Abstract
Interleukin 33 (IL-33) has emerged as a cytokine that can exhibit pleiotropic properties. Here we examine IL-33 for its immunoadjuvant effects in an HPV-associated cancer immune therapy model in which cell-mediated immunity is critical for protection. It is known that two biologically active forms of IL-33 exist: full-length IL-33 and mature IL-33. The potential ability of both isoforms to act as vaccine adjuvants to influence the CD4 Th1 and CD8 T cell immune responses has not been well defined. We show that both isoforms of IL-33 are capable of enhancing potent antigen (Ag)-specific effector and memory T cell immunity in vivo in a DNA vaccine setting. We also show that while both forms of IL-33 drove robust IFN-γ responses, neither form drove high secretion of IL-4 or any elevation of IgE levels. Moreover, both isoforms augmented vaccine-induced Ag-specific polyfunctional CD4+ and CD8+ T cell responses, with a large proportion of CD8+ T cells undergoing cytolytic plurifunctional degranulation. Therapeutic studies indicated that established TC-1-bearing mice undergo rapid and complete regression after therapeutic vaccination with both IL-33 adjuvant isoforms used in conjunction with an HPV DNA vaccine. Furthermore, using the P14 transgenic mouse model, we show that IL-33 can significantly expand the magnitude of Ag-specific CD8+ T cell responses and elicit bonafide effector-memory CD8+ T cells. Overall, the data suggests the potential use of these two IL-33 isoforms as immunoadjuvant candidates in future vaccination against other pathogens and in the context of anti-tumor immune-based therapy.
Keywords: IL-33, adjuvant, HPV, DNA Vaccine, anti-tumor immunity
INTRODUCTION
Adjuvants are critical components of most clinical vaccines and are used to enhance adaptive immune responses to antigen (1). Adjuvants can help shape the quantity and quality of immune responses (1). However, currently available FDA-licensed adjuvants are poor inducers of CD4+ T helper 1 (Th1) and even worse at treating CD8+ T cell responses (2,3). It is important to identify a new generation of potent vaccine adjuvant(s) that can drive and specifically direct both these desired responses. Thus, the inclusion of different molecular adjuvants, such as cytokines, are actively being studied as a way to increase the efficacy of vaccines. Different vaccine platforms have been studied, but the development of DNA-based vaccines in conjunction with cytokine adjuvants, has emerged as a particularly promising for inducing antiviral and anti-tumor cell-mediated immune responses (4,5). Indeed, the potency of DNA-based vaccines co-administered with molecular cytokine adjuvants as part of a vaccine cocktail has been demonstrated to boost the adaptive immune response (5). Recently IL-12 as a vaccine molecular adjuvant has been shown to augment the T cell immunity induced by a DNA vaccine in humans (4). IL-12 was particularly effective in expanding CD4 and CD8 immunity but less effective, in driving strong B cell immunity. Building on this important recent success is an area of great importance. We therefore employed a DNA vaccination approach to investigate the inclusion of Interleukin 33 (IL-33) to further enhance, both arms of the adaptive immune responses.
IL-33 is a member of the IL-1 family of cytokines that is constitutively expressed in the nucleus of epithelial and endothelial cells (6,7). IL-33 is classified as an alarmin-like molecule, whose release during cell injury signals tissue damage to local immune cells (7). Alarmin IL-33 has been shown to have pleiotropic cytokine activities such as mediating diverse pro-inflammatory responses (9,10), activation and recruitment of antigen-presenting cells (11), enhancing adaptive immunity (12,13), and wound healing (14). To date, IL-33 has been studied primarily in the context of T helper type 2 (Th2) immune responses associated with modulating inflammatory disorders such as asthma and atopic dermatitis (6,15,16–18). More recently though, IL-33 has been reported to activate CD8+ T cells and influence the development of protective anti-viral CD8+ T cells against infections in mice (12). However, the role of IL-33 in the induction of vaccine-induced, antigen-specific Th1 and CD8 T cell immunity remains to be determined. Two different biologically active forms of IL-33 exist: full-length IL-33 (proIL-33) and mature IL-33 (mtrIL-33) (18). ProIL-33 is thought to be the most biologically active form promoting inflammation, while the function of mtrIL-33 in modulating immune responses remains more elusive (7,15,18). Therefore, we investigated whether the two isoforms of IL-33 (proIL-33 and mtrIL-33) can function as vaccine adjuvants to augment both Th1 and CD8+ T cell responses and induce anti-tumor immunity using a murine model for HPV-associated cancer.
In this study, we demonstrate that IL-33 can act as a potent cell-mediated adjuvant using the DNA vaccine platform. Its adjuvant activity skews towards the Th1 axis, and not to the Th2 axis. We show that IL-33 can be effective as an adjuvant in either form – its uncleaved “pro” form or its “mature” state, a shorter form that results from cleavage by cellular enzymes (10,17,19). Both IL-33 isoforms when combined with an HPV16 E6/E7-encoded DNA vaccine enhance the adaptive effector and memory immune responses, but proIL-33 was more potent at also expanding the humoral immune response. We show that both immunoadjuvant IL-33 isoforms induce potent anti-tumor immunity and regression of established TC-1 tumor-bearing mice. Using the P14 LCMV DbGP33 transgenic mouse model, we show that immunoadjuvant IL-33 can significantly expand the magnitude of Ag-specific CD8+ T cell responses and elicit potent effector-memory CD8+ T cells. Our findings reveal that IL-33 can be an effective adjuvant to drive CD4 immunity, humoral immunity and to generate effective CD8 mediated protective immunity against cancer and potentially have application in treatment of chronic viral infections.
Materials and Methods
Plasmid Construction
The GenBank sequence NM_001164724.1 for mouse IL-33 was used to synthesize full-length (proIL-33) and mature IL-33 (mtrIL-33) (aa 109–266) plasmid DNA constructs. Each construct had highly efficient immunoglobulin E (IgE) leader sequence inserted at the 5′end of the gene. The constructs were commercially synthesized and optimized as described previously (20). Plasmid expressing HPV 16 ConE6E7 was prepared as previously described (21). The GP33 construct was provided by Dr. Rafi Ahmed of Emory University, Atlanta GA, USA and used as described (22).
Transfection and Expression of Plasmids
ProIL-33 and mtrIL-33 construct expression was confirmed using Western Blot and Immunofluorescence microscopy in RD cells. (Supplementary Materials and Methods).
Animals
Female 8-week-old C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). The P14 mice bearing the DbGP33-specific T-cell receptor were a kind gift from Dr. John Wherry of the University of Pennsylvania. To generate the “P14 chimera” mice, 1.6×105 naïve T- cell receptor transgenic T cells were adoptively transferred into naïve B6 mice. All animals were conducted and maintained in accordance with the National Institutes of Health and the University of Pennsylvania IACUC guidelines.
Immunization/EP of mice
Mice were immunized three times at three-week intervals in the tibialis anterior muscle. In vivo Electroporation (EP) was delivered, with the CELLECTRA adaptive constant current EP device (Inovio Pharmaceuticals, Blue Bell, PA), at the same site immediately following vaccination as described (20). The mice (n=4–5) were immunized with either 5μg pVAX1 or 5μg ConE6E7 alone or with various amounts of proIL-33 and mtrIL-33 constructs, depending on the experiment. The GP33 construct was administered at 5μg. All studies were repeated twice.
ELISpot assays
Spleens were harvested 8 days following the final immunization as previously described (20). After spleens were harvested and processed both IFN-γ and IL-4 ELISpot assays were performed to determine antigen-specific cytokine secretion from immunized mice as described previously (20,21,22).
Flow Cytometry
Lymphocytes were isolated and processed from the spleen and peripheral blood as previously described (20, 23, 24). The antibodies used in the present study are listed in the Supplementary Materials and Methods.
Tumor Cell line
TC-1 cells were purchased from ATCC and cultured as previously described (25). The TC-1 cell line was a graciously given gift from Dr. Yvonne Paterson of the University of Pennsylvania, Philadelphia, PA, USA. The TC-1 cell line is well-characterized, constitutively expresses E6 and E7, and is highly tumorigenic (25, 26). TC-1 cells were prepared and mixed with Matrigel (BD Bioscience) for subcutaneous (s.c.) tumor implantation.
In vivo tumor treatment (regression) study
Female B6 mice were separated into four groups of 10 mice each and 5 × 104 TC-1 cells were s.c. implanted into the flanks of each wild-type female B6 mice. On days 4, (after tumor implantation and when tumors reached 3mm), each group of mice was immunized i.m./EP with pVAX, ConE6E7, ConE6E7 proIL-33 and ConE6E7 mtrIL-33, respectively and boosted on day 11 and 18. Mice were monitored twice a week for tumor growth and were measured as described previously (21,26).
Statistical analysis
Student’s t-test was applied for comparison of the quantitative data of the cellular immune response and tumor diameters. Error bars indicate SEM and all tests were performed using Prism Software (***P < 0.001, **P < 0.01, *P < 0.05 compared with ConE6E7).
RESULTS
Construction and expression of IL-33 isoforms
Two IL-33 adjuvants constructs (pro-IL33 and mtrIL-33) were designed and generated to test our working hypothesis (Fig. 1A). To determine the expression of both IL-33 isoforms, human rhabdomyosarcoma (RD) cells were transfected separately with each construct, and expression was assessed by Western immunoblotting. A ~20kDA protein was observed for mtrIL-33 and a ~30kDA and ~20kDA protein size was observed for proIL-33, in cell lyates harvested 48 hours after transfection using an anti-IL33 monoclonal antibody (mAb) for detection (Fig. 1B). For a comparative control, no protein band could be detected in the negative pVAX control. To examine the cytokine secretion of both isoforms, cell supernatants were obtained 48 hours after transfection in RD cells and the detection of cytokine secretion into the extracellular environment were carried out by enzyme-linked immunosorbent assays (ELISAs). As shown in Fig. 1C, supernatants from mtrIL-33 and proIL-33 transfected RD cells contained mtrIL-33 and proIL-33 at concentrations of roughly 20,000 pg/ml and 600 pg/ml, respectively. Finally, the expression for both IL-33 isoforms was further confirmed using immunoflourescent staining using an anti-IL33 mAb. ProIL-33 can act both as a secreted cytokine and as a nuclear binding factor (19). ProIL-33 nuclear localization is mediated by the nuclear localization signal in its N-terminus, which also contains a chromatin-binding motif (Fig. 1A). However, the cleavage of proIL-33 into mtrIL-33 yields a truncated IL-33 that lacks the nuclear localization signal found in proIL-33. As projected, high nuclear expression with some cytoplasmic expression was observed in the proIL-33 transfected cells (Fig. 1D, bottom). This is an important observation which supports previous findings showing that proIL-33 cytokine can also be expressed and found in the cytoplasm (27). In contrast, only high cytoplasmic expression was visualized in the mtrIL-33 transfected cells shown in Fig. 1D, middle.
Figure 1. Expression and secretion of mtrIL-33 and proIL-33 DNA vaccine constructs.
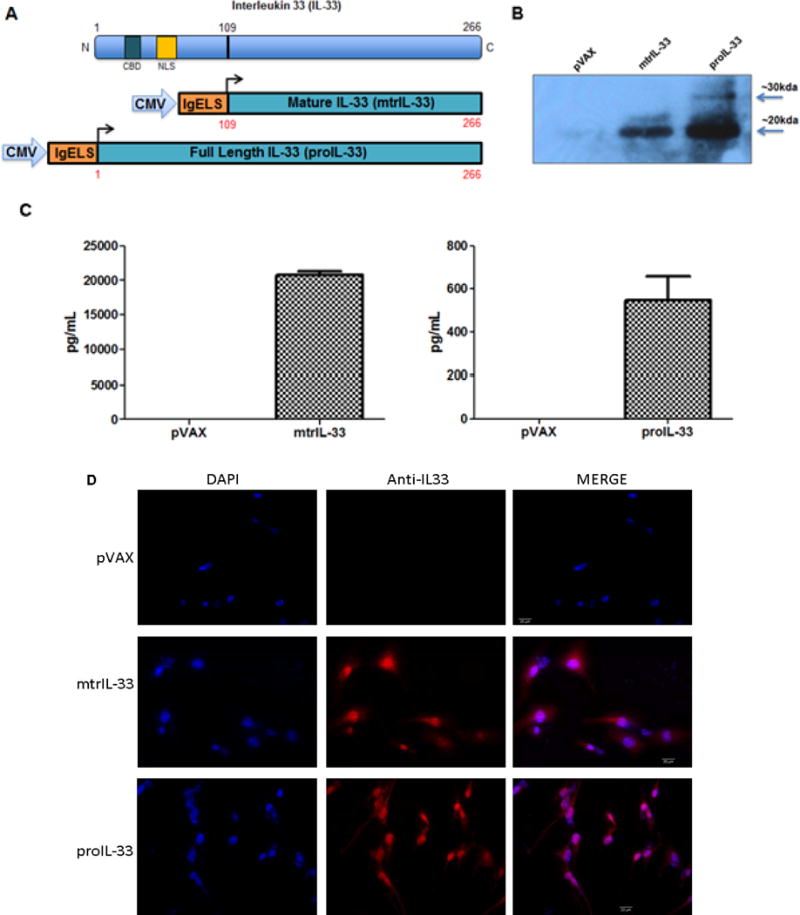
(A) Schematic representation of IL-33 protein and the IL-33 adjuvant constructs encompassing the proIL-33 and mtrIL-33 under the CMV promoter. All constructs contain an IgE leader sequence. The N-terminus domain of IL-33 contains a chromatin-binding motif (CBM) and nuclear localization signal (NLS). (B) Expression of mtrIL-33 and proIL-33 constructs in RD cells as examined by Western blot analysis. Labeled lanes show proteins detected by anti-IL33 mAb. Smaller band represents mtrIL-33, while the larger band represents proIL-33. (C) Secretion of IL-33 from transfected RD cells was confirmed via ELISA. Data shows the means with standard error of the means (SEM) for two replicate assays. (D) Detection of expression of mtrIL-33 and proIL-33 via Immunofluorescence microscopy.
IL-33 adjuvant isoforms enhance potent HPV16 E6/E7-specific cellular immune responses following vaccination
A quantitative ELISpot assay was used to determine the number of antigen-specific IFN-γ secreting cells in response to stimulation with the E6 and E7 peptide pool. As we have reported, electroporation (EP) improves the immunogenicity or potency of DNA vaccines by increasing antigen expression (28,29), thus we performed ConE6E7/EP intramuscular (i.m.) vaccination in C57BL/6 (B6) mice (n=5) with a dosage of 5μg alone or in combination with either mtrIL-33 or proIL-33 at various doses followed by EP. One week after final immunization we monitored the degree of immune responses by isolating splenocytes for further analysis (Fig. 2A). As shown in Fig. 2B, the critical role of IL-33 to drive Th1-polarized immune responses is clearly demonstrated. Co-immunization with both adjuvant cytokine-encoding plasmids induced higher numbers of E6- and E7-specific IFN-γ secreting T cells at all doses when compared with ConE6E7 alone-vaccinated mice (~500 SFU per million splenocytes). As noted in Fig. 2B, the optimal dose of either the mtrIL-33 or proIL-33 (7μg) resulted in a total 4 and 3.5-fold increases in IFN-γ responses, respectively. Due to earlier reports suggesting that IL-33 was a key cytokine in the induction and support of a Th2 response (6,15,16), we assessed whether IL-33 induced the prototypical Th2 cytokine, Interleukin-4 (IL-4) via IL-4 ELISpot. Our data reveals that neither form of IL-33 drove a robust secretion of IL-4 (Fig. 2C). Instead, IL-33 as an adjuvant skewed towards the Th-1 biased axis, and not the Th2 cytokine associated immune responses as originally described (18).
Figure 2. Immunoadjuvants IL-33 isoforms enhance strong HPV16 E6- and E7-specific IFN-γ immune responses, but no IL-4 responses.
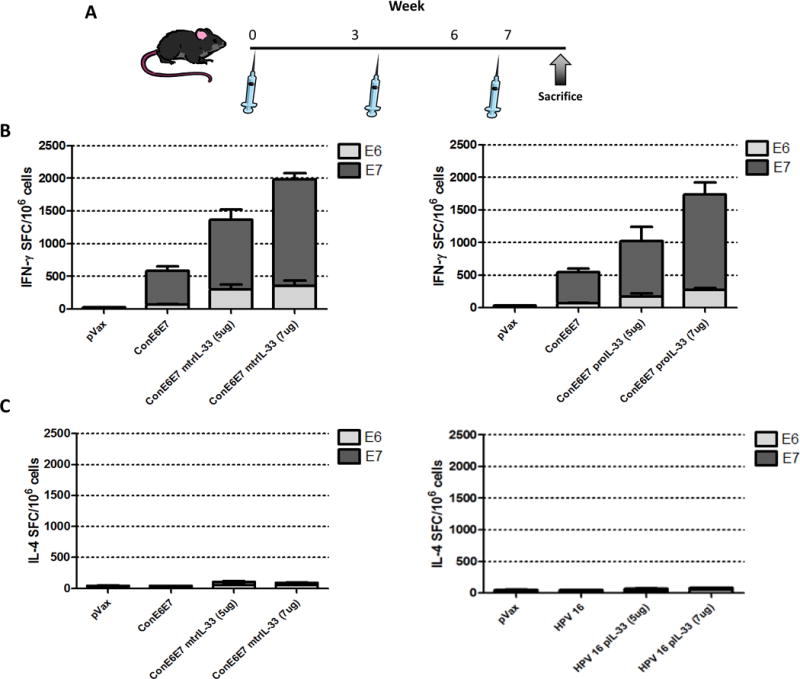
(A) DNA vaccine immunization schedule for adjuvant study. C57BL/6 (B6) mice (n=5 per group) were immunized at weeks 0, 3, and 6 with HPV16 consensus E6/E7 (ConE6E7) construct with or without adjuvant via intramuscular/EP and spleens were harvested one week post final immunization to assess the cellular immune responses. (B) The induction of a Th1 response is shown by the frequency of HPV16 E6 and E7-specific IFN-γ spot-forming units (SFU) per million splenocytes determined by IFN-γ ELISpot assay. (C) Antigen-specific IL-4 responses measured by IL-4 ELISpot assay. Experiments were performed independently at least two times with similar results.
IL-33 enhances HPV antigen-specific CD4+ and CD8+ T cell immunity
We next characterized the antigen (Ag)-specific phenotype and cytokine production profile of memory T cells generated, using the 7μg dose that induced the optimal adjuvant affect as shown in Fig. 2B. Given the importance of multifunctional CD4+ and CD8+ T cell immunity in the elimination of HPV16-infected cells (5, 30–33), we measured the ability of vaccine-induced Ag-specific T cell populations to secrete IFN-γ and TNF-α, in response to ex vivo E6/E7 pooled peptide stimulation in the spleens. Our gating strategy for intracellular cytokine flow-cytometry analysis is depicted in Fig. 3A. Compared with ConE6E7 vaccination alone, the ConE6E7 co-administered with mtrIL-33 and proIL-33 elicited higher frequency of HPV-specific CD4+ T cells producing either total IFN-γ (mtrIL-33: 0.21%; proIL-33: 0.25%), total TNF-α (mtrIL-33: 0.25%; proIL-33: 0.39%) and dual IFN-γ/TNF-α (mtrIL-33: 0.12%; proIL-33: 0.15%) (Fig. 3B–E). In terms of CD8+ T cells, we observed that vaccination with both IL-33 isoforms elicited substantially higher frequencies of HPV-specific CD8+ T cells producing total IFN-γ (mtrIL-33: 3.68%; proIL-33: 3.50%), total TNF-α (mtrIL-33: 3.11%; proIL-33: 3.13%) and dual IFN-γ/TNF-α (mtrIL-33: 2.83%; proIL-33: 2.75%) (Fig. 4A–C). The same trend was seen with the frequency of Ag-specific CD8+ T cells secreting IFN-γ alone and TNF-α alone (Fig. 4D). Overall, both immunoadjuvant IL-33 isoforms produced similar amounts of Ag-specific CD4+ and CD8+ T cell responses, with cytokine production mediated mainly by CD8+ T cells. The high frequencies of effector cells secreting anti-viral cytokines are indicative of the adjuvant effects of IL-33 to enhance vaccine potency.
Figure 3. Cytokine frequencies of specific CD4+ T cells induced by immunoadjuvants mtrIL-33 and proIL-33.
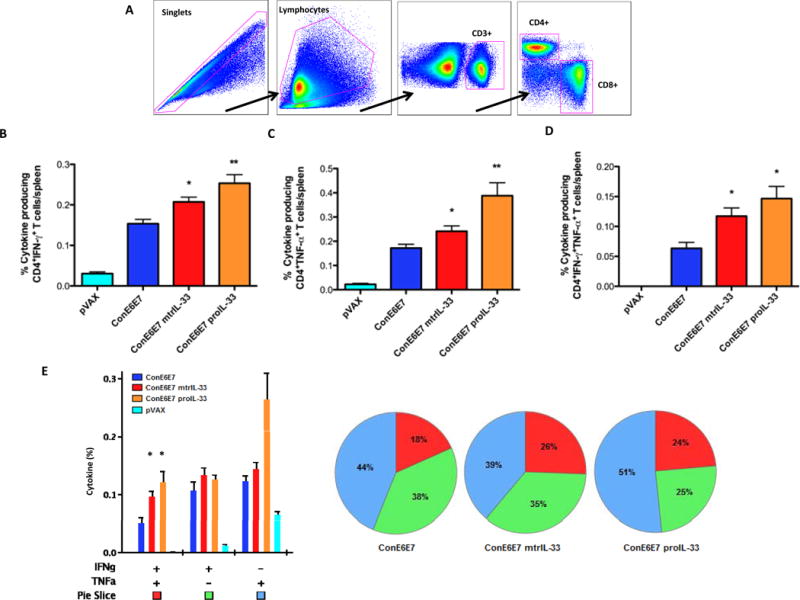
(A) Depicted is the gating strategy used for identifying Ag-specific T cell populations. (B) Column graphs depicting E6/E7-specific CD4+ T cells releasing the cytokines IFN-γ (C) TNF-α and (D) double-positive producing cells (and pVAX control). (E) Column graph shows plurifunctional subpopulations of single- and double-positive CD4+ T cells releasing the cytokine IFN-γ and TNF-α. Pie charts show the relative proportion of each cytokine subpopulation to Ag-specific stimulation. Experiments were performed independently at least two times with similar results with five mice per group.
Figure 4. Cytokine frequencies of specific CD8+ T cells induced by immunoadjuvants mtrIL-33 and proIL-33.
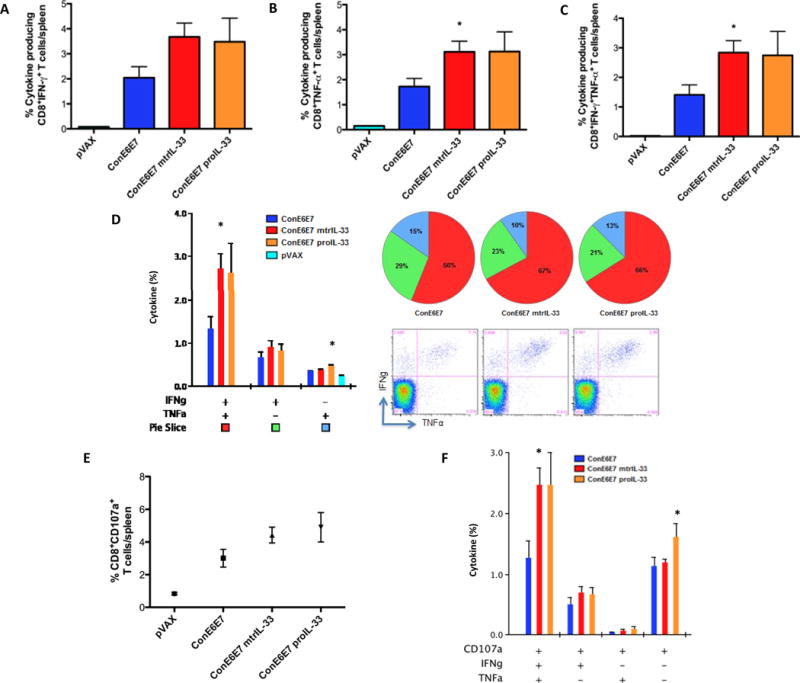
(A) Column graphs depicting E6/E7-specific CD8+ T cells releasing the cytokines IFN-γ (B) TNF-α and (C) double-positive producing cells (and pVAX control). (D) Column graph shows plurifunctional subpopulations of single- and double-positive CD8+ T cells releasing the cytokine IFN-γ and TNF-α. Pie charts show the relative proportion of each cytokine subpopulation to Ag-specific stimulation. Dot plots, representative of four mice is also shown in (D), depicting double positive cytokine expressing CD8+ cells after stimulation with pooled E6/E7 peptide. (E) Antigen-specific cytolytic degranulation T cells were measured by degranulation marker expression, CD107a. (F) Cytokine profile of the cytolytic phenotype. Experiments were performed independently at least two times with similar results with five mice per group.
Given the importance of cytotoxic CD8 T lymphocytes (CTLs) functionality as critical components in protection (34), we characterized the cytotoxic potential of vaccine induced CD8+ T cells undergoing degranulation. CD8+ T cells isolated from mice vaccinated with adjuvant showed a higher frequency of the degranulation marker, CD107a (mtrIL-33: 4.4%; proIL-33: 4.9%), compared to mice that received the ConE6E7 constructs alone (Fig. 4E). More interestingly, the HPV-adjuvanted vaccines elicited substantially higher frequencies of plurifunctional effector CD8+ T cells co-expressing CD107a/IFN-γ/TNF-α (mtrIL-33: 2.5%; proIL-33: 2.5%), compared to the ConE6E7 construct alone (Fig. 4F). These results indicate the adjuvant potential of IL-33 to induce functional effector cytotoxic CTLs, which have a phenotype suggesting the cells ability to clear HPV16 infected cells.
IL-33 role in the induction of humoral responses
Identifying potent adjuvants that not only mediate protective cell-mediated immune responses, but can also induce humoral immune responses, will be ideal for enhancing effective prophylactic and therapeutic vaccines against a variety of microbial infections. Thus, to determine whether mtrIL-33 and proIL-33 influence the level of circulating HPV E6- & E7-specific antibodies, we analyzed humoral responses by ELISA using collected sera obtained one-week post final vaccination. As shown in Fig. 5A, only co-immunization with proIL-33 significantly induced E7-specific total IgG compared to other immunized groups. No E6-specific antibodies were induced or detected (data not shown). In addition, because reports have indicated that IL-33 plays a role in allergic responses we examined E7-specific IgE and total IgE responses in the sera. As illustrated in Fig. 5B and 5C, the adjuvant effects of mtrIL-33 and proIL-33 did not drive enhanced levels of IgE responses compared to control vaccinated groups. This is consistent with the low induction of IL-4 responses shown in Fig. 2C, as IL-4 is known to drive IgE class-switch (35). These results supported that IL-33 adjuvant effects in a DNA vaccination setting do not induce Th2-associated responses. Interesting, only the combination of HPV and proIL-33 increased Ag-specific IgG humoral responses, indicating its role as an effective adjuvant to enhance both Ag-specific cell-mediated and humoral responses.
Figure 5. Humoral responses of ConE6E7 with and without mtrIL-33 and proIL-33 adjuvants.
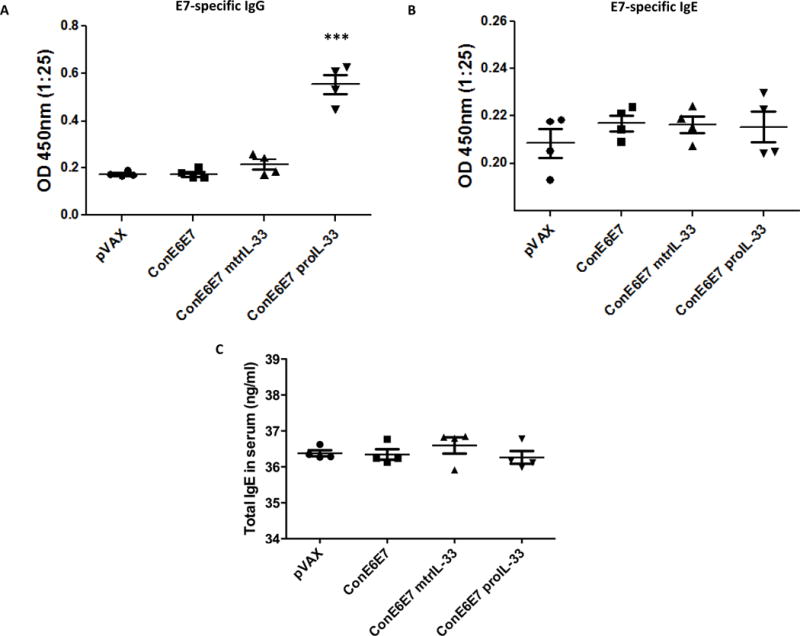
Blood collected from control (pVAX) and immunized mice (n=4) was analyzed for humoral responses via ELISA one week after last immunization. (A) Specific total IgG antibodies against HPV16 E7 (B) Specific IgE antibodies against HPV16 E7. (C) Total IgE antibodies detected in the serum. Experiments were repeated two times with similar results.
IL-33 immunoadjuvants induce potent anti-tumor immunity and regression of established TC-1 tumor-bearing mice
A strong frequency of anti-HPV CD4+ Th1 and CD8+ T cell immunity has been considered a critical characteristic of an effective therapeutic T cell-based vaccine designed to control and eliminate established pre-existing HPV infections and associated lesions (32,36). Given the results that IL-33 acts as a cell-mediated adjuvant eliciting potent HPV Ag-specific Th1-and CD8-biased T cell immune responses, we performed an in vivo tumor therapy study to determine the therapeutic efficacy of IL-33 immunoadjuvants in TC-1 tumor bearing mice. HPV16 E6/E7-expressing TC-1 tumors (5×104) cells were implanted in naïve B6 recipient mice. Four days after TC-1 cell implantation, tumors were measured (tumors had reached a average size of 3 mm) and groups of mice (n=10) were immunized with pVAX, ConE6E7 (5μg) alone, or ConE6E7 co-administered with 7μg of mtrIL-33 or proIL-33, followed with two boosts at one week intervals as outlined in Fig. 6A. As shown in Fig. 6B, tumor growth was substantially rejected in the mtrIL-33 and proIL-33-adjuvanted groups compared with controls. The IL-33 groups remained tumor free until day 42, with the exception of one mouse in the mtrIL-33-adjuvant group. Meanwhile, only 6 mice in the ConE6E7-vaccinated group were tumor free after 42 days and in the control group all mice had died by day 28. Furthermore, as shown in supplementary Fig. 1, both IL-33 isoforms can maintain and elicit anti-tumor memory responses similar to ConE6E7. Clearly, ConE6E7 can easily prevent E6/E7 tumor growth, but have difficulties curing in a tumor therapy study, however the inclusion of IL-33 makes a substantial difference (Fig. 6B). Thus, our data illustrates that HPV-specific T cell immunity induced by both immunoadjuvants provides substantial protective anti-tumor immunity by further delaying or rapidly inducing complete regression of established TC-1 tumors.
Figure 6. Vaccination with IL-33 adjuvants induces regression of established TC-1 tumors.
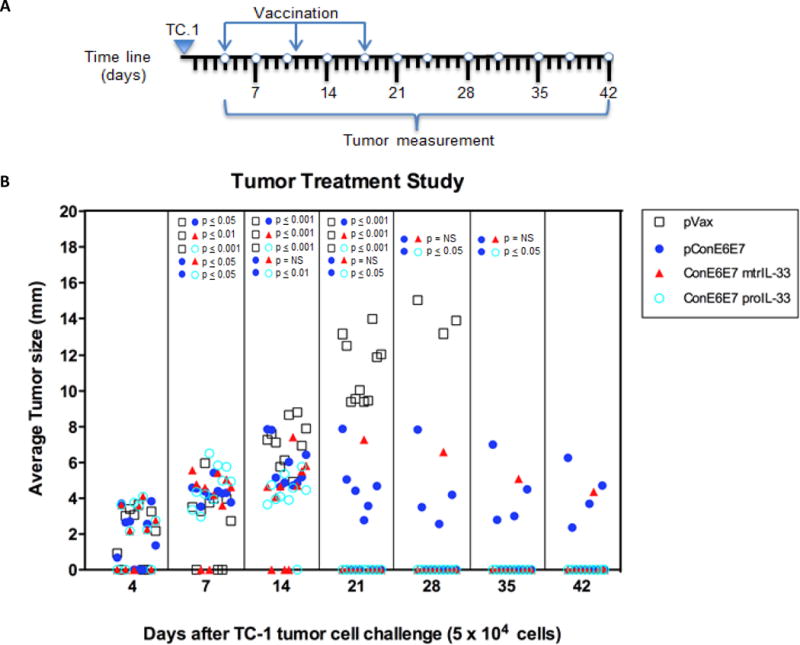
(A) Schematic illustration of the time line of therapeutic study regimen. (B) Groups of B6 mice (10 mice/group) were s.c. challenged with 5×104 TC-1 tumor cells. Tumors were measured twice a week in two dimensions with electronic calipers and data are presented with the average of these values over time for each individual mouse. Mice were sacrificed when tumor diameter reached approximately 2.0 cm. Tumor measurements for each time point are shown only for surviving mice. pVAX immunized mice served as negative control.
IL-33 adjuvant expands Ag-specific CD8+ T cell responses and elicits potent CD62L− KLRG1+ effector-memory CD8+ T cell responses after vaccination
Given the increase of Ag-specific CD8+ T cell responses and the remarkable display of complete tumor regression elicited by immunoadjuvant proIL-33, we examined whether the 100% protective efficacy of proIL-33 was due to its ability to rapidly expand the effector memory CD8+ Ag-specific T cell responses. To achieve this goal, we took advantage of the P14 (DbGP33-specific T cell receptor (TCR)) mouse model, which is a great model for tracking populations of T cell subsets. Therefore, to investigate CD8+ T cell expansion during vaccination with proIL-33, we transferred ~150,000 Ly5.1+ naïve P14 TCR transgenic CD8+ T cells into (n=4/group) naïve wild type recipients to make “P14 chimeric mice” that were subsequently vaccinated with GP33 alone and GP33 coimmunized with proIL-33. The frequency of the Ag-specific CD8+ T cells responses was monitored in the blood during the course of a prime and boost DNA vaccination with or without proIL-33 adjuvant (Fig. 7). As shown in Fig. 7A and Supplementary Fig. 2, the GP33-proIL-33 adjuvanted group dramatically increased the frequency of P14 CD8+Ly5.1+ T cells in the blood, compared to the non-adjuvanted group. This significantly increased frequency (~5-fold) of Ly5.1+ CD8+ T cells peaked at ~14 dpv (days post vaccination) compared to the GP33 immunized group which reached its peak at ~21 dpv. Furthermore, seven days after homologous boosting (48 days after initial vaccination), proIL-33 immunoadjuvant markedly increased the frequency of Ag-specific CD8+ T cells compared to control group (Fig. 7A).
Figure 7. Immunoadjuvant IL-33 expands the frequency and effector memory phenotype of the Ag-specific CD8+ T cells.
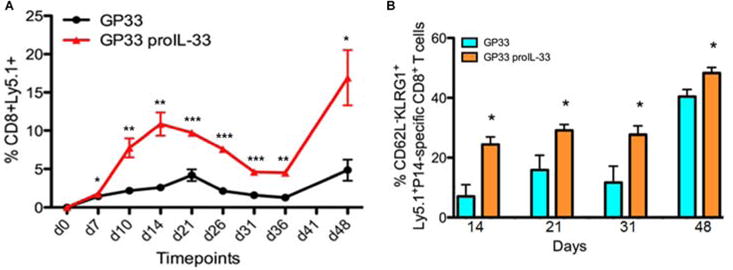
(A) Kinetics of Ly5.1+ expression on P14-specific CD8+ T cells in PBMC following DNA vaccination with a prime at day 0 and a boost at day 41. (B) Distribution of effector memory CD8+ T cell from immunized mice at day 14, 21, 31 after first vaccination and day 48 (day 7 after second vaccination). Data are representative of two independent experiments with four mice per group.
Several studies have suggested that effector CD8+ T cells are the optimal subset for protective immunity and pathogen control (37–39). It has been proposed that a predominant CD62L−KLRG1+ effector-memory T cell response may be a vital prognostic for the efficacy of therapeutic cancer vaccines (40). Thus, starting at 14 dpv we examined the phenotype of the effector CD8 T cells within the vaccine-induced P14-specific CD8+ T cell population based on the cell surface expression markers: Ly5.1, CD62L and KLRG1 (Fig. 7B). As shown in Fig. 7B, the percentages of CD62L−KLRG1+ effector memory cells were significantly higher in the proIL-33 adjuvant group compared to the GP33-only vaccinated group. Secondary memory cells showed a greatly expanded population of KLRG1+ T cells in both groups after homologous DNA boosting, 48 days after initial immunization. The effector-memory responses remained significantly higher in the proIL-33-adjuvanted group compared to GP33-alone group. Together, these results support the notion that IL-33 increases the formation of Ag-specific CD8+ T cells and that IL-33 can enhance clonal expansion of the effector memory pool (12).
Discussion
In this study, we provide insight on the Th1-and CD8-biased adjuvant activity of two isoforms of IL-33-encoding plasmids in a DNA vaccine setting. We demonstrate that IL-33 elicits bonafide Ag-specific Th-1 cell-mediated immune responses to a consensus HPV16 E6/E7 antigen, but not IL-4, nor any elevation of IgE levels as previously described. Clearly both isoforms elicited strong HPV16 Ag-specific polyfunctional CD4+ and CD8+ T cells secreting both anti-viral IFN-γ and/or TNF-α cytokines, and also induced an increase in the Ag-specific cytolytic effector CD8+ T cells undergoing plurifunctional degranulation. More importantly, both IL-33 isoforms were shown to be strong adjuvants when used in conjunction with a therapeutic HPV DNA vaccine to generate robust anti-tumor immunity, facilitating successful tumor regression in established TC-1 tumor-bearing mice.
The major significant difference between proIL-33 and mtrIL-33 was that proIL-33 was able to increase E7-antigen specific IgG levels. However, because mtrIL-33 induced 90% tumor regression, it suggested that T cells mediated the anti-tumor protection, not B-cell responses. Full length IL-33’s dual function property, to act not only as a cytokine, but also as a nuclear transcription factor, may explain the increase in antibody responses by proIL-33. Its nuclear localization may have additive effects on modulating the humoral immune responses. However, the specific transcriptional targets of nuclear IL-33 are still unclear. We are currently pursuing understanding its precise role in the nucleus and its association with modulation of immunogenicity. Although the importance of this finding is not yet clear, the data suggests that proIL-33 could also be useful in vaccine strategies aiming to achieve enhanced antibody responses and cellular immunity. This is an area of further investigation.
The specific roles in the protective responses against HPV infection and associated cancers have been attributed to CD8+ T cell immune responses, and therefore, are the focus for achieving effective immunity by therapeutic treatments against tumors (5,21, 28, 32). Our results reveal that the cytokine secreting T cell responses induced by IL-33 were mainly mediated by eliciting a high frequency of Ag-specific CD8+ T cells co-expressing CD107a/IFN-γ/TNF-α. While Bonilla et al. similarly demonstrated that IL-33 can drive plurifunctional CD8+ T cell responses in a viral infection model (12), we further demonstrated that the delivery of IL-33 as an immunoadjuvant can indeed enhance plurifunctional CD8+ T cell responses, further expanding the pool of information we now know about IL-33. Consistent with this enhanced polyfunctional anti-HPV effector CD8+ and CD4+ T cell immunity, mice vaccinated with IL-33 demonstrated remarkable ability to induce anti-tumor immunity and tumor regression in established TC-1 tumor bearing mice (Fig. 5B). The significantly improved vaccine efficacy offered by IL-33 suggests its potential utility as a vaccine adjuvant. Recently, Luzina et al. demonstrated that mtrIL-33 induced Th2 responses in vivo via a mouse model of pulmonary infection (18). In contrast, we show in vivo that not only proIL-33, but also mtrIL-33, a cleaved form of proIL-33 has pleiotropic properties, and can modulate the immune responses towards a Th1 and CD8 T cell response. It seems that IL-33 may not be a classical Th2 cytokine as originally suggested, but under certain conditions can promote Th1 and CD8 type immunity. It is likely that other immune cells may have accounted for the observed enhancement in Th1 immunity and tumor regression. For instance, IL-33 has been shown to activate Natural Killer (NK) cells (6,41). However, it is unlikely that NK cells could have accounted for the observed enhancement in CD8+ T cell immunity or tumor regression. The HPV E6-E7 vaccine encodes a nuclear antigen that is not lipid based and not targetable by Fc-Receptor bound antibodies directing NK immunity and can only be a target of CD8+ T cells. Furthermore, much prior work in the TC-1 tumor challenge model, including work conducted by our lab, have established that this model is CD8+ T cell dependent for protection (26,32,42–44). Nevertheless, further studies will be needed to elucidate under what conditions IL-33 promotes Th1 and CD8 T cell immunity, and the IL-33 regulatory networks connecting the innate with the adaptive immune response.
It is known that IL-33 exhibits pleiotropic properties and could promote responses other than Th2, such as activating CD8+ T cells (12,13,27). Thus, to investigate the ability of IL-33 to modulate the CD8+ T cells we used the P14 mouse model to monitor the expansion of LCMV DbGP33/Ly5.1+ cells in the P14 chimeras after immunizing mice with a cognate viral Ag. We show in vivo that IL-33 can modulate the expansion of CD8+ T cells in a vaccine setting and observed that inclusion of the IL-33 adjuvant significantly expanded the magnitude of Gp33/Ly5.1+-specific CD8+ T cell responses in the blood. These data demonstrate the overall superiority of immunoadjuvant IL-33 in enhancing the Ag-specific CD8+ T cells in a DNA vaccine. In addition to implying that IL-33 plays an important role in the expansion of CD8+ T cells, it also suggests that IL-33 mediated antitumor immunity and tumor regression in the TC-1 tumor therapy study was probably CD8+ T cell related (Fig. 6). Moreover, as shown in Fig. 7, the peak of CD8 expansion (14 dpv) seemed to correlate with the complete tumor regression mediated by prolL-33, which was 17 days post first vaccination (Fig. 6B). From the increased expansion of CD8 effector T cells elicited by the effects of IL-33 adjuvant properties, we can postulate their important role in providing tumor protection as shown in Fig. 6B. Subsequently, we also demonstrate that a boost vaccination can further expand the formation of Ag-specific CD8+ T cells after a prime vaccination suggesting the potential recall of the established memory CD8+ T cell pool (Fig. 7A). The reasons behind the ability of IL-33 to expand the frequency of CD8+ T cells is not yet entirely clear (12,13,41,45). However, further studies are needed to elucidate these mechanisms.
From a therapeutic point of view, the goal of successful vaccination is the induction of the most potent subsets of CD8+ memory T cell populations to rapidly control infection. Recently, reports have begun to show that the effector-memory KLRG1+CD8+ T cell population can mediate potent protective immunity against certain pathogens (37–39) and might be optimal for immediate regression of established subcutaneous (s.c.) tumors (40). Mice immunized with IL-33 demonstrated robust expansion of activated effector memory CD8+ T cells in the periphery (Fig. 7A), suggesting trafficking of activated CD8+ T cells to the site of Ag stimulation. Our findings support the concept that vaccine-induced effector-memory CD8 T cell responses might be important memory CD8+ T cell subsets for an effective therapeutic vaccine against tumors (40). The high frequency of Ag-specific effector-memory cells in the periphery is consistent with the observation that effector-memory T cells can migrate to the site of infection and initiate immediate effector function (46). Furthermore, these results are in agreement with Bonilla et al., reporting IL-33 is important for primary effector CD8 T cell responses (12). However, they show that IL-33 may not play a role in memory responses, while our findings suggest that in certain cases it may play an important role. The reasons for the differences between the two studies are currently unknown, but may be due to differences in model systems. We also demonstrate that secondary memory cells after boost showed a greater formation of CD62L−KLRG1+ cells in the periphery (Figure 7B). Together, these results indicate that the increase in the frequency and phenotype of the IL-33-adjuvanted vaccine-induced Ag-specific P14 CD8+ T cells after a prime and boost vaccination may be a prediction of the protective correlates of immunity behind the therapeutic efficacy of immunoadjuvant IL-33 against the established TC-1 tumors (Fig. 6B). We are currently investigating the ability of IL-33 to generate central memory immunity, since central memory T cells are important subsets of memory CD8+ T cells that also mediate optimal protective immunity against pathogens (47,48). Overall, understanding the mechanism of action by which IL-33 influences the expansion and development of heterogeneous CD8+ T cell populations in vaccines is an important area for further investigation. Altogether, these results support evidence that IL-33 acts as a potent adjuvant capable of inducing and modulating potent Ag-specific cell-mediated immunity in a variety of pathogens.
In summary, we provide insight into the biological function of proIL-33 and mtrIL-33 and its affects on modulating the adaptive immune responses in vivo, inducing potent Ag-specific anti-viral and anti-tumor Th1 and CD8+ T cell immunity, which resulted in effective tumor regression. This study provides evidence that immunoadjuvant IL-33 elicits its affects by enhancing the formation of the Ag-specific effector CD8+ T cells and markedly amplifying the effector-memory CD8+ T cells responses. These findings, we believe, establish the validity of IL-33 as a new adjuvant for consideration in the context of immune-therapies, in particular, for cancer vaccine therapies.
Supplementary Material
Acknowledgments
The authors thank Dr. Yvonne Paterson for the TC-1 cell line, Dr. John Wherry for providing the transgenic P14 mice and Dr. Rafi Ahmed for the GP33 DNA plasmid. The authors also thank Ms. Rebekah Siefert and Ms. Carolina Pombo for their technical help.
Footnotes
Disclosure: D.B.W. has grant funding, participates in industry collaborations, has received speaking honoraria, and fees for consulting. This service includes serving on scientific review committees and advisory boards. Remuneration includes direct payments or stock or stock options and in the interest of disclosure therefore he notes potential conflicts associated with this work with Pfizer, Bristol Myers Squibb, Inovio, Touchlight, oncosec, Merck, VGXI, and possibly others. Licensing of technology from his laboratory has created over 100 jobs in the private sector in the biotech/pharma industry. The other authors declare no competing financial interests. No writing assistance was utilized in the production of this manuscript.
References
- 1.Miller AM. Role of IL-33 in inflammation and disease. J Inflamm. 2011;8:22. doi: 10.1186/1476-9255-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindblad EB. Aluminium adjuvants–in retrospect and prospect. Vaccine. 2004;22:3658–68. doi: 10.1016/j.vaccine.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 3.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalams SA, Parker SD, Elizaga M, Metch B, Edupuganti S, Hural J, et al. Safety and Comparative Immunogenicity of an HIV-1 DNA Vaccine in Combination with Plasmid Interleukin 12 and Impact of Intramuscular Electroporation for Delivery. J Infect Dis. 2013;208:818–29. doi: 10.1093/infdis/jit236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villarreal DO, Talbott KT, Choo DK, Shedlock DJ, Weiner DB. Synthetic DNA vaccine strategies against persistent viral infections. Expert Rev Vaccines. 2013;12:537–54. doi: 10.1586/erv.13.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liew FY, Pitman NI, McInnes IB. Disease-associated functions of IL-33: the new kid in the IL-1 family. Nat Rev Immunol. 2010;10:103–10. doi: 10.1038/nri2692. [DOI] [PubMed] [Google Scholar]
- 7.Haraldsen G, Balogh J, Pollheimer J, Sponhiem J, Kuchler Interleukin-33 - cytokine of dual function or novel alarmin? Trends Immunol. 2009;30:227–33. doi: 10.1016/j.it.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Cayrol C, Girard JP. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc Natl Acad Sci USA. 2009;106:9021–6. doi: 10.1073/pnas.0812690106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yagami A, Orihara K, Morita H, Futamura K, Hashimoto N, Matsumoto K, et al. IL-33 mediates inflammatory responses in human lung tissue cells. J Immunol. 2010;185:5743–50. doi: 10.4049/jimmunol.0903818. [DOI] [PubMed] [Google Scholar]
- 10.Yagami A, Orihara K, Morita H, Futamura K, Hasimoto N, Matsumoto K, et al. Full-length IL-33 promotes inflammation but not Th2 response in vivo in an ST2-independent fashion. J Immunol. 2012;189:403–10. doi: 10.4049/jimmunol.1200259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rank MA, Kobayashi T, Kozaki H, Bartemes KR, Squillace DL, Kita H. IL-33-activated dendritic cells induce an atypical TH2-type response. J Allergy Clin Immunol. 2009;123:1047–54. doi: 10.1016/j.jaci.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonilla WV, Frohlich A, Senn K, Kallert S, Fernandez M, Johnson S, et al. The alarmin interleukin-33 drives protective antiviral CD8(+) T cell responses. Science. 2012;335:984–9. doi: 10.1126/science.1215418. [DOI] [PubMed] [Google Scholar]
- 13.Yang Q, Li G, Zhu Y, Liu L, Chen E, Turnquist H, et al. IL-33 synergizes with TCR and IL-12 signaling to promote the effector function of CD8+ T cells. Eur J Immunol. 2011;41:3351–60. doi: 10.1002/eji.201141629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moussion C, Ortega N, Girard JP. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One. 2008;3:3331. doi: 10.1371/journal.pone.0003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller AM. Role of IL-33 in inflammation and disease. J Inflamm. 2011;8:22. doi: 10.1186/1476-9255-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer G, Gabay C. Interleukin-33 biology with potential insights into human diseases. Nat Rev Rheumatol. 2011;7:321–9. doi: 10.1038/nrrheum.2011.53. [DOI] [PubMed] [Google Scholar]
- 17.Lefrançais E, Roga S, Gautier V, Gonzalez-de-Peredo A, Monsarrat B, Girard JP, Cayrol C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc Natl Acad Sci USA. 2012;109:1673–8. doi: 10.1073/pnas.1115884109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luzina IG, Pickering EM, Kopach P, Kang PH, Lockatell V, Todd NW, et al. Full-length IL-33 promotes inflammation but not Th2 response in vivo in an ST2-independent fashion. J Immunol. 2012;189:403–10. doi: 10.4049/jimmunol.1200259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci U S A. 2007;104:282–7. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shedlock DJ, Aviles J, Talbott KT, Wong G, Wu SJ, Villarreal DO, et al. Induction of broad cytotoxic T cells by protective DNA vaccination against marburg and ebola. Mol Ther. 2013;21:1432. doi: 10.1038/mt.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan J, ReichenBach DK, Corbitt N, Hokey DA, Ramananthan MP, McKinney KA, et al. Induction of antitumor immunity in vivo following delivery of a novel HPV-16 DNA vaccine encoding an E6/E7 fusion antigen. Vaccine. 2009;27:431–40. doi: 10.1016/j.vaccine.2008.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obeng-Adjei N, Choo DK, Weiner DB. Hydrodynamic immunization leads to poor CD8 T cell expansion, low frequency of memory CTLs and ineffective antiviral protection. Cancer Gene Therapy. 2013;20:552–63. doi: 10.1038/cgt.2013.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrow MP, Pankhong P, Laddy DJ, Schoenly KA, Yan J, Cisper N, et al. Comparative ability of IL-12 and IL-28B to regulate T reg populations and ehance adaptive cellular immunity. Blood. 2009;113:5868–77. doi: 10.1182/blood-2008-11-190520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angelosnato JM, Blackburn SD, Crawford A, Wherry EJ. Progressive Loss of Memory T cell potential and commitment to exhuastion during chronic viral infection. J Virol. 2012;86:8161–70. doi: 10.1128/JVI.00889-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guirnalda PD, Paterson Y. Vaccination with immunotherapeutic Listeria monocytogenes induces IL-17 (+) γδ T cells in a murine model for HPV associated cancer. Oncoimmunology. 2012;1:822–828. doi: 10.4161/onci.20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol. 2001;167:6471–9. doi: 10.4049/jimmunol.167.11.6471. [DOI] [PubMed] [Google Scholar]
- 27.Kakkar R, Hei H, Dobner S, Lee RT. Interleukin 33 as a mechanically responsive cytokine secreted by living cells. J Biol Chem. 2012;287:6941–8. doi: 10.1074/jbc.M111.298703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bagarazzi ML, Yan J, Morrow MP, Shen X, Parker RL, Lee JC, et al. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci Transl Med. 2012;4:155. doi: 10.1126/scitranslmed.3004414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sardesai NY, Weiner DB. Electroporation delivery of DNA vaccines: prospects for success. Curr Opin Immunol. 2011;23:421–9. doi: 10.1016/j.coi.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hung K, Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, Levitsky H. The central role of CD4(+) T cells in the antitumor immune response. J Exp Med. 1998;188:2357–68. doi: 10.1084/jem.188.12.2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamikanra A, Pan ZK, Isaacs SN, Wu TC, Paterson Y. Regression of established human papillomavirus type 16 (HPV-16) immortalized tumors in vivo by vaccinia viruses expressing different forms of HPV-16 E7 correlates with enhanced CD8(+) T-cell responses that home to the tumor site. J Virol. 2001;75:9654–64. doi: 10.1128/JVI.75.20.9654-9664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrow MP, Yan J, Sardesai NY. Human papillomavirus therapeutic vaccines: targeting viral antigens as immunotherapy for precancerous disease and cancer. Expert Rev Vaccines. 2013;12:271–83. doi: 10.1586/erv.13.23. [DOI] [PubMed] [Google Scholar]
- 33.Daniel D, Chiu C, Giraudo E, Inoue M, Mizzen LA, Chu NR, et al. CD4+ T cell-mediated antigen-specific immunotherapy in a mouse model of cervical cancer. Cancer Res. 2005;65:2018–25. doi: 10.1158/0008-5472.CAN-04-3444. [DOI] [PubMed] [Google Scholar]
- 34.Appay V, van Lier RA, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: consensus and issues. Cytometry A. 2008;73:975–83. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- 35.Gould HJ, Sutton BJS. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205–17. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 36.Lin CT, Chang TC, Shaw SW, Cheng PJ, Huang CT, Chao A, et al. Maintenance of CD8 effector T cells by CD4 helper T cells eradicates growing tumors and promotes long-term tumor immunity. Vaccine. 2006;24:6199–207. doi: 10.1016/j.vaccine.2006.05.108. [DOI] [PubMed] [Google Scholar]
- 37.Olson JA, McDonald-Hyman C, Jameson Sc, Hamilton SE. Effector-like CD8(+) T cells in the memory population mediate potent protective immunity. Immunity. 2013;38:1250–60. doi: 10.1016/j.immuni.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye F, Turner J, Flano E. Contribution of pulmonary KLRG1(high) and KLRG1(low) CD8 T cells to effector and memory responses during influenza virus infection. J Immunol. 2012;189:5206–11. doi: 10.4049/jimmunol.1200137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bachmann MF, Wolint P, Schwarz K, Oxenius A. Recall proliferation potential of memory CD8+ T cells and antiviral protection. J Immunol. 2005;175:4677–85. doi: 10.4049/jimmunol.175.7.4677. [DOI] [PubMed] [Google Scholar]
- 40.van Duikeren S, Fransen MF, Redeker A, Wieles B, Platenburg G, Krebber WJ, et al. Vaccine-induced effector-memory CD8+ T cell responses predict therapeutic efficacy against tumors. J Immunol. 2012;189:3397–403. doi: 10.4049/jimmunol.1201540. [DOI] [PubMed] [Google Scholar]
- 41.Bourgeois E, Van LP, Samson M, Diem S, Barra A, Roga S, Gombert JM, et al. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. Eur J Immunol. 2009;39:1046–55. doi: 10.1002/eji.200838575. [DOI] [PubMed] [Google Scholar]
- 42.Indrova M, Bieblova J, Bubenik J, Reinis M. IL-12 immunotherapy of minimal residual disease in murin models of HPV16-associated tumours: induction of immune responses, cytokine production and kinetics of immune cell subsets. Int J Oncol. 2008;32:499–507. [PubMed] [Google Scholar]
- 43.Indrova M, Simova J, Bieblova J, Bubenik J, Reinis M. NK1.1+ cells are important for the development of protective immunity against MHC I-deficient, HPV16-associated tumours. Oncol Rep. 2011;25:281–8. [PubMed] [Google Scholar]
- 44.Khairuddin N, Blake SJ, Firdaus F, Steptoe RJ, Behlke MA, Hertzog PJ, et al. In vivo comparison of local versus systemic delivery of immunostimulating siRNA in HPV-driven tumours. Immunol Cell Biol. 2013 doi: 10.1038/icb.2013.75. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 45.Choi YS, Park JA, Kim J, Rho SS, Park H, Kim YM, Kwon YG. Nuclear IL-33 is a transcriptional regulator of NF-κB p65 and induces endothelial cell activation. Biochem Biophys Res Commun. 2012;421:305–11. doi: 10.1016/j.bbrc.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 46.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–42. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 47.Laouar A, Manocha M, Haridas V, Majuanth N. Concurrent generation of effector and central memory CD8 T cells during vaccinia virus infection. PLoS One. 2008;3:4089. doi: 10.1371/journal.pone.0004089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–34. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


