Summary
Endometriosis is a chronic disease that affects millions of reproductive-age women. Despite the destructive and invasive nature of endometrioses, most cases are perpetually benign or eventually regress; however, atypical endometriosis is a precursor lesion and can lead to certain types of ovarian cancer. Endometriosis induced inflammation and auto- and paracrine production of sex steroid hormones contribute to ovarian tumorigenesis. These changes provide microenvironment necessary to accumulate enough genetic alterations for endometriosis associated malignant transformation. It takes years for endometriosis to undergo the pathophysiological progression that begins with atypical epithelial proliferation (atypical endometriosis and metaplasia), and then is followed by the formation of well-defined borderline tumors, and finally culminates in fully malignant ovarian cancer. This study is a review of the natural history of endometriosis and the role of microenvironments that favor the accumulation of genetic alterations and endometriosis-associated ovarian cancer progression.
Keywords: Endometriosis, Genetic alterations, Inflammation, Ovarian cancer, Pathogenesis
Endometriosis is recognized as an estrogen-dependent disorder, defined by the presence of endometriotic tissue outside of the uterus (ectopic) composed of endometriotic glands and stroma. The accurate diagnosis of endometriosis frequently relies on the histologic recognition of endometriotic tissue. Endometriosis is a common public health problem, affecting an estimated 2% to 8% of reproductive-aged women and 30% of women with infertility (1). Annual incidence of endometriosis by surgical diagnosis is reported to be 1.3 to 1.6 per 1000 women from 15 to 49 years of age (2).
Endometriosis has the unique status of being a benign metastatic disease. Owing to its ability to invade the surrounding tissues, and in some cases, to metastasize to lymph nodes and beyond the abdominal cavity (3), endometriosis closely resembles cancer. Despite its destructive and invasive nature, and its clonal origin from single epithelial cells implanting at abnormal locations (4), endometriosis is not commonly fatal, and endometrial tissue invasion is controlled and eventually stops. However, in some cases, endometriosis can progress to biologically malignant tumors, often in reproductive organs.
Sampson (5) first described the association between endometriosis and ovarian cancer in 1925, and his criteria are still used to identify malignant tumors arising from endometriosis. Scott (6) further defined the diagnosis of endometriosis-associated ovarian carcinoma (endometriotic-associated carcinoma), stating that benign endometriosis should be contiguous with malignant tissue (5). In this study, we review evidence supporting the pathophysiological connection between endometriosis and ovarian cancer. Specifically, we discuss the major steps and mechanisms necessary for endometriotic-associated carcinoma tumorigenesis with regard to natural history; pathologic presentation; and the role of sex steroid hormones, inflammation-related microenvironments, and molecular genetic alterations. We particularly focus on the different stages of endometriotic-associated carcinoma tumor progression, from early endometriosis, atypical endometriosis, and borderline tumors to fully malignant tumors (ie, ovarian carcinoma; Fig. 1).
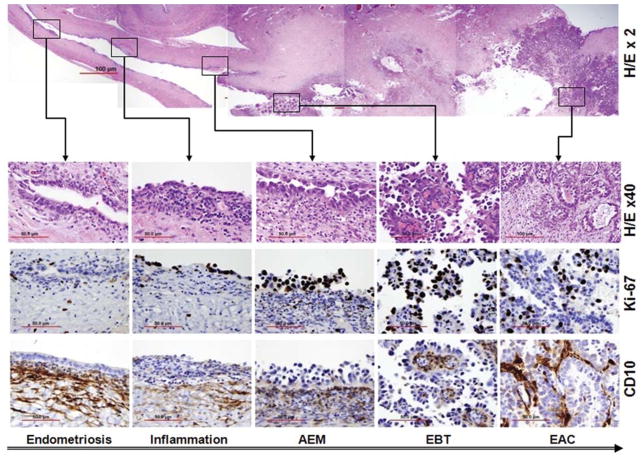
FIG. 1.
Photomicrographs illustrating the morphologic steps of tumor progression from endometriosis to endometriosis-associated carcinoma. Upper panel, photomicrograph of a hematoxylin and eosin (H/E)-stained section of large, cystic, endometriosis-associated clear cell ovarian carcinoma in which different stages of tumor progression are indicated within boxes. Lower panels, magnification of the boxed areas from the ovarian tumor section in the upper panel. From left to right, the sections show the histologic and immunohistochemical features of typical endometriosis, early inflammation, atypical endometriosis, ovarian borderline tumors, and clear cell carcinoma. Ki-67 immunostaining is an indicator of cell proliferation and CD10 immunostaining localizes endometrioid stromal cells.
ENDOMETRIOSIS AND OVARIAN CANCER: NATURAL HISTORY AND PATHOGENESIS
The histogenesis of endometriosis remains controversial and several distinct hypotheses have been proposed. The proposed mechanisms include [for review, please refer to Gazvani and Templeton (7)]: (1) retrograde menstruation; (2) celomic metaplasia (endometriosis originating in the celomic membrane by a process of metaplasia); (3) embryonic cell rests; (4) induction; and (5) lymphatic and vascular dissemination. None of these proposed mechanisms can fully explain the clinical and pathologic nature of endometriosis, however.
If we accept that retrograde menstruation is the primary initiating factor for endometriosis, it is possible that 100% of women have had transient endometriosis at some point in their lifetime (8). It follows that if only 2% to 8% of women have a surgical diagnosis of endometriosis (1), then 92% to 98% of women with endometriosis regress spontaneously. The natural course of endometriosis, starting from ectopic tissue implantation, is thought to start with bleeding that is followed by inflammation, which activates fibrin deposition, adhesion formation, and eventually, the scarring and distortion of peritoneal surfaces of organs and pelvic anatomy.
The risk of developing ovarian carcinoma from endometriosis is generally low. Several large cohort and case control studies have shown that the risk of ovarian cancer from endometriosis ranges between 1.3 and 1.9 (1). The overall frequency of malignant transformation is estimated to be from 0.3% to 0.8% (9,10). In populations of primarily infertile women, the relative risk increases to 2.7. The risk of endometriotic-associated carcinoma is higher in older women [13% in women >50 yr of age; (11)], and in women with genetic predisposition to endometriosis, abnormal inflammatory responses to endometriosis (see discussion below), and long histories of endometriosis [follow-up >10 yr, relative risk of 4.2 for developing ovarian cancer; (11)]. Given the high incidence of endometriosis in reproductive-age women, the number of ovarian cancers arising from endometriosis may also be high. A greater understanding of the pathogenesis of endometriotic-associated carcinoma will better inform research into ovarian cancer prevention strategies.
Studies of the natural history of endometriosis have also been useful for establishing a connection between endometriosis and ovarian cancer. Studies of nonrandomized populations show that endometriosis requires 4 to 10 years for water blister lesions to progress to scarred blue-domed cysts, 7 to 10 years to progress from clear to red to scarred black lesions, and approximately 20 years for the proportion of scarred black lesions to increase from 23% to 63% (12). In some women, advanced ovarian endometriosis itself may not be associated with progression to cancer, but may instead represent highly differentiated tissue. Most endometrioses encounter an unfavorable microenvironment and eventually regress (13) (Fig. 2). Among patients with malignant ovarian tumors, those without endometriosis tend to be significantly older (age, 55 yr) than those with endometriosis elsewhere in the pelvis (age, 45 yr) (14). Large-sized, long-standing endometriosis and rapid growth of ovarian mass are cause for concern and warrant surgical and pathologic evaluation (Fig. 2); however, there is still no tool that can clinically identify patients at risk of developing endometriotic-associated carcinoma.
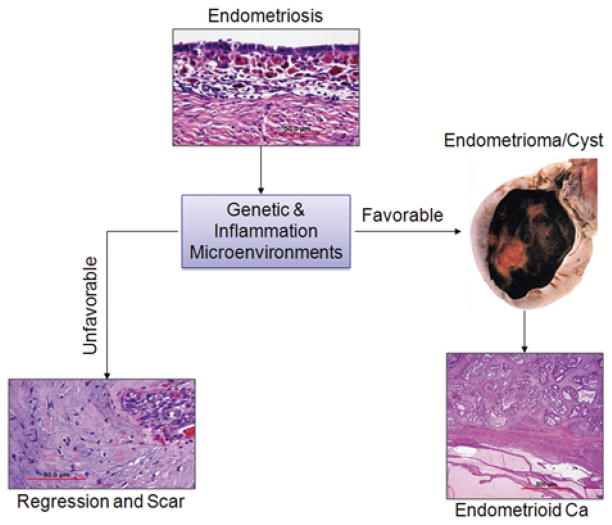
FIG. 2.
The natural history of human endometriosis. Ectopic endometriotic tissues on the ovarian surface either encounter an unfavorable microenvironment (left) and undergo regression with scar formation (fibrosis with minimal endometriotic tissue) or encounter a favorable microenvironment and continue growth (right) to form endometriotic cysts/masses, which have an increased risk of accumulating additional genetic alterations that lead to the development of endometrioid ovarian cancer.
Establishing a reliable model of human endometriosis has been difficult because it remains unclear whether different presentations of endometriosis reflect different histogeneses or just varied forms of pathophysiological outcomes. Endometriosis has very different clinical and pathologic presentations (15). Most endometrioses are mild and diffusely localized, with minimal histologic changes or inflammatory responses (eg, adenomyosis). They typically consist of endometriotic implants on the surface of the pelvic peritoneum, rectovaginal or ovaries (peritoneal endometriosis), and cervix. Less commonly, some lesions may be just a few foci around the ovaries with large ovarian cysts lined by endometriotic tissue (endometriomas) that show significant responses to cycle changes and lead to inflammation and proliferation. Two additional, but rare, forms of endometriosis are described by a complex solid mass (rectovaginal endometriotic nodules) or polypoid endometriotic tissues in the peritoneum or ovaries [polypoid endometriosis (16)]. The risk of ovarian cancer associated with different forms of endometriosis remains to be determined.
The local microenvironment is an important factor that influences the progression of endometriosis (Fig. 2). Recent studies show that, in addition to eutopic endometrium and peritoneal fluid, macroscopically normal peritoneum localized at the pelvic brim is biologically different in women with and without endometriosis (17). Animal models suggest that implanted endometrial tissue in normal pelvic regions yields no endometriosis, unless genetically modified local microenvironments are introduced that enable endometrial implant growth (18). Although some microenvironments support progression to endometriotic-associated carcinoma, unfavorable genetic microenvironments can lead to regression of endometriosis (Fig. 2).
Large cystic endometriotic tissues, characterized by hemorrhage and inflammation, have been the primary models for the study of endometriotic-associated carcinoma tumor progression (Fig. 2). In a study of 324 patients with 5-cm ovarian masses, the frequency of endometriosis in benign, borderline malignant, and malignant tumors was 9.7%, 12.5%, and 11.4%, respectively (19). In endometriotic cysts, the accumulation of molecular-level changes and cell transformation occur in a distinct stepwise manner through atypical endometriosis, ovarian borderline tumor, and then fully malignant tumor (endometriotic-associated carcinoma; Fig. 3).
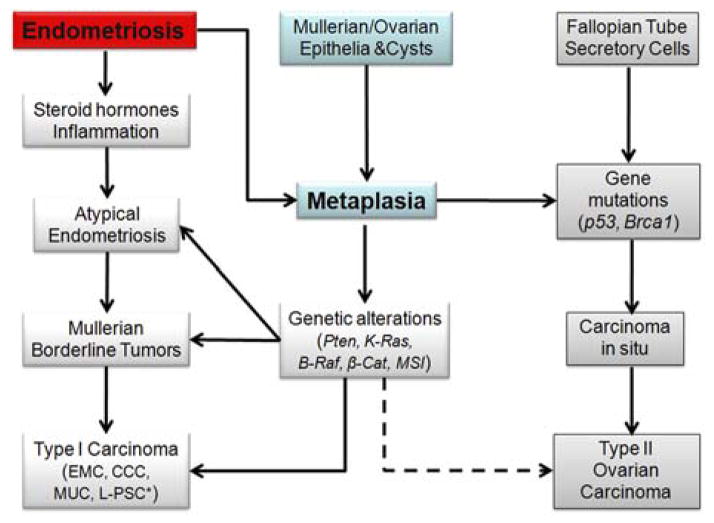
FIG. 3.
The major steps involved in endometriosis-associated tumor progression. Ovarian carcinoma is classified as type I (left bottom) or type II (right bottom). Endometriosis-associated ovarian carcinomas are commonly type I. CCC indicates clear cell carcinoma; EMC, endometrioid carcinoma; L-PSC, low-grade papillary serous carcinoma; MUC, mucinous carcinoma. * Association of low-grade papillary serous carcinoma with endometriosis has not been established.
Atypical Endometriosis
Atypical endometriosis is a recently proposed stage and is defined by dysplastic characteristics that are different from typical endometriosis. Atypical endometriosis are considered to be precancerous and strongly associated with endometriosis-associated ovarian cancer (Fig. 1). However, recognition of atypical endometriosis as diagnostic criteria for pathologic and clinical management remains controversial. Czernobilsky and Morris (20) first described atypical endometriosis by distinguishing between mild and severe atypical endometriosis: (1) mild atypia (reactive): 1 layer of slightly eosinophilic cuboidal or flattened cells with enlarged hyperchromatic and pleomorphic nuclei; and (2) severe atypia (favors precancer lesion): large pleomorphic hyperchromatic or pale nuclei, eosinophilic cytoplasm, occasional squamoid features, tufting, crowding and stratification, intraluminal projections, and bridging in glands. Later, Thomas and Campbell (21) further classified atypical endometriosiss based on features identified in histologic examinations with the following criteria : large hyperchromatic or pale nuclei with moderate-to-marked pleomorphism; increased nuclear-to-cytoplasmic ratio; and cellular crowding and stratification.
In a review of a large series of studies, approximately 8% of endometrioses contain atypical endometriosis (22). In ovarian cancer, atypical endometriosis is present in 23% of endometrioid carcinomas and in 36% of clear cell carcinomas (23). Notably, most atypical endometriosis show direct continuity with endometriotic-associated carcinoma (24,25), but are rarely seen in noncancer endometriosis (26). The spatial and chronological association with endometriotic-associated carcinoma suggest that atypical endometrioses are precancerous lesions, as seen in atypical hyperplasia of the endometrium (26). This has been supported by histologic evidence of transition between endometriosis, atypical endometriosis, and endometriotic-associated carcinoma (24,25) (Fig. 1). In addition, atypical endometriosis represents an early stage of dysplastic endometriotic tissue that contains significant genetic alterations (21,27). The diagnosis of atypical endometriosis thus relies on both histologic features and molecular changes. Increased awareness of the characteristics of atypical endometriosis will improve early detection of patients with endometriosis who are at risk of endometriotic-associated carcinoma.
Of note, endometriotic metaplasia is characterized by histologic changes that are distinct from those of atypical endometriosis. Metaplasia is frequently found in both benign endometriosis and endometriotic-associated carcinoma (28,29) and is usually seen in the precancerous stage (26). The association of endometriotic metaplasia and endometriotic-associated carcinoma is unclear, although limited data suggest that mucinous metaplasia is associated with mucinous borderline tumor (28) (Fig. 3).
Endometriosis-Associated Ovarian Borderline Tumors
Borderline tumors from endometriosis is a well-defined tumor stage and it is the next and last step for the risk of endometriotic-associated carcinoma. Identification of a borderline tumor from endometriosis is critical for study of endometriosis-associated tumorigenesis (Fig. 3) and for clinical management. In contrast to atypical endometriosis with relative uniform histology, ovarian borderline tumors are histologically heterogeneous, including a variety of histologic types and differentiation.
Most knowledge of ovarian borderline tumors is from mucinous and serous borderline tumors, but less from endometrioid or clear cell types. This is in contrast to most endometriotic-associated carcinomas that are from endometrioid and clear cell types (30–32). Ovarian borderline tumors are a well-defined entity as a tumor stage immediately before fully malignant ovarian cancer. Ovarian borderline tumors are characterized by glands lined by specific epithelia that are atypical (stratification and tufting) or cytologically malignant. In general, ovarian borderline tumors, regardless of histotypes, present as part of the spectrum of tumor progression in most low-grade ovarian cancer (33).
Endometrioid borderline tumors were first established as a separate category in endometrioid tumors in the early 1970s by the International Federation of Gynecology and Obstetrics and the World Health Organization (34). Most authorities follow the guidelines of the World Health Organization, which define them as tumors composed of glands lined by endometrioid-type epithelium that is atypical or cytologically malignant, but lacking obvious stromal invasion (35). Borderline tumors from endometriosis share the same criteria proposed in endometrioid and other types of ovarian borderline tumor. Studies of ovarian borderline tumors in association with endometriosis are very scant in the literature. Limited studies show that endometriosis-associated ovarian borderline tumor have molecular changes closely linked to downstream change of endometriotic-associated carcinoma (36).
Mucinous and serous borderline tumors are the most common one seen in ovary. For example, in a large retrospective study from a Japanese population, the majority of the borderline tumors (73.2%) were of the mucinous type, followed by serous type (only 16.9%). The other types such as endometrioid (2.8%), Brenner (1.4%), and mixed type (5.6%) were much rarer (37). Mucinous and serous borderline tumors in association with endometriosis have not been established. Both tumor types have characteristic molecular and histologic features (33).
Recent studies may change our view of ovarian/ mullerian mucinous borderline tumors (28,38). Fukunaga and Ushigome (28) suggested the possibility that endometriosis with mucinous metaplasia and epithelial hyperplasia might contribute to the histogenesis of mullerian mucinous borderline tumors (28). On the basis of its frequent association with endometriosis and its morphologic similarity with atypical papillary proliferation of endometrioid epithelium, mullerian mucinous borderline tumors were considered a low-grade tumor on the spectrum of endometrioid tumors (38). In other words, mullerian mucinous borderline tumors could be interpreted as a low-grade endometrioid tumor with extremely extensive mucinous papillary metaplastic change. Someone has also suggested that a malignant counterpart of mullerian mucinous borderline tumors is likely to be on the spectrum of endometrioid tumors. Recent studies have shown that there are similar immunohistochemical expression profiles between mullerian mucinous borderline tumors and low-grade endometrioid tumors (38), suggesting that a group of low-grade mullerian tumors (most endometrioid, mucinous, clear cell, and some serous) are precancer lesions of endometriotic-associated carcinoma (Fig. 3).
On the basis of our clinical experience, pure endometriotic-associated borderline tumors seemed to be uncommon; rather, borderline tumors are frequently seen as part of endometriotic-associated carcinoma (Fig. 1). In fact, the incidence of endometriosis may be higher in borderline tumors than that in carcinoma. In a retrospective analysis of 160 malignant and 23 borderline ovarian tumors, the incidence of endometriosis in borderline tumors (13%, 3/23) was higher than that in ovarian cancer (6.9%, 11/160) (39). Identification of borderline tumors in endometriotic-associated carcinoma will not change the outcome of patient management, but will substantially impact our study of molecular pathways as a pathologic landmark for the tumorigenesis of this particular type of ovarian cancer development.
Endometriosis-Associated Ovarian Carcinoma
The prevalence of endometriotic-associated carcinoma varies based on the specific histologic type of ovarian cancer. Somigliana et al. (1) reviewed 11 studies on the frequency of endometriosis in patients with ovarian cancers according to histologic type, and endometriotic-associated carcinoma was most commonly seen in clear cell carcinoma (35% in 390 cases), followed by endometrioid carcinoma (27% in 648 cases), serous carcinoma (5% in 1372 cases), and mucinous carcinoma (4% in 614 cases). The study concluded that: (1) endometriotic cells might undergo somatic mutations leading to malignant potential; and (2) endometriosis and ovarian cancer might represent 2 distinct biologic entities of a different set of causative molecular events.
According to Kurman and Shih’s classification (33), in which ovarian cancers are designated as type I and type II based on morphologic, molecular, and histogenic characteristics, endometriosis is commonly linked to the tumorigenesis of type I ovarian carcinomas (31,32), but is rarely seen in type II ovarian carcinomas (Fig. 3).
Type I ovarian tumors are slow growing, generally confined to the ovary or peritoneum at the time of diagnosis, and develop from well-established precursor lesions. Type I tumors include endometrioid, clear cell, mucinous, and low-grade serous carcinomas. They are genetically stable and are characterized by mutations and dysfunction of K-Ras, B-Raf, Pten, and β-catenin/Wnt, and microsatellite instability (40–42).
Type II tumors are rapidly growing, highly aggressive neoplasms, and before they become invasive cancer, they are believed to have poorly defined precursor lesions that develop de novo (43). Type II tumors are often considered to have originated from a single layer of ovarian surface epithelial cells or inclusion cyst cells in the ovary (44). Recent studies have suggested that epithelial cells in the fallopian tubes play an important role in spreading pelvic serous carcinoma, which originated from serous tubal intraepithelial carcinoma. Serous tubal intraepithelial carcinoma has been found in the fimbria of fallopian tubes and its molecular mechanisms are currently under intensive study (45). Type II tumors include high-grade serous carcinoma, malignant mixed mullerian tumors, and undifferentiated carcinomas. This group of tumors has a high level of genetic instability and is characterized by mutation of p53 (46) (Fig. 3).
Although ovarian endometrioid and clear cell carcinomas are the most common tumors associated with endometriosis, only a proportion of these ovarian cancer types are found to be associated with endometriosis (30–32). The apparently low prevalence of endometriotic-associated carcinoma, however, may be due to underdetection of endometriosis in these patients. Currently, there is no imaging system available that is capable of providing a reliable measure of the level and severity of endometriosis. As an example, one study showed that sonography diagnosed endometriosis in only 60% of patients with an endometrioma at least 2 cm in diameter (47).
Histologic sampling may also contribute to underscoring of endometriotic-associated carcinoma in patients with ovarian cancer. For example, most pathologic reports are just focused on the presence or absence of endometriosis based on available sections. Little or no attention has been paid to the areas of transition between endometriosis, atypical endometriosis, and endometriotic-associated carcinoma. Van Gorp et al. (22) classified ovarian cancer associated with endometriosis into 3 categories: (C1) ovarian cancers with histologic proof of transition from endometriosis to ovarian cancer based on the definition of Sampson and Scott (5,6); (C2) ovarian cancers with endometriosis in the same ovary, but without histologic proof of transition; and (C3) ovarian cancers with concomitant endometriosis at any location in the pelvis. On the basis of these categories, most histologic analyses identify C3. More extensive sampling of ovarian endometrioid or clear cell carcinomas will likely increase the probability of detecting C1 endometriotic-associated carcinoma or C2 endometriosis. Pathologic examination has provided a morphologic approach to defining the spectrum of cellular alterations of type I ovarian cancers associated with endometriosis, from benign-appearing endometriotic tissue to fully malignant carcinoma (Fig. 1).
Fifty percent of women with ovarian endometrioid carcinomas will also have a simultaneous endometrial adenocarcinoma; 2% to 8% of patients with endometrial adenocarcinomas will have synchronous ovarian carcinoma; approximately 90% of synchronous tumors from ovary and endometrium will be endometrioid. Studies have shown that there is a high prevalence of coexisting endometriosis in these concurrent ovarian and endometrial carcinoma (48,49). The findings suggest that genetic background plays an important role in the tumorigenesis of endometriotic-associated carcinoma, in addition to inflammation/microenvironment.
In addition, we may also rely on the molecular biomarkers for the further evaluation of clinical cases in which endometriotic-associated carcinoma cannot be determined by histologic examination only. For example, Ki-67 reacts with a nuclear nonhistone protein expressed in the nuclei of proliferating cells. It can be used to predict the premalignant potential of atypical endometriosis. Ogawa et al. (31) found statistical differences between the Ki-67 indices of typical endometriosis, atypical endometriosis, and ovarian cancer (2.7±0.90, 9.9±1.73, and 23.1±3.29, respectively). CD10 can be used to evaluate endometriotic tissue (positive for endometriotic stromal cells). CD10 can help to identify endometriosis in those cases in which endometriosis is difficult to be appreciated on histology (Fig. 1).
ENDOMETRIOSIS AND OVARIAN CANCER: MOLECULAR GENETICS
Certain women clustered in families seem to be genetically susceptible to endometriosis. Several studies have suggested a familial tendency for endometriosis with a genetic basis (50–52). Furthermore, family history of ovarian cancer and colon cancer is significantly higher in women with endometriosis (53). Genetic linkage analyses have defined several potential candidate genetic loci and genes associated with endometriosis. Treloar et al. (51) analyzed 1176 families (sister pairs) by linkage analysis and identified a susceptibility locus at 10q26 that has a high lod score of >3.0 for endometriosis. Although the researchers failed to find a direct link between 2 candidate genes (EMX2 and PTEN) in this (54), other studies have provided strong evidence in favor of a genetic basis of endometriosis. Identification of early genetic alterations responsible for the initiation of endometriosis and endometriotic-associated carcinoma is a major goal of current research efforts.
Endometriosis is clonal. The patterns of clonality and loss of heterozygosity (LOH) between endometriosis, atypical endometriosis, and endometriotic-associated carcinoma were exactly the same in the cases examined by Campbell’s study (21). In particular, the patterns of genetic alteration are related to the proximity between endometriosis and endometrioid carcinoma, indicative of the spectrum of tumor progression (21) (Table 1).
TABLE 1.
Frequencies of major genetic alterations/mutations in different stages of EAC
| Gene mutations | EMS (%) | AEM (%) | EBT (%) | EAC (%) | Reference | |
|---|---|---|---|---|---|---|
| PTEN | 0 | 0 | ~10 | 21 ~5 |
EMC CCC |
(36,41,55–58) |
| K-RAS | 0 | 0 | ~10 | ~20 ~7 |
EMC CCC |
(18,40,57–60) |
| B-RAF | 0 | 0 | 0 | ~17 ~1 |
EMC CCC |
(57,61) |
| β-Catenin | Low | ~90 | ~25 0 |
EMC CCC |
(40,62) | |
| ARID1A | ~60 30 |
CCC EMC |
(63) | |||
| PIK3CA | ~46 11 |
CCC EMC |
(57,58,64) | |||
| P53 | 15 8 |
CCC EMC |
(58,64) | |||
| LOH | ~20 | ~25 | ~40 | ~50 | (21,27,51,65,55,66) | |
| MSI | ~80 | ~75 | ? | ~60 | (67) |
AEM indicates atypical endometriosis; CCC, clear cell carcinoma; EAC, endometriosis-associated ovarian cancer; EBT, endometriosis-associated borderline tumor; EMC, endometrioid carcinoma; EMS, endometriosis; MSI, microsatellite instability.
Genetically engineered animal models are important tools in analyzing the genetic basis of this disease, allowing an examination of the roles of specific genes or microenvironments in the histogenesis of endometriosis and the risk of endometriotic-associated carcinoma. Dinulescu et al. (18) injected an adenoviral Cre recombinases construct in the ovarian bursa, which led to tissue-specific expression of active mutant K-Ras, and resulted in benign lesions reminiscent of endometriosis. In the mice harboring an oncogenic allele of K-Ras, a subsequent conditional deletion of Pten caused the development of ovarian cancer (24). This mouse model provides direct evidence that genetic alterations can lead to the transformation of endometriosis into cancer. Although animal models of K-Ras mutations can be valuable in studying endometriosis, the mechanism of disease progression in mice does not relate directly to the initiation and early development of human endometriosis (68,69).
Examination of global genomic alterations in women with endometriosis can provide us with useful genetic fingerprints of those susceptible to developing endometriosis and/or progression to endometriotic-associated carcinoma. The most commonly used strategies include searching for genomic alterations or genomic differences between eutopic and ectopic endometria, and examining different stages of endometriosis. Molecular tools used for the studies include comparative genomic hybridization, single nucleotide polymorphism, and LOH. A number of studies have been conducted recently with variable results, likely due to different clinical settings, sample sizes, platforms, and analytical tools. A few genetic alterations, however, seem to be strongly associated with endometriosis and shared among the studies (Table 1). By microsatellite analysis, LOH is present in 10% to 20% of endometriosis cases, commonly involving 9p, 11q, and 22q (27). The patterns of LOH in endometriotic-associated carcinoma are similar to those found in endometriosis, but the rates of LOH are significantly higher (20%–60%; Table 1). The data from comparative genomic hybridization studies revealed similar findings (65). LOH in the Xq region is not present in endometriosis, but is as high as 38% in endometriotic-associated carcinoma (27). The rate of LOH in chromosome 10q suggests that it may be a region potentially associated with endometriosis and endometriotic-associated carcinoma. For example, in endometriosis, association studies show that 10q26 is significantly associated with endometriosis (51), but no LOH is found (21). In atypical endometriosis, LOH of 10q23 is found in 40% of cases, whereas in endometriotic-associated carcinoma, LOH of 10q23 can be as high as 45% (55).
Thus far, based on the knowledge of genomic alterations, it has been hypothesized that: (1) endometriosis presents significant evidence of somatic cell and molecular damages and these damages are monoclonal; (2) in a proportion of cases, this damage may be sustaining the progression of the disease; (3) areas of additional chromosomal losses (6p, 17p, and Xq) may contain important tumor suppressor genes; and (4) in a very small proportion of cases, the disease progresses to an endometrioid tumor (66) (Fig. 3).
Several molecular pathways have been identified that contribute to the tumorigenesis of endometrioid carcinomas, including RAS, PTEN, ARID1A, PIK3 CA, and β-catenin, and rarely, P53 (Table 1). Currently, only very few studies have examined the changes in these pathways or gene mutations that occur during the progression of endometriosis to atypical endometriosis, ovarian borderline tumor, and endometriotic-associated carcinoma. PTEN is a tumor suppressor gene that is commonly lost in endometrioid carcinoma (41). Genomic alteration/ mutation of the PTEN gene can be found in endometriosis, before transformation. Studies showed that although LOH at the PTEN locus (10q23) is infrequent in endometriosis, somatic mutations at the PTEN gene can be as high as 57% (13 of 23) in solitary endometriotic cysts (55). Martini et al. (56) stated that reduced Pten protein expression was detected in 15% (7 of 46) of endometriosis. PTEN mutations in atypical endometriosis remain to be determined (41). In endometrioid borderline tumors, approximately 12% of tumors showed Pten mutations (36,57).
In both endometrioid and clear cell ovarian endometriotic-associated carcinoma, LOH and PTEN mutations are frequently seen. LOH at 10q23.3 was found in 42.1% of ovarian endometrioid carcinomas and 27.3% of clear cell carcinomas carcinomas (55), whereas PTEN mutations were found in approximately 21% of ovarian endometrioid carcinoma but not in clear cell carcinoma (41). More studies and larger sample sizes are needed to further study the rate of PTEN mutations in different stages of tumor progression from endometriosis. Current studies suggest that Pten mutations may be an important mechanism associated with endometriotic-associated carcinoma progression (Fig. 3 and Table 1).
K-Ras is another gene frequently mutated in type I ovarian tumors. From limited studies, it seems that K-Ras gene mutations are not common in endometriosis, atypical endometriosis, or endometriosis-associated borderline tumors. In endometriotic-associated carcinoma, K-Ras gene mutations were frequently found, but the rate of gene mutations varied depending on the studies. Overall, K-Ras mutations are found in 10% to 20% of endometriotic-associated carcinoma (40,59,60) (Table 1). If we include mucinous ovarian carcinoma, the K-Ras gene mutations are present in as many as 50% of tumors. These findings suggest that K-Ras mutations may be a later and less common event of tumorigenesis in endometriotic-associated carcinoma. Mullerian borderline tumors from endometriosis seemed to have a higher rate of K-Ras mutation rate and 69% of mullerian borderline tumors had K-Ras mutations in small series (70). Of note, this seems to be different in the mouse endometriosis model, in which K-Ras mutations play a major role in the development of endometriosis, and subsequent PTEN mutations promote endometriotic-associated carcinoma transformation (18). We are still in the early stages of studying whether the development of endometriotic-associated carcinoma requires sequential genetic alterations of tumor suppressors or oncogenes; we currently lack critical information about the stages of tumor progression.
β-catenin mutations and overexpression are very common in ovarian endometrioid carcinoma; approximately 50% of endometrioid carcinoma have β-catenin alterations (40). Endometrioid carcinoma containing β-catenin mutations are low grade and associated with better prognosis. As many as 90% of endometrioid borderline tumors harbor β-catenin mutations (40) (Table 1). These findings suggest that β-catenin mutation is an early event in the tumorigenesis of endometrioid carcinoma in the ovary. Given that most endometriotic-associated carcinoma are low grade and have a good prognosis, clinical use of β-catenin as a marker for detecting early-stage endometriotic-associated carcinoma should be further investigated.
CA125 is one of the few biomarkers used to monitor cancer development. In a study of nonserous ovarian carcinoma (mucinous, endometrioid, and clear cell types), approximately half of the patients showed a bordering elevation of CA125 (35<CA125 <65U/mL) within a period of 3.8 years. Seventy-five percent of serous carcinomas show a sudden elevation of CA125 (71). CA125 may be a valuable biomarker for monitoring endometriotic-associated carcinoma development, particularly in borderline ovarian tumors associated with endometriosis.
ARID1A mutations are newly identified in ovarian clear cell carcinoma. Wiegand et al. (63) conducted ARID1A mutation analysis in large numbers of endometriosis-associated carcinomas, and ARID1A mutations were seen in 55 of 119 ovarian clear cell carcinomas (46%), in 10 of 33 endometrioid carcinomas (30%), and in none of the 76 high-grade serous ovarian carcinomas. The findings provide new molecular tools for the study of endometriosis-associated carcinoma. Currently, no mutation analysis has been performed in either atypical endometriosis or endometriosis-associated borderline tumor.
Currently, we are still in the early stages of characterizing the molecular changes that occur during the progression of endometriotic-associated carcinoma. Global gene expression profiling analysis may be useful for identifying potential molecular markers of the different stages of endometriotic-associated carcinoma. Studies have shown that ectopic and eutopic endometria contain a sizable number of common genomic alterations, indicating similarities in endometriotic tissue. However, the data from the gene profiles can differentiate ectopic from eutopic endometria and classify different subtypes of endometrioses from the peritoneum and ovary (72). Information about the differential expression of genes between eutopic and ectopic endometrial tissues may provide clues about the histogenesis of endometriosis (73), but may be less informative with regard to endometriosis-associated tumor progression. Future studies should examine the differences in gene expression in each stage of endometriosis-associated tumor progression. The search for potential target genes should be carried out for each stage of tumor progression in selected cases in which the morphologic spectrum of tumor progression is well characterized (Fig. 1).
MicroRNAs (miRNAs) are newly identified small noncoding RNAs that have been found to be significantly associated with human carcinomas. Several studies have reported that deregulation of miRNAs is associated with endometriosis (74–76). These miRNAs potentially regulate the putative molecular pathways constituted by their targets, suggesting that miRNAs may play an important role in endometriotic lesion development. Ohlsson et al. (74) further proposed that endometriosis-associated miRNAs may have regulatory functions in hypoxia, inflammation, tissue repair, transforming growth factor (TGF)-β-regulated pathways, cell growth, cell proliferation, apoptosis, extracellular matrix remodeling, and angiogenesis. In addition, specific miRNAs may be associated with malignant progression in endometriosis (74). Therefore, miRNAs may be useful as biomarkers for tumor classification or to differentiate between endometriotic-associated carcinoma and nonendometriotic-associated carcinoma. The role of miRNAs for evaluating endometriotic-associated carcinoma remains to be determined.
ENDOMETRIOSIS AND OVARIAN CANCER: INFLAMMATION
It is increasingly evident that chronic inflammation, coupled with genetic alterations, promotes the progression of a malignant endometriotic-associated carcinoma phenotype. The immune system plays paradoxical roles in endometriotic-associated carcinoma. Endometrial cells are considered to be dysfunctional cells that chronically stimulate the immune system. Unless the immune system can detect and eradicate the abnormally located endometrial tissue (immunosurveillance), chronic inflammation results in immune-mediated changes referred to as immunosculpting, a process that includes both permanent (eg, mutations) and nonpermanent (eg, reversible ligand-induced cytokine production) events. As a result, the immune system may induce alterations in the tumor that make it acceptable to the immune system (immunoediting). These alterations may be associated with immune escape, such as transformation and increased aggressiveness [ie, inflammation-induced tumor promotion; (77)].
Inflammatory Milieu in Early-Stage Endometriosis
In endometriosis, proinflammatory interferon-γ and interleukin (IL)-2 from Th1, and the anti-inflammatory cytokines IL-4 and IL-10 from Th2, are found to be increased in peritoneal fluids. Th1 cytokines are known to induce activation of T cell-mediated, delayed-type hypersensitivity and macrophage activation (classic M1) with production of significant amounts of reactive nitrogen and oxygen intermediates. Th2 cytokines stimulate antibody-mediated immunity by B-cell differentiation to plasma cells, inhibit the Th1-mediated response, and activate macrophages (78) (Fig. 4, alternative M2). Increased proliferation of endometrial tissue and retrograde menstruation lead to high levels of hemoglobin, heme, and iron (79,80). As a consequence, high levels of oxidative stress may further enhance adhesion of refluxed endometrial cells onto the peritoneum during menstruation and perpetuate the progression of endometriosis.
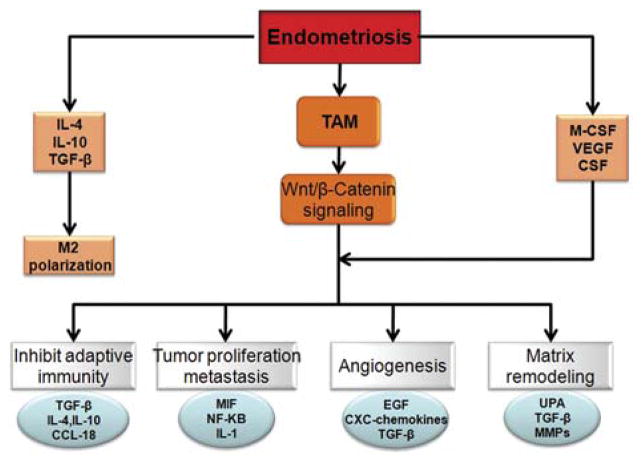
FIG. 4.
Protomural role of tumor-associated macrophages (TAM). Endometriosis favors Th2 cell production and M2 polarization, which lead to an increase in TAM. Through the Wnt/β-catenin signaling pathway and other unknown pathways, TAM promotes tumorigenesis. Mechanisms include suppression of the adaptive immune response by the release of chemokines such as CCL-8 that recruit T-cell subsets devoid of cytotoxic ability (eg, Th2, T reg), which in turn release immunosuppressive mediators including interleukin (IL)-10 and transforming growth factor (TGF)-β. TAM signaling also promotes angiogenesis through production of epithelial growth factor, TGF-β, and chemokines, and stimulates tumor cell growth and survival factors such as NF-κB, migration inhibitory factor (MIF), and IL-1. TAM also plays a role in degradation of extracellular matrix and tissue remodeling activity through expression and release of matrix metallopeptidases 2 and 9 (MMPs) and urokinase plasminogen activator (UPA), which digest tumor basement membrane and facilitate metastasis.
In endometriosis, accumulation of iron affects a wide range of cell types, including endometrial cells (stromal and epithelial), promoting proliferation through catalysis of key DNA synthesis reactions, and increasing adhesion by damaging the mesothelium and creating new adhesion sites. Iron overload also exacerbates activation of peritoneal macrophages, the major cells involved in degradation of erythrocytes and iron metabolism. Thus, iron sustains the state of chronic inflammation and modulates multiple mechanisms involved in the progression of endometriosis (81,82).
Protumorigenic Cytokines and Major Cells in Progressive and Atypical Endometriosis
Macrophages are the main population of peritoneal leukocytes. Increased cell counts may be attributable to elevated levels of macrophage colony-stimulating factor (83) and monocyte chemotactic protein 1 (84) derived from endometrial/endometriotic cells or peritoneal macrophages, respectively.
In vitro studies revealed that peritoneal macrophages derived from patients with endometriosis produce increased levels of the cytokines IL-6, IL-1, and tumor necrosis factor (TNF)-α (85), compared with peritoneal macrophages of women with other benign gynecologic disorders. These factors promote the adhesion of endometrial cells to peritoneum in patients with endometriosis (86).
Similar to CD4+ T cells, macrophages can contribute to tumor destruction or facilitate tumor growth and metastasis, depending on their phenotype. Macrophages are differentially activated by various cytokines and other factors, and become either tumor promoting (M2) or tumoricidal (M1). Some of the molecules produced by M2 macrophages attract additional proinflammatory mediators to the lesion site, thereby amplifying the inflammatory microenvironment. Macrophages activated through the “alternative” pathway with IL-4, IL-13, and/or TGF-β promote tumor progression by enhancing angiogenesis and producing type 2 cytokines and chemokines (87) (Fig. 4).
Peritoneal macrophages also are a major source for hepatocyte growth factor (HGF) production. HGF behaves similar to a pleiotropic growth factor and has mitogenic, motogenic, and morphogenic functions in vitro on various epithelial cells derived from rodents and humans (88). The concentrations of HGF in women with advanced endometriosis are significantly higher than those from women without endometriosis.
Most progressively growing ovarian tumors are infiltrated by large numbers of macrophages. These tumor-associated macrophages (TAMS) are a key component of the tumor stroma and are essential for angiogenesis and matrix remodeling that support progressively growing neoplasms (Fig. 4). In human ovarian cancer, TAMS promote tumor progression by blocking the activation of tumor-specific T cells by their expression of a negative regulator of T-cell activation (89,90). Gene profiling has confirmed that TAMS and M2 macrophages are intermediate in the continuum of macrophage phenotypes (91). In mucinous and serous ovarian tumors, TAMS and M2 cells preferentially express CD163 and CD204. In addition, their expression in borderline and malignant tumors was significantly higher than in benign tumors and correlated well with the histologic gradient of malignancy (Fig. 4).
In addition to TNF-α, IL-1, and IL-8, macrophage migration inhibitory factor (MIF) seems to be crucial in generating a milieu for tumor progression (Fig. 4). MIF is a key regulator of immune and inflammatory responses, is produced by a variety of cells and tissues, and is a key inducer of inflammatory cytokines such as TNF-α and IL-1 (92). MIF binds to the extracellular domain of CD74, the cell-surface form of the major histocompatibility complex class-II-associated invariant chain (93). CD74 may be the long sought-after MIF receptor, or it may be a docking molecule that is involved in the presentation of MIF to its as-yet-unidentified receptor. An elevation of MIF also was observed in the peritoneal fluid of women with endometriosis and increased expression of MIF protein and activity have been reported in active ectopic endometrial implants (94). Ovarian borderline tumor, ovarian carcinomas, and malignant ascites of patients with ovarian cancer express significant MIF protein.
MIF affects protumorigenic pathways in many different ways. Importantly, MIF sustains macrophage viability, which sustains inflammation through TAMS activation, and leads to tumor progression and the development of metastases (95). Loss of MIF seems to disrupt certain DNA damage checkpoints and Skp1-Cullin-F-box-dependent degradation of specific cell cycle regulators, leading to tumorigenesis. MIF upregulates COX-2 synthesis and Prostaglandin E2 (PGE2) secretion in ectopic endometrial cells through p38 and ERK MAPK signaling pathways (96). COX-2 is thought to contribute to tumor cell proliferation, survival, and angiogenesis. In the ovary, COX-2 is implicated in early events of neoplastic transformation; it is rarely found in normal ovarian surface epithelia but is present in endometriosis and in ovarian inclusion cysts considered to be premalignant [(96); Fig. 4].
IL-1 secreted by peritoneal macrophages plays a major role in induction of COX-2 expression. In ectopic endometrial implants, it is 100 times more sensitive compared with eutopic loci. IL-1 acts rapidly on endometriotic cells and stimulates MIF secretion. This interaction between IL-1 and MIF may have an important impact on endometriotic cell growth and endometriosis pathophysiology. IL-1 can also upregulate the expression of the steroidogenic enzyme 11βHSD-1 and suppress the GnRH receptor, thus inducing glucocorticoids and reducing progesterone responsiveness. The latter effect may also trigger proliferation (94). As a protumorigenic cytokine, IL-1 is likely to stimulate a continuing chemical conversation between the developing tumor and its supportive stroma (97).
IL-8 is a macrophage-derived protein that is highly expressed in ectopic endometrial cells, in peritoneal fluid of women with endometriosis, and in the cyst fluid of endometriomas and ovarian carcinomas (98). IL-8 is a CXC chemokine, initially identified as a regulator for the recruitment and trafficking of leukocytes to sites of inflammation. IL-8 induces ovarian cancer cell proliferation partly through increasing ERα activity, and induces proliferation of endometrial stromal cells by acting as an autocrine growth factor in normal endometrioma and in endometriotic cells (99). IL-8 concentration was shown to be among the most informative ovarian cancer serum biomarkers in a multianalyte profiling study (100).
TGF-β activity is also elevated in the peritoneal fluid of women with endometriosis, and women with a higher stage of endometriosis also have a higher concentration of TGF-β in their peritoneal fluid (101). TGF-β has been implicated in ovarian tumorigenesis. TGF-β is a dominant, indirect, proinvasive factor for epithelial cancer cells (102). Under the influence of TGF-β, CD4+ T cells develop into T regulatory cells that actively block tumor immunity by suppressing tumoricidal CD8+ T cells.
In summary, inflammation is considered to be a hallmark of endometriosis, with local and systemic implications (Fig. 4). Local inflammatory reactions at the endometriotic implant site elicit the release of proinflammatory cytokines by associated immune cells and endometriotic cells. The chronic aberrant expression of these proinflammatory cytokines alters regulatory signaling pathways, thereby changing the physiological homeostasis and inducing progressive transcriptional changes that drive sustained proliferation, and increase the rate of DNA repair and the likelihood of accumulation of genetic mutations in endometriotic cells.
ENDOMETRIOSIS AND OVARIAN CANCER: SEX STEROID HORMONES
Estrogen seems to be a mitogen both for endometriosis and ovarian cancer. Direct and indirect epidemiologic evidence suggest that estrogen may be carcinogenic to the ovary (103). However, laboratory evidence does not support this concept, and a plausible mechanism that links estrogen to ovarian carcinogenesis has not yet been developed. There is evidence supporting a role of estrogen in the etiology of endometriosis and enhancement of inflammation.
Estrogen Overproduction in Endometriosis
Estrogen production plays a key role in the pathology of endometriosis because its inhibition by GnRH analogs, oral contraceptives, progestins, or aromatase inhibitors significantly reduces pelvic disease and pain (104). Steroidogenic acute regulatory protein (STAR) facilitates the initial step of estrogen formation (105). The key step, the conversion of C19 steroids to estrogens, is catalyzed by CYP19A1 (aromatase), inhibition of which effectively eliminates all estrogen production.
Three major body sites produce estrogen in women with endometriosis. First, estradiol is secreted by the ovary. Second, aromatase enzyme in peripheral adipose and skin tissue catalyzes the conversion of circulating androstenedione to estrone. Finally, cholesterol is converted to estradiol locally in endometriosis. Peripheral and/or local aromatase activity in endometriosis may be particularly important because a combination of a GnRH agonist and an aromatase inhibitor is significantly superior to only GnRH agonist for providing long-lasting pain relief in patients with severe endometriosis (106).
Estrogen and Inflammation
Two basic pathologic processes, namely cell survival and inflammation, are responsible for the primary symptoms of endometriosis. Estrogen enhances the survival or persistence of endometriotic tissue, whereas prostaglandins and cytokines mediate pain, inflammation, and infertility (107,108). A link between inflammation and estrogen production in endometriosis was recently uncovered and describes a positive feedback cycle that favors overexpression of key steroidogenic genes, most notably aromatase, overexpression of COX2, and continuous local production of estradiol and PGE2 in endometriotic tissue (109–112).
PGE2 coordinately stimulates the expression of all necessary steroidogenic genes, thus enabling the endometrial stromal cell to synthesize estradiol from cholesterol (105). Among the 6 steroidogenic genes, the regulation of STAR and aromatase has been characterized extensively. Both endometriotic and endometrial stromal cells express similar levels of the PGE2 receptor subtypes (113). PGE2 or a cAMP analog stimulates STAR and aromatase levels and activity in endometriotic cells, but not in endometrial stromal cells (105,109,111). Thus, PGE2-cAMP-dependent steroidogenesis in endometriotic stromal cells requires downstream effectors and its absence in endometrial stromal cells is mediated by inhibitory mechanisms or a lack of stimulatory effectors downstream of cAMP.
The nuclear receptor NR5A1, which is present in endometriosis and absent in endometrium, serves as the key transcription factor responsible for mediating the PGE2-cAMP-dependent induction of STAR, aromatase, and possibly other steroidogenic genes in endometriotic stromal cells. The absence of NR5A1 in endometrial cells plays a major role in the lack of responsiveness of steroidogenic genes to PGE2 or cAMP analogs. In addition, the redundant presence of a number of transcriptional inhibitors of STAR and aromatase promoters in endometrial cells provides a fail-safe system for silencing these steroidogenic genes. In the absence of NR5A1, a transcriptional complex that consists of these repressors occupies the steroidogenic promoters and suppresses them in endometrial cells (105).
In summary, on PGE2 induction, coordinated recruitment of NR5A1 to the promoters of the essential steroidogenic genes is the key event for estradiol synthesis in endometriotic stromal cells. A suitable therapeutic target among the steroidogenic enzymes is aromatase, which is encoded by a single gene, because its inhibition blocks all estradiol biosynthesis. Aromatase inhibitors diminish or eradicate endometriotic implants and associated pain that is refractory to currently available treatments (114).
CONCLUSION AND FUTURE DIRECTIONS
Endometriosis-associated ovarian cancer has been and will continue to be a major focus of research efforts into the causes of ovarian cancer. Several recent review articles have discussed endometriosis and ovarian cancer (1,21,22,115–118). This review discussed the stepwise tumor progression of endometriotic-associated carcinoma and focused on studies in the roles of sex steroid hormones, inflammatory processes, and genetic alterations in the progression from endometriosis, atypical endometriosis, ovarian borderline tumor, and endometriotic-associated carcinoma. The World Endometriosis Society and the World Endometriosis Research Foundation recently endorsed a workshop to develop a global consensus statement of research directions and priorities in endometriosis (the 10th World Congress on Endometriosis held in Melbourne, Australia, March 2008). One of the major recommendations from the workshop with regard to basic research is to identify the mechanisms and risk factors underlying the transformation of ovarian endometriosis to ovarian cancer. This task first requires a better understanding of the pathophysiological processes of endometriosis, including histology, immunohistochemistry, and molecular and proteomics approaches. Development of new biomarkers based on genetic alterations identified in endometriotic-associated carcinoma will eventually benefit the clinical evaluation, diagnosis, and management of patients at various stages along the continuum of the endometriosis-associated ovarian carcinoma.
Acknowledgments
Grant support: This study was supported by a Northwestern Memorial Foundation Dixon Translational Research Grant to J-J.W.
Footnotes
The authors declare no conflict of interest.
References
- 1.Somigliana E, Vigano P, Parazzini F, et al. Association between endometriosis and cancer: a comprehensive review and a critical analysis of clinical and epidemiological evidence. Gynecol Oncol. 2006;101:331–41. doi: 10.1016/j.ygyno.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 2.Missmer SA, Cramer DW. The epidemiology of endometriosis. Obstet Gynecol Clin North Am. 2003;30:1–19. vii. doi: 10.1016/s0889-8545(02)00050-5. [DOI] [PubMed] [Google Scholar]
- 3.Abrao MS, Podgaec S, Dias JA, Jr, et al. Deeply infiltrating endometriosis affecting the rectum and lymph nodes. Fertil Steril. 2006;86:543–7. doi: 10.1016/j.fertnstert.2006.02.102. [DOI] [PubMed] [Google Scholar]
- 4.Yano T, Jimbo H, Yoshikawa H, et al. Molecular analysis of clonality in ovarian endometrial cysts. Gynecol Obstet Invest. 1999;47(suppl 1):41–5. doi: 10.1159/000052858. discussion 6. [DOI] [PubMed] [Google Scholar]
- 5.Sampson J. Endometrial carcinoma of the ovary arising in endometrial tissue in that organ. Arch Surg [report] 1925;10:1–72. [Google Scholar]
- 6.Scott R. Malignant change in endometriosis. Obstet Gynecol. 1953;2:293–9. [PubMed] [Google Scholar]
- 7.Gazvani R, Templeton A. New considerations for the pathogenesis of endometriosis. Int J Gynaecol Obstet. 2002;76:117–26. doi: 10.1016/s0020-7292(01)00577-x. [DOI] [PubMed] [Google Scholar]
- 8.Evers JL. Endometriosis does not exist; all women have endometriosis. Hum Reprod. 1994;9:2206–9. doi: 10.1093/oxfordjournals.humrep.a138421. [DOI] [PubMed] [Google Scholar]
- 9.Heaps JM, Nieberg RK, Berek JS. Malignant neoplasms arising in endometriosis. Obstet Gynecol. 1990;75:1023–8. [PubMed] [Google Scholar]
- 10.Moll UM, Chumas JC, Chalas E, et al. Ovarian carcinoma arising in atypical endometriosis. Obstet Gynecol. 1990;75(3 Pt 2):537–9. [PubMed] [Google Scholar]
- 11.Brinton LA, Gridley G, Persson I, et al. Cancer risk after a hospital discharge diagnosis of endometriosis. Am J Obstet Gynecol. 1997;176:572–9. doi: 10.1016/s0002-9378(97)70550-7. [DOI] [PubMed] [Google Scholar]
- 12.Koninckx PR, Barlow D, Kennedy S. Implantation versus infiltration: the Sampson versus the endometriotic disease theory. Gynecol Obstet Invest. 1999;47(suppl 1):3–9. doi: 10.1159/000052853. discussion -10. [DOI] [PubMed] [Google Scholar]
- 13.Zafrakas M, Tarlatzis BC, Streichert T, et al. Genome-wide microarray gene expression, array-CGH analysis, and telomerase activity in advanced ovarian endometriosis: a high degree of differentiation rather than malignant potential. Int J Mol Med. 2008;21:335–44. [PubMed] [Google Scholar]
- 14.Kobayashi H, Sumimoto K, Moniwa N, et al. Risk of developing ovarian cancer among women with ovarian endometrioma: a cohort study in Shizuoka, Japan. Int J Gynecol Cancer. 2007;17:37–43. doi: 10.1111/j.1525-1438.2006.00754.x. [DOI] [PubMed] [Google Scholar]
- 15.Garry R. Is insulin resistance an essential component of PCOS?: The endometriosis syndromes: a clinical classification in the presence of aetiological confusion and therapeutic anarchy. Hum Reprod. 2004;19:760–8. doi: 10.1093/humrep/deh147. [DOI] [PubMed] [Google Scholar]
- 16.Parker RL, Dadmanesh F, Young RH, et al. Polypoid endometriosis: a clinicopathologic analysis of 24 cases and a review of the literature. Am J Surg Pathol. 2004;28:285–97. doi: 10.1097/00000478-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Kyama CM, Overbergh L, Mihalyi A, et al. Endometrial and peritoneal expression of aromatase, cytokines, and adhesion factors in women with endometriosis. Fertil Steril. 2008;89:301–10. doi: 10.1016/j.fertnstert.2007.02.057. [DOI] [PubMed] [Google Scholar]
- 18.Dinulescu DM, Ince TA, Quade BJ, et al. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med. 2005;11:63–70. doi: 10.1038/nm1173. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi K, Kurioka H, Irikoma M, et al. Benign or malignant ovarian neoplasms and ovarian endometriomas. J Am Assoc Gynecol Laparosc. 2001;8:278–84. doi: 10.1016/s1074-3804(05)60591-9. [DOI] [PubMed] [Google Scholar]
- 20.Czernobilsky B, Morris WJ. A histologic study of ovarian endometriosis with emphasis on hyperplastic and atypical changes. Obstet Gynecol. 1979;53:318–23. [PubMed] [Google Scholar]
- 21.Thomas EJ, Campbell IG. Molecular genetic defects in endometriosis. Gynecol Obstet Invest. 2000;50(suppl 1):44–50. doi: 10.1159/000052878. [DOI] [PubMed] [Google Scholar]
- 22.Van Gorp T, Amant F, Neven P, et al. Endometriosis and the development of malignant tumours of the pelvis: a review of literature. Best Pract Res Clin Obstet Gynaecol. 2004;18:349–71. doi: 10.1016/j.bpobgyn.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Fukunaga M, Nomura K, Ishikawa E, et al. Ovarian atypical endometriosis: its close association with malignant epithelial tumours. Histopathology. 1997;30:249–55. doi: 10.1046/j.1365-2559.1997.d01-592.x. [DOI] [PubMed] [Google Scholar]
- 24.LaGrenade A, Silverberg SG. Ovarian tumors associated with atypical endometriosis. Hum Pathol. 1988;19:1080–4. doi: 10.1016/s0046-8177(88)80090-x. [DOI] [PubMed] [Google Scholar]
- 25.Stern RC, Dash R, Bentley RC, et al. Malignancy in endometriosis: frequency and comparison of ovarian and extraovarian types. Int J Gynecol Pathol. 2001;20:133–9. doi: 10.1097/00004347-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Prefumo F, Todeschini F, Fulcheri E, et al. Epithelial abnormalities in cystic ovarian endometriosis. Gynecol Oncol. 2002;84:280–4. doi: 10.1006/gyno.2001.6529. [DOI] [PubMed] [Google Scholar]
- 27.Jiang X, Hitchcock A, Bryan EJ, et al. Microsatellite analysis of endometriosis reveals loss of heterozygosity at candidate ovarian tumor suppressor gene loci. Cancer Res. 1996;56:3534–9. [PubMed] [Google Scholar]
- 28.Fukunaga M, Ushigome S. Epithelial metaplastic changes in ovarian endometriosis. Mod Pathol. 1998;11:784–8. [PubMed] [Google Scholar]
- 29.Fukunaga M. Smooth muscle metaplasia in ovarian endometriosis. Histopathology. 2000;36:348–52. doi: 10.1046/j.1365-2559.2000.00845.x. [DOI] [PubMed] [Google Scholar]
- 30.Lim MC, Lee DO, Kang S, et al. Clinical manifestations in patients with ovarian clear cell carcinoma with or without coexisting endometriosis. Gynecol Endocrinol. 2009;25:435–40. doi: 10.1080/09513590902770131. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa S, Kaku T, Amada S, et al. Ovarian endometriosis associated with ovarian carcinoma: a clinicopathological and immunohistochemical study. Gynecol Oncol. 2000;77:298–304. doi: 10.1006/gyno.2000.5765. [DOI] [PubMed] [Google Scholar]
- 32.Christie M, Oehler MK. Molecular pathology of epithelial ovarian cancer. J Br Menopause Soc. 2006;12:57–63. doi: 10.1258/136218006777525794. [DOI] [PubMed] [Google Scholar]
- 33.Kurman RJ, Shih Ie M. Pathogenesis of ovarian cancer: lessons from morphology and molecular biology and their clinical implications. Int J Gynecol Pathol. 2008;27:151–60. doi: 10.1097/PGP.0b013e318161e4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obstetrics IFoGa. Classification and staging of malignant tumours in the female pelvis. Acta Obstet Gynecol Scand. 1971;50:1–7. doi: 10.3109/00016347109157278. [DOI] [PubMed] [Google Scholar]
- 35.Bell DA, Scully RE. Atypical and borderline endometrioid adenofibromas of the ovary: a report of 27 cases. Am J Surg Pathol. 1985;9:205–14. doi: 10.1097/00000478-198503000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Oliva E, Sarrio D, Brachtel EF, et al. High frequency of beta-catenin mutations in borderline endometrioid tumours of the ovary. J Pathol. 2006;208:708–13. doi: 10.1002/path.1923. [DOI] [PubMed] [Google Scholar]
- 37.Nakashima N, Nagasaka T, Oiwa N, et al. Ovarian epithelial tumors of borderline malignancy in Japan. Gynecol Oncol. 1990;38:90–8. doi: 10.1016/0090-8258(90)90017-f. [DOI] [PubMed] [Google Scholar]
- 38.Yasunaga M, Ohishi Y, Oda Y, et al. Immunohistochemical characterization of mullerian mucinous borderline tumors: possible histogenetic link with serous borderline tumors and low-grade endometrioid tumors. Hum Pathol. 2009;40:965–74. doi: 10.1016/j.humpath.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Oral E, Ilvan S, Tustas E, et al. Prevalence of endometriosis in malignant epithelial ovary tumours. Eur J Obstet Gynecol Reprod Biol. 2003;109:97–101. doi: 10.1016/s0301-2115(03)00047-2. [DOI] [PubMed] [Google Scholar]
- 40.Geyer JT, Lopez-Garcia MA, Sanchez-Estevez C, et al. Pathogenetic pathways in ovarian endometrioid adenocarcinoma: a molecular study of 29 cases. Am J Surg Pathol. 2009;33:1157–63. doi: 10.1097/PAS.0b013e3181a902e1. [DOI] [PubMed] [Google Scholar]
- 41.Obata K, Morland SJ, Watson RH, et al. Frequent PTEN/ MMAC mutations in endometrioid but not serous or mucinous epithelial ovarian tumors. Cancer Res. 1998;58:2095–7. [PubMed] [Google Scholar]
- 42.Shih Ie M, Kurman RJ. Molecular pathogenesis of ovarian borderline tumors: new insights and old challenges. Clin Cancer Res. 2005;11:7273–9. doi: 10.1158/1078-0432.CCR-05-0755. [DOI] [PubMed] [Google Scholar]
- 43.Bell DA. Origins and molecular pathology of ovarian cancer. Mod Pathol. 2005;18(suppl 2):S19–32. doi: 10.1038/modpathol.3800306. [DOI] [PubMed] [Google Scholar]
- 44.Bell DA, Scully RE. Early de novo ovarian carcinoma: a study of fourteen cases. Cancer. 1994;73:1859–64. doi: 10.1002/1097-0142(19940401)73:7<1859::aid-cncr2820730714>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 45.Roh MH, Kindelberger D, Crum CP. Serous tubal intraepithelial carcinoma and the dominant ovarian mass: clues to serous tumor origin? Am J Surg Pathol. 2009;33:376–86. doi: 10.1097/PAS.0b013e3181868904. [DOI] [PubMed] [Google Scholar]
- 46.Shih Ie M, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164:1511–8. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Asch E, Levine D. Variations in appearance of endometriomas. J Ultrasound Med. 2007;26:993–1002. doi: 10.7863/jum.2007.26.8.993. [DOI] [PubMed] [Google Scholar]
- 48.Zaino R, Whitney C, Brady MF, et al. Simultaneously detected endometrial and ovarian carcinomas—a prospective clinicopathologic study of 74 cases: a gynecologic oncology group study. Gynecol Oncol. 2001;83:355–62. doi: 10.1006/gyno.2001.6400. [DOI] [PubMed] [Google Scholar]
- 49.Falkenberry SS, Steinhoff MM, Gordinier M, et al. Synchronous endometrioid tumors of the ovary and endometrium. A clinicopathologic study of 22 cases. J Reprod Med. 1996;41:713–8. [PubMed] [Google Scholar]
- 50.Treloar S, Hadfield R, Montgomery G, et al. The International Endogene Study: a collection of families for genetic research in endometriosis. Fertil Steril. 2002;78:679–85. doi: 10.1016/s0015-0282(02)03341-1. [DOI] [PubMed] [Google Scholar]
- 51.Treloar SA, Wicks J, Nyholt DR, et al. Genomewide linkage study in 1176 affected sister pair families identifies a significant susceptibility locus for endometriosis on chromosome 10q26. Am J Hum Genet. 2005;77:365–76. doi: 10.1086/432960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kashima K, Ishimaru T, Okamura H, et al. Familial risk among Japanese patients with endometriosis. Int J Gynaecol Obstet. 2004;84:61–4. doi: 10.1016/s0020-7292(03)00340-0. [DOI] [PubMed] [Google Scholar]
- 53.Matalliotakis IM, Cakmak H, Krasonikolakis GD, et al. Endometriosis related to family history of malignancies in the Yale series. Surg Oncol. 2010;19:33–7. doi: 10.1016/j.suronc.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 54.Treloar SA, Zhao ZZ, Le L, et al. Variants in EMX2 and PTEN do not contribute to risk of endometriosis. Mol Hum Reprod. 2007;13:587–94. doi: 10.1093/molehr/gam023. [DOI] [PubMed] [Google Scholar]
- 55.Sato N, Tsunoda H, Nishida M, et al. Loss of heterozygosity on 10q23. 3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res. 2000;60:7052–6. [PubMed] [Google Scholar]
- 56.Martini M, Ciccarone M, Garganese G, et al. Possible involvement of hMLH1, p16(INK4a) and PTEN in the malignant transformation of endometriosis. Int J Cancer. 2002;102:398–406. doi: 10.1002/ijc.10715. [DOI] [PubMed] [Google Scholar]
- 57.Kuo KT, Mao TL, Jones S, et al. Frequent activating mutations of PIK53CA in ovarian clear cell carcinoma. Am J Pathol. 2009;174:1597–601. doi: 10.2353/ajpath.2009.081000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ayhan A, Kurman RJ, Yemelyanova A, et al. Defining the cut point between low-grade and high-grade ovarian serous carcinomas: a clinicopathologic and molecular genetic analysis. Am J Surg Pathol. 2009;33:1220–4. doi: 10.1097/PAS.0b013e3181a24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mayr D, Hirschmann A, Lohrs U, et al. KRAS and BRAF mutations in ovarian tumors: a comprehensive study of invasive carcinomas, borderline tumors and extraovarian implants. Gynecol Oncol. 2006;103:883–7. doi: 10.1016/j.ygyno.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 60.Auner V, Kriegshauser G, Tong D, et al. KRAS mutation analysis in ovarian samples using a high sensitivity biochip assay. BMC Cancer. 2009;9:111. doi: 10.1186/1471-2407-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ueda M, Toji E, Noda S. Germ line and somatic mutations of BRAF V599E in ovarian carcinoma. Int J Gynecol Cancer. 2007;17:794–7. doi: 10.1111/j.1525-1438.2006.00853.x. [DOI] [PubMed] [Google Scholar]
- 62.Catasus L, Bussaglia E, Rodrguez I, et al. Molecular genetic alterations in endometrioid carcinomas of the ovary: similar frequency of beta-catenin abnormalities but lower rate of microsatellite instability and PTEN alterations than in uterine endometrioid carcinomas. Hum Pathol. 2004;35:1360–8. doi: 10.1016/j.humpath.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 63.Wiegand KC, Shah SP, Al-Agha OM, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–43. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Willner J, Wurz K, Allison KH, et al. Alternate molecular genetic pathways in ovarian carcinomas of common histological types. Hum Pathol. 2007;38:607–13. doi: 10.1016/j.humpath.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 65.Gogusev J, Bouquet de Joliniere J, Telvi L, et al. Detection of DNA copy number changes in human endometriosis by comparative genomic hybridization. Hum Genet. 1999;105:444–51. doi: 10.1007/s004390051129. [DOI] [PubMed] [Google Scholar]
- 66.Campbell IG, Thomas EJ. Endometriosis: candidate genes. Hum Reprod Update. 2001;7:15–20. doi: 10.1093/humupd/7.1.15. [DOI] [PubMed] [Google Scholar]
- 67.Ali-Fehmi R, Khalifeh I, Bandyopadhyay S, et al. Patterns of loss of heterozygosity at 10q23. 3 and microsatellite instability in endometriosis, atypical endometriosis, and ovarian carcinoma arising in association with endometriosis. Int J Gynecol Pathol. 2006;25:223–9. doi: 10.1097/01.pgp.0000192274.44061.36. [DOI] [PubMed] [Google Scholar]
- 68.Amemiya S, Sekizawa A, Otsuka J, et al. Malignant transformation of endometriosis and genetic alterations of K-ras and microsatellite instability. Int J Gynaecol Obstet. 2004;86:371–6. doi: 10.1016/j.ijgo.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 69.Otsuka J, Okuda T, Sekizawa A, et al. K-ras mutation may promote carcinogenesis of endometriosis leading to ovarian clear cell carcinoma. Med Electron Microsc. 2004;37:188–92. doi: 10.1007/s00795-004-0252-5. [DOI] [PubMed] [Google Scholar]
- 70.Kim KR, Choi J, Hwang JE, et al. Endocervical-like (Mullerian) mucinous borderline tumours of the ovary are frequently associated with the KRAS mutation. Histopathology. 2010;57:587–96. doi: 10.1111/j.1365-2559.2010.03673.x. [DOI] [PubMed] [Google Scholar]
- 71.Kobayashi H, Ooi H, Yamada Y, et al. Serum CA125 level before the development of ovarian cancer. Int J Gynaecol Obstet. 2007;99:95–9. doi: 10.1016/j.ijgo.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 72.Wu Y, Kajdacsy-Balla A, Strawn E, et al. Transcriptional characterizations of differences between eutopic and ectopic endometrium. Endocrinology. 2006;147:232–46. doi: 10.1210/en.2005-0426. [DOI] [PubMed] [Google Scholar]
- 73.Matsuzaki S, Canis M, Pouly JL, et al. Differential expression of genes in eutopic and ectopic endometrium from patients with ovarian endometriosis. Fertil Steril. 2006;86:548–53. doi: 10.1016/j.fertnstert.2006.02.093. [DOI] [PubMed] [Google Scholar]
- 74.Ohlsson Teague EM, Print CG, Hull ML. The role of microRNAs in endometriosis and associated reproductive conditions. Hum Reprod Update. 2010;16:142–65. doi: 10.1093/humupd/dmp034. [DOI] [PubMed] [Google Scholar]
- 75.Burney RO, Hamilton AE, Aghajanova L, et al. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol Hum Reprod. 2009;15:625–31. doi: 10.1093/molehr/gap068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pan Q, Chegini N. MicroRNA signature and regulatory functions in the endometrium during normal and disease states. Semin Reprod Med. 2008;26:479–93. doi: 10.1055/s-0028-1096128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reiman JM, Kmieciak M, Manjili MH, et al. Tumor immunoediting and immunosculpting pathways to cancer progression. Semin Cancer Biol. 2007;17:275–87. doi: 10.1016/j.semcancer.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Podgaec S, Abrao MS, Dias JA, Jr, et al. Endometriosis: an inflammatory disease with a Th2 immune response component. Hum Reprod. 2007;22:1373–9. doi: 10.1093/humrep/del516. [DOI] [PubMed] [Google Scholar]
- 79.Dassen H, Kamps R, Punyadeera C, et al. Haemoglobin expression in human endometrium. Hum Reprod. 2008;23:635–41. doi: 10.1093/humrep/dem430. [DOI] [PubMed] [Google Scholar]
- 80.Gupta S, Agarwal A, Krajcir N, et al. Role of oxidative stress in endometriosis. Reprod Biomed Online. 2006;13:126–34. doi: 10.1016/s1472-6483(10)62026-3. [DOI] [PubMed] [Google Scholar]
- 81.Defrere S, Lousse JC, Gonzalez-Ramos R, et al. Potential involvement of iron in the pathogenesis of peritoneal endometriosis. Mol Hum Reprod. 2008;14:377–85. doi: 10.1093/molehr/gan033. [DOI] [PubMed] [Google Scholar]
- 82.Van Langendonckt A, Casanas-Roux F, Dolmans MM, et al. Potential involvement of hemoglobin and heme in the pathogenesis of peritoneal endometriosis. Fertil Steril. 2002;77:561–70. doi: 10.1016/s0015-0282(01)03211-3. [DOI] [PubMed] [Google Scholar]
- 83.Fukaya T, Sugawara J, Yoshida H, et al. The role of macrophage colony stimulating factor in the peritoneal fluid in infertile patients with endometriosis. Tohoku J Exp Med. 1994;172:221–6. doi: 10.1620/tjem.172.221. [DOI] [PubMed] [Google Scholar]
- 84.Akoum A, Lemay A, McColl SR, et al. Increased monocyte chemotactic protein-1 level and activity in the peripheral blood of women with endometriosis. Le Groupe d’Investigation en Gynecologie. Am J Obstet Gynecol. 1996;175:1620–5. doi: 10.1016/s0002-9378(96)70115-1. [DOI] [PubMed] [Google Scholar]
- 85.Keenan JA, Chen TT, Chadwell NL, et al. Interferon-gamma (IFN-gamma) and interleukin-6 (IL-6) in peritoneal fluid and macrophage-conditioned media of women with endometriosis. Am J Reprod Immunol. 1994;32:180–3. doi: 10.1111/j.1600-0897.1994.tb01111.x. [DOI] [PubMed] [Google Scholar]
- 86.Beliard A, Noel A, Goffin F, et al. Adhesion of endometrial cells labeled with 111Indium-tropolonate to peritoneum: a novel in vitro model to study endometriosis. Fertil Steril. 2003;79(suppl 1):724–9. doi: 10.1016/s0015-0282(02)04819-7. [DOI] [PubMed] [Google Scholar]
- 87.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 88.Khan KN, Kitajima M, Hiraki K, et al. Immunopathogenesis of pelvic endometriosis: role of hepatocyte growth factor, macrophages and ovarian steroids. Am J Reprod Immunol. 2008;60:383–404. doi: 10.1111/j.1600-0897.2008.00643.x. [DOI] [PubMed] [Google Scholar]
- 89.Kryczek I, Zou L, Rodriguez P, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–81. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Allavena P, Sica A, Solinas G, et al. The inflammatory microenvironment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 91.Mantovani A, Sozzani S, Locati M, et al. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 92.Toh ML, Aeberli D, Lacey D, et al. Regulation of IL-1 and TNF receptor expression and function by endogenous macrophage migration inhibitory factor. J Immunol. 2006;177:4818–25. doi: 10.4049/jimmunol.177.7.4818. [DOI] [PubMed] [Google Scholar]
- 93.Leng L, Metz CN, Fang Y, et al. MIF signal transduction initiated by binding to CD74. J Exp Med. 2003;197:1467–76. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Herrmann Lavoie C, Fraser D, Therriault MJ, et al. Interleukin-1 stimulates macrophage migration inhibitory factor secretion in ectopic endometrial cells of women with endometriosis. Am J Reprod Immunol. 2007;58:505–13. doi: 10.1111/j.1600-0897.2007.00471.x. [DOI] [PubMed] [Google Scholar]
- 95.Mitchell RA, Liao H, Chesney J, et al. Macrophage migration inhibitory factor (MIF) sustains macrophage proinflammatory function by inhibiting p53: regulatory role in the innate immune response. Proc Natl Acad Sci USA. 2002;99:345–50. doi: 10.1073/pnas.012511599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Carli C, Metz CN, Al-Abed Y, et al. Up-regulation of cyclooxygenase-2 expression and prostaglandin E2 production in human endometriotic cells by macrophage migration inhibitory factor: involvement of novel kinase signaling pathways. Endocrinology. 2009;150:3128–37. doi: 10.1210/en.2008-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 98.Darai E, Detchev R, Hugol D, et al. Serum and cyst fluid levels of interleukin (IL) -6, IL-8 and tumour necrosis factor-alpha in women with endometriomas and benign and malignant cystic ovarian tumours. Hum Reprod. 2003;18:1681–5. doi: 10.1093/humrep/deg321. [DOI] [PubMed] [Google Scholar]
- 99.Furuya M, Suyama T, Usui H, et al. Up-regulation of CXC chemokines and their receptors: implications for proinflammatory microenvironments of ovarian carcinomas and endometriosis. Hum Pathol. 2007;38:1676–87. doi: 10.1016/j.humpath.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 100.Bertenshaw GP, Yip P, Seshaiah P, et al. Multianalyte profiling of serum antigens and autoimmune and infectious disease molecules to identify biomarkers dysregulated in epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2008;17:2872–81. doi: 10.1158/1055-9965.EPI-08-0464. [DOI] [PubMed] [Google Scholar]
- 101.Oosterlynck DJ, Meuleman C, Waer M, et al. Transforming growth factor-beta activity is increased in peritoneal fluid from women with endometriosis. Obstet Gynecol. 1994;83:287–92. [PubMed] [Google Scholar]
- 102.Denys H, Derycke L, Hendrix A, et al. Differential impact of TGF-beta and EGF on fibroblast differentiation and invasion reciprocally promotes colon cancer cell invasion. Cancer Lett. 2008;266:263–74. doi: 10.1016/j.canlet.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 103.Lacey JV, Jr, Mink PJ, Lubin JH, et al. Menopausal hormone replacement therapy and risk of ovarian cancer. JAMA. 2002;288:334–41. doi: 10.1001/jama.288.3.334. [DOI] [PubMed] [Google Scholar]
- 104.Olive DL, Pritts EA. Treatment of endometriosis. N Engl J Med. 2001;345:266–75. doi: 10.1056/NEJM200107263450407. [DOI] [PubMed] [Google Scholar]
- 105.Bulun SE, Lin Z, Imir G, et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57:359–83. doi: 10.1124/pr.57.3.6. [DOI] [PubMed] [Google Scholar]
- 106.Soysal S, Soysal M, Ozer S, et al. The effects of post-surgical administration of goserelin plus anastrozole compared to goserelin alone in patients with severe endometriosis: a prospective randomized trial. Hum Reprod. 2004;19:160–7. doi: 10.1093/humrep/deh035. [DOI] [PubMed] [Google Scholar]
- 107.Ryan IP, Taylor RN. Endometriosis and infertility: new concepts. Obstet Gynecol Surv. 1997;52:365–71. doi: 10.1097/00006254-199706000-00021. [DOI] [PubMed] [Google Scholar]
- 108.Bruner KL, Matrisian LM, Rodgers WH, et al. Suppression of matrix metalloproteinases inhibits establishment of ectopic lesions by human endometrium in nude mice. J Clin Invest J1-jci. 1997;99:2851–7. doi: 10.1172/JCI119478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Noble LS, Takayama K, Putman JM, et al. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab. 1997;82:600–6. doi: 10.1210/jcem.82.2.3783. [DOI] [PubMed] [Google Scholar]
- 110.Tsai SJ, Wu MH, Lin CC, et al. Regulation of steroidogenic acute regulatory protein expression and progesterone production in endometriotic stromal cells. J Clin Endocrinol Metab. 2001;86:5765–73. doi: 10.1210/jcem.86.12.8082. [DOI] [PubMed] [Google Scholar]
- 111.Sun HS, Hsiao KY, Hsu CC, et al. Transactivation of steroidogenic acute regulatory protein in human endometriotic stromal cells is mediated by the prostaglandin EP2 receptor. Endocrinology. 2003;144:3934–42. doi: 10.1210/en.2003-0289. [DOI] [PubMed] [Google Scholar]
- 112.Bulun SE, Yang S, Fang Z, et al. Role of aromatase in endometrial disease. J Steroid Biochem Mol Biol. 2001;79:19–25. doi: 10.1016/s0960-0760(01)00134-0. [DOI] [PubMed] [Google Scholar]
- 113.Zeitoun K, Bulun S. Aromatase: a key molecule in the pathophysiology of endometriosis and a therapeutic target. Fertil Steril. 1999;72:961–9. doi: 10.1016/s0015-0282(99)00393-3. [DOI] [PubMed] [Google Scholar]
- 114.Attar E, Bulun SE. Aromatase inhibitors: the next generation of therapeutics for endometriosis? Fertil Steril. 2006;85:1307–18. doi: 10.1016/j.fertnstert.2005.09.064. [DOI] [PubMed] [Google Scholar]
- 115.Obata K, Hoshiai H. Common genetic changes between endometriosis and ovarian cancer. Gynecol Obstet Invest. 2000;50(suppl 1):39–43. doi: 10.1159/000052877. [DOI] [PubMed] [Google Scholar]
- 116.Vigano P, Somigliana E, Parazzini F, et al. Bias versus causality: interpreting recent evidence of association between endometriosis and ovarian cancer. Fertil Steril. 2007;88:588–93. doi: 10.1016/j.fertnstert.2006.11.180. [DOI] [PubMed] [Google Scholar]
- 117.Nezhat F, Datta MS, Hanson V, et al. The relationship of endometriosis and ovarian malignancy: a review. Fertil Steril. 2008;90:1559–70. doi: 10.1016/j.fertnstert.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 118.Kobayashi H, Kajiwara H, Kanayama S, et al. Molecular pathogenesis of endometriosis-associated clear cell carcinoma of the ovary (Review) Oncol Rep. 2009;22:233–40. [PubMed] [Google Scholar]