Summary
We compared different techniques for measuring gut HIV reservoirs and assessed for HIV in non-CD4+T cells. HIV DNA levels were similar when measured from rectal biopsies and isolated rectal cells, while HIV RNA tended to be higher in rectal cells. HIV DNA levels in total rectal cells were greater than those predicted from levels in sorted CD4+T cells, suggesting a reservoir in non-CD4+T cells, and HIV DNA was detected in sorted myeloid cells (7/7 subjects).
Keywords: HIV, HIV-1, Intestines, Rectum, Myeloid Cells
Introduction
In HIV-infected patients receiving antiretroviral therapy (ART), most infected cells persist in the lymphoid tissues and gut[1-12], which serve as major obstacles to eradication. However, it is unclear what types of tissue samples are best for measuring HIV levels, and whether all of the HIV is found in CD4+T cells. The goals of this study were: 1) to compare HIV levels in intact biopsies, dissociated cells, and sorted cells; 2) to determine whether the above measurements are impacted by the method used to measure cell equivalents; 3) to assess for HIV in non-CD4+T cells by comparing HIV levels measured in sorted CD4+T cells and unsorted total gut cells; and 4) to measure HIV levels in sorted myeloid cells.
Methods
Blood and rectal biopsies were obtained from 7 ART-suppressed HIV+ subjects with CD4+T cell count≥350 cells/μl and viral load<50 copies/ml for ≥6 months. The study was approved by the local Institutional Review Board. All participants provided informed consent. Six biopsies were immediately frozen and 18 were dissociated to total gut cells by collagenase digestion [11]. Of these total gut cells, 40% were frozen and 60% were sorted for CD4+T cells (CD3+CD4+) and non-T leukocytes (CD45+CD3-) expressing CD13+ (aminopeptidase N, found on myeloid cells)[13]. DNA and RNA were extracted from intact biopsies, total gut cells, and sorted cells using Trireagent[13]. RNA was DNase-treated and purified using QIAgen RNeasy columns[13]. HIV levels were measured by qPCR for the LTR[13]. The cell equivalents in the extracted nucleic acid were determined by: 1) DNA mass (assuming 1μg=160,000 cells) or RNA mass (assuming 1μg =106 cells[14]), as measured by Nanodrop; and 2) qPCR for TERT DNA or GAPDH RNA[12, 13]. Statistical comparisons (Wilcoxon signed rank test) and correlations (Spearman test) were analyzed using GraphPad Prism 5.0.
Results
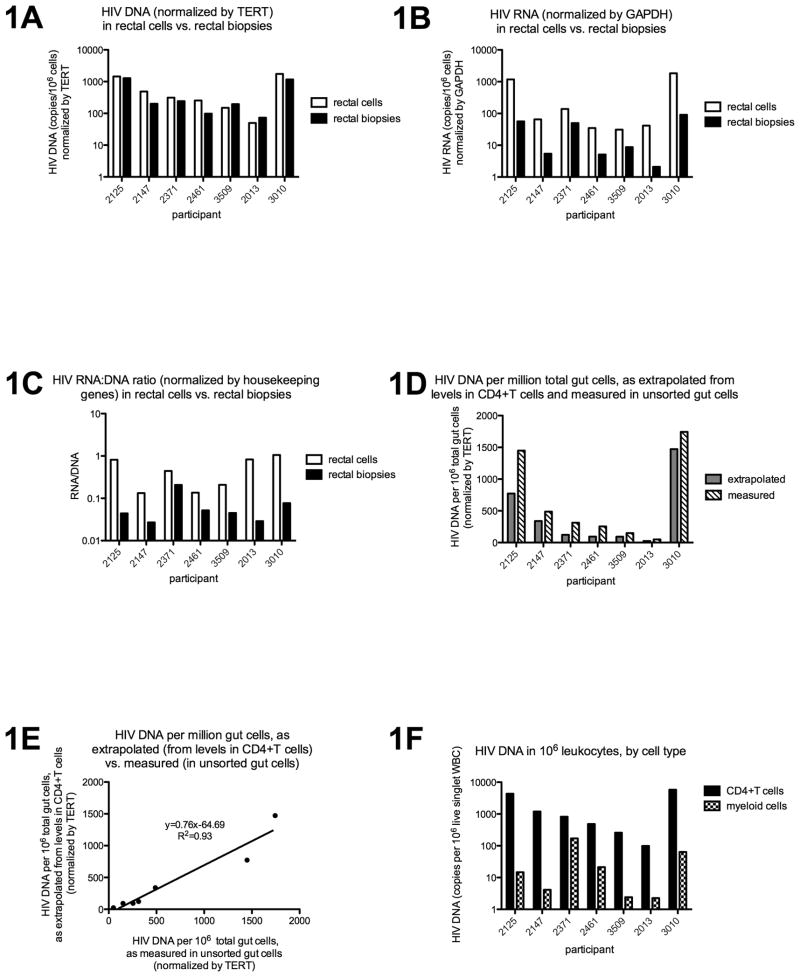
There was a linear correlation between HIV DNA as normalized by DNA mass and by TERT (r=0.97, p<0.0001), and between HIV RNA as normalized by RNA mass and GAPDH (r=0.90, p<0.0001). HIV DNA levels were not significantly different when measured from intact rectal biopsies and isolated rectal cells (Figure 1A), and the two measures correlated strongly (r=0.89, p=0.012). In contrast, HIV RNA levels tended to be higher in rectal cells than in biopsies (Figure 1B; p=0.016), although the two measures tended to correlate (r=0.75, p=0.066). The average HIV transcription per infected cell also tended to be higher in rectal cells (Figure 1C; p=0.016), although no significant correlation was observed.
Figure 1.
DNA and RNA were extracted from intact rectal biopsies, total rectal cells, sorted rectal CD4+T cells, and sorted rectal myeloid cells using Trireagent. HIV DNA and RNA levels were measured by qPCR or qRT-pCR and then normalized to cell equivalents using TERT or GAPDH. HIV DNA (1A) was not significantly different when measured from rectal cells (white bars) and rectal biopsies (black bars), while HIV RNA (1B) and RNA:DNA (1C) tended to be greater in rectal cells compared to biopsies. The measured HIV levels in sorted CD4+T cells were used in combination with the measured frequency of these cells (percent of all gut cells) to extrapolate HIV levels in total gut cells (grey bars, 1D), which were then compared to actual HIV levels measured in total gut cells (dashed bars, 1D). The two measures tended to correlate (1E), although extrapolation from CD4+T cells consistently underestimated the HIV DNA measured in total gut cells (1E, slope = 0.76), suggesting a reservoir in non-CD4+T cells. HIV DNA was also detected in myeloid cells in all 7 subjects (1F). The HIV DNA levels in sorted CD4+T cells (black bars) and myeloid cells (granulated bars) were normalized to cell number (copies/million cells of that type) using TERT, and then further normalized by the frequency of each cell type (as percent of live singlet WBC) to obtain the HIV DNA per million WBC. For 1A-C and 1F, results are plotted on a log scale.
When HIV DNA levels measured in sorted rectal CD4+T cells were used in combination with the measured rectal CD4+T cell frequencies to calculate the HIV DNA levels in total rectal cells, the results correlated well with those measured in unsorted rectal cells (r=1.0, p=0.0004). However, the HIV DNA as extrapolated from CD4+T cells consistently underestimated the HIV DNA in unsorted rectal cells (Figure 1D: 7/7 subjects, p=0.016; Figure 1E: m=0.76). In contrast, extrapolation from CD4+T cells tended to overestimate the HIV RNA in unsorted rectal cells (6/7 subjects; p=0.16) and no significant correlation was observed.
Despite low yields of sorted cells, HIV DNA was detected in rectal myeloid cells in 7/7 subjects (Figure 1F) and HIV RNA was detected in one subject. However, when normalized by cell frequencies (copies/million live singlet WBC), myeloid cells accounted for an average of only 4% of the total HIV DNA.
Discussion
Gut HIV DNA levels were not significantly different when measured in intact biopsies and isolated gut cells, while HIV RNA levels and RNA:DNA ratios tended to be higher in the isolated cells. Collagenase digestion and cell straining may result in disproportionate loss of certain cell types, and new infection may occur during cell isolation. However, either explanation should result in differences in HIV DNA. Alternatively, cell isolation may induce selective transcription of HIV or degradation of cellular RNA. The use of whole biopsies may avoid changes due to sample processing, while tissue digestion allows for flow cytometry and cell sorting, which allows more extensive phenotyping, easier normalization to CD4+T cell numbers, and assessment of HIV levels in different cell types.
HIV DNA levels in total rectal cells were greater than those predicted from levels in sorted CD4+T cells, suggesting a reservoir in non-CD4+T cells. HIV has been detected in many other cell types[15]. This finding is subject to several caveats: 1) flow cytometric measurements of CD4+T cell frequencies may not reflect the composition of the unsorted cells; 2) HIV-infected CD4+T cells may die or downregulate CD4 prior to sorting. However, these factors should cause parallel changes in HIV DNA and RNA, while extrapolation from CD4+T cells tended to overestimate the HIV RNA in total cells. The latter result could also reflect selective induction of HIV RNA transcription or degradation of cellular RNA during cell sorting.
To further assess for HIV in non-CD4+T cells, we sorted myeloid cells. HIV DNA was detected in rectal CD13+WBC in 7/7 subjects. Purity checks performed on other HIV+ patients showed <0.5% contamination with CD4+T cells. Also, the CD13+WBC population should contain neutrophils, so the measured levels may underestimate the levels in macrophages. While some studies have suggested that intestinal macrophages are less permissive[16, 17] or non-permissive[18-20] to HIV infection in vitro, other studies have detected HIV RNA, p24, and virions on gut macrophages from untreated patients[21, 22], while p24 has been detected on duodenal macrophages from ART-suppressed patients[23]. HIV-infected monocytes may home to the gut and differentiate into infected macrophages[24]. However, both neutrophils and macrophages could harbor phagocytosed proviral DNA. Also, CD13+WBC accounted for an average of only 4% of the total HIV DNA, suggesting a reservoir other than CD4+T cells or myeloid cells.
Further study is needed to confirm that HIV can persist in tissue cells other than CD4+T cells. These studies are challenging because of the difficulties in isolating cells from tissue and the fact that cell isolation can perturb the sample. Nevertheless, such studies are critical because the tissues are the largest reservoirs for HIV-infected cells, and tissue reservoirs and mechanisms of persistence may differ from those that govern HIV persistence in the blood.
Acknowledgments
We thank the following people and institutions: 1) the study participants; 2) the staff at the GI Procedures Unit at San Francisco General Hospital; and 3) the staff at the UCSF Core Immunology Lab, including Alex Carvidi. This work was supported by the U.S. Department of Veterans Affairs [1 IK2 CX000520-01 (to SY), I01 BX000192 (to JW)]; the Delaney AIDS Research Enterprise (DARE) [U19AI096109]; the UCSF-Gladstone Center for AIDS Research (CFAR) [P30-AI027763]; the National Cancer Institute [K23 CA157929 (to MS)]; and the National Institute of Allergy and Infectious Diseases at the National Institutes of Health [R56AI091573 [JW, SY, AS, DH] and K24 AI069994 (to SD)].
Sources of support include: the U.S. Department of Veterans Affairs [1 IK2 CX000520-01 (to SY), I01 BX000192 (to JW)]; the Delaney AIDS Research Enterprise (DARE) [U19AI096109]; the UCSF-Gladstone Center for AIDS Research (CFAR) [P30-AI027763]; the National Cancer Institute [K23 CA157929 (to MS)]; and the National Institute of Allergy and Infectious Diseases at the National Institutes of Health [R56AI091573 [JW, SY, AS, DH] and K24 AI069994 (to SD)].
Footnotes
Request for reprints should be made to: Steven A. Yukl, San Francisco VA Medical Center, 4150 Clement St, 111W3, San Francisco, CA 94121, steven.yukl@ucsf.edu
Justification for author number: Though this was a small pilot study, it would not have been possible without the combined efforts of many people, and all of the authors made significant contributions. Therefore, the corresponding author felt it would be unfair not to recognize the efforts of these twelve individuals.
No authors have a commercial or other association that might pose a conflict of interest.
This data was presented in part at the 20th Conference on Retroviruses and Opportunistic Infections, March 3-6, 2013, Atlanta, Georgia, USA (Abstract D-152; poster #372; session #15/80).
Author Contributions: SAY and HH conceived the study; ES devised the sorting strategy. HH, PWH, and SGD recruited subjects. MS performed endoscopies. SAY and MK processed gut and blood samples. SAY performed extractions and assays for HIV and housekeeping genes; PL contributed reagents. SAY, HH, ES, VG, LE, SGD, and JKW analyzed data. SAY wrote the first draft of the manuscript. SAY, HH, PH, MS, DH, SGD, JKW, and ES contributed to writing the manuscript.
References
- 1.Talal AH, Monard S, Vesanen M, Zheng Z, Hurley A, Cao Y, et al. Virologic and immunologic effect of antiretroviral therapy on HIV-1 in gut-associated lymphoid tissue. J Acquir Immune Defic Syndr. 2001;26:1–7. doi: 10.1097/00126334-200101010-00001. [DOI] [PubMed] [Google Scholar]
- 2.Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, et al. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J Virol. 2003;77:11708–11717. doi: 10.1128/JVI.77.21.11708-11717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anton PA, Mitsuyasu RT, Deeks SG, Scadden DT, Wagner B, Huang C, et al. Multiple measures of HIV burden in blood and tissue are correlated with each other but not with clinical parameters in aviremic subjects. AIDS. 2003;17:53–63. doi: 10.1097/00002030-200301030-00008. [DOI] [PubMed] [Google Scholar]
- 4.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, et al. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J Exp Med. 2004;200:749–759. doi: 10.1084/jem.20040874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poles MA, Boscardin WJ, Elliott J, Taing P, Fuerst MM, McGowan I, et al. Lack of decay of HIV-1 in gut-associated lymphoid tissue reservoirs in maximally suppressed individuals. J Acquir Immune Defic Syndr. 2006;43:65–68. doi: 10.1097/01.qai.0000230524.71717.14. [DOI] [PubMed] [Google Scholar]
- 6.Belmonte L, Olmos M, Fanin A, Parodi C, Bare P, Concetti H, et al. The intestinal mucosa as a reservoir of HIV-1 infection after successful HAART. AIDS. 2007;21:2106–2108. doi: 10.1097/QAD.0b013e3282efb74b. [DOI] [PubMed] [Google Scholar]
- 7.Chun TW, Nickle DC, Justement JS, Meyers JH, Roby G, Hallahan CW, et al. Persistence of HIV in Gut-Associated Lymphoid Tissue despite Long-Term Antiretroviral Therapy. J Infect Dis. 2008 doi: 10.1086/527324. [DOI] [PubMed] [Google Scholar]
- 8.Avettand-Fenoel V, Prazuck T, Hocqueloux L, Melard A, Michau C, Kerdraon R, et al. HIV-DNA in rectal cells is well correlated with HIV-DNA in blood in different groups of patients, including long-term non-progressors. AIDS. 2008;22:1880–1882. doi: 10.1097/QAD.0b013e32830fbdbc. [DOI] [PubMed] [Google Scholar]
- 9.Lafeuillade A, Cheret A, Hittinger G, Bernardini D, Cuquemelle C, Jullian E, et al. Rectal cell-associated HIV-1 RNA: a new marker ready for the clinic. HIV Clin Trials. 2009;10:324–327. doi: 10.1310/hct1005-324. [DOI] [PubMed] [Google Scholar]
- 10.Tincati C, Biasin M, Bandera A, Violin M, Marchetti G, Piacentini L, et al. Early initiation of highly active antiretroviral therapy fails to reverse immunovirological abnormalities in gut-associated lymphoid tissue induced by acute HIV infection. Antivir Ther. 2009;14:321–330. [PubMed] [Google Scholar]
- 11.Yukl SA, Gianella S, Sinclair E, Epling L, Li Q, Duan L, et al. Differences in HIV burden and immune activation within the gut of HIV-positive patients receiving suppressive antiretroviral therapy. J Infect Dis. 2010;202:1553–1561. doi: 10.1086/656722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eriksson S, Graf EH, Dahl V, Strain MC, Yukl SA, Lysenko ES, et al. Comparative analysis of measures of viral reservoirs in HIV-1 eradication studies. PLoS Pathog. 2013;9:e1003174. doi: 10.1371/journal.ppat.1003174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yukl SA, Shergill A, Ho T, Killian M, Girling V, Epling L, et al. The distribution of HIV DNA and RNA in cell subsets differs in gut and blood of HIV+ patients on ART: implications for viral persistence. J Infect Dis. 2013 doi: 10.1093/infdis/jit308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer M, Huber W, Kallivroussis A, Ott P, Opravil M, Luthy R, et al. Highly sensitive methods for quantitation of human immunodeficiency virus type 1 RNA from plasma, cells, and tissues. J Clin Microbiol. 1999;37:1260–1264. doi: 10.1128/jcm.37.5.1260-1264.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexaki A, Liu Y, Wigdahl B. Cellular reservoirs of HIV-1 and their role in viral persistence. Curr HIV Res. 2008;6:388–400. doi: 10.2174/157016208785861195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith PD, Meng G, Shaw GM, Li L. Infection of gastrointestinal tract macrophages by HIV-1. J Leukocyte Biol. 1997;62:72–77. doi: 10.1002/jlb.62.1.72. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Meng G, Graham MF, Shaw GM, Smith PD. Intestinal macrophages display reduced permissiveness to human immunodeficiency virus 1 and decreased surface CCR5. Gastroenterology. 1999;116:1043–1053. doi: 10.1016/s0016-5085(99)70007-7. [DOI] [PubMed] [Google Scholar]
- 18.Meng G, Sellers MT, Mosteller-Barnum M, Rogers TS, Shaw GM, Smith PD. Lamina propria lymphocytes, not macrophages, express CCR5 and CXCR4 and are the likely target cell for human immunodeficiency virus type 1 in the intestinal mucosa. J Infect Dis. 2000;182:785–791. doi: 10.1086/315790. [DOI] [PubMed] [Google Scholar]
- 19.Smith PD, Meng G, Sellers MT, Rogers TS, Shaw GM. Biological parameters of HIV-1 infection in primary intestinal lymphocytes and macrophages. J Leukocyte Biol. 2000;68:360–365. [PubMed] [Google Scholar]
- 20.Shen R, Richter HE, Clements RH, Novak L, Huff K, Bimczok D, et al. Macrophages in vaginal but not intestinal mucosa are monocyte-like and permissive to human immunodeficiency virus type 1 infection. J Virol. 2009;83:3258–3267. doi: 10.1128/JVI.01796-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewin-Smith M, Wahl SM, Orenstein JM. Human immunodeficiency virus-rich multinucleated giant cells in the colon: a case report with transmission electron microscopy, immunohistochemistry, and in situ hybridization. Modern Pathol. 1999;12:75–81. [PubMed] [Google Scholar]
- 22.Wang TH, Donaldson YK, Brettle RP, Bell JE, Simmonds P. Identification of shared populations of human immunodeficiency virus type 1 infecting microglia and tissue macrophages outside the central nervous system. J Virol. 2001;75:11686–11699. doi: 10.1128/JVI.75.23.11686-11699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zalar A, Figueroa MI, Ruibal-Ares B, Bare P, Cahn P, de Bracco MM, et al. Macrophage HIV-1 infection in duodenal tissue of patients on long term HAART. Antivir Res. 2010;87:269–271. doi: 10.1016/j.antiviral.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Smith PD, Meng G, Salazar-Gonzalez JF, Shaw GM. Macrophage HIV-1 infection and the gastrointestinal tract reservoir. J Leukocyte Biol. 2003;74:642–649. doi: 10.1189/jlb.0503219. [DOI] [PubMed] [Google Scholar]