Abstract
Objectives
Ovarian cancer is the most lethal gynecological malignancy in North America. Although survival rates are high when the disease is diagnosed at an early stage, this decreases exponentially in late-stage diagnoses. As such, there is a need for novel early detection biomarkers. Through an integrated approach to ovarian cancer biomarker discovery that combines proteomics with transcriptomics and bioinformatics, our laboratory has identified folate-receptor 1 (FOLR1) and Dickkopf-related protein 3 (Dkk-3) as putative biomarkers. The objective of this study was to measure the levels of FOLR1 and Dkk-3 in the serum of patients with ovarian cancer, benign gynecological conditions and healthy women.
Design and Methods
FOLR1 and Dkk-3 were analyzed in serum of 100 ovarian cancer patients, 100 patients with benign gynecological conditions, and 100 healthy women using enzyme-linked immunosorbent assays (ELISAs). All specimens were analyzed in triplicate.
Results
FOLR1 was significantly elevated in the serum of ovarian cancer patients compared to serum of both healthy controls (p < 0.0001) and patients with benign gynecological conditions (p < 0.0001). Furthermore, FOLR1 was strongly correlated with CA125 as both were elevated in the serous histotype and in late-stage disease. FOLR1 did not outperform CA125 in receiver operating characteristic curve analysis and there was no significant complementarity between the two markers. Dkk-3 was not significantly different between the three serum cohorts and was not correlated with CA125.
Conclusions
FOLR1 is a new biomarker for ovarian cancer which correlates closely with CA125. The role of FOLR1 in the pathogenesis of ovarian cancer warrants further investigation.
Keywords: ovarian cancer, biomarker, FOLR1, Dkk-3, CA125, ELISA, diagnostic
INTRODUCTION
Ovarian cancer is the most lethal gynecological malignancy and the fifth-leading cause of mortalities due to cancer in North American women. Although the 5-year survival rate for cases diagnosed at an early stage (I–II) is approximately 80–90%, this decreases to 20–30% in late stage diagnoses (III–IV) (1). Unfortunately, no reliable mode of screening currently exists and in conjunction with the disease being asymptomatic during its early stages, early detection remains a major unmet clinical need for ovarian cancer. Standard methods for identifying suspected cases of ovarian cancer revolve mostly around physical pelvic examinations, ultrasonography, and measurements of serum carbohydrate antigen 125 (CA125). CA125 still remains the best serum tumour marker for ovarian cancer and is approved by the Food and Drug Administration (FDA) for both monitoring treatment with chemotherapy and differential diagnosis of patients presenting with a pelvic mass. However, increased serum CA125 levels are associated with other pathological states such as peritonitis, pericarditis, malignancies of the uterus, fallopian tubes, colon, and stomach, and benign gynecological conditions (2–4). Moreover, serum CA125 is above the reference interval in only half of the patients who present with clinically detectable early stage ovarian cancer (5). As a result, CA125 suffers from poor sensitivity for early-stage cancer and poor specificity overall. For these reasons, CA125 is approved neither for ovarian cancer screening nor for early detection. Taken together, there is a crucial need to identify novel serum biomarkers with higher specificity and sensitivity for the management of ovarian cancer.
It is now recognized that because ovarian cancer is a loose term that actually encompasses a heterogeneous group of distinct diseases, no single biomarker can provide sufficient clinical information. Instead, biomarker discovery is now focusing on the identification of multiple biomarkers, where concurrent examination of the markers will provide a more accurate view of the disease and thus, facilitate improved disease management. To accommodate these recent findings, we have developed an integrated systems biology platform that utilizes transcriptomics and bioinformatics to complement our proteomic analyses of biological fluids relevant to the disease (6). The rationale for this approach is that examining ovarian cancer across different aspects will help to identify more potential biomarkers that may have otherwise been missed based on a single approach. Our laboratory has previously deciphered the subproteome of ovarian cancer ascites with over 450 proteins identified (7). To prioritize the proteins according to their potential to be novel serological biomarkers for ovarian cancer, filtering criteria based on different sources of data were applied to the candidate proteins as highlighted by Kulasingam et al (6). This integrated approach was highly efficient, as 25 known ovarian cancer serum biomarkers were identified subsequent to candidate prioritization. In addition to the known serum biomarkers, 32 candidates were identified as having high potential to be novel ovarian cancer serum biomarkers. To perform an initial assessment of clinical utility, some of the candidates were screened in a small serum cohort of 10 apparently healthy controls and 10 late-stage ovarian cancer patients. From this initial screening, several candidates were identified as having strong potential to be novel serum biomarkers, including nidogen-2 (NID2), Dickkopf-related protein 3 (Dkk-3) and folate-receptor 1 (FOLR1). NID2 has been validated in an independent cohort and was found to be significantly elevated in the serum of ovarian cancer patients compared to healthy controls and correlated strongly with serum CA125 (8).
Dkk-3 belongs to the Dickkopf family of secreted proteins that, for the most part, are antagonists of the Wnt signal transduction pathway (9;10). However, Dkk-3 appears to be a divergent member of the Dickkopf family because unlike Dkk-1/2/4, Dkk-3 does not bind effectors of the Wnt pathway and as such, has not been shown to be a direct regulator of the embryogenic pathway (11;12). Instead, it has been proposed that Dkk-3 is a potential tumour suppressor since its associated gene locus is often deleted in cancers (13–17). Epigenetic inactivation of Dkk-3 expression has been reported in malignancies such as lung, prostate, bladder, and renal cell carcinoma (18–21). For example, Kobayashi et al. (22) and Lodygin et al. (23) observed that hypermethylation of the REIC/DKK-3 gene promoter was associated with occurrence of prostate and other cancers. The role of Dkk-3 in ovarian cancer, however, remains obscure, as only a few studies on the implications of this molecule in gynecological carcinogenesis exist. A recent study by Jiang et al. found that Dkk-3 displayed differential expression in the serum of patients with gynecological malignancies compared to healthy controls (24). For ovarian cancer, Dkk-3 was significantly decreased in ovarian cancer sera and the authors concluded that Dkk-3 may represent a novel detection and diagnostic marker for ovarian cancer. Unfortunately, the study only consisted of a small cohort of ovarian cancer cases (n = 36) and to our knowledge, there exists no validation studies examining the utility of Dkk-3 as a serum biomarker for ovarian cancer with sufficient statistical power.
FOLR1 is a 38 kDa glycosylphosphatidylinositol-anchored glycoprotein with strong binding affinity for folic acid and its derivatives (25). The receptor mediates folate transportation through receptor-mediated endocytosis upon ligand binding. In healthy individuals, FOLR1 expression is often limited to the apical surfaces of epithelium in the lung, kidney and choroid plexus but is overexpressed in a variety of solid tumours such as ovarian cancer, non-small cell lung cancer, breast cancer, kidney cancer and high-grade osteosarcoma (26–29). It is hypothesized that FOLR1 upregulation in solid malignancies is due to the increased metabolic requirements for folates to fuel nucleic acid synthesis and cellular growth (30). The biological relevance of folates, to carcinogenesis has thus made FOLR1 an attractive therapeutic target in ovarian cancer, especially those of epithelial etiology, where FOLR1 is upregulated in approximately 90% of the cases (28). In 2010, Konner et al. reported the positive responses observed in patients with epithelial ovarian cancer to intravenous farletuzumab, a humanized monoclonal antibody directed against FOLR1 (31). Serum CA125 and tumour size progressively decreased with each cycle of farletuzumab treatment and overall, the chemotherapeutic was well-tolerated. The use of FOLR1 targeting has even been applied to targeted delivery of chemotherapeutics through folate-conjugated liposomes, where binding of the liposomes to folate receptors induced receptor-mediated endocytosis, allowing the chemotherapeutics to enter the tumour-associated macrophages (32). Aside from chemotherapeutics, FOLR1 has implications in the imaging aspects of ovarian cancer management as well. In 2011, van Dam et al. were able to exploit the overexpression of FOLR1 in epithelial ovarian cancers by developing tumour-specific fluorescence imaging of ovarian carcinomas through conjugating folate to fluorescein isothiocyanate (33). In terms of serum biomarker studies, Basal et al. measured functional FOLR1 in the blood of ovarian cancer patients and found that it was significantly elevated in the serum of ovarian cancer patients compared to healthy controls (34). Unfortunately, this was performed in a small cohort (n = 30 for healthy controls and ovarian cancer patients) and furthermore, FOLR1 was not measured by enzyme-linked immunosorbent assay (ELISA) which is the gold standard for protein quantification in biological fluids. Thus, to our knowledge there exist no studies that have validated FOLR1 as a reliable serum biomarker for ovarian cancer in a large enough cohort.
In this study, we investigated the levels of Dkk-3 and FOLR1 in the serum of apparently healthy women, patients with benign gynecological conditions and ovarian cancer patients. Dkk-3 was not elevated in the serum of ovarian cancer patients. FOLR1, on the other hand, was found to be elevated in the serum of ovarian cancer patients and this was mostly associated with patients diagnosed with serous and late-stage ovarian carcinoma. Additionally, FOLR1 expression correlated with CA125 levels. Taken together, FOLR1 may represent a novel serum biomarker of ovarian carcinoma but its clinical utility needs to be addressed in larger serum cohorts.
METHODS
Patients and specimens
All patients in this study were of Japanese origin and were identified through 212 hospitals in the region of Shizuoka, Hamamatsu, Japan. Samples were collected with informed consent and Institutional Review Board approval and stored at −80 °C until analysis. From the samples available, we selected 100 serum samples from ovarian cancer patients, 100 serum samples from normal, apparently healthy women, and 100 serum samples from women with benign gynecological conditions. Of the 100 ovarian carcinoma patients, 37 were stage I, 19 were stage II, 23 were stage III, 3 were stage IV and 18 cases were unknown. In terms of histopathology, 50 samples were from serous, 14 from mucinous, 7 from endometrioid and 11 from clear cell carcinomas of the ovary with 18 samples of unknown histological origin. The cases of ovarian cancer were staged according to the International Federation of Gynecology and Obstetrics criteria. Patients with benign gynecological conditions were diagnosed with uterine leiomyomas (n = 45), adenomyosis (n = 18) and ovarian cysts (n = 37).
Measurement of CA125, FOLR1, and Dkk-3 in serum
CA125 was measured with a commercially available immunoassay method (Roche). The precision of this assay was <10%.
The concentration of FOLR1 in serum was measured using an adapted protocol from a commercially-available ELISA kit by R&D Systems (Minneapolis, MN). For FOLR1, mouse anti-human FOLR1 capture antibody was immobilized in a 96-well clear polystyrene plate by incubating 100 µL of 2.0 ng/µL capture antibody in PBS (137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4,1.5 mM KH2PO4, pH 7.4) overnight. The plates were washed three times with washing buffer (5 mmol/L Tris, 150 mmol/L NaCl, 0.05% Tween® 20, pH 7.8), after which the plate was blocked by adding 250 µL of Reagent Diluent (1% BSA in PBS) to each well and incubated with shaking at room temperature for 60 minutes. The plates were then washed three times with washing buffer and incubated with 100 µL per well of FOLR1 standards or serum samples with shaking at room temperature for 2 hours. FOLR1 standards and serum samples were diluted in Reagent Diluent with all serum samples diluted 5-fold. After incubation, the plates were washed six times with washing buffer and incubated with 100 µL per well of biotinylated goat antihuman FOLR1 detection antibody solution (100 pg/µL detection antibody in Reagent Diluent) with shaking at room temperature for 2 hours. After washing the plates six times with washing buffer, 100 µL of streptavidin-conjugated horseradish peroxidase solution (diluted 200-fold in Reagent Diluent) was added to each well and incubated for 15 minutes with shaking at room temperature. A final wash of six times with washing buffer was followed by the addition of 100 µL of 3,3’,5,5’-Tetramethylbenzidine (Sigma Aldrich) per well and incubated with shaking at room temperature for 15 minutes. The chromogenic reaction was stopped with the addition of 50 µL of 2 mol/L hydrochloric acid solution per well. Subsequently, the absorbance of each well was measured with the Wallac Envision 2103 Multilabel Reader (Perkin Elmer) at 450 nm, standardized to background absorbance at 540 nm. Final serum concentrations were calculated by multiplying with the dilution factor.
The concentrations of Dkk-3 in serum were also measured using an adapted protocol from a commercially-available ELISA kit by R&D Systems (Minneapolis, MN). The protocol was identical to that for FOLR1 with the exception of the capture antibody solution being at 4.0 ng/µL, the detection antibody solution being at 200 pg/µL, and the serum samples being diluted 200-fold. All samples were analyzed in triplicate.
Data analysis and statistics
Statistical analysis was performed using SPSS software, version 15.0 (SPSS Inc., Chicago, IL) and R (R Foundation for Statistical Computing). Normal distribution fit was assessed by the Shapiro-Wilk test and inspection of the Q-Q plot. The Kruskal-Wallis test was performed for comparison between more than two groups and Mann-Whitney U test with Bonferroni’s correction was performed for comparison between two groups. A P-value less than 0.05 was considered to be statistically significant. Spearman’s rank correlation coefficient was used to assess the correlations between CA125, FOLR1, and Dkk-3.
Logistic regression was performed to calculate odds ratios (OR) that defines the relation between biomarkers and cases, benign or control subjects. OR were calculated on log-transformed biomarkers and were represented with their 95% confidence interval (CI) and two-sided P-values. Furthermore, the diagnostic value of the markers was evaluated using receiver operating characteristic (ROC) curves. ROC curves were constructed by plotting sensitivity against 1-specificity and for each ROC curve, the area under the curve (AUC) was calculated using the bootstrap method to determine the AUC confidence intervals. Multi-parametric models were constructued using a logistic regression model on the log-transformed biomarker levels. ROC curve analysis was first performed on individual markers, and then in combination, to determine whether a multi-marker panel could lead to improved performance. Regression, OR, and ROC curve analyses were performed using R and pROC software package (35).
RESULTS
Association of Biomarkers with Ovarian Cancer
The distributions of CA125, FOLR1 and Dkk-3 in the three serum cohorts studied are shown in Figure 1. Both CA125 and FOLR1 were elevated in the serum of ovarian cancer patients compared to patients with benign gynecological conditions and healthy controls; these elevations were found to be statistically significant through the Mann-Whitney test (P < 0.0001 for both markers). Dkk-3, however, was not elevated in the serum of ovarian cancer patients and it was determined that there were no significant differences in serum Dkk-3 between any of the three serum cohorts (P = 0.976 for normal compared with cancer; P = 0.833 for benign compared with cancer). Comparisons between the different serum cohorts for the biomarkers are further detailed in Table 1. The elevations in CA125 and FOLR1 remained significant (P < 0.0001 for CA125; P = 0.0011 for FOLR1) after subdividing the cancer cohort into early (stages I–II) and late cancer diagnoses (stage III–IV) in which the serum biomarker levels were higher in the late cancer subgroup (data not shown).
Figure 1. Distribution of serum markers across the patient cohorts.
Distribution of serum CA125 (A), FOLR1 (B), and Dkk-3 (C) in the three cohorts of healthy controls, patients with benign gynecological conditions, and patients with ovarian cancer. The horizontal line represents the median value for each cohort. The differences between the normal or benign cohorts and cancer cohort are highly significant (p < 0.0001) for CA125 and FOLR1, but not for Dkk-3.
TABLE 1.
Distribution of age and serum CA125, Dkk-3, and FOLR1 in normal controls, patients with benign disease, and patients with ovarian cancer.
| Comparison | Markera | Disease | Nb | Median | P valuec |
|---|---|---|---|---|---|
| Age | Normal | 89 | 50 | - | |
| Benign | 93 | 38 | - | ||
| Cancer | 76 | 57 | - | ||
| Normal vs. Cancer |
CA125 | Normal | 95 | 12 | - |
| Cancer | 100 | 597 | <0.0001 | ||
| Early | 56 | 194 | <0.0001 | ||
| Late | 44 | 1063.5 | <0.0001 | ||
| Dkk-3 | Normal | 100 | 83.4 | - | |
| Cancer | 100 | 85.6 | 0.98 | ||
| Early | 56 | 89.2 | 0.24 | ||
| Late | 44 | 83.9 | 0.19 | ||
| FOLR1 | Normal | 100 | 0.5 | - | |
| Cancer | 100 | 1.5 | <0.0001 | ||
| Early | 56 | 1.1 | <0.0001 | ||
| Late | 44 | 2.2 | <0.0001 | ||
| Benign vs. Cancer |
CA125 | Benign | 99 | 14 | - |
| Cancer | 100 | 597 | <0.0001 | ||
| Early | 56 | 194 | <0.0001 | ||
| Late | 44 | 1063.5 | <0.0001 | ||
| Dkk-3 | Benign | 99 | 80.9 | - | |
| Cancer | 100 | 85.6 | 0.83 | ||
| Early | 56 | 89.2 | 0.21 | ||
| Late | 44 | 83.9 | 0.27 | ||
| FOLR1 | Benign | 99 | 0.6 | - | |
| Cancer | 100 | 1.5 | <0.0001 | ||
| Early | 56 | 1.1 | <0.0001 | ||
| Late | 44 | 2.2 | <0.0001 | ||
| Early vs. Late Cancer |
CA125 | Early | 56 | 194 | - |
| Late | 44 | 1063.5 | <0.0001 | ||
| Dkk-3 | Early | 56 | 89.2 | - | |
| Late | 44 | 83.9 | 0.055 | ||
| FOLR1 | Early | 56 | 1.1 | - | |
| Late | 44 | 2.2 | 0.0011 | ||
Age is in years. CA125 values are in U/mL. Dkk-3 and FOLR1 values are in µg/L.
Number of samples.
Kruskal-Wallis Test.
Correlations Among Biomarkers
Spearman’s rank correlation coefficients were used to assess the correlations among markers for the normal, benign, and cancer serum cohorts. CA125 was not correlated with Dkk-3 in the normal (Spearman r = −0.022, P = 0.832), benign (Spearman r = −0.115, P = 0.257), or cancer serum cohorts (Spearman r = −0.191, P = 0.057) (data not shown). CA125 was not correlated with FOLR1 in the normal serum cohort (Spearman r = 0.068, P = 0.511) but was weakly correlated with FOLR1 in the benign serum cohort (Spearman r = 0.206, P = 0.040) and strongly correlated with FOLR1 in the cancer serum cohort (Spearman r = 0.764, P < 0.0001). Overall, FOLR1 and CA125 correlated relatively strongly (Spearman r = 0.609, P < 0.0001). Figure 2 displays the correlation between FOLR1 and CA125 for all samples.
Figure 2. Correlation between CA125 and FOLR1.
Correlation between CA125 and FOLR1 across the three serum cohorts. The Spearman correlation coefficient was significant (r = 0.609; p < 0.0001).
Diagnostic Value of Biomarkers
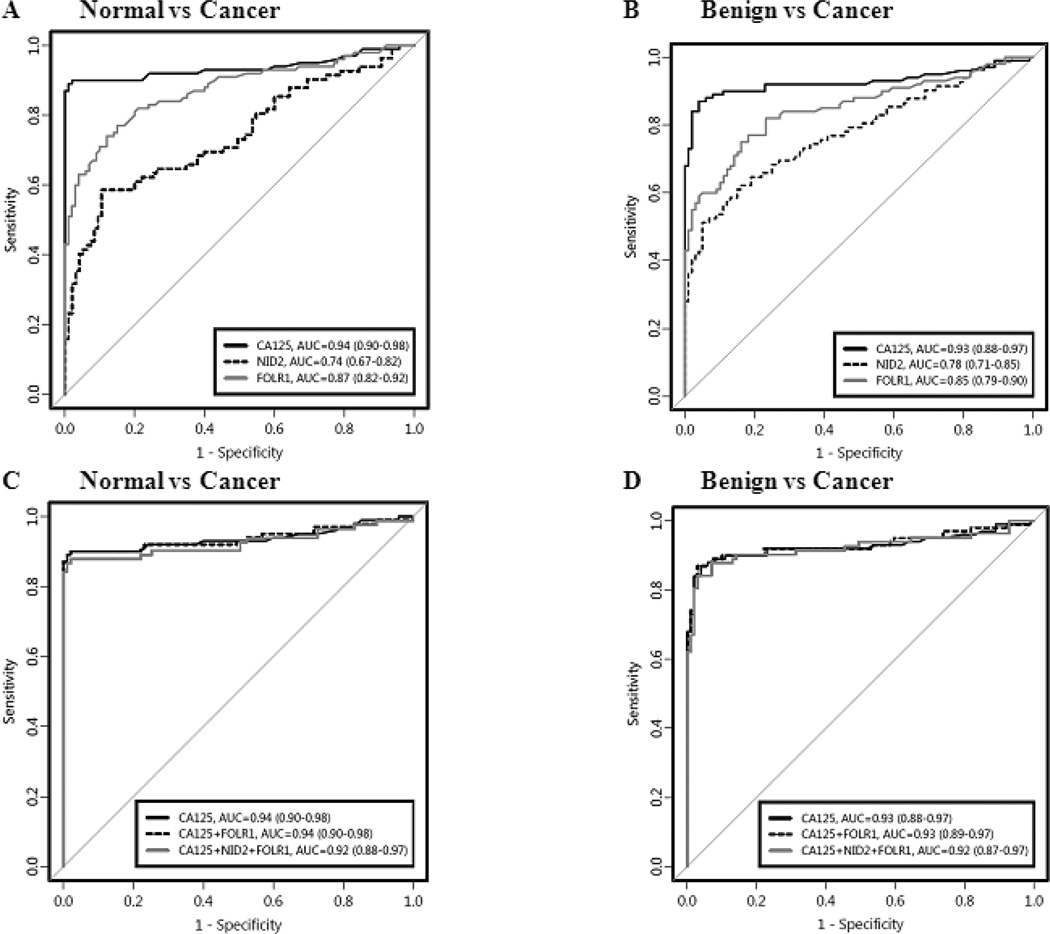
ROC curve analysis was used to quantify the diagnostic value of the three markers as well as nidogen-2 (NID2), a marker our laboratory previously validated (8). As seen in Figures 3A and 3B, FOLR1 surpassed both Dkk-3 and NID2 in its ability to discriminate normal sera from cancer sera and benign sera from cancer sera with an AUC of 0.87 (95% CI [0.82, 0.92]) and 0.85 (95% CI [0.79, 0.90]), respectively (Dkk3 – data not shown). However, CA125 was superior to FOLR1 in its diagnostic performance for all serum cohort comparisons. The diagnostic strength of CA125 was reiterated when combinations of the four markers were examined. As seen in Figures 3C and 3D, the addition of FOLR1 and the addition of FOLR1+NID2 to CA125 did not improve the ability of CA125 alone to discriminate normal or benign sera from cancer sera. Furthermore, a combination of our three identified putative markers (FOLR1, Dkk-3, and NID2) yielded AUCs of 0.86 (95% CI [0.80, 0.92]) and 0.84 (95% CI [0.79, 0.90]) for normal versus cancer and benign versus cancer, respectively and thus, did not outperform CA125 alone (data not shown). The superiority of FOLR1 over Dkk-3 and NID2, but not CA125, was observed again when the sensitivities of the markers and combinations of markers at fixed specificities of 80% and 90% were examined (Table 2).
Figure 3. Receiver operating characteristic curve analysis for serum markers.
Receiver operating characteristic (ROC) curve analysis for CA125, FOLR1, Dkk-3 and a previously studied marker, NID2. Panels (A) and (B) display the ROC curves for the biomarkers alone while panels (C) and (D) display the ROC curves for various biomarker combinations. Panels (A) and (C) compare the healthy controls with the ovarian cancer patients while panels (B) and (D) compare the patients with benign gynecological conditions with the ovarian cancer patients.
TABLE 2.
Receiver operating characteristic (ROC) curve analysis for biomarkers and biomarker combinations.
| Marker | AUCa | 95% CIb | Sensitivity |
|
|---|---|---|---|---|
| 80% Set Specificity |
90% Set Specificity |
|||
| Normal (n = 100) vs. Cancer (n = 100) | ||||
| CA125 | 0.94 | (0.90 – 0.98) | 90% | 90% |
| FOLR1 | 0.87 | (0.67 – 0.82) | 81% | 71% |
| NID2 | 0.72 | (0.67 – 0.82) | 60% | 52% |
| CA125 + FOLR1 | 0.94 | (0.90 – 0.98) | 90% | 90% |
| CA125 + FOLR1 + NID2 | 0.92 | (0.88 – 0.97) | 88% | 88% |
| Benign (n = 99) vs. Cancer (n = 100) | ||||
| CA125 | 0.93 | (0.88 – 0.97) | 90% | 89% |
| FOLR1 | 0.85 | (0.79 – 0.90) | 77% | 62% |
| NID2 | 0.79 | (0.71 – 0.85) | 65% | 54% |
| CA125 + FOLR1 | 0.93 | (0.89 – 0.97) | 91% | 89% |
| CA125 + FOLR1 + NID2 | 0.92 | (0.87 – 0.97) | 89% | 88% |
Area under the curve.
Confidence interval.
Association of Biomarkers with Age
Logistic regression models were used to determine associations between the markers and ovarian cancer with and without adjustment for age. Similar to the previous models, elevations in CA125, NID2 and FOLR1 were associated with the presence of ovarian cancer and were highly significant even after adjusting for age (Table 3). These associations were observed in both normal versus cancer and benign versus cancer comparisons. However, serum Dkk-3 was not associated with the presence of ovarian cancer in any of the comparisons (data not shown).
TABLE 3.
Odds ratio with and without adjusting for age.
| Comparison | Marker | No adjustment |
Age adjusted |
||||
|---|---|---|---|---|---|---|---|
| ORa | 95% CIb | P value | ORa | 95% CIb | P value | ||
| Normal vs Cancer | CA125 | 2.87 | (2.04, 4.04) | <0.0001 | 2.89 | (2.02, 4.14) | <0.0001 |
| NID2 | 2.98 | (1.98, 4.47) | <0.0001 | 2.80 | (1.81, 4.32) | <0.0001 | |
| FOLR1 | 3.17 | (2.27, 4.44) | <0.0001 | 3.13 | (2.12, 4.61) | <0.0001 | |
| Benign vs Cancer | CA125 | 2.25 | (1.80, 2.82) | <0.0001 | 2.02 | (1.59, 2.58) | <0.0001 |
| NID2 | 3.81 | (2.44, 5.96) | <0.0001 | 3.55 | (2.01, 6.26) | <0.0001 | |
| FOLR1 | 2.84 | (2.08, 3.87) | <0.0001 | 2.54 | (1.74, 3.70) | <0.0001 | |
Odds ratio.
Confidence interval
DISCUSSION
Ovarian cancer biomarkers for various applications such as early diagnosis, prognosis, monitoring and prediction of therapeutic response remain an important unmet clinical need. Currently, CA125 is the most widely used serum biomarker for monitoring ovarian cancer but due to its poor specificity, the use of CA125 for screening or early detection often results in high false positive rates. CA125 is elevated in benign gynecological conditions and pathologies of organs derived from coelomic epithelium (3). Additionally, CA125 displays poor sensitivity for early disease and thus, novel biomarkers with high sensitivity and high specificity are needed to improve the management of ovarian cancer.
The role of folic acid and its derivatives in carcinogenesis has been well-defined since the first chemotherapeutics were developed. The need for folates in nucleic acid synthesis coupled with observations of increased DNA synthesis and metabolism in tumoural cells made folates a potential chemotherapeutic target. This led to the creation of aminopterin and later, the less toxic antifolate, methotrexate, which is still used currently to treat various malignancies including leukemia, lymphoma and osteosarcoma. Since folates are transported via its associated receptors – namely, FOLR1 – and expression of these receptors is highly restricted in normal tissues but overexpressed in epithelial malignancies, FOLR1 has become a particularly interesting molecule to examine in ovarian cancer.
In this study, FOLR1 was elevated in the serum of patients with ovarian carcinoma compared to patients with benign gynecological conditions and apparently healthy controls. ROC curve analysis demonstrated that FOLR1 has potential as a novel serum biomarker and it surpassed the diagnostic value of NID2, a basement membrane protein we previously reported as a novel ovarian cancer serum biomarker (8). Spearman correlation showed that serum FOLR1 correlates strongly with CA125 in patients with ovarian carcinoma (Spearman r = 0.609, P < 0.001) and similar to CA125, FOLR1 appears to be more specific to the serous adenocarcinoma histotype. Taken together, these observations are consistent with the notion that as an epithelial cell surface receptor, FOLR1 or moieties of the receptor can be shed into the circulation and therefore, could serve as a serum marker of ovarian carcinoma. Indeed, Basal et al. reported elevation of functional FOLR1 in the serum of ovarian cancer patients albeit through immunoblotting and a radiolabeled folic acid-based microfiltration binding assay and as well, in a relatively small cohort (n = 30 ovarian cancer patients; n = 30 healthy controls) (34). Targeting of FOLR1 has shown some clinical utility with respect to ovarian cancer, as seen in anti-FOLR1 immunotherapeutics, in folate-conjugated liposomes which exploit FOLR1-mediated endocytosis to deliver chemotherapeutics contained within tumour-associated cells (32), and in folate-conjugated fluorescent dyes for the imaging of ovarian carcinomas (33). An interesting hypothesis to investigate would be to determine if serum FOLR1 is an indicator of a patient’s response to such treatment modalities – for example, would elevated serum FOLR1 predict a positive response to folate-conjugated liposomes containing chemotherapeutics in patients diagnosed with serous ovarian carcinoma? Undoubtedly, the recent advances in folate-based treatments in ovarian cancer warrants further investigation into the possible clinical applications of FOLR1 as a novel serum marker.
Unlike FOLR1, Dkk-3 was not found to be significantly elevated in the serum of ovarian cancer patients. Contrary to the results of Jiang et al., we found no significant differences in serum Dkk-3 levels between any of the three cohorts; although Dkk-3 was slightly decreased in late-cancer patients compared to early-cancer patients (see Table 1). It is possible that the observed lower level of Dkk-3 in the serum of ovarian cancer patients by the former study were as a result of population variation, biological artifacts, and the lack of validation in a larger, independent cohort.
As stated earlier, there is now an increased focus on identifying multi-marker panels for the management of ovarian cancer rather than the identification of single biomarkers that will exceed the performance of CA125. Recently, the FDA granted approval for serum human epididymal protein 4 (HE4), and the serum-based Risk of Malignancy Algorithm (ROMA) and OVA1 test to be used for clinical management of ovarian cancer – although these new biomarkers have seen some success leading up to FDA-approval, there are conflicting results as to how well they truly perform compared to CA125. However, the emergence of the ROMA and OVA1 tests are a clear indication of the paradigm shift from single biomarkers to multiparametric biomarker panels for the purposes of ovarian cancer management. Thus, while FOLR1 did not perform better than CA125 alone or in combination, the idea that other biomarkers may complement both FOLR1 and CA125 to yield a sensitivity and specificity greater than any marker alone is worth examining in the future.
Highlights.
Ovarian cancer (OvCa) is the most lethal gynecological malignancy.
No adequate screening or diagnostic tools exist for early stage OvCa diagnosis.
FOLR1 appears to be a promising novel serological biomarker for ovarian cancer.
ACKNOWLEDGEMENTS
This work is supported by Proteomic Methods Inc., Toronto, Canada. E.P. Diamandis is funded by the Early Detection Research Network of NIH (EDRN; Grant #5U01CA152755).
Abbreviations
- CA125
carbohydrate antigen 125
- FDA
Food and Drug Administration
- NID2
nidogen-2
- Dkk-3
Dickkopf-related protein 3
- FOLR1
folate-receptor 1
- ELISA
enzyme-linked immunosorbent assay
- OR
odds ratio
- CI
confidence interval
- ROC
receiver operating characteristic
- AUC
area under the curve
- ROMA
Risk of Malignancy Algorithm
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None declared.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Sevinc A, Adli M, Kalender ME, Camci C. Benign causes of increased serum CA-125 concentration. Lancet Oncol. 2007;8:1054–1055. doi: 10.1016/S1470-2045(07)70357-1. [DOI] [PubMed] [Google Scholar]
- 3.Sevinc A, Buyukberber S, Sari R, Kiroglu Y, Turk HM, Ates M. Elevated serum CA-125 levels in hemodialysis patients with peritoneal, pleural, or pericardial fluids. Gynecol Oncol. 2000;77:254–257. doi: 10.1006/gyno.2000.5776. [DOI] [PubMed] [Google Scholar]
- 4.Zuckerman E, Lanir A, Sabo E, Rosenvald-Zuckerman T, Matter I, Yeshurun D, Eldar S. Cancer antigen 125: a sensitive marker of ascites in patients with liver cirrhosis. Am J Gastroenterol. 1999;94:1613–1618. doi: 10.1111/j.1572-0241.1999.01152.x. [DOI] [PubMed] [Google Scholar]
- 5.Liede A, Karlan BY, Baldwin RL, Platt LD, Kuperstein G, Narod SA. Cancer incidence in a population of Jewish women at risk of ovarian cancer. J Clin Oncol. 2002;20:1570–1577. doi: 10.1200/JCO.2002.20.6.1570. [DOI] [PubMed] [Google Scholar]
- 6.Kulasingam V, Pavlou MP, Diamandis EP. Integrating high-throughput technologies in the quest for effective biomarkers for ovarian cancer. Nat Rev Cancer. 2010;10:371–378. doi: 10.1038/nrc2831. [DOI] [PubMed] [Google Scholar]
- 7.Kuk C, Kulasingam V, Gunawardana CG, Smith CR, Batruch I, Diamandis EP. Mining the ovarian cancer ascites proteome for potential ovarian cancer biomarkers. Mol Cell Proteomics. 2009;8:661–669. doi: 10.1074/mcp.M800313-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuk C, Gunawardana CG, Soosaipillai A, Kobayashi H, Li L, Zheng Y, Diamandis EP. Nidogen-2: a new serum biomarker for ovarian cancer. Clin Biochem. 2010;43:355–361. doi: 10.1016/j.clinbiochem.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krupnik VE, Sharp JD, Jiang C, Robison K, Chickering TW, Amaravadi L, et al. Functional and structural diversity of the human Dickkopf gene family. Gene. 1999;238:301–313. doi: 10.1016/s0378-1119(99)00365-0. [DOI] [PubMed] [Google Scholar]
- 10.Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25:7469–7481. doi: 10.1038/sj.onc.1210054. [DOI] [PubMed] [Google Scholar]
- 11.Wu W, Glinka A, Delius H, Niehrs C. Mutual antagonism between dickkopf1 and dickkopf2 regulates Wnt/beta-catenin signalling. Curr Biol. 2000;10:1611–1614. doi: 10.1016/s0960-9822(00)00868-x. [DOI] [PubMed] [Google Scholar]
- 12.Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 13.Hsieh SY, Hsieh PS, Chiu CT, Chen WY. Dickkopf-3/REIC functions as a suppressor gene of tumor growth. Oncogene. 2004;23:9183–9189. doi: 10.1038/sj.onc.1208138. [DOI] [PubMed] [Google Scholar]
- 14.Tsuji T, Miyazaki M, Sakaguchi M, Inoue Y, Namba M. A REIC gene shows downregulation in human immortalized cells and human tumor-derived cell lines. Biochem Biophys Res Commun. 2000;268:20–24. doi: 10.1006/bbrc.1999.2067. [DOI] [PubMed] [Google Scholar]
- 15.Tsuji T, Nozaki I, Miyazaki M, Sakaguchi M, Pu H, Hamazaki Y, et al. Antiproliferative activity of REIC/Dkk-3 and its significant down-regulation in non-small-cell lung carcinomas. Biochem Biophys Res Commun. 2001;289:257–263. doi: 10.1006/bbrc.2001.5972. [DOI] [PubMed] [Google Scholar]
- 16.Nozaki I, Tsuji T, Iijima O, Ohmura Y, Andou A, Miyazaki M, et al. Reduced expression of REIC/Dkk-3 gene in non-small cell lung cancer. Int J Oncol. 2001;19:117–121. doi: 10.3892/ijo.19.1.117. [DOI] [PubMed] [Google Scholar]
- 17.Kurose K, Sakaguchi M, Nasu Y, Ebara S, Kaku H, Kariyama R, et al. Decreased expression of REIC/Dkk-3 in human renal clear cell carcinoma. J Urol. 2004;171:1314–1318. doi: 10.1097/01.ju.0000101047.64379.d4. [DOI] [PubMed] [Google Scholar]
- 18.Yue W, Sun Q, Dacic S, Landreneau RJ, Siegfried JM, Yu J, Zhang L. Downregulation of Dkk3 activates beta-catenin/TCF-4 signaling in lung cancer. Carcinogenesis. 2008;29:84–92. doi: 10.1093/carcin/bgm267. [DOI] [PubMed] [Google Scholar]
- 19.Zenzmaier C, Untergasser G, Hermann M, Dirnhofer S, Sampson N, Berger P. Dysregulation of Dkk-3 expression in benign and malignant prostatic tissue. Prostate. 2008;68:540–547. doi: 10.1002/pros.20711. [DOI] [PubMed] [Google Scholar]
- 20.Urakami S, Shiina H, Enokida H, Hirata H, Kawamoto K, Kawakami T, et al. Wnt antagonist family genes as biomarkers for diagnosis, staging, and prognosis of renal cell carcinoma using tumor and serum DNA. Clin Cancer Res. 2006;12:6989–6997. doi: 10.1158/1078-0432.CCR-06-1194. [DOI] [PubMed] [Google Scholar]
- 21.Urakami S, Shiina H, Enokida H, Kawakami T, Kawamoto K, Hirata H, et al. Combination analysis of hypermethylated Wnt-antagonist family genes as a novel epigenetic biomarker panel for bladder cancer detection. Clin Cancer Res. 2006;12:2109–2116. doi: 10.1158/1078-0432.CCR-05-2468. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi K, Ouchida M, Tsuji T, Hanafusa H, Miyazaki M, Namba M, et al. Reduced expression of the REIC/Dkk-3 gene by promoter-hypermethylation in human tumor cells. Gene. 2002;282:151–158. doi: 10.1016/s0378-1119(01)00838-1. [DOI] [PubMed] [Google Scholar]
- 23.Lodygin D, Epanchintsev A, Menssen A, Diebold J, Hermeking H. Functional epigenomics identifies genes frequently silenced in prostate cancer. Cancer Res. 2005;65:4218–4227. doi: 10.1158/0008-5472.CAN-04-4407. [DOI] [PubMed] [Google Scholar]
- 24.Jiang T, Huang L, Wang S, Zhang S. Clinical significance of serum Dkk-3 in patients with gynecological cancer. J Obstet Gynaecol Res. 2010;36:769–773. doi: 10.1111/j.1447-0756.2010.01234.x. [DOI] [PubMed] [Google Scholar]
- 25.Elnakat H, Ratnam M. Distribution, functionality and gene regulation of folate receptor isoforms: implications in targeted therapy. Adv Drug Deliv Rev. 2004;56:1067–1084. doi: 10.1016/j.addr.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Bueno R, Appasani K, Mercer H, Lester S, Sugarbaker D. The alpha folate receptor is highly activated in malignant pleural mesothelioma. J Thorac Cardiovasc Surg. 2001;121:225–233. doi: 10.1067/mtc.2001.111176. [DOI] [PubMed] [Google Scholar]
- 27.Hartmann LC, Keeney GL, Lingle WL, Christianson TJ, Varghese B, Hillman D, et al. Folate receptor overexpression is associated with poor outcome in breast cancer. Int J Cancer. 2007;121:938–942. doi: 10.1002/ijc.22811. [DOI] [PubMed] [Google Scholar]
- 28.Toffoli G, Russo A, Gallo A, Cernigoi C, Miotti S, Sorio R, et al. Expression of folate binding protein as a prognostic factor for response to platinum-containing chemotherapy and survival in human ovarian cancer. Int J Cancer. 1998;79:121–126. doi: 10.1002/(sici)1097-0215(19980417)79:2<121::aid-ijc4>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 29.Yang R, Kolb EA, Qin J, Chou A, Sowers R, Hoang B, et al. The folate receptor alpha is frequently overexpressed in osteosarcoma samples and plays a role in the uptake of the physiologic substrate 5-methyltetrahydrofolate. Clin Cancer Res. 2007;13:2557–2567. doi: 10.1158/1078-0432.CCR-06-1343. [DOI] [PubMed] [Google Scholar]
- 30.Kelemen LE, Sellers TA, Keeney GL, Lingle WL. Multivitamin and alcohol intake and folate receptor alpha expression in ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:2168–2172. doi: 10.1158/1055-9965.EPI-05-0260. [DOI] [PubMed] [Google Scholar]
- 31.Konner JA, Bell-McGuinn KM, Sabbatini P, Hensley ML, Tew WP, Pandit-Taskar N, et al. Farletuzumab, a humanized monoclonal antibody against folate receptor alpha, in epithelial ovarian cancer: a phase I study. Clin Cancer Res. 2010;16:5288–5295. doi: 10.1158/1078-0432.CCR-10-0700. [DOI] [PubMed] [Google Scholar]
- 32.Turk MJ, Waters DJ, Low PS. Folate-conjugated liposomes preferentially target macrophages associated with ovarian carcinoma. Cancer Lett. 2004;213:165–172. doi: 10.1016/j.canlet.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 33.van Dam GM, Themelis G, Crane LM, Harlaar NJ, Pleijhuis RG, Kelder W, et al. Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptoralpha targeting: first in-human results. Nat Med. 2011;17:1315–1319. doi: 10.1038/nm.2472. [DOI] [PubMed] [Google Scholar]
- 34.Basal E, Eghbali-Fatourechi GZ, Kalli KR, Hartmann LC, Goodman KM, Goode EL, et al. Functional folate receptor alpha is elevated in the blood of ovarian cancer patients. PLoS One. 2009;4:e6292. doi: 10.1371/journal.pone.0006292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. [Accessed September 13 2012];pROC: an open source package for R and S+ to analyze and compare ROC curves. doi: 10.1186/1471-2105-12-77. http://www.biomedcentral.com/1471-2105/12/77/. [DOI] [PMC free article] [PubMed]