid food data that has resulted in more comprehensive flavonoid databases.104,132,133
Additional errors in exposure assessment may have arisen from underestimates of flavonoid content due to the use of food frequency questionnaires which grouped several foods together into a single category (such as melons grouped with berries in early food intake studies). Although food frequency questionnaires may have limitations for assessing intakes of flavonoids, they are widely used in epidemiological studies of the type we reviewed here. In the future, the use of food frequency questionnaires that are constructed to target flavonoid food sources more precisely may provide better information on flavonoid intakes and cardiovascular outcomes.
Biomarkers of intakes were not used in these studies. Although there are several recent studies of flavonoid biomarkers134–139 and several studies validating isoflavone intakes140–153 very few validation studies154–157 of other flavonoids have been done that examined blood or urine samples for biomarkers.158,159 Thus, the errors in flavonoid intake assessment may have been considerable. Earlier investigations were probably too imprecise to uncover consistent statistically significant associations with cardiovascular endpoints if they were present.
Comparisons across studies were also challenging because of differences from study to study in age, sex, length of followup, health status and the cardiovascular endpoints studied. In most studies diet was assessed only once, so that consistency in diet over time could not be measured or accounted for in analyses. Eight of the studies included less than 200 cases63,65–68,71,76,78 limiting statistical power. If the associations between these compounds and risk do exist but are relatively small, larger studies and/or pooled analyses will be required to demonstrate these differences.
The European and US cohorts reviewed herein appeared to be quite similar in their flavonoid intakes. In contrast, flavonoid intakes of European and US cohorts differ strikingly from profiles reported for Asian populations in at least one respect; that the US and European populations ate lesser amounts of isoflavones (<1 mg/day) than Asians (~29 mg/day).111 Differences between European and US diets2–8 and Asian diets140,160 in other flavonoid classes are not well documented. Explorations of the associations between intakes of isoflavones or other flavonoids in Asian diets and cardiovascular disease outcomes in Asian populations in were beyond the scope of this paper, but they also deserve attention. High levels of specific flavonoids such as those observed in Asian cohorts111 or in studies of populations with greater intakes of these and other flavonoids (e.g. vegetarians) may be needed to replicate findings in Asian cohorts.161,162
The studies reviewed here were primarily of older men and women who may have had advanced cardiovascular disease already. If the effects of the flavonoids occur earlier in the disease process, younger populations might be more suitable. Additional research is warranted on flavonoid and cardiovascular disease prevention and survival, since several flavonoids, including the anthocyanins, flavones, flavan-3-ols and proanthocyanidins may have blood pressure lowering effects2,30,122,163,164 and may have beneficial effects on other cardiovascular disease risk factors as well.48,60,113,118,165 The studies reviewed here estimated flavonoid intakes from foods, which contain numerous flavonoids as well as other nutritive and non-nutritive compounds. Thus the potential for confounding by other nutrients and bioactive compounds exists. It is therefore not known, based on the existing literature, whether flavonoid supplements in larger doses are safe166–174 or would afford meaningful protection.175,176
In spite of the limitations of many of the existing studies, particularly the earlier studies, evidence is building that some flavonoid classes (flavonols, anthocyanidins, flavanones, and possibly flavan-3-ols) appear to be associated with lower coronary heart disease mortality in these European and US cohorts. Randomized controlled trials for the effects of flavonoid containing foods on cardiovascular disease outcomes would be difficult and perhaps impractical to conduct, although studies of their effects on surrogate markers may be possible. The flavanones and flavonols were also inversely related to stroke incidence in three out of seven cohorts. The overall findings from these cohorts suggest but do not prove that higher flavonoid consumption may be associated both with primary prevention of cardiovascular disease and, perhaps even more so, with a lower risk of cardiovascular disease mortality.
CONCLUSION
There is intriguing but not yet compelling evidence that relatively small amounts of certain of the dietary flavonoids may lower risk of coronary heart disease mortality in European and US countries. More research is needed to establish that cardioprotective relationships exist with these bioactive compounds and, if they prove to be protective, what consumption levels may be required to achieve health benefits. Future studies are needed that allow for more direct comparison of research findings using more complete and comprehensive flavonoid databases, more standardized and comprehensive dietary assessment methods, more information on the age, sex, health status and other characteristics of populations studied, more complete cardiovascular outcome measures, and longer lengths of follow-up that will allow for more direct comparison of research findings.
Acknowledgements
Funding and sponsorship: This work was supported in part with resources from the NIH’s National Heart, Lung and Blood Institute grant R21HL087217 (PJ, JD, JP) and the US Department of Agriculture Cooperative State Research, Education, and Extension Service grant #2006-35200-17259 and the US Department of Agriculture, Agricultural Research Service, under agreement No. 58-1950-7-707 (JP). Any opinions, findings, conclusions, or recommendations expressed here are those of the authors and do not necessarily reflect the view of the US Department of Agriculture.
Footnotes
Authorship: JP did the data collection, interpretation, analysis as well as the writing. JD participated fully in the writing of the paper, PJ and MM participated in the writing and critical review of the manuscript. No other individuals were involved in writing and producing the paper
Declaration of Interest: The authors state no competing interests or conflicts of interest.
References
- 1.Crozier A, Jaganath IB, Clifford MN. Dietary phenolics: chemistry, bioavailability and effects on health. Natural Products Reports. 2009;26:1001–1043. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- 2.Cassidy A, O’Reilly EJ, Kay C, et al. Habitual intake of flavonoid subclasses and incident hypertension in adults. American Journal of Clinical Nutrition. 2011;93:338–347. doi: 10.3945/ajcn.110.006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chun OK, Chung SJ, Song WO. Estimated dietary flavonoid intake and major food sources of U.S. Adults. Journal of Nutrition. 2007;137:1244–1252. doi: 10.1093/jn/137.5.1244. [DOI] [PubMed] [Google Scholar]
- 4.Fink BN, Steck SE, Wolff MS, Kabat GC, Gammon MD. Construction of a flavonoid database for assessing intake in a population-based sample of women on Long Island, New York. Nutrition & Cancer. 2006;56:57–66. doi: 10.1207/s15327914nc5601_8. [DOI] [PubMed] [Google Scholar]
- 5.Ovaskainen ML, Torronen R, Koponen JM, et al. Dietary intake and major food sources of polyphenols in Finnish adults. Journal of Nutrition. 2008;138:562–566. doi: 10.1093/jn/138.3.562. [DOI] [PubMed] [Google Scholar]
- 6.Perez-Jimenez J, Fezeu L, Touvier M, et al. Dietary intake of 337 polyphenols in French adults. American Journal of Clinical Nutrition. 2011;93:1220–1228. doi: 10.3945/ajcn.110.007096. [DOI] [PubMed] [Google Scholar]
- 7.Song WO, Chun OK. Tea is the major source of flavan-3-ol and flavonol in the U.S. diet. Journal of Nutrition. 2008;138:1543S–1547S. doi: 10.1093/jn/138.8.1543S. [DOI] [PubMed] [Google Scholar]
- 8.Zamora-Ros R, Andres-Lacueva C, Lamuela-Raventos RM, et al. Estimation of dietary sources and flavonoid intake in a Spanish adult population (EPIC-Spain) Journal of the American Dietetic Association. 2010;110:390–398. doi: 10.1016/j.jada.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 9.Tribolo S, Lodi F, Connor C, et al. Comparative effects of quercetin and its predominant human metabolites on adhesion molecule expression in activated human vascular endothelial cells. Atherosclerosis. 2008;197:50–56. doi: 10.1016/j.atherosclerosis.2007.07.040. [DOI] [PubMed] [Google Scholar]
- 10.Xia M, Ling W, Zhu H, et al. Anthocyanin attenuates CD40-mediated endothelial cell activation and apoptosis by inhibiting CD40-induced MAPK activation. Atherosclerosis. 2009;202:41–47. doi: 10.1016/j.atherosclerosis.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Xia M, Ling W, Zhu H, et al. Anthocyanin prevents CD40-activated proinflammatory signaling in endothelial cells by regulating cholesterol distribution. Arteriosclerosis, Thrombosis & Vascular Biology. 2007;27:519–524. doi: 10.1161/01.ATV.0000254672.04573.2d. [DOI] [PubMed] [Google Scholar]
- 12.Kawai Y, Nishikawa T, Shiba Y, et al. Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries: implication in the anti-atherosclerotic mechanism of dietary flavonoids. Journal of Biological Chemistry. 2008;283:9424–9434. doi: 10.1074/jbc.M706571200. [DOI] [PubMed] [Google Scholar]
- 13.Tu YC, Lian TW, Yen JH, Chen ZT, Wu MJ. Antiatherogenic effects of kaempferol and rhamnocitrin. Journal of Agricultural & Food Chemistry. 2007;55:9969–9976. doi: 10.1021/jf0717788. [DOI] [PubMed] [Google Scholar]
- 14.Yamagata K, Miyashita A, Matsufuji H, Chino M. Dietary flavonoid apigenin inhibits high glucose and tumor necrosis factor alpha-induced adhesion molecule expression in human endothelial cells. Journal of Nutritional Biochemistry. 2010;21:116–124. doi: 10.1016/j.jnutbio.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Arab L, Liebeskind DS. Tea, flavonoids and stroke in man and mouse. Archives of Biochemistry & Biophysics. 2010;501:31–36. doi: 10.1016/j.abb.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 16.Frei B, Higdon JV. Antioxidant activity of tea polyphenols in vivo: evidence from animal studies. Journal of Nutrition. 2003;133:3275S–3284S. doi: 10.1093/jn/133.10.3275S. [DOI] [PubMed] [Google Scholar]
- 17.Gandhi C, Upaganalawar A, Balaraman R. Protection against in vivo focal myocardial ischemia/reperfusion injury-induced arrhythmias and apoptosis by hesperidin. Free Radical Research. 2009;43:817–827. doi: 10.1080/10715760903071656. [DOI] [PubMed] [Google Scholar]
- 18.Karthikeyan K, Bai BR, Devaraj SN. Efficacy of grape seed proanthocyanidins on cardioprotection during isoproterenol-induced myocardial injury in rats. Journal of Cardiovascular Pharmacology. 2009;53:109–115. doi: 10.1097/FJC.0b013e3181970c01. [DOI] [PubMed] [Google Scholar]
- 19.Loke WM, Proudfoot JM, Hodgson JM, et al. Specific dietary polyphenols attenuate atherosclerosis in apolipoprotein E-knockout mice by alleviating inflammation and endothelial dysfunction. Arteriosclerosis, Thrombosis & Vascular Biology. 2010;30:749–757. doi: 10.1161/ATVBAHA.109.199687. [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki K, Makino K, Iwadate E, Deguchi Y, Ishikawa F. Anthocyanins from purple sweet potato Ipomoea batatas cultivar Ayamurasaki suppress the development of atherosclerotic lesions and both enhancements of oxidative stress and soluble vascular cell adhesion molecule-1 in apolipoprotein E-deficient mice. Journal of Agricultural & Food Chemistry. 2008;56:11485–11492. doi: 10.1021/jf801876n. [DOI] [PubMed] [Google Scholar]
- 21.Ramesh E, Geraldine P, Thomas PA. Regulatory effect of epigallocatechin gallate on the expression of C-reactive protein and other inflammatory markers in an experimental model of atherosclerosis. Chemico-Biological Interactions. 2010;183:125–132. doi: 10.1016/j.cbi.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 22.Register TC. Primate models in women's health: inflammation and atherogenesis in female cynomolgus macaques (Macaca fascicularis) American Journal of Primatology. 2009;71:766–775. doi: 10.1002/ajp.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheng R, Gu ZL, Xie ML, Zhou WX, Guo CY. EGCG inhibits proliferation of cardiac fibroblasts in rats with cardiac hypertrophy. Planta Medica. 2009;75:113–120. doi: 10.1055/s-0028-1088387. [DOI] [PubMed] [Google Scholar]
- 24.Tiwari R, Mohan M, Kasture S, Maxia A, Ballero M. Cardioprotective potential of myricetin in isoproterenol-induced myocardial infarction in Wistar rats. Phytotherapy Research. 2009;23:1361–1366. doi: 10.1002/ptr.2688. [DOI] [PubMed] [Google Scholar]
- 25.Atteritano M, Marini H, Minutoli L, et al. Effects of the phytoestrogen genistein on some predictors of cardiovascular risk in osteopenic, postmenopausal women: a two-year randomized, double-blind, placebo-controlled study. Journal of Clinical Endocrinology & Metabolism. 2007;92:3068–3075. doi: 10.1210/jc.2006-2295. [DOI] [PubMed] [Google Scholar]
- 26.Aubertin-Leheudre M, Lord C, Khalil A, Dionne IJ. Effect of 6 months of exercise and isoflavone supplementation on clinical cardiovascular risk factors in obese postmenopausal women: a randomized, double-blind study. Menopause. 2007;14:624–629. doi: 10.1097/gme.0b013e31802e426b. [DOI] [PubMed] [Google Scholar]
- 27.Chong MF, Macdonald R, Lovegrove JA. Fruit polyphenols and CVD risk: a review of human intervention studies. British Journal of Nutrition. 2010;104(Suppl 3):S28–S39. doi: 10.1017/S0007114510003922. [DOI] [PubMed] [Google Scholar]
- 28.Clerici C, Setchell KD, Battezzati PM, et al. Pasta naturally enriched with isoflavone aglycons from soy germ reduces serum lipids and improves markers of cardiovascular risk. Journal of Nutrition. 2007;137:2270–2278. doi: 10.1093/jn/137.10.2270. [DOI] [PubMed] [Google Scholar]
- 29.Greany KA, Nettleton JA, Wangen KE, Thomas W, Kurzer MS. Consumption of isoflavone-rich soy protein does not alter homocysteine or markers of inflammation in postmenopausal women. European Journal of Clinical Nutrition. 2008;62:1419–1425. doi: 10.1038/sj.ejcn.1602885. [DOI] [PubMed] [Google Scholar]
- 30.Hooper L, Kroon PA, Rimm EB, et al. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. American Journal of Clinical Nutrition. 2008;88:38–50. doi: 10.1093/ajcn/88.1.38. [DOI] [PubMed] [Google Scholar]
- 31.Jia L, Liu X, Bai YY, et al. Short-term effect of cocoa product consumption on lipid profile: a meta-analysis of randomized controlled trials. American Journal of Clinical Nutrition. 2010;92:218–225. doi: 10.3945/ajcn.2009.28202. [DOI] [PubMed] [Google Scholar]
- 32.Marini H, Bitto A, Altavilla D, et al. Efficacy of genistein aglycone on some cardiovascular risk factors and homocysteine levels: A follow-up study. Nutrition Metabolism & Cardiovascular Diseases. 2010;20:332–340. doi: 10.1016/j.numecd.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Maskarinec G, Oum R, Chaptman AK, Ognjanovic S. Inflammatory markers in a randomized soya intervention among men. British Journal of Nutrition. 2009;101:1740–1744. doi: 10.1017/S0007114508147389. [DOI] [PubMed] [Google Scholar]
- 34.Mulvihill EE, Huff MW. Antiatherogenic properties of flavonoids: implications for cardiovascular health. Canadian Journal of Cardiology. 2010;26(Suppl A):17A–21A. doi: 10.1016/s0828-282x(10)71056-4. [DOI] [PubMed] [Google Scholar]
- 35.Oyama J, Maeda T, Kouzuma K, et al. Green tea catechins improve human forearm endothelial dysfunction and have antiatherosclerotic effects in smokers. Circulation Journal. 2010;74:578–588. doi: 10.1253/circj.cj-09-0692. [DOI] [PubMed] [Google Scholar]
- 36.Rios DR, Rodrigues ET, Cardoso AP, Montes MB, Franceschini SA, Toloi MR. Lack of effects of isoflavones on the lipid profile of Brazilian postmenopausal women. Nutrition. 2008;24:1153–1158. doi: 10.1016/j.nut.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 37.Rotondo S, Krauze-Brzosko K, Manarini S, et al. Inhibition by soya isoflavones of human polymorphonuclear leukocyte function: possible relevance for the beneficial effects of soya intake. British Journal of Nutrition. 2008;99:240–247. doi: 10.1017/S0007114507797052. [DOI] [PubMed] [Google Scholar]
- 38.Shenouda SM, Vita JA. Effects of flavonoid-containing beverages and EGCG on endothelial function. Journal of the American College of Nutrition. 2007;26:366S–372S. doi: 10.1080/07315724.2007.10719625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taku K, Umegaki K, Sato Y, Taki Y, Endoh K, Watanabe S. Soy isoflavones lower serum total and LDL cholesterol in humans: a meta-analysis of 11 randomized controlled trials. American Journal of Clinical Nutrition. 2007;85:1148–1156. doi: 10.1093/ajcn/85.4.1148. [DOI] [PubMed] [Google Scholar]
- 40.Villa P, Costantini B, Suriano R, et al. The differential effect of the phytoestrogen genistein on cardiovascular risk factors in postmenopausal women: relationship with the metabolic status. Journal of Clinical Endocrinology & Metabolism. 2009;94:552–558. doi: 10.1210/jc.2008-0735. [DOI] [PubMed] [Google Scholar]
- 41.Arts IC, Hollman PC. Polyphenols and disease risk in epidemiologic studies. American Journal of Clinical Nutrition. 2005;81(1 Suppl):317S–325S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 42.Cano A, Garcia-Perez MA, Tarin JJ. Isoflavones and cardiovascular disease. Maturitas. 2010;67:219–226. doi: 10.1016/j.maturitas.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 43.de Lange DW, Verhoef S, Gorter G, Kraaijenhagen RJ, van de Wiel A, Akkerman JW. Polyphenolic grape extract inhibits platelet activation through PECAM-1: an explanation for the French paradox. Alcoholism: Clinical & Experimental Research. 2007;31:1308–1314. doi: 10.1111/j.1530-0277.2007.00439.x. [DOI] [PubMed] [Google Scholar]
- 44.Erdman JW, Jr, Carson L, Kwik-Uribe C, Evans EM, Allen RR. Effects of cocoa flavanols on risk factors for cardiovascular disease. Asia Pacific Journal of Clinical Nutrition. 2008;17(Suppl 1):284–287. [PubMed] [Google Scholar]
- 45.Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Critical Reviews in Food Science & Nutrition. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 46.Huntley AL. The health benefits of berry flavonoids for menopausal women: cardiovascular disease, cancer and cognition. Maturitas. 2009;63:297–301. doi: 10.1016/j.maturitas.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Kar P, Laight D, Shaw KM, Cummings MH. Flavonoid-rich grapeseed extracts: a new approach in high cardiovascular risk patients? International Journal of Clinical Practice. 2006;60:1484–1492. doi: 10.1111/j.1742-1241.2006.01038.x. [DOI] [PubMed] [Google Scholar]
- 48.Landberg R, Sun Q, Rimm EB, et al. Selected dietary flavonoids are associated with markers of inflammation and endothelial dysfunction in U.S. women. Journal of Nutrition. 2011;141:618–625. doi: 10.3945/jn.110.133843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manach C, Mazur A, Scalbert A. Polyphenols and prevention of cardiovascular diseases. Current Opinion in Lipidology. 2005;16:77–84. doi: 10.1097/00041433-200502000-00013. [DOI] [PubMed] [Google Scholar]
- 50.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacological Reviews. 2000;52:673–751. [PubMed] [Google Scholar]
- 51.Rasmussen SE, Frederiksen H, Struntze Krogholm K, Poulsen L. Dietary proanthocyanidins: occurrence, dietary intake, bioavailability, and protection against cardiovascular disease. Molecular Nutrition & Food Research. 2005;49:159–174. doi: 10.1002/mnfr.200400082. [DOI] [PubMed] [Google Scholar]
- 52.Sacks FM, Lichtenstein A, Van Horn L, Harris W, Kris-Etherton P, Winston M. American Heart Association Nutrition Committee. Soy protein, isoflavones, and cardiovascular health: an American Heart Association Science Advisory for professionals from the Nutrition Committee. Circulation. 2006;113:1034–1044. doi: 10.1161/CIRCULATIONAHA.106.171052. [DOI] [PubMed] [Google Scholar]
- 53.Sbarouni E, Iliodromitis EK, Zoga A, Theodorakis GN, Kremastinos DT. The effect of the phytoestrogen genistein on myocardial protection and preconditioning in hypercholesterolemia. Cardiovascular Drugs & Therapy. 2007;21:399–400. doi: 10.1007/s10557-007-6048-9. [DOI] [PubMed] [Google Scholar]
- 54.Basu A, Rhone M, Lyons TJ. Berries: emerging impact on cardiovascular health. Nutrition Reviews. 2010;68:168–177. doi: 10.1111/j.1753-4887.2010.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Erdman JW, Jr, Balentine D, Arab L, et al. Flavonoids and heart health: proceedings of the ILSI North America Flavonoids Workshop, May 31–June 1, 2005, Washington, DC. Journal of Nutrition. 2007;137(Suppl 1)(3):718S–737S. doi: 10.1093/jn/137.3.718S. [DOI] [PubMed] [Google Scholar]
- 56.Robbins RJ, Kwik-Uribe C, Hammerstone JF, Schmitz HH. Analysis of flavanols in foods: what methods are required to enable meaningful health recommendations? Journal of Cardiovascular Pharmacology. 2006;47(Suppl 2):S110–S118. doi: 10.1097/00005344-200606001-00004. [DOI] [PubMed] [Google Scholar]
- 57.Rudkowska I. Functional foods for cardiovascular disease in women. Menopause International. 2008;14:63–69. doi: 10.1258/mi.2008.008002. [DOI] [PubMed] [Google Scholar]
- 58.Rudkowska I, Jones PJ. Functional foods for the prevention and treatment of cardiovascular diseases: cholesterol and beyond. Expert Review of Cardiovascular Therapy. 2007;5:477–490. doi: 10.1586/14779072.5.3.477. [DOI] [PubMed] [Google Scholar]
- 59.Scheid L, Reusch A, Stehle P, Ellinger S. Antioxidant effects of cocoa and cocoa products ex vivo and in vivo: is there evidence from controlled intervention studies? Current Opinion in Clinical Nutrition & Metabolic Care. 2010;13:737–742. doi: 10.1097/MCO.0b013e32833ec45c. [DOI] [PubMed] [Google Scholar]
- 60.Schroeter H, Heiss C, Spencer JP, Keen CL, Lupton JR, Schmitz HH. Recommending flavanols and procyanidins for cardiovascular health: current knowledge and future needs. Molecular Aspects of Medicine. 2010;31:546–557. doi: 10.1016/j.mam.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 61.Sies H. Polyphenols and health: update and perspectives. Archives of Biochemistry & Biophysics. 2010;501:2–5. doi: 10.1016/j.abb.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 62.Steinberg FM, Bearden MM, Keen CL. Cocoa and chocolate flavonoids: implications for cardiovascular health. Journal of the American Dietetic Association. 2003;103:215–223. doi: 10.1053/jada.2003.50028. [DOI] [PubMed] [Google Scholar]
- 63.Arts IC, Hollman PC, Feskens EJ, Bueno de Mesquita HB, Kromhout D. Catechin intake might explain the inverse relation between tea consumption and ischemic heart disease: the Zutphen Elderly Study. American Journal of Clinical Nutrition. 2001;74:227–232. doi: 10.1093/ajcn/74.2.227. [DOI] [PubMed] [Google Scholar]
- 64.Arts IC, Jacobs DR, Jr, Harnack LJ, Gross M, Folsom AR. Dietary catechins in relation to coronary heart disease death among postmenopausal women. Epidemiology. 2001;12:668–675. doi: 10.1097/00001648-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 65.Geleijnse JM, Launer LJ, Van der Kuip DA, Hofman A, Witteman JC. Inverse association of tea and flavonoid intakes with incident myocardial infarction: the Rotterdam Study. American Journal of Clinical Nutrition. 2002;75:880–886. doi: 10.1093/ajcn/75.5.880. [DOI] [PubMed] [Google Scholar]
- 66.Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 67.Hertog MG, Feskens EJ, Kromhout D. Antioxidant flavonols and coronary heart disease risk. Lancet. 1997;349:699. doi: 10.1016/S0140-6736(05)60135-3. [DOI] [PubMed] [Google Scholar]
- 68.Hertog MG, Sweetnam PM, Fehily AM, Elwood PC, Kromhout D. Antioxidant flavonols and ischemic heart disease in a Welsh population of men: the Caerphilly Study. American Journal of Clinical Nutrition. 1997;65:1489–1494. doi: 10.1093/ajcn/65.5.1489. [DOI] [PubMed] [Google Scholar]
- 69.Hirvonen T, Pietinen P, Virtanen M, et al. Intake of flavonols and flavones and risk of coronary heart disease in male smokers. Epidemiology. 2001;12:62–67. doi: 10.1097/00001648-200101000-00011. [DOI] [PubMed] [Google Scholar]
- 70.Hirvonen T, Virtamo J, Korhonen P, Albanes D, Pietinen P. Intake of flavonoids, carotenoids, vitamins C and E, and risk of stroke in male smokers. Stroke. 2000;31:2301–2306. doi: 10.1161/01.str.31.10.2301. [DOI] [PubMed] [Google Scholar]
- 71.Keli SO, Hertog MG, Feskens EJ, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Archives of Internal Medicine. 1996;156:637–642. [PubMed] [Google Scholar]
- 72.Knekt P, Isotupa S, Rissanen H, et al. Quercetin intake and the incidence of cerebrovascular disease. European Journal of Clinical Nutrition. 2000;54:415–417. doi: 10.1038/sj.ejcn.1600974. [DOI] [PubMed] [Google Scholar]
- 73.Knekt P, Jarvinen R, Reunanen A, Maatela J. Flavonoid intake and coronary mortality in Finland: a cohort study. BMJ. 1996;312:478–481. doi: 10.1136/bmj.312.7029.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Knekt P, Kumpulainen J, Jarvinen R, et al. Flavonoid intake and risk of chronic diseases. American Journal of Clinical Nutrition. 2002;76:560–568. doi: 10.1093/ajcn/76.3.560. [DOI] [PubMed] [Google Scholar]
- 75.Lin J, Rexrode KM, Hu F, et al. Dietary intakes of flavonols and flavones and coronary heart disease in US women. American Journal of Epidemiology. 2007;165:1305–1313. doi: 10.1093/aje/kwm016. [DOI] [PubMed] [Google Scholar]
- 76.Marniemi J, Alanen E, Impivaara O, et al. Dietary and serum vitamins and minerals as predictors of myocardial infarction and stroke in elderly subjects. Nutrition Metabolism & Cardiovascular Diseases. 2005;15:188–197. doi: 10.1016/j.numecd.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 77.Mink PJ, Scrafford CG, Barraj LM, et al. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. American Journal of Clinical Nutrition. 2007;85:895–909. doi: 10.1093/ajcn/85.3.895. [DOI] [PubMed] [Google Scholar]
- 78.Mursu J, Voutilainen S, Nurmi T, Tuomainen TP, Kurl S, Salonen JT. Flavonoid intake and the risk of ischemic stroke and CVD mortality in middle-aged Finnish men: the Kuopio Ischemic Heart Disease Risk Factor Study. British Journal of Nutrition. 2008;100:890–895. doi: 10.1017/S0007114508945694. [DOI] [PubMed] [Google Scholar]
- 79.Rimm EB, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Relation between intake of flavonoids and risk for coronary heart disease in male health professionals. Annals of Internal Medicine. 1996;125:384–389. doi: 10.7326/0003-4819-125-5-199609010-00005. [DOI] [PubMed] [Google Scholar]
- 80.Sesso HD, Gaziano JM, Liu S, et al. Flavonoid intake and the risk of cardiovascular disease in women. American Journal of Clinical Nutrition. 2003;77:1400–1408. doi: 10.1093/ajcn/77.6.1400. [DOI] [PubMed] [Google Scholar]
- 81.van der Schouw YT, Kreijkamp-Kaspers S, Peeters PH, Keinan-Boker L, Rimm EB, Grobbee DE. Prospective study on usual dietary phytoestrogen intake and cardiovascular disease risk in Western women. Circulation. 2005;111:465–471. doi: 10.1161/01.CIR.0000153814.87631.B0. [DOI] [PubMed] [Google Scholar]
- 82.Yochum L, Kushi LH, Meyer K, Folsom AR. Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. American Journal of Epidemiology. 1999;149:943–949. doi: 10.1093/oxfordjournals.aje.a009738. [DOI] [PubMed] [Google Scholar]
- 83.Lagiou P, Samoli E, Lagiou A, et al. Intake of specific flavonoid classes and coronary heart disease--a case-control study in Greece. European Journal of Clinical Nutrition. 2004;58:1643–1648. doi: 10.1038/sj.ejcn.1602022. [DOI] [PubMed] [Google Scholar]
- 84.Tavani A, Spertini L, Bosetti C, et al. Intake of specific flavonoids and risk of acute myocardial infarction in Italy. Public Health Nutrition. 2006;9:369–374. doi: 10.1079/phn2006859. [DOI] [PubMed] [Google Scholar]
- 85.Willett W. Nutritional Epidemiology. 2nd ed. New York: Oxford University Press; 1998. [Google Scholar]
- 86.Hertog MG. PhD dissertation. The Hague: Koninklijke Bibliotheek; 1994. Flavonols and flavones in foods and their relation with cancer and coronary heart disease risk. [Google Scholar]
- 87.Hertog MGL, Hollman PCH, Katan MB. Content of potentially anticarcinogenic flavonoids of 28 vegetables and 9 fruits commonly consumed in the Netherlands. Journal of Agricultural & Food Chemistry. 1992;40:2379–2383. [Google Scholar]
- 88.Hertog MG, Hollman PC, Katan MB, Kromhout D. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands. Nutrition & Cancer. 1993;20(1):21–29. doi: 10.1080/01635589309514267. [DOI] [PubMed] [Google Scholar]
- 89.Hertog MGL, Hollman PCH, van de Putte B. Content of potentially anticarcinogenic flavonoids in tea infusions, wine and fruit juices. Journal of Agricultural & Food Chemistry. 1993;41:1242–1246. [Google Scholar]
- 90.Hertog MGL, Hollman PCH, Venema DP. Optimization of a quantitative HPLC determination of potentially anticarcinogenic flavonoids in vegetables and fruits. Journal of Agricultural & Food Chemistry. 1992;40:1591–1598. [Google Scholar]
- 91.Arts ICW, Hollman PCH. Optimization of a quantitative method for the determination of catechins in fruits and legumes. Journal of Agricultural and Food Chemistry. 1998;46:5156–5162. [Google Scholar]
- 92.Arts IC, van de Putte B, Hollman PC. Catechin contents of foods commonly consumed in The Netherlands. 1. Fruits, vegetables, staple foods, and processed foods. Journal of Agricultural & Food Chemistry. 2000;48:1746–1751. doi: 10.1021/jf000025h. [DOI] [PubMed] [Google Scholar]
- 93.Arts IC, van De Putte B, Hollman PC. Catechin contents of foods commonly consumed in The Netherlands. 2. Tea,wine, fruit juices, and chocolate milk. Journal of Agricultural & Food Chemistry. 2000;48:1752–1757. doi: 10.1021/jf000026+. [DOI] [PubMed] [Google Scholar]
- 94.Boker LK, Van der Schouw YT, De Kleijn MJ, Jacques PF, Grobbee DE, Peeters PH. Intake of dietary phytoestrogens by Dutch women. Journal of Nutrition. 2002;132(6):1319–1328. doi: 10.1093/jn/132.6.1319. [DOI] [PubMed] [Google Scholar]
- 95.U.S. Department of Agriculture. USDA Database for the Flavonoid Content of Selected Foods. Beltsville MD: Agricultural Research Service, Nutrient Data Laboratory; 2003. [Google Scholar]
- 96.US Department of Agriculture (USDA) USDA–Iowa State University database on the isoflavone content of foods. 1999
- 97.U.S. Department of Agriculture. USDA database for the proanthocyanidin content of selected foods. Beltsville, MD: Agricultural Research Service, Nutrient Data Laboratory; 2004. [Google Scholar]
- 98.Hakkinen SH, Karenlampi SO, Heinonen IM, Mykkanen HM, Torronen AR. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. Journal of Agricultural & Food Chemistry. 1999;47(6):2274–2279. doi: 10.1021/jf9811065. [DOI] [PubMed] [Google Scholar]
- 99.Starke H, Herrmann K. [The phenolics of fruits. VIII. Changes in flavonol concentrations during fruit development (authors transl)] [German] Zeitschrift fur Lebensmittel-Untersuchung und -Forschung. 1976;161(2):131–135. doi: 10.1007/BF01112856. [DOI] [PubMed] [Google Scholar]
- 100.Wildanger W, Herrmann K. Die phenolischen Inhaltsstoffe des Obstes. II. Die Flavonole des Obstes. Zeitschrift fur Lebensmittel-Untersuchen und -Forschung. 1973;151:103–108. [Google Scholar]
- 101.Mattila P, Astola J, Kumpulainen J. Determination of flavonoids in plant material by HPLC with diode-array and electro-array detections. Journal of Agricultural & Food Chemistry. 2000;48(12):5834–5841. doi: 10.1021/jf000661f. [DOI] [PubMed] [Google Scholar]
- 102.Sampson L, Rimm E, Hollman PC, de Vries JH, Katan MB. Flavonol and flavone intakes in US health professionals. Journal of the American Dietetic Association. 2002;102(10):1414–1420. doi: 10.1016/s0002-8223(02)90314-7. [DOI] [PubMed] [Google Scholar]
- 103.U.S. Department of Agriculture, Agricultural Research Service. USDA Database for the Isoflavone Content of Selected Foods, Release 2.0. Nutrient Data Laboratory Home Page; 2008. http://www.ars.usda.gov/nutrientdata/isoflav. [Google Scholar]
- 104.U.S Department of Agriculture. USDA Database for the Flavonoid Content of Selected Foods. Beltsville MD: Agricultural Research Service, Nutrient Data Laboratory; 2007. [Google Scholar]
- 105.World Health Organization. Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death, 8th Revision. Geneva, Switzerland: World Health Organization; 1965. [Google Scholar]
- 106.World Health Organization. Based on recommendations of the 9th revision conference, 1975, and adopted by the 29th World Health Assembly. Geneva: WHO; 1977–1978. International classification of diseases, injuries, and causes of death. [Google Scholar]
- 107.World Health Organization. International Statistical Classification of Diseases and Related Health Problems, Tenth Revision. 10th ed. Geneva: WHO; 1992. [PubMed] [Google Scholar]
- 108.Rose GA, Blackburn H. Cardiovascular Survey Methods. Geneva: World Health Organization; 1982. [PubMed] [Google Scholar]
- 109.World Health Organization. Ischaemic heart disease registers: report of the Fifth Working Group, including a second revision of the operating protocol: Copenhagen, 26–29 April 1971. Copenhagen: Regional Office for Europe, World Health Organization; 1971. [Google Scholar]
- 110.Huxley RR, Neil HA. The relation between dietary flavonol intake and coronary heart disease mortality: a meta-analysis of prospective cohort studies. European Journal of Clinical Nutrition. 2003;57:904–908. doi: 10.1038/sj.ejcn.1601624. [DOI] [PubMed] [Google Scholar]
- 111.Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutrition & Cancer. 2006;55:1–12. doi: 10.1207/s15327914nc5501_1. [DOI] [PubMed] [Google Scholar]
- 112.Hollman PC, Geelen A, Kromhout D. Dietary flavonol intake may lower stroke risk in men and women. Journal of Nutrition. 2010;140:600–604. doi: 10.3945/jn.109.116632. [DOI] [PubMed] [Google Scholar]
- 113.Geleijnse JM, Hollman PCH. Flavonoids and cardiovascular health: which compounds, what mechanisms? American Journal of Clinical Nutrition. 2008;88:12–13. doi: 10.1093/ajcn/88.1.12. [DOI] [PubMed] [Google Scholar]
- 114.Yap S, Qin C, Woodman OL. Effects of resveratrol and flavonols on cardiovascular function: Physiological mechanisms. Biofactors. 2010;36:350–359. doi: 10.1002/biof.111. [DOI] [PubMed] [Google Scholar]
- 115.McCarty MF. Scavenging of peroxynitrite-derived radicals by flavonoids may support endothelial NO synthase activity, contributing to the vascular protection associated with high fruit and vegetable intakes. Medical Hypotheses. 2008;70:170–181. doi: 10.1016/j.mehy.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 116.Zafra-Stone S, Yasmin T, Bagchi M, Chatterjee A, Vinson JA, Bagchi D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Molecular Nutrition & Food Research. 2007;51:675–683. doi: 10.1002/mnfr.200700002. [DOI] [PubMed] [Google Scholar]
- 117.Balzer J, Rassaf T, Heiss C, et al. Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients a double-masked, randomized, controlled trial. Journal of the American College of Cardiology. 2008;51:2141–2149. doi: 10.1016/j.jacc.2008.01.059. [DOI] [PubMed] [Google Scholar]
- 118.Bayard V, Chamorro F, Motta J, Hollenberg NK. Does flavanol intake influence mortality from nitric oxide-dependent processes? Ischemic heart disease, stroke, diabetes mellitus, and cancer in Panama. International Journal of Medical Sciences. 2007;4(1):53–58. doi: 10.7150/ijms.4.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cesarone MR, Di Renzo A, Errichi S, Schonlau F, Wilmer JL, Blumenfeld J. Improvement in circulation and in cardiovascular risk factors with a proprietary isotonic bioflavonoid formula OPC-3. Angiology. 2008;59:408–414. doi: 10.1177/0003319708321801. [DOI] [PubMed] [Google Scholar]
- 120.Farouque HM, Leung M, Hope SA, et al. Acute and chronic effects of flavanol-rich cocoa on vascular function in subjects with coronary artery disease: a randomized double-blind placebo-controlled study. Clinical Science. 2006;111:71–80. doi: 10.1042/CS20060048. [DOI] [PubMed] [Google Scholar]
- 121.Ghosh D, Scheepens A. Vascular action of polyphenols. Molecular Nutrition & Food Research. 2009;53:322–331. doi: 10.1002/mnfr.200800182. [DOI] [PubMed] [Google Scholar]
- 122.Vlachopoulos C, Alexopoulos N, Stefanadis C. Effect of dark chocolate on arterial function in healthy individuals: cocoa instead of ambrosia? Current Hypertension Reports. 2006;8:205–211. doi: 10.1007/s11906-006-0052-5. [DOI] [PubMed] [Google Scholar]
- 123.Webb CM, Hayward CS, Mason MJ, Ilsley CD, Collins P. Coronary vasomotor and blood flow responses to isoflavone-intact soya protein in subjects with coronary heart disease or risk factors for coronary heart disease. Clinical Science. 2008;115:353–359. doi: 10.1042/CS20070443. [DOI] [PubMed] [Google Scholar]
- 124.Booyse FM, Pan W, Grenett HE, et al. Mechanism by which alcohol and wine polyphenols affect coronary heart disease risk. Annals of Epidemiology. 2007;17(5 Suppl):S24–S31. doi: 10.1016/j.annepidem.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 125.de Gaetano G, De Curtis A, di Castelnuovo A, Donati MB, Iacoviello L, Rotondo S. Antithrombotic effect of polyphenols in experimental models: a mechanism of reduced vascular risk by moderate wine consumption. Annals of the New York Academy of Sciences. 2002;957:174–188. doi: 10.1111/j.1749-6632.2002.tb02915.x. [DOI] [PubMed] [Google Scholar]
- 126.Maron DJ. Flavonoids for reduction of atherosclerotic risk. Current Atherosclerosis Reports. 2004;6:73–78. doi: 10.1007/s11883-004-0119-1. [DOI] [PubMed] [Google Scholar]
- 127.Ostertag LM, O'Kennedy N, Kroon PA, Duthie GG, de Roos B. Impact of dietary polyphenols on human platelet function--a critical review of controlled dietary intervention studies. Molecular Nutrition & Food Research. 2010;54:60–81. doi: 10.1002/mnfr.200900172. [DOI] [PubMed] [Google Scholar]
- 128.Garcia-Lafuente A, Guillamon E, Villares A, Rostagno MA, Martinez JA. Flavonoids as anti-inflammatory agents: implications in cancer and cardiovascular disease. Inflammation Research. 2009;58:537–552. doi: 10.1007/s00011-009-0037-3. [DOI] [PubMed] [Google Scholar]
- 129.Heiss C, Schroeter H, Balzer J, et al. Endothelial function, nitric oxide, and cocoa flavanols. Journal of Cardiovascular Pharmacology. 2006;47(Suppl 2):S128–S135. S172–S176. doi: 10.1097/00005344-200606001-00007. [DOI] [PubMed] [Google Scholar]
- 130.Vita JA. Polyphenols and cardiovascular disease: effects on endothelial and platelet function. American Journal of Clinical Nutrition. 2005;81(1 Suppl):292S–297S. doi: 10.1093/ajcn/81.1.292S. [DOI] [PubMed] [Google Scholar]
- 131.Ishizawa K, Yoshizumi M, Kawai Y, et al. Pharmacology in health food: metabolism of quercetin in vivo and its protective effect against arteriosclerosis. Journal of Pharmacological Sciences. 2011;115:466–470. doi: 10.1254/jphs.10r38fm. [DOI] [PubMed] [Google Scholar]
- 132.Neveu V, Perez-Jimenez J, Vos F, et al. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database: The Journal of Biological Databases and Curation. 2010 doi: 10.1093/database/bap024. bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gry J, Black L, Eriksen FD, et al. EuroFIR-BASIS - a combined composition and biological activity database for bioactive compounds in plant-based foods. Trends in Food Science & Technology. 2007;18(8):434–444. [Google Scholar]
- 134.Cao J, Zhang Y, Chen W, Zhao X. The relationship between fasting plasma concentrations of selected flavonoids and their ordinary dietary intake. British Journal of Nutrition. 2010;103:249–255. doi: 10.1017/S000711450999170X. [DOI] [PubMed] [Google Scholar]
- 135.Linseisen J, Rohrmann S. Biomarkers of dietary intake of flavonoids and phenolic acids for studying diet-cancer relationship in humans. European Journal of Nutrition. 2008;47(Suppl 2):60–68. doi: 10.1007/s00394-008-2007-x. [DOI] [PubMed] [Google Scholar]
- 136.Loke WM, Jenner AM, Proudfoot JM, et al. A metabolite profiling approach to identify biomarkers of flavonoid intake in humans. Journal of Nutrition. 2009;139:2309–2314. doi: 10.3945/jn.109.113613. [DOI] [PubMed] [Google Scholar]
- 137.Mennen LI, Sapinho D, Ito H, Galan P, Hercberg S, Scalbert A. Urinary excretion of 13 dietary flavonoids and phenolic acids in free-living healthy subjects - variability and possible use as biomarkers of polyphenol intake. European Journal of Clinical Nutrition. 2008;62:519–525. doi: 10.1038/sj.ejcn.1602744. [DOI] [PubMed] [Google Scholar]
- 138.Perez-Jimenez J, Hubert J, Hooper L, et al. Urinary metabolites as biomarkers of polyphenol intake in humans: a systematic review. American Journal of Clinical Nutrition. 2010;92:801–809. doi: 10.3945/ajcn.2010.29924. [DOI] [PubMed] [Google Scholar]
- 139.Scott EN, Gescher AJ, Steward WP, Brown K. Development of dietary phytochemical chemopreventive agents: biomarkers and choice of dose for early clinical trials. Cancer Prevention Research. 2009;2:525–530. doi: 10.1158/1940-6207.CAPR-08-0223. [DOI] [PubMed] [Google Scholar]
- 140.Arai Y, Uehara M, Sato Y, et al. Comparison of isoflavones among dietary intake, plasma concentration and urinary excretion for accurate estimation of phytoestrogen intake. Journal of Epidemiology. 2000;10:127–135. doi: 10.2188/jea.10.127. [DOI] [PubMed] [Google Scholar]
- 141.Chan SG, Ho SC, Kreiger N, et al. Validation of a food frequency questionnaire for assessing dietary soy isoflavone intake among midlife Chinese women in Hong Kong. Journal of Nutrition. 2008;138:567–573. doi: 10.1093/jn/138.3.567. [DOI] [PubMed] [Google Scholar]
- 142.Frankenfeld CL, Lampe JW, Shannon J, et al. Frequency of soy food consumption and serum isoflavone concentrations among Chinese women in Shanghai. Public Health Nutrition. 2004;7:765–772. doi: 10.1079/phn2004614. [DOI] [PubMed] [Google Scholar]
- 143.Frankenfeld CL, Patterson RE, Horner NK, et al. Validation of a soy food-frequency questionnaire and evaluation of correlates of plasma isoflavone concentrations in postmenopausal women. American Journal of Clinical Nutrition. 2003;77:674–680. doi: 10.1093/ajcn/77.3.674. [DOI] [PubMed] [Google Scholar]
- 144.Frankenfeld CL, Patterson RE, Kalhorn TF, Skor HE, Howald WN, Lampe JW. Validation of a soy food frequency questionnaire with plasma concentrations of isoflavones in US adults. Journal of the American Dietetic Association. 2002;102:1407–1413. doi: 10.1016/s0002-8223(02)90313-5. [DOI] [PubMed] [Google Scholar]
- 145.French MR, Thompson LU, Hawker GA. Validation of a phytoestrogen food frequency questionnaire with urinary concentrations of isoflavones and lignan metabolites in premenopausal women. Journal of the American College of Nutrition. 2007;26:76–82. doi: 10.1080/07315724.2007.10719588. [DOI] [PubMed] [Google Scholar]
- 146.Heald CL, Bolton-Smith C, Ritchie MR, Morton MS, Alexander FE. Phyto-oestrogen intake in Scottish men: use of serum to validate a self-administered food-frequency questionnaire in older men. European Journal of Clinical Nutrition. 2006;60:129–135. doi: 10.1038/sj.ejcn.1602277. [DOI] [PubMed] [Google Scholar]
- 147.Huang MH, Harrison GG, Mohamed MM, et al. Assessing the accuracy of a food frequency questionnaire for estimating usual intake of phytoestrogens. Nutrition & Cancer. 2000;37:145–154. doi: 10.1207/S15327914NC372_5. [DOI] [PubMed] [Google Scholar]
- 148.Jaceldo-Siegl K, Fraser GE, Chan J, Franke A, Sabate J. Validation of soy protein estimates from a food-frequency questionnaire with repeated 24-h recalls and isoflavonoid excretion in overnight urine in a Western population with a wide range of soy intakes. American Journal of Clinical Nutrition. 2008;87:1422–1427. doi: 10.1093/ajcn/87.5.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kirk P, Patterson RE, Lampe J. Development of a soy food frequency questionnaire to estimate isoflavone consumption in US adults. Journal of the American Dietetic Association. 1999;99:558–563. doi: 10.1016/S0002-8223(99)00139-X. [DOI] [PubMed] [Google Scholar]
- 150.Tseng M, Olufade T, Kurzer MS, et al. Food frequency questionnaires and overnight urines are valid indicators of daidzein and genistein intake in U.S. women relative to multiple 24-h urine samples. Nutrition & Cancer. 2008;60:619–626. doi: 10.1080/01635580801993751. 2008. [DOI] [PubMed] [Google Scholar]
- 151.Verkasalo PK, Appleby PN, Allen NE, et al. Validation study of soya intake and plasma isoflavone levels among British women. IARC Scientific Publications. 2002;156:135–136. [PubMed] [Google Scholar]
- 152.Williams AE, Maskarinec G, Hebshi S, Oshiro C, Murphy S, Franke AA. Validation of a soy questionnaire with repeated dietary recalls and urinary isoflavone assessments over one year. Nutrition & Cancer. 2003;47:118–125. doi: 10.1207/s15327914nc4702_2. [DOI] [PubMed] [Google Scholar]
- 153.Yamamoto S, Sobue T, Sasaki S, et al. Validity and reproducibility of a self-administered food-frequency questionnaire to assess isoflavone intake in a Japanese population in comparison with dietary records and blood and urine isoflavones. Journal of Nutrition. 2001;131:2741–2747. doi: 10.1093/jn/131.10.2741. [DOI] [PubMed] [Google Scholar]
- 154.Hakim IA, Hartz V, Harris RB, et al. Reproducibility and relative validity of a questionnaire to assess intake of black tea polyphenols in epidemiological studies. Cancer Epidemiology, Biomarkers & Prevention. 2001;10:667–678. [PubMed] [Google Scholar]
- 155.Mullie P, Clarys P, Deriemaeker P, Hebbelinck M. Estimation of daily human intake of food flavonoids. Plant Foods for Human Nutrition. 2007;62:93–98. doi: 10.1007/s11130-007-0047-7. [DOI] [PubMed] [Google Scholar]
- 156.Mullie P, Clarys P, Deriemaeker P, Hebbelinck M. Estimation of daily human intake of food flavonoids. International Journal of Food Sciences & Nutrition. 2008;59:291–298. doi: 10.1080/09687630701539293. [DOI] [PubMed] [Google Scholar]
- 157.Ranka S, Gee JM, Biro L, et al. Development of a food frequency questionnaire for the assessment of quercetin and naringenin intake. European Journal of Clinical Nutrition. 2008;62:1131–1138. doi: 10.1038/sj.ejcn.1602827. [DOI] [PubMed] [Google Scholar]
- 158.Brantsaeter AL, Haugen M, Rasmussen SE, Alexander J, Samuelsen SO, Meltzer HM. Urine flavonoids and plasma carotenoids in the validation of fruit, vegetable and tea intake during pregnancy in the Norwegian Mother and Child Cohort Study (MoBa) Public Health Nutrition. 2007;10:838–847. doi: 10.1017/S1368980007339037. [DOI] [PubMed] [Google Scholar]
- 159.Mikkelsen TB, Olsen SF, Rasmussen SE, Osler M. Relative validity of fruit and vegetable intake estimated by the food frequency questionnaire used in the Danish National Birth Cohort. Scandinavian Journal of Public Health. 2007;35:172–179. doi: 10.1080/14034940600975625. [DOI] [PubMed] [Google Scholar]
- 160.Zhang Y, Li Y, Cao C, et al. Dietary flavonol and flavone intakes and their major food sources in Chinese adults. Nutrition & Cancer. 2010;62:1120–1127. doi: 10.1080/01635581.2010.513800. [DOI] [PubMed] [Google Scholar]
- 161.Kokubo Y, Iso H, Ishihara J, Okada K, Inoue M, Tsugane S. JPHC Study Group. Association of dietary intake of soy, beans, and isoflavones with risk of cerebral and myocardial infarctions in Japanese populations: the Japan Public Health Center-based (JPHC) study cohort I. Circulation. 2007;116(22):2553–2562. doi: 10.1161/CIRCULATIONAHA.106.683755. [DOI] [PubMed] [Google Scholar]
- 162.Nagata C. Ecological study of the association between soy product intake and mortality from cancer and heart disease in Japan. International Journal of Epidemiology. 2000;29:832–836. doi: 10.1093/ije/29.5.832. [DOI] [PubMed] [Google Scholar]
- 163.Desch S, Schmidt J, Kobler D, et al. Effect of cocoa products on blood pressure: systematic review and meta-analysis. American Journal of Hypertension. 2010;23:97–103. doi: 10.1038/ajh.2009.213. [DOI] [PubMed] [Google Scholar]
- 164.Perez-Vizcaino F, Duarte J, Jimenez R, Santos-Buelga C, Osuna A. Antihypertensive effects of the flavonoid quercetin. Pharmacological Reports: PR. 2009;61:67–75. doi: 10.1016/s1734-1140(09)70008-8. [DOI] [PubMed] [Google Scholar]
- 165.Phang M, Lazarus S, Wood LG, Garg M. Diet and thrombosis risk: nutrients for prevention of thrombotic disease. Seminars in Thrombosis & Hemostasis. 2011;37:199–208. doi: 10.1055/s-0031-1273084. [DOI] [PubMed] [Google Scholar]
- 166.Akazome Y, Kametani N, Kanda T, Shimasaki H, Kobayashi S. Evaluation of safety of excessive intake and efficacy of long-term intake of beverages containing apple polyphenols. Journal of Oleo Science. 2010;59:321–338. doi: 10.5650/jos.59.321. [DOI] [PubMed] [Google Scholar]
- 167.Basu A, Wilkinson M, Penugonda K, Simmons B, Betts NM, Lyons TJ. Freeze-dried strawberry powder improves lipid profile and lipid peroxidation in women with metabolic syndrome: baseline and post intervention effects. Nutrition Journal. 2009;8:43. doi: 10.1186/1475-2891-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Daniele C, Mazzanti G, Pittler MH, Ernst E. Adverse-event profile of Crataegus spp.: a systematic review. Drug Safety. 2006;29:523–535. doi: 10.2165/00002018-200629060-00005. [DOI] [PubMed] [Google Scholar]
- 169.Galati G, O'Brien PJ. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radical Biology & Medicine. 2004;37:287–303. doi: 10.1016/j.freeradbiomed.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 170.Munro IC, Harwood M, Hlywka JJ, et al. Soy isoflavones: a safety review. Nutrition Reviews. 2003;61:1–33. doi: 10.1301/nr.2003.janr.1-33. [DOI] [PubMed] [Google Scholar]
- 171.Prasain JK, Carlson SH, Wyss JM. Flavonoids and age-related disease: risk, benefits and critical windows. Maturitas. 2010;66:163–171. doi: 10.1016/j.maturitas.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schonthal AH. Adverse effects of concentrated green tea extracts. Molecular Nutrition and Food Research. 2011;55:874–885. doi: 10.1002/mnfr.201000644. [DOI] [PubMed] [Google Scholar]
- 173.Song WO, Chun OK, Hwang I, et al. Soy isoflavones as safe functional ingredients. Journal of Medicinal Food. 2007;10:571–580. doi: 10.1089/jmf.2006.0620. [DOI] [PubMed] [Google Scholar]
- 174.Wuttke W, Jarry H, Seidlova-Wuttke D. Isoflavones--safe food additives or dangerous drugs? Ageing Research Reviews. 2007;6:150–188. doi: 10.1016/j.arr.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 175.Bast A, Haenen GR, Bruynzeel AM, Van der Vijgh WJ. Protection by flavonoids against anthracycline cardiotoxicity: from chemistry to clinical trials. Cardiovascular Toxicology. 2007;7:154–159. doi: 10.1007/s12012-007-0018-0. [DOI] [PubMed] [Google Scholar]
- 176.Chan YH, Lau KK, Yiu KH, et al. Reduction of C-reactive protein with isoflavone supplement reverses endothelial dysfunction in patients with ischaemic stroke. European Heart Journal. 2008;29:2800–2807. doi: 10.1093/eurheartj/ehn409. [DOI] [PubMed] [Google Scholar]