Abstract
Secoisolariciresinol diglucoside (SDG) is the major lignan in wholegrain flaxseed. However, extraction methods are complex and are associated with low yield and high costs. Using a novel synthetic pathway, our group succeeded in chemically synthesizing SDG (S,S and R,R enantiomers), which faithfully recapitulates the properties of their natural counterparts, possessing strong antioxidant and free radical scavenging properties. This study further extends initial findings by now investigating the DNA-radioprotective properties of the synthetic SDG enantiomers compared to the commercial SDG. DNA radioprotection was assessed by cell-free systems such as: (a) plasmid relaxation assay to determine the extent of the supercoiled (SC) converted to open-circular (OC) plasmid DNA (pBR322) after exposure of the plasmid to gamma radiation; and (b) determining the extent of genomic DNA fragmentation. Exposure of plasmid DNA to 25 Gy of γ radiation resulted in decreased supercoiled form and increased open-circular form, indicating radiation-induced DNA damage. Synthetic SDG (S,S) and SDG (R,R), and commercial SDG at concentrations of 25–250 μM significantly and equipotently reduced the radiation-induced supercoiled to open-circular plasmid DNA in a dose-dependent conversion. In addition, exposure of calf thymus DNA to 50 Gy of gamma radiation resulted in DNA fragments of low-molecular weight (<6,000 bps), which was prevented in a dose-dependence manner by all synthetic and natural SDG enantomers, at concentrations as low as 0.5 μM. These novel results demonstrated that synthetic SDG (S,S) and SDG (R,R) isomers and commercial SDG possess DNA-radioprotective properties. Such properties along with their antioxidant and free radical scavenging activity, reported earlier, suggest that SDGs are promising candidates for radioprotection for normal tissue damage as a result of accidental exposure during radiation therapy for cancer treatment.
INTRODUCTION
In radioactive decay, three types of radiations can be produced: alpha particles (α, positive charge); beta particles (β, negative charge); and gamma rays (γ, no charge) (1). Gamma radiation has a very small wavelength (<0.005 nm) and therefore has high energy, which is capable of ionizing molecules and atoms. In biological systems or in solution, ionizing radiation generates hydroxyl radicals (•OH) by water radiolysis (2, 3). These hydroxyl radicals (•OH) are the predominant source of ionizing radiation-induced damage to cellular components including lipids, proteins and genomic DNA. The hydroxyl radicals (•OH) produced by gamma radiation result in single-strand and double-strand breaks in DNA. The hydroxyl radicals (•OH) damage DNA by abstracting H-atoms from the deoxyribose, purine and pyrimidine bases or by adding to the double bonds of the bases (4), these reactions result in DNA strand breaks (5).
Compounds with antioxidant and free radical scavenging properties could potentially function as radioprotectors and prevent radiation-induced DNA damage. In view of these needs, we have synthesized enantiomers of secoisolariciresinol diglucoside (SDG), which is the major lignan phenolic in flaxseed (6). Due to complex extraction, purification and enrichment methods to isolate SDG from natural resources (7, 8), plus the associated high costs, variability and difficulty of producing large quantities of SDG for preclinical and clinical testing, we decided to chemically synthesize SDG (9). Using the natural compounds vanillin and glucose, we successfully synthesized two enantiomers of SDG, SDG (S,S) and SDG (R,R), which we have shown to possess potent antioxidant properties.
Our group and other investigators have shown in many studies that SDG is a potent antioxidant agent and a potent free radical scavenger (10–13). Importantly, in a recent study, we showed that the synthetic SDG enantiomers (synthesized by our group) also possess strong antioxidant and free radical scavenging characteristics (14). In the study presented here we evaluated the radioprotective properties of the synthesized SDG enantiomers SDG (S,S) and SDG (R,R) versus commercial SDG. The radioprotective characteristics of the three compounds were assessed using the plasmid DNA relaxation assay by determining the ability of the SDGs to prevent the supercoil (SC) to open-circle (OC) plasmid DNA conversion after exposure of the plasmid to gamma radiation as well as by evaluating inhibition of genomic DNA fragmentation after exposure of DNA to gamma radiation. SDG is metabolized by intestinal bacteria to produce secoisolariciresinol (SECO), enterodiol (ED) and enterolactone (EL) (15). Therefore, we also evaluated the effect of these metabolites of SDG on gamma radiation-induced fragmentation of genomic DNA.
MATERIAL AND METHODS
Chemicals
Plasmid DNA (pBR322), ethidium bromide, UltraPure™ 10× TAE buffer and 1 kb plus DNA ladder were purchased from Invitrogen (Life Technologies, Carlsbad, CA). Agarose (UltraPure) and calf thymus DNA were purchased from Sigma-Aldrich (St. Louis, MO). Secoisolariciresinol diglucoside (commercial), Secoisolariciresinol (SECO), enterodiol (ED) and enterolactone (EL) were purchased from Chromadex (Irvine, CA). Adjusted purities for SDG, SECO, ED and EL were 97.6%, 97.2%, 93.1% and 99.2%, respectively. All compounds were reconstituted in phosphate buffered saline (PBS).
Synthesis of Secoisolariciresinol Diglucoside
Synthetic SDG (S,S) and SDG (R,R) stereoisomers were synthesized by our group (9). The synthesis of the secoisolariciresinol core was performed by a novel, scalable route that has been previously described (16). The purities of synthetic SDG (S,S) and SDG (R,R) stereoisomers were >95%, as determined by NMR spectroscopy. The details and specific steps of the chemical synthesis and quality determination are presented in our recent publication (9).
Exposure of Plasmid DNA and Calf Thymus DNA to Gamma Radiation
Plasmid DNA (pBR322) or calf thymus DNA samples with or without varying concentrations of SDG (R,R), SDG (S,S) and SDG (commercial) were exposed to gamma radiation with a Mark I cesium (Cs-137) irradiator (J.L. Shepherd, San Fernando, CA) at a dose rate of 1.7 Gy/min in PBS, pH 7.4.
Determination of Radiation-Induced Plasmid DNA Relaxation
The effect of test compounds on radiation-induced strand breaks and supercoil to open-circle conversion was determined using plasmid DNA (pBR322) (Life Technologies). Plasmid DNA (500 ng) in PBS (pH 7.4) was mixed with various concentrations (25–250 μM) of SDG (R,R), SDG (S,S) and SDG (commercial) and exposed to 25 Gy of radiation in PBS. At 30 min postirradiation, samples were mixed with loading dye and subjected to agarose (1%) gel electrophoresis in TAE buffer (pH 8.3) at 100 V. The gel was stained with ethidium bromide (0.5 μg/ml) for 40 min, washed for 20 min and then visualized on a UV transilluminator (Bio-Rad, Hercules, CA). The captured gel images were scanned and the density of the open-circle and supercoiled plasmid DNA bands determined by Gel-doc image analyzer program. The density of the SC and OC plasmid DNA was expressed as percentage of the total density (OC + SC).
Determination of Radiation-Induced DNA Fragmentation
The effect of test compounds on radiation-induced strand breaks in DNA was determined using calf thymus DNA (Sigma, St. Louis, MO). DNA (500 ng) in PBS (pH 7.4) was mixed with varying concentrations (25–250 μM) of SDG (R,R), SDG (S,S) and SDG (commercial) and 50 Gy irradiated for 30 min. A second series of experiments were performed at varying concentrations ranging from 0.5–10 μM. Samples were mixed with loading dye and subjected to agarose (1%) gel electrophoresis in TAE buffer (pH 8.3) at 100 V. The gel was stained with ethidium bromide (0.5 μg/ml) for 40 min, washed for 20 min and then visualized on a UV transilluminator. The captured gel images were scanned and the density of the calf thymus DNA fragments was determined using the Gel-Pro image analyzer program (Media Cybernetics, Silver Spring, MD). The density of the low (<6,000 bps) and high (>6,000 bps) molecular weight fragments of calf thymus DNA were expressed as the percentage of the total density (low mol. wt. + high mol. wt.).
Analysis of the Data
Data obtained are presented as mean values ± standard deviation. The data were subjected to one-way analysis of variance (ANOVA) with post hoc comparison using Bonferroni correction (StatView, SAS, Cary, NC). P ≤ 0.05 was considered significant.
RESULTS
The radioprotective potential of synthetic SDG (R,R), SDG (S,S) and SDG (commercial) was determined using plasmid DNA (pBR322). The radioprotection assay used in this study is based on the principle that plasmid DNA after exposure to gamma radiation moves slower than the unexposed plasmid DNA. This is due to the supercoiled plasmid DNA moving faster in the agarose gel due to its compact size. In comparison, the radiation-induced nicks in the plasmid DNA unravel supercoil resulting in a relatively larger size circular plasmid, which moves slower in the gel. Therefore, determining the density of the open-circular compared to the supercoiled plasmid DNA reflects the extent of radiation-induced damage.
Radiation Causes a Dose-Dependent Supercoil to Open-Circular DNA Plasmid Conversion
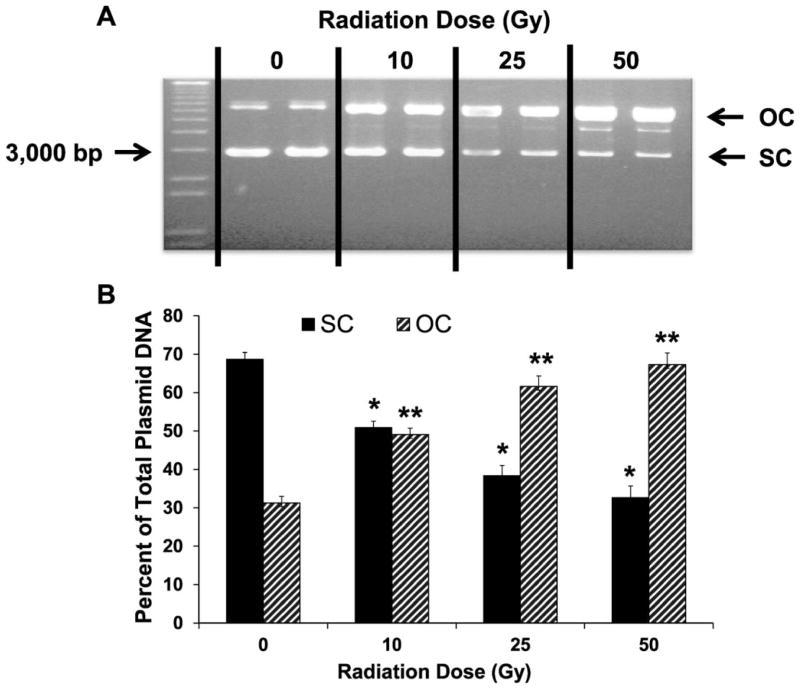
To select a radiation dose that causes significant DNA damage yet allows for a therapeutic window to test our radiation-mitigating agent, we exposed plasmid DNA to 10, 25 and 50 Gy of gamma radiation. The results presented in Fig. 1A show that there is a radiation dose-dependent increase in OC form as well as a radiation dose-dependent decrease in SC form of the plasmid DNA. The distribution of SC and OC (Fig. 1B) shows that the percentage of SC decreased from 68.73 ± 2.54% to 50.91 ± 2.31%, 38.37 ± 3.73% and 35.66 ± 4.24% (P < 0.05), when exposure to 0, 10, 25 and 50 Gy of radiation, respectively. At the same time, the percentage of OC increased from 31.26 ± 2.50% to 49.08 ± 2.31%, 61.62 ± 3.73% and 67.33 ± 4.24% (P < 0.05), when exposure to 0, 10, 25 and 50 Gy of radiation, respectively. Based on these initial experiments, a radiation dose of 25 Gy (at which considerable and clearly demonstrable damage was achieved) was selected for the subsequent experiments to determine the radioprotecting characteristic of the different SDGs.
FIG. 1.
Effect of increasing doses of gamma radiation on plasmid (pBR322) DNA relaxation. Supercoiled (SC) represents the compact form and open-circular (OC) represents the relaxed or damaged form of the plasmid DNA. Panel A: The SC form is seen as the lower prominent band (at 3,000 bps) while the OC form is the upper prominent band. Lane 1: 1 kb DNA standard ladder; lanes 2 and 3: untreated plasmid DNA; lanes 4 and 5: plasmid DNA exposed to 10 Gy of radiation; lanes 6 and 7: plasmid DNA exposed to 25 Gy of radiation; and lanes 8 and 9: plasmid DNA exposed to 50 Gy of radiation. Panel B: SC and OC forms are presented as percentage of total plasmid DNA. For each condition, all samples were run in duplicate. The data are presented as mean ± standard deviation. P < 0.05 was considered significant. *Indicate a significant difference compared to untreated *SC and **OC forms.
Radioprotective Activity of Synthetic SDG Using Plasmid DNA Relaxation Assay
Plasmid DNA was exposed to the selected dose of 25 Gy of gamma radiation (see Fig. 1) and the percentage inhibition of DNA damage (SC to OC formation) was determined for each of the SDG agents (synthetic and commercial) at various concentrations (25–250 μM).
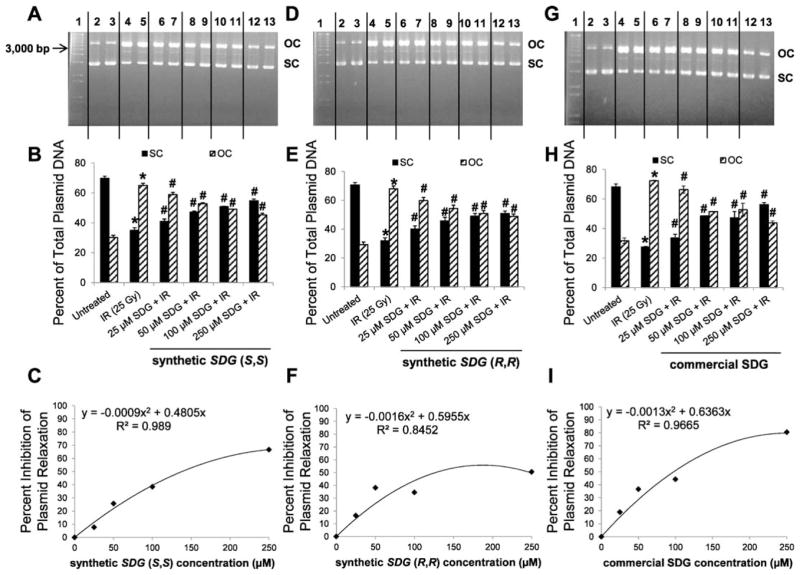
A representative gel blot of plasmid DNA after exposure to 25 Gy of radiation in the presence of 25, 50, 100 and 250 μM SDG (S,S) is shown in Fig. 2A and semiquantitative densitometric analysis is shown in Fig. 2B, while percentage inhibition compared to control is shown in Fig. 2C. Interestingly, increasing the concentrations of SDG (S,S) (25, 50, 100 and 250 μM) increased the proportion of the SC form and the density of OC form decreased significantly (P < 0.05) in a dose-dependent manner. Using the percentage inhibition plot (Fig. 2C), the EC50 value can be determined for each agent [i.e., the effective concentration (EC) needed to prevent 50% of plasmid relaxation at 25 Gy] and is 141.77 μM for SDG (S,S), the EC50 value for preventing plasmid DNA relaxation is comparable to the EC50 value for scavenging DPPH free radicals (14). These results demonstrate the radioprotective characteristic of our synthetic SDG (S,S) enantiomer. Similar results were shown for the SDG (R,R) enantiomer (Fig. 2D–F) and SDG (commercial) (Fig. 2G–I) with an EC50 of 127.96 μM and 98.38 μM, respectively. These values for preventing plasmid DNA relaxation are comparable to the respective EC50 value for scavenging DPPH free radicals (14). These results demonstrate the radioprotective characteristics of both the synthetic and the commercially available, natural SDG.
FIG. 2.
Effect of increasing concentration of synthetic SDG (S,S), SDG (R,R) and SDG (commercial) on gamma-radiation-induced plasmid (pBR322) DNA relaxation. All samples were exposed to a 25 Gy dose of γ radiation. SDGs concentrations were 25, 50, 100 and 250 μM. Panels A, D and G: Representative agarose gel scans of plasmid DNA after exposure to 25 Gy of radiation in the presence of 25, 50, 100 and 250 μM SDG (S,S), SDG (R,R) and SDG (commercial) are shown. Lane 1: 1 kb DNA standard ladder; lanes 2 and 3: untreated plasmid DNA; lanes 4 and 5: 25 μM; lanes 6 and 7: 50 μM; lanes 8 and 9: 100 μM; and lanes 10 and 11: 250 μM SDGs. Panels B, E and H: SC and OC forms are presented as percentage of total plasmid DNA. For each condition, all samples were run in duplicate. The data are presented as mean ± standard deviation. P < 0.05 was considered significant. Significant difference compared to untreated *SC and #OC forms. **,##Significant differences compared to samples exposed to 25 Gy of radiation without SDGs. Panels C, F and I: SDGs-dependent inhibition of plasmid DNA relaxation is shown. EC50 values were determined from the quadratic equations shown under the curves.
Radiation Causes Dose-Dependent DNA Fragmentation from High- to Low-Molecular-Weight Fragments
Radiation induces an increase in DNA fragmentation as shown in the DNA gel in Fig. 3A. Based on size, the calf thymus DNA fragments were divided into two groups: high-molecular-weight (>6,000 bps) size and low-molecular-weight (<6,000 bps) size. The distribution (Fig. 3B) of the high- and low-molecular-weight fragments show that the percentage of the high-molecular-weight DNA decreased from 88.16 ± 0.50% to 67.82 ± 7.89% and 34.94 ± 4.45% (P < 0.05) at 25 and 50 Gy exposures, respectively. At the same time, the proportion of low-molecular-weight fragments increased from 11.83 ± 0.50% to 32.17 ± 7.89% and 65.05 ± 4.45% (P < 0.05) at 25 and 50 Gy exposures, respectively. Our results (Fig. 3B) show a significant decrease in high-molecular-weight DNA and a significant increase in low-molecular-weight DNA fragments indicating damage to DNA at 50 Gy exposure. Based on these initial experiments, a radiation dose of 50 Gy (at which a clearly demonstrable calf thymus DNA fragmentation was observed) was selected for the following experiments determining the radioprotection characteristic of different SDGs.
FIG. 3.
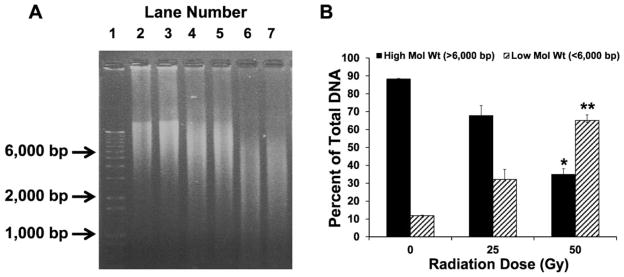
Effect of increasing doses of gamma radiation on calf thymus DNA fragmentation. DNA exposed to gamma radiation generates fragments of small molecular weights, which move faster than the high-molecular-weight DNA. Determining the density of the low-molecular-weight DNA fragments (<6,000 bps) compared to the high-molecular-weight DNA (>6,000 bps) reflects the extent of radiation-induced damage. Panel A: Lane 1: 1 kb DNA standard ladder; lanes 2 and 3: untreated calf thymus DNA; lanes 4 and 5: DNA exposed to 25 Gy; and lanes 6 and 7: DNA exposed to 50 Gy of radiation. Panel B: High- and low-molecular-weight DNA forms are shown as percentage of total DNA. For each condition, all samples were run in duplicate. The data are shown as mean ± standard deviation. P < 0.05 was considered significant. *,**Indicate significant differences compared to the untreated forms, respectively.
Radioprotective Activity of Synthetic SDG Using Calf Thymus DNA Fragmentation Assay
The radioprotective potential of synthetic SDG (R,R), SDG (S,S) and SDG (commercial) was determined using radiation-induced fragmentation of calf thymus DNA as described above.
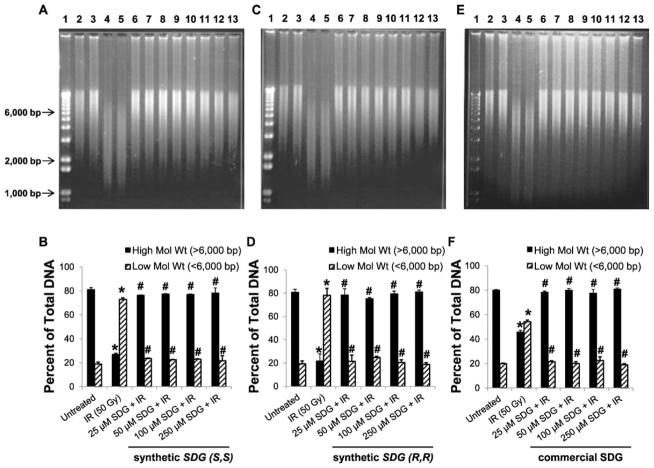
High SDG Concentration (25–250 μM)
Figure 4A shows a representative DNA gel of calf thymus DNA after exposure to 50 Gy in the presence of 25, 50, 100 and 250 μM SDG (S,S). In the presence of increasing concentrations of SDG (S,S) (25, 50, 100 and 250 μM), the proportion of the high-molecular-weight DNA form increased significantly (P < 0.05) after radiation exposure while the low-molecular-weight fragments decreased. The distribution of high- and low-molecular-weight DNA forms in the presence of various concentrations of SDG (S,S) are shown in Fig. 4B. These results demonstrate the radioprotective characteristic of our synthetic SDG (S,S) enantiomer using calf thymus genomic DNA. Similarly, results presented in Fig. 4C–F show the radioprotective property of synthetic SDG (R,R) and SDG (commercial), respectively. These results demonstrate the radioprotective characteristic of our synthetic SDG (R,R) and (S,S) enantiomers using calf thymus genomic DNA.
FIG. 4.
Effect of increasing concentration of synthetic SDG (S,S), SDG (R,R) and SDG (commercial) on gamma-radiation-induced calf thymus DNA fragmentation. All samples were exposed to a 50 Gy dose of gamma radiation. SDG concentrations were 25, 50, 100 and 250 μM. Panels A, C and E: Representative agarose gel scans of calf thymus DNA after exposure to 50 Gy in the presence of 25, 50, 100 and 250 μM SDG (S,S), SDG (R,R) and SDG (commercial) are shown. Lane 1: 1 kb DNA standard ladder; lanes: 2 and 3, untreated DNA; lanes 4 and 5: 25 μM; lanes 6 and 7: 50 μM; lanes 8 and 9: 100 μM; and lanes 10 and 11: 250 μM SDGs. Panels B, D and F: High- and low-molecular-weight DNA forms are shown as percentage of total DNA. For each condition, all samples were run in duplicate. The data are shown as mean ± standard deviation. P < 0.05 was considered significant. *Significant difference compared to untreated DNA. #Significant difference compared to samples exposed to 50 Gy without SDGs.
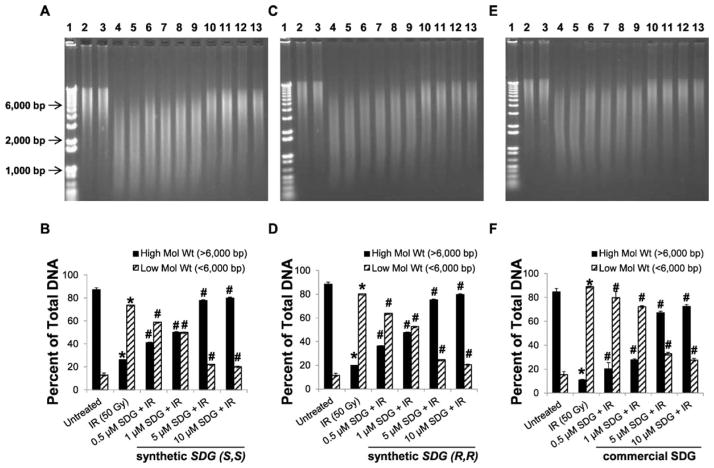
To further determine the lower limits of SDG in DNA protection, we performed a series of DNA fragmentation experiments testing lower concentrations of all 3 SDGs, ranging from 0.5–10 μM.
Low SDG Concentration (0.5–10 μM)
The results of experiments performed at low concentrations of SDG (S,S), SDG (R,R) and SDG (commercial) compared to their EC50 values for antioxidant and free radical scavenging activity are shown in Fig. 5. Similar to the higher SDG concentrations, the results presented in this section using calf thymus DNA fragmentation assay demonstrate that our synthetic SDG (S,S) and SDG (R,R) enantiomers possess a strong radioprotection characteristic even at low concentrations.
FIG. 5.
Effect of very low concentrations of synthetic SDG (S,S), SDG (R,R) and SDG (commercial) on gamma-radiation-induced calf thymus DNA fragmentation. All samples were exposed to a 50 Gy dose of gamma radiation. SDG concentrations were 0.5, 1.0, 5.0 and 10 μM. Panels A, C and E: Representative agarose gel scans of calf thymus DNA after exposure to 50 Gy of radiation in the presence of 0.5, 1.0, 5.0 and 10 μM SDG (S,S), SDG (R,R) and SDG (commercial) are shown. Lane 1: 1 kb DNA standard ladder; lanes 2 and 3: untreated DNA; lanes 4 and 5: 0.5 μM; lanes 6 and 7: 1.0 μM; lanes 8 and 9: 5.0 μM; and lanes 10 and 11: 10 μM SDGs. Panels B, D and F: High- and low-molecular-weight DNA forms are shown as percentage of total DNA. For each condition, all samples were run in duplicate. The data is shown as mean ± standard deviation. P < 0.05 was considered significant. *Significant difference compared to untreated DNA. #Significant difference compared to samples exposed to 50 Gy of radiation without SDGs.
Radioprotective Activity of SDG Metabolites Using Calf Thymus DNA Fragmentation Assay
The radioprotective potential of SDG metabolites SECO, ED and EL was determined and compared with SDG using radiation-induced fragmentation of calf thymus DNA as described above. The concentration of 10 μM of each test agent was selected based on previous findings shown above as a median effective dose. The results are shown in the Supplementary Fig. S1 (http://dx.doi.org/10.1667/RR13635.1.S1). The data demonstrate that SDG and its metabolites, SECO, ED and EL, are equipotent with respect to their radioprotective properties.
DISCUSSION
The results of this study show that our synthetic SDG (S,S) and SDG (R,R) enantiomers possess a strong radioprotection capacity. The radioprotection potential of these enantiomers, as determined using plasmid DNA (pBR322), increased as their concentration increased. These synthetic SDG (S,S) and SDG (R,R) enantiomers prevent radiation-induced damage to plasmid DNA in a concentration-dependent manner. The radioprotection potential of the synthetic isomers of SDG was comparable to the commercial SDG. The synthetic enantiomers SDG (S,S) and SDG (R,R) also prevented the radiation-induced DNA fragmentation of calf thymus genomic DNA. At the lowest concentration tested, SDG (S,S) and SDG (R,R) completely prevented the radiation-induced generation of low-molecular-weight fragments of calf thymus DNA, demonstrating a strong radioprotective characteristic of our synthetic SDG (S,S) and SDG (R,R) enantiomers. Results of low concentrations of SDG (S,S), SDG (R,R) and SDG (commercial) indicated that the concentration required for protecting calf thymus DNA from gamma-radiation damage is much lower as compared to the EC50 values for their antioxidant and free radical scavenging activity. Importantly, the mammalian lignan metabolites of SDG, SECO, ED and EL showed equally potent DNA-protective properties.
Flavonoids possess strong antioxidant activity (17), and specifically, such polyphenols possess free radical-scavenging activity and are known to be more effective antioxidants in vitro than vitamins E and C (18, 19). Dietary and medicinal plants possessing antioxidant properties are also known to prevent many human diseases associated with oxidative stress (19) and are useful radioprotectors (20). Antioxidants, including vitamins and minerals, suppressed the levels of clastogenic factors in Chernobyl workers many years after radiation exposure (21).
Our group has been investigating the role of whole grain dietary flaxseed (11, 22), which is rich in lignan polyphenols, as well as flaxseed lignan formulations enriched in SDG (23, 24), in radiation-induced damage using a mouse model of thoracic radiation damage. We have shown that flaxseed ameliorated the radiation-induced inflammation and oxidative stress in mice when administered both prior to and after radiation exposure. We also demonstrated that irradiated mice fed diets containing only the lignan component of flaxseed, enriched in the lignan biphenol SDG, also showed significantly improved hemodynamic measurements and survival in addition to improvement in lung inflammation and oxidative tissue damage. These studies indicated that flaxseed through the actions of the lignan SDG is protective against radiation-induced tissue damage in vivo.
Increased generation of reactive oxygen species (ROS) such as superoxide anion (O2–), hydroxyl radical (•OH) and hydrogen peroxide leads to tissue damage under various experimental and pathological conditions. Reactive oxygen species result in cellular damage by oxidative modification of cellular membrane lipids, proteins and the genomic DNA (25). A number of studies have shown that extracted, purified or synthetic flaxseed SDG is a potent antioxidant in vitro as well as in vivo (10, 12, 26). Therefore, SDG as an antioxidant may have therapeutic potential under various experimental and disease conditions including radiation-induced tissue damage in patients undergoing radiation therapy.
Polyphenols commonly occur as glycosides in plants and possess antioxidant properties (27). Flavonoids, as antioxidants, interfere with the activities of enzymes involved in the generation of reactive oxygen species, quench of free radicals, chelate transition metals and rendering them redox inactive in the Fenton reaction (28). Secoisolariciresinol is the major lignan in flaxseed and has been shown to be a potent antioxidant in vitro as well as in vivo. In exploring the therapeutic potential of flaxseed lignan SDG previously, we synthesized SDG by a novel chemical reaction using vanillin as a precursor molecule and determined the antioxidant properties of the synthetic SDG (R,R) and SDG (S,S) by assessing their reducing power, metal chelating potential, and free radical scavenging activity for hydroxyl, peroxyl and DPPH radicals (14). In the current study, we have investigated the radioprotective characteristics of our synthetic SDG (R,R) and SDG (S,S) enantiomers and a commercially available SDG (as control) by assessing their potential for preventing gamma radiation-induced damage to plasmid DNA (pBR322) and calf thymus DNA. Radiation-induced damage to plasmid DNA was assessed by the increase in open-circular form of plasmid DNA and decrease in supercoiled form of the plasmid DNA. Radiation-induced damage to genomic DNA was assessed by determining the level of DNA fragmentation. In this study, we have examined the efficacy of synthetic SDG (R,R), SDG (S,S) and commercial SDG against radiation-induced DNA damage in a cell-free system.
The antioxidant properties of the SDG molecule have been previously demonstrated by us and others (10, 12–14, 26). We have previously shown that natural, commercially available SDG has potent free-radical scavenging properties in cells exposed to gamma radiation (11). However, the radioprotective characteristics of the novel synthetic SDG enantiomers have not yet been investigated. In our previous study, we investigated the antioxidant and free radical scavenging characteristics of these synthetic SDG (R,R) and SDG (S,S) enantiomers and demonstrated that these compounds possess strong reducing power, high metal-ion chelating potential and high free radical scavenging activity for hydroxyl, peroxyl and DPPH radicals (14, 29). These characteristics of the synthetic SDG (R,R) and SDG (S,S) indicate that these molecules show strong potential for modulating cellular redox state, decreasing metal-ion concentration and scavenging oxygen free radicals. Further, these characteristics suggest an ability to function by acting at and preventing all the three steps of initiation, propagation as well as termination of the free radical reaction. We propose that these underlying mechanisms are potentially responsible for the radioprotective characteristics of the SDG (R,R) and SDG (S,S) enantiomers in vivo.
An important observation we made is that the maximum radioprotection of genomic DNA by SDG is already achieved at approximately 5.0 μM concentration, well below the EC50 values for their free radical scavenging and antioxidant activity, which are in the range of 130–200 μM (10, 14). These differences in effective concentrations indicate that the radioprotection of genomic DNA by SDG molecules is potentially due to mechanism(s) in addition to their free radical scavenging and antioxidant activity.
Although speculative at this stage, there could be several potential mechanisms by which SDG might protect DNA from gamma-radiation-induced damage, first, by scavenging hydroxyl free radicals and preventing their generation, since radiation-induced radiolysis of water generates hydroxyl radicals, which are considered to be the major contributor for DNA damage (30), and second, by associating with DNA base pairs, since several flavonoids are known to do so. This is currently being further explored in our laboratory. Specifically, the two benzene ring structures (planar configuration) within the SDG molecule may provide a basis for association with the DNA base pairs. This has been observed for other flavonoids such as luteolin, kempferol and quercetin (29, 31, 32), and third, by blocking abstraction of protons or addition of •OH radicals on the purine and pyrimidine bases, especially at C5, C6 and C8, and at the deoxyribose sites. These mechanisms have been proposed for protection from free radical-induced DNA damage (5, 33–35). Therefore, SDG as an antioxidant and free radical scavenger can function as a DNA radioprotector and potentially as a radiation mitigator. Therapeutic potential of flaxseed lignan as an antioxidant, primarily as a hydroxyl radical scavenger, anticancer, antidiabetic, antiviral, bactericidal, anti-inflammatory and anti-atherosclerotic agent, has been previously discussed (36–42), however, its role as a radioprotector has recently been recognized (11, 43–45).
In summary, in this study, we have demonstrated that our synthetic SDG (S,S) and SDG (R,R) enantiomers possess a strong radioprotection characteristic. The radioprotection potential of these enantiomers was determined using plasmid DNA (pBR322) and calf thymus DNA. Our synthetic SDG (S,S) and SDG (R,R) enantiomers prevented the radiation-induced damage to plasmid DNA in a concentration-dependent manner. Synthetic enantiomers SDS (S,S) and SDG (R,R) also prevented the radiation-induced fragmentation of calf thymus genomic DNA. At the concentration of 5 μM, SDG (R,R) and SDG (S,S) completely prevented the radiation-induced generation of low-molecular-weight fragments of calf thymus DNA, demonstrating a strong radioprotective capacity of these enantionmers. Our current results establish our synthetic SDG (R,R) and SDG (S,S) enantiomers as strong radioprotectors for potential use in vivo.
Supplementary Material
Effect of SDG, SECO, ED and EL on gamma-radiation-induced calf thymus DNA fragmentation. All samples were exposed to a 50 Gy dose of gamma radiation. SDG, SECO, ED and EL were used at 10 μM concentration. Panel A: Representative agarose gel scans of calf thymus DNA after exposure to 50 Gy in the presence of 10 μM SDG, SECO, ED and EL are shown. Lane 1: 1 kb DNA standard ladder; lanes 2 and 3: untreated DNA; lanes 4–6: 50 Gy of ionizing irradiation; lanes 7 and 8: SDG; lanes 9 and 10: SECO; lanes 11 and 12: ED; and lanes 13 and 14: EL. Panel B: High- and low-molecular-weight DNA forms are shown as percentage of total DNA. For each condition, all samples were run in duplicates. The data are shown as mean ± standard deviation. P < 0.05 was considered significant. *Significant difference as compared to untreated DNA. #Significant difference compared to samples exposed to 50 Gy alone.
Acknowledgments
This work was funded in part by: NIH-R01 CA133470 (MCS), NIH-RC1AI081251 (MCS), the University of Pennsylvania Research Foundation (MCS) and by pilot project support from 1P30 ES013508-02 awarded to MCS (the content is solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH).
References
- 1.Lahtz C, Bates SE, Jiang Y, Li AX, Wu X, Hahn MA, et al. Gamma irradiation does not induce detectable changes in DNA methylation directly following exposure of human cells. PLoS One. 2012;7:e44858. doi: 10.1371/journal.pone.0044858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feldberg RS, Carew JA. Water radiolysis products and nucleotide damage in gamma-irradiated DNA. Int J Radiat Biol Relat Stud Phys Chem Med. 1981;40:11–7. [PubMed] [Google Scholar]
- 3.Kuipers GK, Lafleur MV. Characterization of DNA damage induced by gamma-radiation-derived water radicals, using DNA repair enzymes. Int J Radiat Biol. 1998;74:511–9. doi: 10.1080/095530098141384. [DOI] [PubMed] [Google Scholar]
- 4.Cadet J, Douki T, Ravanat JL. Oxidatively generated base damage to cellular DNA. Free Radic Biol Med. 2010;49:9–21. doi: 10.1016/j.freeradbiomed.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 5.Spotheim-Maurizot M, Davidkova M. Radiation damage to DNA in DNA-protein complexes. Mutat Res. 2011;711:41–8. doi: 10.1016/j.mrfmmm.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Axelson M, Sjovall J, Gustafsson BE, Setchell KD. Origin of lignans in mammals and identification of a precursor from plants. Nature. 1982;298:659–60. doi: 10.1038/298659a0. [DOI] [PubMed] [Google Scholar]
- 7.Hosseinian FS, Beta T. Patented techniques for the extraction and isolation of secoisolariciresinol diglucoside from flaxseed. Recent Pat Food Nutr Agric. 2009;1:25–31. doi: 10.2174/2212798410901010025. [DOI] [PubMed] [Google Scholar]
- 8.Lehraiki A, Attoumbre J, Bienaime C, Matifat F, Bensaddek L, Nava-Saucedo E, et al. Extraction of lignans from flaxseed and evaluation of their biological effects on breast cancer MCF-7 and MDA-MB-231 cell lines. J Med Food. 2010;13:834–41. doi: 10.1089/jmf.2009.0172. [DOI] [PubMed] [Google Scholar]
- 9.Mishra OP, Simmons N, Tyagi S, Pietrofesa R, Shuvaev V, Heretsch P, et al. Synthesis and antioxidant evaluation of (S,S)-and (R,R)-secoisolariciresinol diglucosides (SDGs) Bioorg Med Chem Lett. 2013;19:5325–8. doi: 10.1016/j.bmcl.2013.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moree S. Secoisolariciresinol Diglucoside - A Phytoestrogen Nutraceutical of Flaxseed: Synthesis and Evaluation of Antioxidant Potency. Free Radicals Antioxidants. 2011;1:31–8. [Google Scholar]
- 11.Lee JC, Krochak R, Blouin A, Kanterakis S, Chatterjee S, Arguiri E, et al. Dietary flaxseed prevents radiation-induced oxidative lung damage, inflammation and fibrosis in a mouse model of thoracic radiation injury. Cancer Biol Ther. 2009;8:47–53. doi: 10.4161/cbt.8.1.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu C, Yuan YV, Kitts DD. Antioxidant activities of the flaxseed lignan secoisolariciresinol diglucoside, its aglycone secoisolariciresinol and the mammalian lignans enterodiol and enterolactone in vitro. Food Chem Toxicol. 2007;45:2219–27. doi: 10.1016/j.fct.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 13.Kitts DD, Yuan YV, Wijewickreme AN, Thompson LU. Antioxidant activity of the flaxseed lignan secoisolariciresinol diglycoside and its mammalian lignan metabolites enterodiol and enterolactone. Molec Cell Biochem. 1999;202:91–100. doi: 10.1023/a:1007022329660. [DOI] [PubMed] [Google Scholar]
- 14.Mishra OP, Simmons N, Tyagi S, Pietrofesa R, Shuvaev VV, Valiulin RA, et al. Synthesis and antioxidant evaluation of (S,S)-and (R,R)-secoisolariciresinol diglucosides (SDGs) Bioorg Med Chem Lett. 2013;23:5325–8. doi: 10.1016/j.bmcl.2013.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Setchell KD, Brown NM, Zimmer-Nechemias L, Wolfe B, Jha P, Heubi JE. Metabolism of secoisolariciresinol-diglycoside the dietary precursor to the intestinally derived lignan enterolactone in humans. Food Funct. 2014;5:491–501. doi: 10.1039/c3fo60402k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee E, Ahamed VS, Kumar MS, Rhee SW, Moon SS, Hong IS. Synthesis and evaluation of cytotoxic effects of hanultarin and its derivatives. Bioorg Med Chem Lett. 2011;21:6245–8. doi: 10.1016/j.bmcl.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Rice-Evans CA, Miller NJ. Antioxidant activities of flavonoids as bioactive components of food. Biochem Soc Trans. 1996;24:790–5. doi: 10.1042/bst0240790. [DOI] [PubMed] [Google Scholar]
- 18.Rice-Evans CA, Miller NJ, Bolwell PG, Bramley PM, Pridham JB. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic Res. 1995;22:375–83. doi: 10.3109/10715769509145649. [DOI] [PubMed] [Google Scholar]
- 19.Scalbert A, Johnson IT, Saltmarsh M. Polyphenols. antioxidants and beyond. Am J Clin Nutr. 2005;81:215S–7S. doi: 10.1093/ajcn/81.1.215S. [DOI] [PubMed] [Google Scholar]
- 20.Weiss JF, Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology. 2003;189:1–20. doi: 10.1016/s0300-483x(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 21.Emerit I, Filipe P, Meunier P, Auclair C, Freitas J, Deroussent A, et al. Clastogenic activity in the plasma of scleroderma patients: a biomarker of oxidative stress. Dermatology. 1997;194:140–6. doi: 10.1159/000246083. [DOI] [PubMed] [Google Scholar]
- 22.Christofidou-Solomidou M, Tyagi S, Tan KS, Hagan S, Pietrofesa R, Dukes F, et al. Dietary flaxseed administered post thoracic radiation treatment improves survival and mitigates radiation-induced pneumonopathy in mice. BMC Cancer. 2011;11:269. doi: 10.1186/1471-2407-11-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christofidou-Solomidou M, Tyagi S, Pietrofesa R, Dukes F, Arguiri E, Turowski J, et al. Radioprotective role in lung of the flaxseed lignan complex enriched in the phenolic secoisolariciresinol diglucoside (SDG) Radiat Res. 2012;178:568–80. doi: 10.1667/RR2980.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pietrofesa R, Turowski J, Tyagi S, Dukes F, Arguiri E, Busch TM, et al. Radiation mitigating properties of the lignan component in flaxseed. BMC Cancer. 2013;13:179. doi: 10.1186/1471-2407-13-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halliwell B. Protection against tissue damage in vivo by desferrioxamine: what is its mechanism of action? Free Radic Biol Med. 1989;7:645–51. doi: 10.1016/0891-5849(89)90145-7. [DOI] [PubMed] [Google Scholar]
- 26.Moree SS, Rajesha J. Investigation of in vitro and in vivo antioxidant potential of secoisolariciresinol diglucoside. Mol Cell Biochem. 2013;373:179–87. doi: 10.1007/s11010-012-1487-4. [DOI] [PubMed] [Google Scholar]
- 27.Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000;63:1035–42. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 28.Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002;13:572–84. doi: 10.1016/s0955-2863(02)00208-5. [DOI] [PubMed] [Google Scholar]
- 29.Rusak G, Piantanida I, Masic L, Kapuralin K, Durgo K, Kopjar N. Spectrophotometric analysis of flavonoid-DNA interactions and DNA damaging/protecting and cytotoxic potential of flavonoids in human peripheral blood lymphocytes. Chem Biol Interact. 2010;188:181–9. doi: 10.1016/j.cbi.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Kuipers GK, Lafleur MV. Characterization of DNA damage induced by gamma-radiation-derived water radicals, using DNA repair enzymes. Int J Radiat Biol. 1998;74:511–519. doi: 10.1080/095530098141384. [DOI] [PubMed] [Google Scholar]
- 31.Zhang S, Ling B, Qu F, Sun X. Investigation on the interaction between luteolin and calf thymus DNA by spectroscopic techniques. Spectrochim Acta A Mol Biomol Spectrosc. 2012;97:521–5. doi: 10.1016/j.saa.2012.06.040. [DOI] [PubMed] [Google Scholar]
- 32.Marinic M, Piantanida I, Rusak G, Zinic M. Interactions of quercetin and its lanthane complex with double stranded DNA/RNA and single stranded RNA: spectrophotometric sensing of poly G. J Inorg Biochem. 2006;100:288–98. doi: 10.1016/j.jinorgbio.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 33.Cadet J, Douki T, Ravanat JL. Oxidatively generated base damage to cellular DNA. Free Radic Biol Med. 2010;49:9–21. doi: 10.1016/j.freeradbiomed.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 34.Kumar A, Pottiboyina V, Sevilla MD. Hydroxyl radical (OH*) reaction with guanine in an aqueous environment: a DFT study. J Phys Chem B. 2011;115:15129–37. doi: 10.1021/jp208841q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cadet J, Wagner JR. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb Perspect Biol. 2013;5 doi: 10.1101/cshperspect.a012559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zanwar AA, Hegde MV, Bodhankar SL. Cardioprotective activity of flax lignan concentrate extracted from seeds of Linum usitatissimum in isoprenalin induced myocardial necrosis in rats. Interdiscip Toxicol. 2011;4:90–7. doi: 10.2478/v10102-011-0016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajesha J, Murthy KN, Kumar MK, Madhusudhan B, Ravishankar GA. Antioxidant potentials of flaxseed by in vivo model. J Agric Food Chem. 2006;54:3794–9. doi: 10.1021/jf053048a. [DOI] [PubMed] [Google Scholar]
- 38.Chen J, Stavro PM, Thompson LU. Dietary flaxseed inhibits human breast cancer growth and metastasis and downregulates expression of insulin-like growth factor and epidermal growth factor receptor. Nutr Cancer. 2002;43:187–92. doi: 10.1207/S15327914NC432_9. [DOI] [PubMed] [Google Scholar]
- 39.Prasad K. Oxidative stress as a mechanism of diabetes in diabetic BB prone rats: effect of secoisolariciresinol diglucoside (SDG) Mol Cell Biochem. 2000;209:89–96. doi: 10.1023/a:1007079802459. [DOI] [PubMed] [Google Scholar]
- 40.Collins TF, Sprando RL, Black TN, Olejnik N, Wiesenfeld PW, Babu US, et al. Effects of flaxseed and defatted flaxseed meal on reproduction and development in rats. Food Chem Toxicol. 2003;41:819–34. doi: 10.1016/s0278-6915(03)00033-4. [DOI] [PubMed] [Google Scholar]
- 41.Kinniry P, Amrani Y, Vachani A, Solomides CC, Arguiri E, Workman A, et al. Dietary flaxseed supplementation ameliorates inflammation and oxidative tissue damage in experimental models of acute lung injury in mice. J Nutr. 2006;136:1545–51. doi: 10.1093/jn/136.6.1545. [DOI] [PubMed] [Google Scholar]
- 42.Prasad K. Hydroxyl radical-scavenging property of secoisolariciresinol diglucoside (SDG) isolated from flax-seed. Mol Cell Biochem. 1997;168:117–23. doi: 10.1023/a:1006847310741. [DOI] [PubMed] [Google Scholar]
- 43.Christofidou-Solomidou M, Tyagi S, Pietrofesa R, Dukes F, Arguiri E, Turowski J, et al. Radioprotective role in lung of the flaxseed lignan complex enriched in the phenolic secoisolariciresinol diglucoside (SDG) Radiat Res. 2012;178:568–80. doi: 10.1667/RR2980.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Christofidou-Solomidou M, Tyagi S, Tan KS, Hagan S, Pietrofesa R, Dukes F, et al. Dietary flaxseed administered post thoracic radiation treatment improves survival and mitigates radiation-induced pneumonopathy in mice. BMC Cancer. 2011;11:269. doi: 10.1186/1471-2407-11-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pietrofesa R, Turowski J, Tyagi S, Dukes F, Arguiri E, Busch TM, et al. Radiation mitigating properties of the lignan component in flaxseed. BMC Cancer. 2013;13:179. doi: 10.1186/1471-2407-13-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of SDG, SECO, ED and EL on gamma-radiation-induced calf thymus DNA fragmentation. All samples were exposed to a 50 Gy dose of gamma radiation. SDG, SECO, ED and EL were used at 10 μM concentration. Panel A: Representative agarose gel scans of calf thymus DNA after exposure to 50 Gy in the presence of 10 μM SDG, SECO, ED and EL are shown. Lane 1: 1 kb DNA standard ladder; lanes 2 and 3: untreated DNA; lanes 4–6: 50 Gy of ionizing irradiation; lanes 7 and 8: SDG; lanes 9 and 10: SECO; lanes 11 and 12: ED; and lanes 13 and 14: EL. Panel B: High- and low-molecular-weight DNA forms are shown as percentage of total DNA. For each condition, all samples were run in duplicates. The data are shown as mean ± standard deviation. P < 0.05 was considered significant. *Significant difference as compared to untreated DNA. #Significant difference compared to samples exposed to 50 Gy alone.