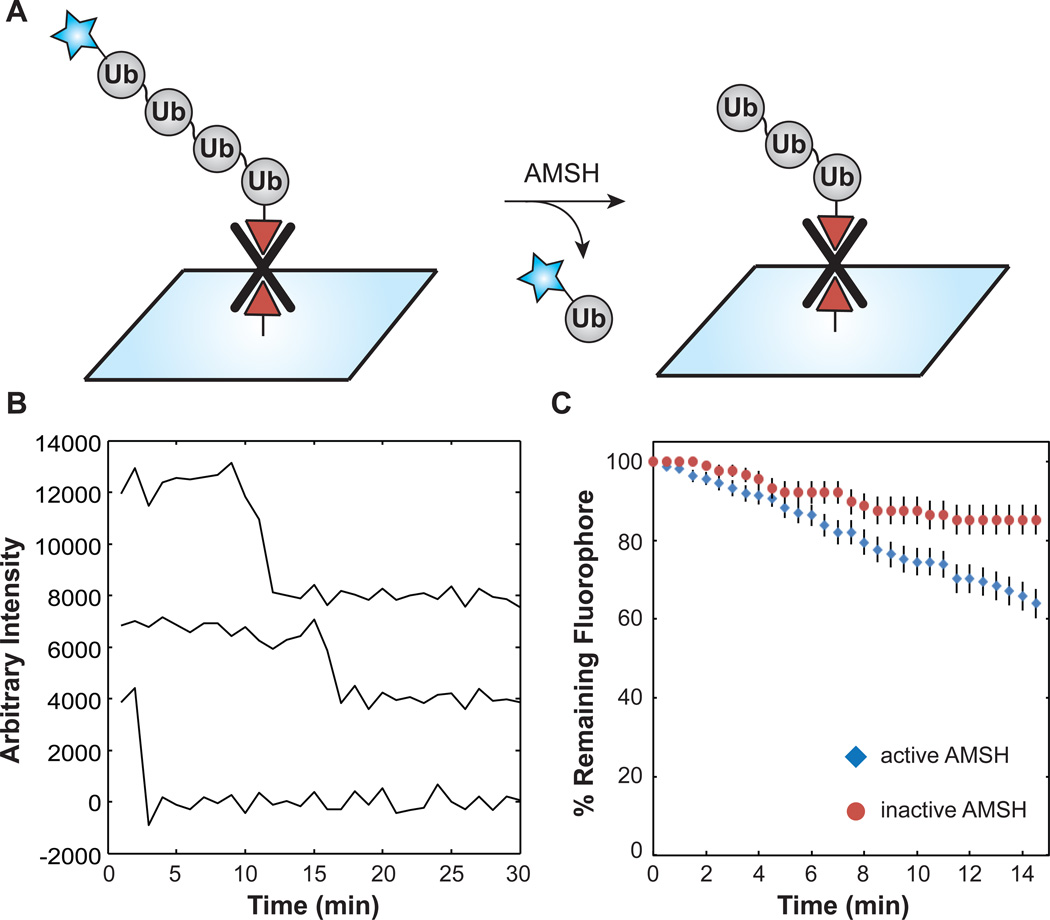
Figure 6.
Detection of DUB activity at the single-molecule level. (A) Reaction scheme. (B) Representative single molecule intensity time courses from AMSH hydrolysis assays. (C) Remaining fluorescence versus time. Consistent with DUB activity, the greatest loss in surface fluorescence occurred in reactions containing both dually functionalized 63-linked tetra-Ub chains and active AMSH ( , N = 160 molecules). This rate was ~2-fold greater than the photobleaching rate measured in the presence of 1,10-phenanthroline-inactivated AMSH (
, N = 160 molecules). This rate was ~2-fold greater than the photobleaching rate measured in the presence of 1,10-phenanthroline-inactivated AMSH ( , N = 88 molecules). Error bars represent the sampling error calculated as a binomial proportion confidence interval.
, N = 88 molecules). Error bars represent the sampling error calculated as a binomial proportion confidence interval.