Abstract
Purpose. This study evaluated the efficacy of 50% autologous serum eye drops in ocular surface diseases not improved by conventional therapy. Methods. We analyzed two groups: (1) acute eye pathologies (e.g., chemical burns) and (2) chronic eye pathologies (e.g., recurrent corneal erosion, neurotropic keratitis, and keratoconjunctivitis sicca). The patients were treated for surface instability after conventional therapy. The patients received therapy 5 times a day until stabilization of the framework; they then reduced therapy to 3 times a day for at least 3 months. We analyzed the best corrected visual acuity, epithelial defects, inflammation, corneal opacity, and corneal neovascularization. We also analyzed symptoms such as tearing, burning, sense of foreign body or sand, photophobia, blurred vision, and difficulty opening the eyelids. Results. We enrolled 15 eyes in group 1 and 11 eyes in group 2. The average therapy period was 16 ± 5.86 weeks in group 1 and 30.54 ± 20.33 weeks in group 2. The epithelial defects all resolved. Signs and symptoms improved in both groups. In group 2, the defect recurred after the suspension of therapy in 2 (18%) patients; in group 1, no defects recurred. Conclusions. Autologous serum eye drops effectively stabilize and improve signs and symptoms in eyes previously treated with conventional therapy.
1. Introduction
The ocular surface is a morphofunctional unit that owes its action to the perfect cooperation of all its structures (i.e., conjunctival and corneal epithelium, lacrimal apparatus, and eyelids) [1]. In particular, tears are important in maintaining the stability of the ocular surface because of its lubricant, mechanical, epitheliotropic, and antimicrobial functions [2]. A qualitative and quantitative deficiency of tears can lead to the persistent and progressive damage of the ocular surface with a compromised wound-healing process [3–5]. In this situation, the conventional therapeutic options are intensive artificial tears, punctual occlusion, contact lenses, and appropriate management of adnexa diseases [2]. However, these therapies are limited in supplying the neurotrophic factors, vitamins, and immunoglobulins necessary for the health of the ocular surface [2]. Just with this target arises the use of autologous serum (AS) eye drops. In 1970, the use of AS for treating ocular surface disorders began when it was used to treat ocular alkali burns [6]. However, only later—first with Fox et al. [7] and then with Tsubota et al. [8]—did this therapy enter clinical practice for the treatment of different ocular surface diseases. Since its introduction, this treatment has become increasingly popular and the indications for its use have expanded rapidly [9]. To date, AS is used for the treatment of persistent epithelial defects [10–15], dry eyes [1, 8, 16–27], neurotrophic keratopathy [28], recurrent erosion syndrome [29, 30], superior limbic keratoconjunctivitis [31], and chemical injuries [32, 33].
The rationale for the use of AS arises from its strong similarity to tears, which contains growth factors, cytokines, vitamins, and bactericidal components that provide the necessary nutritional factors to maintain cellular tropism and reduce the risk of contamination and infection during epithelial repair processes [2]. In fact, human serum contains substances such as epithelial growth factor (EGF), which speeds epithelial cell migration and has antiapoptotic effects [34]; transforming growth factor β (TGF-β), which is involved in the epithelial and stromal repair process [35]; vitamin A, which seems to prevent epithelium squamous metaplasia [36] and modulates the expression of thrombospondin 1 (TSP1) [37], thrombospondin 2 (TSP2), vascular endothelial growth factor A (VEGF-A), metallopeptidase 9, and TGF-β to promote wound healing [38]; albumin, which has antiapoptotic activity [39]; α-2 macroglobulin, which exhibits anticollagenase activity; and fibronectin, which is important in cell migration [11, 40]. Autologous serum also contains neuronal factors such as substance P (SP) and insulin-like growth factor 1 (IGF-1), which seem to have a role in corneal epithelium migration and adhesion [41]. In addition, AS contains immunoglobulins (Ig) such as IgG and IgA and lysozyme that provide bactericidal and bacteriostatic effects [2, 42]. Furthermore, AS is superior to artificial tears in maintaining corneal epithelium health because it is free of preservatives [43] (which potentially induce toxic or allergic reactions [44]) and its osmolality and biomechanical properties are similar to those of natural tears. The aim of this study was to evaluate the efficacy of 50% autologous serum eye drops in the treatment of symptoms and objective signs in different ocular surface diseases that are not improved by conventional therapy.
2. Materials and Methods
This single-center prospective study was conducted from January 2008 to January 2013. We enrolled patients who came to our department because of ocular surface dysfunction related to trophic deficiency (e.g., recurrent corneal erosion, neurotrophic keratitis, and keratoconjunctivitis sicca [Sjögren and non-Sjögren-related]) and chemical burns. Table 1 lists the inclusion and exclusion criteria of the study.
Table 1.
Inclusion and exclusion criteria of the study.
| Criteria | Inclusion criteria | Exclusion criteria |
|---|---|---|
| General | Inability of venous blood sampling because of (i) venous access being not available,, (ii) anemia, (iii) cerebrovascular or cardiovascular disorders, (iv) being positive for viral markers (HBV, HCV, and HIV), (v) being bacterial active, (vi) women who are pregnant or breast-feeding, (vii) patients unable to provide informed consent, (viii) age under 18 years. |
|
|
| ||
| Chemical eye burn |
(i) Persistent epithelial defect (ii) Inflammation and/or opacity and/or corneal neovascularization |
(i) Corneal perforation/melting (ii) Limbal stem cell deficit (iii) Patients with active infections of the eye or eyelid (iv) Abnormalities of the eyelid |
| Severe dry eye syndrome | (i) Symptoms of dry eye with daily activity limitation (ii) Time to break-up <5 sec (iii) Schirmer's test without anaesthesia <5 mm after 5 minutes (iv) Fluorescein staining positive with or without epithelial defect (v) Patients refractory to conventional therapy |
|
| Neurotrophic keratitis | (i) Patients with persistent epithelial defects (ii) Patients refractory to conventional therapy |
|
HBV = hepatitis B virus; HCV = hepatitis C virus; HIV = human immunodeficiency virus.
Written informed consent was obtained from all subjects before their enrollment after they received an explanation of the nature and possible consequences of the study. The institutional review board of the Spedali Civili Hospital (Brescia, Italy) ethics committee approved the study. The Declaration of Helsinki was followed. All patients had a screening visit. The best corrected visual acuity (BCVA) was measured using a Snellen-type acuity in all patients.
Specialized ophthalmologists collected clinical data by slit-lamp examination (using corneal fluorescein staining to study epithelial defects) and evaluated epithelial defects, inflammation, corneal opacity, and corneal neovascularization. A grading scale of 1 to 3 was used to identify the severity of these signs in which grade 1 was “absence of signs”; grade 2 was “slight”; and grade 3 was “severe.”
A similar scale was used for subjective symptoms. We recorded data on tearing, burning, sense of foreign body or sand, photophobia, blurred vision, and difficulty in opening the eyelids on a scale graded from 1 to 4 in which grade 1 was “no symptom,” grade 2 was “slight,” grade 3 was “moderate,” and grade 4 was “severe.” When possible, a picture of the anterior segment of the eye was obtained.
Patients were divided into 2 groups: (1) patients with an acute illness (e.g., chemical burns) and (2) patients with chronic disease (e.g., recurrent corneal erosion, neurotropic keratitis, and keratoconjunctivitis sicca (non-Sjögren and Sjögren-related)).
Group 1 eyes had chemical burns [grades II and III chemical injuries (based on the Dua classification [45])]. We treated the eyes with first-aid therapy, which included irrigation with normal saline or glucose to normalize the ocular surface pH, topical anti-inflammatory drugs, and antibiotic with eye bandage or contact lens. The treatment with AS eye drops was initiated after an average of 7 days of corticosteroid therapy in patients with persistent inflammation, epithelial defects, or any type of ocular surface instability without significant stem cell deficiency (i.e., more than one quadrant). The group was treated 5–6 times per day for 1 month in association with anti-inflammatory therapy (which was progressively decreased during the month) and then was reduced to 3 times daily until the absence of symptoms for at least 3 months without support therapy.
Group 2 included patients with chronic diseases that were unresponsive to conventional therapy (e.g., lubricating drops and ointments, punctal plug, bandage contact lenses, tarsorrhaphy, and gold eyelid weight). The treatment in this group was 5 times a day for 3 months and then was reduced progressively to 3 times a day for 3 months until the absence of symptoms. An antibiotic (moxifloxacin/netilmicin/tobramycin) was administered 4 times a day until the closing of the epithelial defect. No other supportive therapies were used during the treatment period. For each patient, the specialist could modify the therapy whenever necessary. The change was registered and justified.
Autologous serum drops were produced in the following manner. A total of 200 mL of blood was procured by venipuncture and collected in a sterile container. The blood was allowed to stand for 24–48 h at 4°C to allow clotting. The blood was centrifuged at 4000 rpm for 10 min. The serum was separated from the blood and diluted with saline to 50% in a laminar flow cabinet. At this point, the product was quarantined until the outcome of the sterility test performed by the Laboratory of Microbiology of the AO Civil Hospital of Brescia (Brescia, Italy). The final product was formed from an average of 288 single-dose eye drops for venipuncture. Single-application packs were packed in bags of 20 and marked with a label plate.
At −30°C, the product is preserved for 6 months from the date of withdrawal. From the time of delivery, the product is stored at a destination (e.g., home freezer) at −20°C for a period not exceeding 3 months from the date of delivery and no later than the expiration date stated on the label. If the entire production process is successful, the eye drops are validated, which takes into account negative serology examinations, group control transmitted and validated by the Emonet management system (i.e., computer system with records of the personal data of patients and the procedure to be performed), and negative results of sterility control.
Statistical Analysis. The data were recorded on a predesigned pro forma and managed on a spreadsheet using the Microsoft Excel 2013 software (Microsoft Corp., Redmond, WA). All entries were checked for errors. Appropriate statistical tests were applied to analyze the results. The t-test was used to determine the significance of changes in subjective symptoms, BCVA, epithelial defects, corneal neovascularization, opacity, and inflammation before and after AS eye inoculation therapy in the 2 groups of patients. The significance (p) was defined as a probability of error <0.01.
3. Results
3.1. Patients
In this study 28 eyes of 28 patients were enrolled. Two patients dropped out of the study because of the impossibility of obtaining blood samples: the first patient was seropositive for the human immunodeficiency virus and the second patient did not have venous access.
Of the 26 eyes of the 26 patients treated with AS, 18 patients were men and 8 were women. The mean age was 39.6 ± 16.47 years (range, 19–81 years) in group 1 and 57.63 ± 16.59 years (range, 37–80 years) in group 2 (Table 2).
Table 2.
Summary of the 26 eyes treated with autologous serum eye drops.
| Eye | Sex | Age (y) | Etiology | Weeks of serum therapy | Comorbidity |
|---|---|---|---|---|---|
| 1 | M | 41 | CB | 24 | None |
| 2 | M | 22 | CB | 24 | None |
| 3 | M | 59 | CB | 12 | None |
| 4 | F | 81 | CB | 12 | Diabetes mellitus |
| 5 | M | 29 | CB | 24 | None |
| 6 | M | 41 | CB | 12 | None |
| 7 | M | 30 | CB | 48 | None |
| 8 | M | 32 | CB | 12 | None |
| 9 | M | 38 | CB | 12 | None |
| 10 | M | 55 | CB | 12 | None |
| 11 | M | 31 | CB | 12 | None |
| 12 | M | 50 | CB | 24 | None |
| 13 | M | 19 | CB | 24 | None |
| 14 | M | 23 | CB | 12 | None |
| 15 | M | 43 | CB | 12 | None |
| 16 | F | 71 | SS | 24 | None |
| 17 | F | 37 | NK | 24 | s/p PK |
| 18 | F | 64 | NK | 12 | s/p trabeculectomy |
| 19 | M | 71 | DE | 12 | None |
| 20 | M | 80 | DE | 24 | None |
| 21 | M | 60 | NK | 12 | Trigeminal neuralgia |
| 22 | F | 79 | SS | 48 | None |
| 23 | F | 44 | NK | 48 | s/p trabeculectomy |
| 24 | M | 37 | NK | 72 | Acoustic neuroma |
| 25 | F | 40 | NK | 12 | None |
| 26 | F | 51 | NK | 48 | Lagophthalmos |
CB = chemical burn; DE = dry eye syndrome; NK = neurotrophic keratopathy; s/p PK = status postpenetrating keratoplasty; s/p trabeculectomy = status posttrabeculectomy; SS = Sjogren's syndrome.
Group 1 consisted of 15 eyes (58% of all patients in the study) injured by chemical agents of different natures. Group 2 (i.e., patients with chronic eye diseases) consisted of 11 eyes as follows: 6 eyes with neurotrophic keratopathy, 3 eyes with keratoconjunctivitis sicca not Sjögren, and 2 eyes with keratoconjunctivitis sicca, Sjögren-related.
The average therapy period was 22.15 ± 15.44 weeks (range, 12–72 weeks), 16 ± 5.86 weeks in group 1 and 30.54 ± 20.33 weeks in group 2. Two patients, both in group 2, remain under treatment.
3.2. Clinical Data
The visual acuity in group 1 went from 2.4/10 ± 1.91/10 (mean ± standard deviation) to 6.25/10 ± 3.25/10 (P < 0.01) after treatment with an average gain of 4 Snellen lines. In group 2, visual acuity went from 2.96/10 ± 2.04/10 to 4.7/10 ± 3.37/10 (P < 0.01) with an average gain of 2 Snellen lines. The visual acuity improved in 100% of patients (Figures 3(a) and 3(b)). The 55% percent of patients with chronic eye diseases had associated eye pathologies affecting the final visual acuity such as maculopathy, cataracts, and diabetic retinopathy, whereas no acute patient had concomitant pathology. The signs that were evaluated in the clinical examination were the presence of epithelial defects, neovascularization, corneal opacity, and the degree of inflammation.
Figure 3.
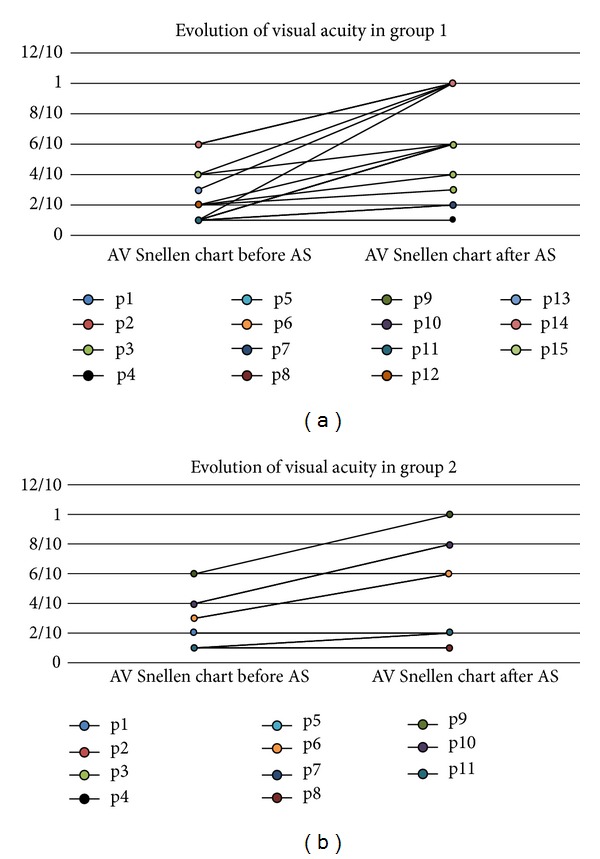
Evolution of visual acuity in the patients in the 2 groups. AS = autologous serum; p1–p15 = patient 1–patient 15.
In group 1 before treatment, 100% of patients had inflammation (it extended to the entire eye in 73% of patients and was grade 2 in 27% of patients); 100% of patients had corneal opacity (grade 3 in 93% of patients and grade 2 in 7% of patients); and 60% of patients had neovascularization (grade 3 in 33% of patients and grade 2 in 27% of patients).
In group 2 before treatment, 100% of patients had corneal inflammation (grade 3 in 64% of patients and grade 2 in 36% of patients); 82% of patients had corneal opacity (grade 3 in 60% of patients and grade 2 in 40% of patients); and 55% of patients had corneal neovascularization (grade 3 in 36% of patients, grade 2 in 27% of patients, and grade 1 in 37% of patients) (Table 3).
Table 3.
Summary of the distribution and evolution of the clinical signs in the 2 groups of patients.
| Signs | Presence of sign PRE-AS gr 1 | Presence of sign POST-AS gr 1 | Distribution of grades | Distribution of grades |
Average of the grades PRE-AS GR.1 |
Average of the grades POST-AS GR.1 |
P
value |
|---|---|---|---|---|---|---|---|
| Epithelial defect | 100% | 0% | GRADE 3: 73% | GRADE 3: 0% | 2.73 ± 0.46 | 1 | <0.01 |
| GRADE 2: 27% | GRADE 2: 0% | ||||||
| GRADE 1: 0% | GRADE 1: 100% | ||||||
|
| |||||||
| Inflammation | 100% | 14% | GRADE 3: 73% | GRADE 3: 0% | 2.73 ± 0.46 | 1.13 ± 0.35 | <0.01 |
| GRADE 2: 27% | GRADE 2: 13% | ||||||
| GRADE 1: 0% | GRADE 1: 87% | ||||||
|
| |||||||
| Corneal opacity | 100% | 27% | GRADE 3: 84% | GRADE 3: 0% | 2.93 ± 0.26 | 1.40 ± 0.51 | <0.01 |
| GRADE 2: 6% | GRADE 2: 40% | ||||||
| GRADE 1: 0% | GRADE 1: 60% | ||||||
|
| |||||||
| Neovascularization | 60% | 40% | GRADE 3: 33% | GRADE 3: 0% | 1.93 ± 0.88 | 1.20 ± 0.41 | <0.01 |
| GRADE 2: 27% | GRADE 2: 27% | ||||||
| GRADE 1: 40% | GRADE 1: 73% | ||||||
| Epithelial defect | 100% | 0% | GRADE 3: 73% | GRADE 3: 0% | 2.73 ± 0.46 | 1 | <0.01 |
| GRADE 2: 27% | GRADE 2: 0% | ||||||
| GRADE 1: 0% | GRADE 1: 100% | ||||||
|
| |||||||
| Inflammation | 100% | 10% | GRADE 3: 64% | GRADE 3: 0% | 2.64 ± 0.51 | 1.09 ± 0.30 | <0.01 |
| GRADE 2: 36% | GRADE 2: 19% | ||||||
| GRADE 1: 0% | GRADE 1: 81% | ||||||
|
| |||||||
| Corneal opacity | 88% | 28% | GRADE 3: 45% | GRADE 3: 0% | 2.27 ± 0.79 | 1.27 ± 0.47 | <0.01 |
| GRADE 2: 36% | GRADE 2: 45% | ||||||
| GRADE 1: 19% | GRADE 1: 55% | ||||||
|
| |||||||
| Neovascularization | 55% | 45% | GRADE 3: 36% | GRADE 3: 0% | 1.67 ± 0.52 | 1.18 ± 0.40 | <0.01 |
| GRADE 2: 18% | GRADE 2: 27% | ||||||
| GRADE 1: 46% | GRADE 1: 73% | ||||||
Post-AS = postautologous serum treatment; pre-AS = preautologous serum treatment.
Evolution of the Clinical Data (Figure 2)
Figure 2.
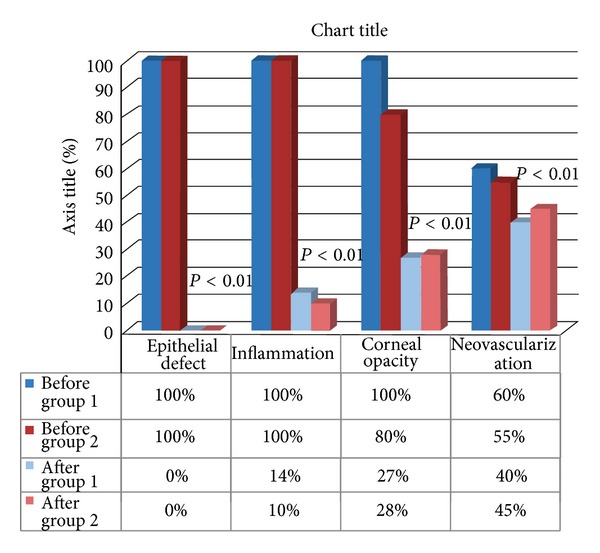
Evolution of clinical signs in group 1 and group 2. All data significantly improved.
Epithelial Defects. Epithelial defects were completely resolved (grade 1) by the end of followup in all patients. In 2 patients in group 2, the epithelial defect recurred after the suspension of therapy. One patient was administered a new cycle of AS eye drops that resolved the defect. In the second patient, a tarsorrhaphy was necessary owing to the severity of the ocular disease and the impossibility of restarting AS therapy because of the inability to perform blood sampling due to the patient's worsening condition.
Inflammation. After treatment in group 1, the inflammation was grade 1 (i.e., absent) in 87% of patients and grade 2 in 13% of patients with an improvement of 2 degrees in 58% of patients and 1 degree in 42% of patients. In group 2, inflammation was grade 1 (i.e., absent) in 81% of patients and grade 2 in 19% of patients with an improvement of 2 degrees in 55% of patients and 1 degree in 45% of patients. All patients improved. The average improvement was 1.60 ± 0.49 degrees in group 1 (P < 0.01) and 1.54 ± 0.50 degrees in group 2 (P < 0.01).
Opacity. After treatment in group 1, opacity became grade 1 in 60% of patients and grade 2 in the remaining 40% with an improvement of 2 degrees in 35% of patients and 1 degree in 54% of patients. In group 2, opacity became grade 1 in 55% of patients and grade 2 in 45% of patients. The improvement was 2 degrees in 18% of patients and 1 degree in 64% of patients; by contrast, 2 patients remained stable at grade 2 (these were diabetic patients with neurotrophic keratopathy).
The average improvement was 1.53 degrees ±0.52 in group 1 (P < 0.01) and 0.82 degrees ±0.60 in group 2 (P < 0.01).
Neovascularization. After treatment in group 1, neovascularization became grade 1 in 73% of patients and grade 2 in 27% of patients with an improvement of 2 degrees in 29% of patients and 1 degree in 64% of patients. In group 2, neovascularization became grade 1 in 73% of patients and grade 2 in 27% of patients with an improvement of 2 degrees in 27% of patients and 1 degree in 45% of patients, whereas there was no change in 1 diabetic patient with neurotrophic keratopathy. The average improvement was 1.11 degrees ±0.60 in group 1 (P < 0.01) and 1.17 degrees ±0.75 in group 2 (P < 0.01).
3.3. Symptoms
The symptoms reported in group 1 were (in decreasing frequency) burning, feeling a foreign body/sand in the eyes, tearing, photophobia, and blurred vision.
Tearing was present in 42% of patients at the first visit: 73% of patients had grade 4 and 27% of patients had grade 3.
Burning was present in 73% of patients at first visit: 79% of patients had grade 4, 11% of patients had grade 3, and 10% of patients had grade 2.
The sense of sand in the eyes was present in 78% of patients at the first visit: 55% of patients had grade 4 and 45% of patients had grade 3.
Photophobia was present in 65% of patients at the first visit: 53% of patients had grade 4, 37% of patients had grade 3, and 10% of patients had grade 2.
Blurred vision was present in 73% of patients at the first visit: 48% of patients had grade 4, 48% of patients had grade 3, and 4% of patients had grade 2.
Difficulty opening the eyelids was present in 42% of patients at the first visit: 64% of patients had grade 4, 27% of patients had grade 3, and 9% of patients had grade 2.
The most severe symptom was eye burning. The symptoms reported in group 2 (in decreasing frequency) were a sense of a foreign body in the eyes, blurred vision, burning, and photophobia. The feeling of sand in the eyes and blurred vision were the most severe symptoms (Table 3).
Evolution of Symptoms. Tearing at the end of followup was grade 1 in 55% of patients and grade 2 in 45% of patients. There was an improvement of 3 degrees in 36% of patients, 2 degrees in 55% of patients, and 1 degree in 9% of patients. All patients improved. The average improvement was 2.43 ± 0.49 degrees in group 1 (P < 0.01) and 2 ± 0.70 degrees in group 2 (P < 0.01).
Burning at the end of followup was grade 1 in 74% of patients, grade 2 in 21% of patients, and grade 3 in 5% of patients. There was an improvement of 3 degrees in 53% of patients, 2 degrees in 32% of patients, and 1 degree in 15% of patients (i.e., 100% of patients improved). The average improvement was 2.42 ± 0.76 degrees in group 1 (P < 0.01) and 2.28 ± 0.70 degrees in group 2 (P < 0.01).
The sense of sand in the eyes at the end of followup was grade 1 in 75% of patients, grade 2 in 15% of patients, and grade 3 in 10% of patients. There was an improvement of 3 degrees in 40% of patients, 2 degrees in 45% of patients, and 1 degree in 10% of patients; however, 1 (5%) patient with neurotrophic keratopathy showed no improvement. The average improvement was 2.45 ± 0.50 degrees in group 1 (P < 0.01) and 1.89 ± 0.99 degrees in group 2 (P < 0.01).
Photophobia at the end of followup was grade 1 in 71% of patients and grade 1 in 29% of patients.
There was an improvement of 3 degrees in 35% of patients, 2 degrees in 47% of patients, and 1 degree in 18% of patients (i.e., 100% of patients improved). The average improvement was 2.36 ± 0.77 degrees in group 1 (P < 0.01) and 1.83 ± 037 degrees in group 2 (P < 0.01).
Blurred vision at the end of followup was grade 1 in 63% of patients, grade 2 in 21% of patients, and grade 3 in 16% of patients. There was an improvement of 3 degrees in 32% of patients, 2 degrees in 37% of patients, 1 degree in 21% of patients, and no improvement in only 2 (10%) patients (the first patient had a chemical eye injury with a slight degree of injury and the second patient had Sjögren syndrome eye with a mild degree of injury). The average improvement was 1.91 ± 0.99 degrees in group 1 (P < 0.01) and 1.87 ± 0.92 degrees in group 2 (P < 0.01).
Difficulty opening the eyelids at the end of followup was grade 1 in 91% of patients and grade 2 in 9% of patients. There was an improvement of 3 degrees in 64% of patients, 2 degrees in 18% of patients, and 1 degree in 18% of patients (i.e., 100% of patients improved). The average improvement was 2.5 ± 0.76 degrees in group 1 (P < 0.01) and 2.25 ± 0.83 degrees in group 2 (P < 0.01).
4. Discussion
Autologous serum eye drops are actually used in the treatment of many ocular diseases, and many studies have demonstrated the effectiveness of AS eye drops in treating different conditions such as superior limbic keratoconjunctivitis [31], recurrent corneal erosion [12, 30], neurotropic keratopathy [10], and Sjogren's syndrome [8].
Despite clinical evidence of the efficacy of AS, a shared protocol for the preparation and the administration of this therapy is lacking because of the bureaucratic and technical difficulties of handling biological materials. The European Union (European Parliament and Council) has issued several directives concerning AS eye drops (1965/65, 1975/139, and 1975/318). However, in the European Union, individual countries regulate the manufacture and distribution of pharmaceuticals, and the use of serum eye drops remains an experimental approach [20]. The marketing authorization for a drug normally depends on proof of efficacy in clinical trials, implementation of quality control, reports of adverse effects, evidence of expert knowledge, and other regulatory issues. A doctor who makes or prescribes a specific medical product to treat a patient on a nominal basis is exempt from the requirement to obtain authorization as a “professional authorized by law to prescribe or administer drugs or devices so the responsibility in preparation and administration is entirely of the prescriber.” This has justified the limited use of AS eye drops [20, 46–48].
In addition, there have been few reports that show the efficacy of some blood products such as platelet-rich plasma (PRP) in treating chemical burns [32, 49]. Hence, we started using AS eye drops for different ocular surface diseases that were not responding to conventional therapies. Our study investigated a heterogeneous group of pathologies with different pathogeneses to understand better the role and potentialities of AS.
We chose to use 50% AS eye drops instead of other concentrations (e.g., 20% or 100%) used in some works in the literature [48]. This decision is based on the significant effect that 50% AS has shown in several studies [1, 14, 50] and on the hypothesis that at higher concentrations some components can become harmful. In fact, the concentration of biologically active molecules is different in serum and tear fluids. There are no data on which concentration of AS is most appropriate for treating ocular surface diseases. For example, Gupta et al. [51] report that the TGF-β concentration in human serum is approximately 50 ng/mL, which is 5 times higher than the amount in tears, and TGF-β has antiproliferative effects and high concentrations of this molecule may suppress wound healing of the epithelium [51, 52]. Jeng and Dupps Jr. [14] say that in their series 100% AS has a very high concentration of serum proteins that could alter the osmolality and pH of the preparation; in addition, patients enjoy the extra viscosity of 50% AS, compared to 20% AS. In addition, we must consider that the frequency of venipuncture and the amount of blood needed are doubled with the use of 100% AS drops [11]. Therefore, even if 100% AS drops were more effective, we believed 50% AS eye drops were safer and more manageable.
The main focus of the study was to show the clinical success of AS eye drops in the treatment of different ocular surface diseases. All collected data were analyzed complexly and subsequently in 2 distinct groups of pathologies—acute eye pathology (i.e., group 1) and chronic eye pathology (i.e., group 2)—with the aim of understanding the potential and the efficacy of this therapy.
The data analysis showed that all patients achieved a significant improvement in symptoms and all patients had excellent compliance with the treatment. No patient in the current study has reported any adverse effects to date; however, some studies have reported adverse effects [39]. In our patients, the treatment was safe and no patients reported allergy intolerance, deposits, or infection.
Best corrected visual acuity improved in all patients, which was in contrast to the study of Ziakas et al. [30] (Figures 3(a) and 3(b)). The average improvement was greater in patients with acute diseases, but this difference occurred because of a higher rate of eye comorbidity, which was also influenced by the age of the patients.
In primary or secondary tear deficiency, the AS supplies the lacking factors and reestablishes a correct ocular surface balance. This is the situation with dry eyes, Sjögren and non-Sjögren-related. For mild dry eyes, artificial tears, when frequently applied, are usually effective since they are able to reduce symptoms and prevent complications and the progression of damage [53, 54]. However, in more severe cases, artificial tears may be unable to stabilize the framework, and the damage to the ocular surface worsens with dramatic consequences such as eye ulcers to eye perforation [55]. In 1984, Fox et al. [7] were the first to report the beneficial effects of applying AS eye drops to dry eye in Sjogren's syndrome. Tsubota et al. [8] later revealed increased numbers of goblet cells and decreased squamous epithelium metaplasia after AS therapy. Compared to patients treated with nonpreserved artificial tears, Kojima et al. [39] found a significant improvement in tear stability, ocular surface vital staining scores, and pain symptom scores in patients treated with AS.
In our study, the patients with dry eye syndrome started therapy with AS eye drops because the symptom had not resolved with conventional therapy and they had a long history of chronic inflammation and recurrent epithelial defects leading to infection, opacity, and visual capacity damage [56]. All patients improved in objective and subjective symptoms. Four patients are currently without AS treatment but maintain artificial tears therapy. They are all followed up regularly and show a stable framework with no recurrence. However, the therapy suspension period remains short and does not allow stating the stability of the framework. It seems that, in cases of medium to serious ocular dryness, AS therapy is able to give stability, even if the duration must be verified.
In neurotropic keratopathy, a different mechanism occurs [57]. In this disease, there is not a single deficiency of tears but rather an imbalance with the production of harmful substance and an increased need for trophic factors. Numerous ocular and systemic diseases may lead to neurotropic keratopathy. In these diseases, neural factors such as acetylcholine or SP are depleted from the cornea. Nishida et al. [41] emphasize the importance of SP and IGF for a normal wound-healing response and Matsumoto et al. [28] report efficiency in the treatment of neurotropic keratopathy with 20% AS eye drops. Matsumoto showed that AS contains nerve growth factor and SP levels that are several times higher than the levels in tears and harbors IGF-1. It is their belief that AS helps healing in neurotropic keratopathy by providing lubrication and nerve healing and epithelialization. In another study, López-García et al. [58] report that, in aniridic keratopathy, AS eye drops improve the ocular surface and give more comfort compared to artificial tears. In our neurotropic keratopathy series patients, 3 patients had postherpetic keratitis, 2 patients had trigeminal nerve injury, and 1 patient had aniridic keratopathy. All patients had long-term therapy with tear substitutes and a history of recurrent trophic ulcers, significant neovascularization, and corneal scarring. On average, these patients had frameworks more severe than previous cases of dry eye, particularly when the deficit involved facial paralysis and anomalies in the dynamics of the eyelids. In all patients, we obtained stabilization of the framework, which was characterized by epithelial stability (i.e., more regular epithelium without defects) and a reduction in neovascularization, inflammation, and corneal opacity (Figure 1). One patient with trigeminal injury remains in therapy after 2 years, whereas the other patient, after discontinuing AS therapy, has relapsed with a trophic ulcer at risk of perforation. This experience allows us to believe that, especially in severe cases of neurotropic damage, the contribution of AS factors should be continuous and a program of chronic therapy can be scheduled.
Figure 1.
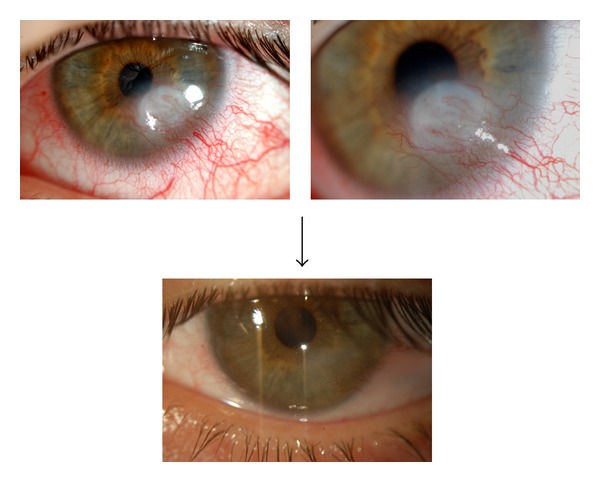
Clinical evolution of a patient with neurotrophic keratitis due to trigeminal nerve injury that occurred during acoustic neuroma excision.
The patients with ocular burn represent a different framework in which a single traumatic event, the acid burn, leads to an ocular surface injury with an action at term, but persistent damage. Few studies are available in the literature on chemical burns, and used as a treatment PRP [32], umbilical cord serum [33], or amniotic membranes [59]. Corneal chemical burns cause corneal infection, ulceration, opacity, and neovascularization. Irrespective of the source of regenerating epithelium, the rate of migration after chemical injury is reduced [60–63]. Therefore, the primary aims of therapy are the promotion of epithelialization as fast as possible, reduction of inflammation, support of reparative processes, and prevention of complications with the least permanent damage. Subconjunctival autologous regenerative factor-rich plasma in ocular alkali burns is able to appreciably reduce corneal and conjunctival epithelialization time, sick leave duration, and healing time [64]. In a small sample, Panda et al. [32] showed significant improvement in the healing of epithelial defects, corneal clarity, and BCVA with the inoculation of PRP. Márquez-de-Aracena et al. [65] showed a shorter corneal healing time with the use of subconjunctival platelet concentrate autologous injection in comparison to conventional therapy. They also state that it is unnecessary to activate PRP and suggest using it topically [65, 66].
The rationale of using AS in chemical burns derives from the fact that it contains antiproteases such as alpha 2 macroglobulin (which reduces collagenase) and vitamin A (which modulates the normal growth and differentiation of the epithelium) [38, 67]. It modulates the expression of TSP1 to accelerate epithelialization [65] and inhibits VEGF-A [38, 68].
In our casuistry, 5–7 days after injury, all patients had evident signs of corneal suffering with epithelial instability and inflammation that was caused by trophic damage on the cornea and conjunctiva, despite limited limbal deficiency. The study of clinical signs showed that in each patient we attained the primary objective, which was the stabilization of the condition in the absence of inflammation, and a complete restitution of epithelial integrity. The regression of neovascularization in most patients may be because of an increase in trophic factors and a decrease in inflammatory factors. In fact, neovascularization that is formed to provide nourishment disappears quickly if the stimulus ceases. Opacity declined because of the reduction of inflammation and because of stromal remodeling supported by serum factors such as EGF, fibronectin, TGF-β, retinoic acid, and nerve growth factor that are able to promote proliferation and differentiation of limbal corneal epithelium cells.
All our patients obtained these results, although with different timings between acute and chronic diseases. Chemical burns had a shorter average treatment time. This suggests that chemical injury is reversible and the ocular surface stability can be self-maintained if inflammation is properly reduced and growth factors are rebalanced. We suggest that our patients had a limited limbal ischemia with a largely preserved limbus. None of the chemically burned patients remain in therapy and all patients have a healthy ocular surface.
A limitation of our study is the lack of a control arm for the acute group patients. A future goal will be to followup with a control arm treated with anti-inflammatory drugs and artificial tears to make direct comparisons between both therapies in chemical injury.
5. Conclusions
In our casuistry, AS eye drops have been effective in improving and stabilizing signs and symptoms in patients who do not improve with conventional therapy. We believe that a reconfirmation of our findings will be desirable in a larger group of patients in a prospective controlled trial setting. Studies aimed at clarifying the beneficial effects and risks of prolonged application of AS drops at different AS concentrations should also be the subject of future investigations.
In addition, a future goal will be to conduct examinations, especially in the composition of tears. This can help scientists understand what factors are decreased in the tears of these patients and how they are decreased and how long AS components can effectively remain in tears after therapy.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Noble BA, Loh RSK, MacLennan S, et al. Comparison of autologous serum eye drops with conventional therapy in a randomised controlled crossover trial for ocular surface disease. British Journal of Ophthalmology. 2004;88(5):647–652. doi: 10.1136/bjo.2003.026211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quinto GG, Campos M, Behrens A. Autologous serum for ocular surface diseases. Arquivos Brasileiros de Oftalmologia. 2009;71(6):47–54. [PubMed] [Google Scholar]
- 3.Stern ME, Beuerman RW, Fox RI, Gao J, Mircheff AK, Pflugfelder SC. The pathology of dry eye: the interaction between the ocular surface and lacrimal glands. Cornea. 1998;17(6):584–589. doi: 10.1097/00003226-199811000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Stern ME, Gao J, Siemasko KF, Beuerman RW, Pflugfelder SC. The role of the lacrimal functional unit in the pathophysiology of dry eye. Experimental Eye Research. 2004;78(3):409–416. doi: 10.1016/j.exer.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 5.Rolando M. Sjögren’s syndrome as seen by an ophthalmologist. Scandinavian Journal of Rheumatology, Supplement. 2001;30(115):27–33. doi: 10.1080/030097401300232619. [DOI] [PubMed] [Google Scholar]
- 6.Ralph RA, Doane MG, Dohlman CH. Clinical experience with a mobile ocular perfusion pump. Archives of Ophthalmology. 1975;93(10):1039–1043. doi: 10.1001/archopht.1975.01010020815015. [DOI] [PubMed] [Google Scholar]
- 7.Fox RI, Chan R, Michelson JB, Belmont JB, Michelson PE. Beneficial effect of artificial tears made with autologous serum in patients with keratoconjunctivitis sicca. Arthritis and Rheumatism. 1984;27(4):459–461. doi: 10.1002/art.1780270415. [DOI] [PubMed] [Google Scholar]
- 8.Tsubota K, Goto E, Fujita H, et al. Treatment of dry eye by autologous serum application in Sjogren’s syndrome. British Journal of Ophthalmology. 1999;83(4):390–395. doi: 10.1136/bjo.83.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeng BH. Use of autologous serum in the treatment of ocular surface disorders. Archives of Ophthalmology. 2011;129(12):1610–1612. doi: 10.1001/archophthalmol.2011.336. [DOI] [PubMed] [Google Scholar]
- 10.Tsubota K, Goto E, Shimmura S, Shimazaki J. Treatment of persistent corneal epithelial defect by autologous serum application. Ophthalmology. 1999;106(10):1984–1989. doi: 10.1016/S0161-6420(99)90412-8. [DOI] [PubMed] [Google Scholar]
- 11.Poon AC, Geerling G, Dart JKG, Fraenkel GE, Daniels JT. Autologous serum eyedrops for dry eyes and epithelial defects: clinical and in vitro toxicity studies. British Journal of Ophthalmology. 2001;85(10):1188–1197. doi: 10.1136/bjo.85.10.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young AL, Cheng ACO, Ng HK, Cheng LL, Leung GYS, Lam DSC. The use of autologous serum tears in persistent corneal epithelial defects. Eye. 2004;18(6):609–614. doi: 10.1038/sj.eye.6700721. [DOI] [PubMed] [Google Scholar]
- 13.Schrader S, Wedel T, Moll R, Geerling G. Combination of serum eye drops with hydrogel bandage contact lenses in the treatment of persistent epithelial defects. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2006;244(10):1345–1349. doi: 10.1007/s00417-006-0257-y. [DOI] [PubMed] [Google Scholar]
- 14.Jeng BH, Dupps WJ., Jr. Autologous serum 50% eyedrops in the treatment of persistent corneal epithelial defects. Cornea. 2009;28(10):1104–1108. doi: 10.1097/ICO.0b013e3181a2a7f6. [DOI] [PubMed] [Google Scholar]
- 15.Choi JA, Chung S-H. Combined application of autologous serum eye drops and silicone hydrogel lenses for the treatment of persistent epithelial defects. Eye and Contact Lens. 2011;37(6):370–373. doi: 10.1097/ICL.0b013e318233c9bb. [DOI] [PubMed] [Google Scholar]
- 16.Rocha EM, Pelegrino F, De Paiva CS, Vigorito AC, De Souza ÇA. GVHD dry eyes treated with autologous serum tears. Bone Marrow Transplantation. 2000;25(10):1101–1103. doi: 10.1038/sj.bmt.1702334. [DOI] [PubMed] [Google Scholar]
- 17.Tananuvat N, Daniell M, Sullivan LJ, et al. Controlled study of the use of autologous serum in dry eye patients. Cornea. 2001;20(8):802–806. doi: 10.1097/00003226-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Takamura E, Shinozaki K, Hata H, Yukari J, Hori S. Efficacy of autologous serum treatment in patients with severe dry eye. Advances in Experimental Medicine and Biology. 2002;506:1247–1250. doi: 10.1007/978-1-4615-0717-8_179. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa Y, Okamoto S, Mori T, et al. Autologous serum eye drops for the treatment of severe dry eye in patients with chronic graft-versus-host disease. Bone Marrow Transplantation. 2003;31(7):579–583. doi: 10.1038/sj.bmt.1703862. [DOI] [PubMed] [Google Scholar]
- 20.Geerling G, MacLennan S, Hartwig D. Autologous serum eye drops for ocular surface disorders. British Journal of Ophthalmology. 2004;88(11):1467–1474. doi: 10.1136/bjo.2004.044347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kojima T, Ishida R, Dogru M, et al. The effect of autologous serum eyedrops in the treatment of severe dry eye disease: a prospective randomized case-control study. American Journal of Ophthalmology. 2005;139(2):242–246. doi: 10.1016/j.ajo.2004.08.040. [DOI] [PubMed] [Google Scholar]
- 22.Leite SC, de Castro RS, Alves M, et al. Risk factors and characteristics of ocular complications, and efficacy of autologous serum tears after haematopoietic progenitor cell transplantation. Bone Marrow Transplantation. 2006;38(3):223–227. doi: 10.1038/sj.bmt.1705426. [DOI] [PubMed] [Google Scholar]
- 23.Noda-Tsuruya T, Asano-Kato N, Toda I, Tsubota K. Autologous serum eye drops for dry eye after LASIK. Journal of Refractive Surgery. 2006;22(1):61–66. doi: 10.3928/1081-597X-20060101-13. [DOI] [PubMed] [Google Scholar]
- 24.Yoon K-C, Heo H, Im S-K, You I-C, Kim Y-H, Park Y-G. Comparison of autologous serum and umbilical cord serum eye drops for dry eye syndrome. American Journal of Ophthalmology. 2007;144(1):e86–e92. doi: 10.1016/j.ajo.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 25.Lee GA, Chen SX. Autologous serum in the management of recalcitrant dry eye syndrome. Clinical and Experimental Ophthalmology. 2008;36(2):119–122. doi: 10.1111/j.1442-9071.2008.01680.x. [DOI] [PubMed] [Google Scholar]
- 26.Urzua CA, Vasquez DH, Huidobro A, Hernandez H, Alfaro J. Randomized double-blind clinical trial of autologous serum versus artificial tears in dry eye syndrome. Current Eye Research. 2012;37(8):684–688. doi: 10.3109/02713683.2012.674609. [DOI] [PubMed] [Google Scholar]
- 27.Phasukkijwatana N, Lertrit P, Liammongkolkul S, Prabhasawat P. Stability of epitheliotrophic factors in autologous serum eye drops from chronic Stevens-Johnson syndrome dry eye compared to non-autoimmune dry eye. Current Eye Research. 2011;36(9):775–781. doi: 10.3109/02713683.2011.587935. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto Y, Dogru M, Goto E, et al. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology. 2004;111(6):1115–1120. doi: 10.1016/j.ophtha.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 29.del Castillo JM, de la Casa JM, Sardiña RC, et al. Treatment of recurrent corneal erosions using autologous serum. Cornea. 2002;21(8):781–783. doi: 10.1097/00003226-200211000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Ziakas NG, Boboridis KG, Terzidou C, et al. Long-term follow up of autologous serum treatment for recurrent corneal erosions. Clinical and Experimental Ophthalmology. 2010;38(7):683–687. doi: 10.1111/j.1442-9071.2010.02304.x. [DOI] [PubMed] [Google Scholar]
- 31.Goto E, Shimmura S, Shimazaki J, Tsubota K. Treatment of superior limbic keratoconjunctivitis by application of autologous serum. Cornea. 2001;20(8):807–810. doi: 10.1097/00003226-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Panda A, Jain M, Vanathi M, Velpandian T, Khokhar S, Dada T. Topical autologous platelet-rich plasma eyedrops for acute corneal chemical injury. Cornea. 2012;31(9):989–993. doi: 10.1097/ICO.0b013e3182114661. [DOI] [PubMed] [Google Scholar]
- 33.Sharma N, Goel M, Velpandian T, Titiyal JS, Tandon R, Vajpayee RB. Evaluation of umbilical cord serum therapy in acute ocular chemical burns. Investigative Ophthalmology and Visual Science. 2011;52(2):1087–1092. doi: 10.1167/iovs.09-4170. [DOI] [PubMed] [Google Scholar]
- 34.Wilson SE, Chen L, Mohan RR, Liang Q, Janice L. Expression of HGF, KGF, EGF and receptor messenger RNAs following corneal epithelial wounding. Experimental Eye Research. 1999;68(4):377–397. doi: 10.1006/exer.1998.0603. [DOI] [PubMed] [Google Scholar]
- 35.Kokawa N, Sotozono C, Nishida K, Kinoshita S. High total TGF-β2 levels in normal human tears. Current Eye Research. 1996;15(3):341–343. doi: 10.3109/02713689609007630. [DOI] [PubMed] [Google Scholar]
- 36.Tseng SCG, Farazdaghi M, Rider AA. Conjunctival transdifferentiation induced by systemic vitamin A deficiency in vascularized rabbit corneas. Investigative Ophthalmology and Visual Science. 1987;28(9):1497–1504. [PubMed] [Google Scholar]
- 37.Uno K, Kuroki M, Hayashi H, Uchida H, Kuroki M, Oshima K. Impairment of thrombospondin-1 expression during epithelial wound healing in corneas of vitamin A-deficient mice. Histology and Histopathology. 2005;20(2):493–499. doi: 10.14670/HH-20.493. [DOI] [PubMed] [Google Scholar]
- 38.Kim EC, Kim TK, Park SH, Kim MS. The wound healing effects of vitamin A eye drops after a corneal alkali burn in rats. Acta Ophthalmologica. 2012;90(7):e540–e546. doi: 10.1111/j.1755-3768.2012.02496.x. [DOI] [PubMed] [Google Scholar]
- 39.Kojima T, Higuchi A, Goto E, Matsumoto Y, Dogru M, Tsubota K. Autologous serum eye drops for the treatment of dry eye diseases. Cornea. 2008;27(1):S25–S30. doi: 10.1097/ICO.0b013e31817f3a0e. [DOI] [PubMed] [Google Scholar]
- 40.Costagliola C, Romano V, Forbice E, et al. Corneal oedema and its medical treatment. Clinical and Experimental Optometry. 2013;96(6):529–535. doi: 10.1111/cxo.12060. [DOI] [PubMed] [Google Scholar]
- 41.Nishida T, Nakamura M, Ofuji K, Reid T, Mannis M, Murphy C. Synergistic effects of substance P with insulin-like growth factor-1 on epithelial migration of the cornea. Journal of Cellular Physiology. 1996;169(1):159–166. doi: 10.1002/(SICI)1097-4652(199610)169:1<159::AID-JCP16>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 42.Liu L, Hartwig D, Harloff S, et al. Corneal epitheliotrophic capacity of three different blood-derived preparations. Investigative Ophthalmology and Visual Science. 2006;47(6):2438–2444. doi: 10.1167/iovs.05-0876. [DOI] [PubMed] [Google Scholar]
- 43.Geerling G, Daniels JT, Dart JKG, Cree IA, Khaw PT. Toxicity of natural tear substitutes in a fully defined culture model of human corneal epithelial cells. Investigative Ophthalmology and Visual Science. 2001;42(5):948–956. [PubMed] [Google Scholar]
- 44.Noecker R. Effects of common ophthalmic preservatives on ocular health. Advances in Therapy. 2001;18(5):205–215. doi: 10.1007/BF02853166. [DOI] [PubMed] [Google Scholar]
- 45.Dua HS, King AJ, Joseph A. A new classification of ocular surface burns. British Journal of Ophthalmology. 2001;85(11):1379–1383. doi: 10.1136/bjo.85.11.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu L, Hartwig D, Harloff S, Herminghaus P, Wedel T, Geerling G. An optimised protocol for the production of autologous serum eyedrops. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2005;243(7):706–714. doi: 10.1007/s00417-004-1106-5. [DOI] [PubMed] [Google Scholar]
- 47.López-García JS, García-Lozano I. Use of containers with sterilizing filter in autologous serum eyedrops. Ophthalmology. 2012;119(11):2225–2230. doi: 10.1016/j.ophtha.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 48.Cho YK, Huang W, Kim GY, Lim BS. Comparison of autologous serum eye drops with different diluents. Current Eye Research. 2013;38(1):9–17. doi: 10.3109/02713683.2012.720340. [DOI] [PubMed] [Google Scholar]
- 49.Marquez De Aracena Del Cid R, Montero De Espinosa Escoriaza I. Subconjunctival application of regenerative factor-rich plasma for the treatment of ocular alkali burns. European Journal of Ophthalmology. 2009;19(6):909–915. doi: 10.1177/112067210901900603. [DOI] [PubMed] [Google Scholar]
- 50.Fischer KR, Opitz A, Böeck M, Geerling G. Stability of serum eye drops after storage of 6 months. Cornea. 2012;31(11):1313–1318. doi: 10.1097/ICO.0b013e3182542085. [DOI] [PubMed] [Google Scholar]
- 51.Gupta A, Monroy D, Ji Z, Yoshino K, Huang A, Pflugfelder SC. Transforming growth factor beta-1 and beta-2 in human tear fluid. Current Eye Research. 1996;15(6):605–614. doi: 10.3109/02713689609008900. [DOI] [PubMed] [Google Scholar]
- 52.Terai K, Call MK, Liu H, et al. Crosstalk between TGF-β and MAPK signaling during corneal wound healing. Investigative Ophthalmology and Visual Science. 2011;52(11):8208–8215. doi: 10.1167/iovs.11-8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aragona P, Papa V, Micali A, Santocono M, Milazzo G. Long term treatment with sodium hyaluronate-containing artificial tears reduces ocular surface damage in patients with dry eye. British Journal of Ophthalmology. 2002;86(2):181–184. doi: 10.1136/bjo.86.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jirsova K, Brejchova K, Krabcova I, et al. The application of autologous serum eye drops in severe dry eye patients; subjective and objective parameters before and after treatment. Current Eye Research. 2014;39(1):21–30. doi: 10.3109/02713683.2013.824987. [DOI] [PubMed] [Google Scholar]
- 55.Mantelli F, Tranchina L, Lambiase A, Bonini S. Ocular surface damage by ophthalmic compounds. Current Opinion in Allergy and Clinical Immunology. 2011;11(5):464–470. doi: 10.1097/ACI.0b013e32834a95c9. [DOI] [PubMed] [Google Scholar]
- 56.Tincani A, Andreoli L, Cavazzana I, et al. Novel aspects of Sjögren’s syndrome in 2012. BMC Medicine. 2013;11(1, article 93) doi: 10.1186/1741-7015-11-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Semeraro F, Angi M, Romano M, Filippelli M, Di Iorio R, Costagliola C. Neurotrophic keratitis. Ophthalmologica. 2014;231(4):191–197. doi: 10.1159/000354380. [DOI] [PubMed] [Google Scholar]
- 58.López-García JS, Rivas L, García-Lozano I, Murube J. Autologous serum eyedrops in the treatment of aniridic keratopathy. Ophthalmology. 2008;115(2):262–267. doi: 10.1016/j.ophtha.2007.03.087. [DOI] [PubMed] [Google Scholar]
- 59.Clare G, Suleman H, Bunce C, Dua H. Amniotic membrane transplantation for acute ocular burns. Cochrane Database of Systematic Reviews. 2012;9 doi: 10.1002/14651858.CD009379.pub2.CD009379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wagoner MD, Kenyon KR, Gipson IK. Polymorphonuclear neutrophils delay corneal epithelial wound healing in vitro. Investigative Ophthalmology and Visual Science. 1984;25(10):1217–1220. [PubMed] [Google Scholar]
- 61.Pfister RR, Burstein N. The effects of ophthalmic drugs, vehicles, and preservatives on corneal epithelium: a scanning electron microscope study. Investigative Ophthalmology. 1976;15(4):246–259. [PubMed] [Google Scholar]
- 62.Berman M, Manseau E, Law M, Aiken D. Ulceration is correlated with degradation of fibrin and fibronectin at the corneal surface. Investigative Ophthalmology and Visual Science. 1983;24(10):1358–1366. [PubMed] [Google Scholar]
- 63.Hayashi K, Berman M, Smith D, El-Ghatit A, Pease S, Kenyon KR. Pathogenesis of corneal epithelial defects: role of plasminogen activator. Current Eye Research. 1991;10(5):381–398. doi: 10.3109/02713689109001747. [DOI] [PubMed] [Google Scholar]
- 64.Berman M, Leary R, Gage J. Evidence for a role of the plasminogen activator-plasmin system in corneal ulceration. Investigative Ophthalmology and Visual Science. 1980;19(10):1204–1221. [PubMed] [Google Scholar]
- 65.Márquez-de-Aracena R, Montero-de-Espinosa I, Muñoz M, Pereira G. Subconjunctival application of plasma platelet concentrate in the treatment of ocular burns. Preliminary results. Archivos de la Sociedad Espanola de Oftalmologia. 2007;82(8):475–481. doi: 10.4321/s0365-66912007000800005. [DOI] [PubMed] [Google Scholar]
- 66.Granelli-Piperno A, Vassalli JD, Reich E. Secretion of plasminogen activator by human polymorphonuclear leukocytes. Modulation by glucocorticoids and other effectors. Journal of Experimental Medicine. 1977;146(6):1693–1706. doi: 10.1084/jem.146.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, Kinoshita S. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Progress in Retinal and Eye Research. 2000;19(1):113–129. doi: 10.1016/s1350-9462(99)00007-5. [DOI] [PubMed] [Google Scholar]
- 68.Kobayashi TK, Tsubota K, Takamura E, Sawa M, Ohashi Y, Usui M. Effect of retinol palmitate as a treatment for dry eye: a cytological evaluation. Ophthalmologica. 1997;211(6):358–361. doi: 10.1159/000310829. [DOI] [PubMed] [Google Scholar]


