Abstract
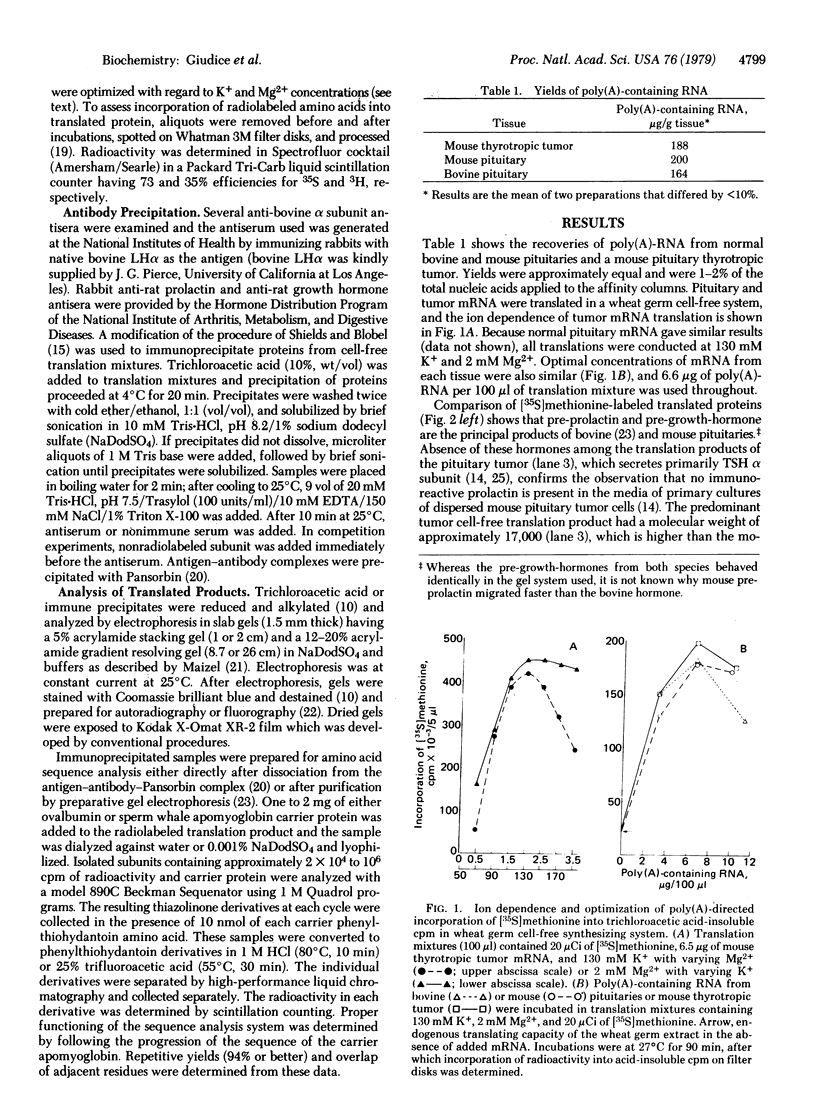
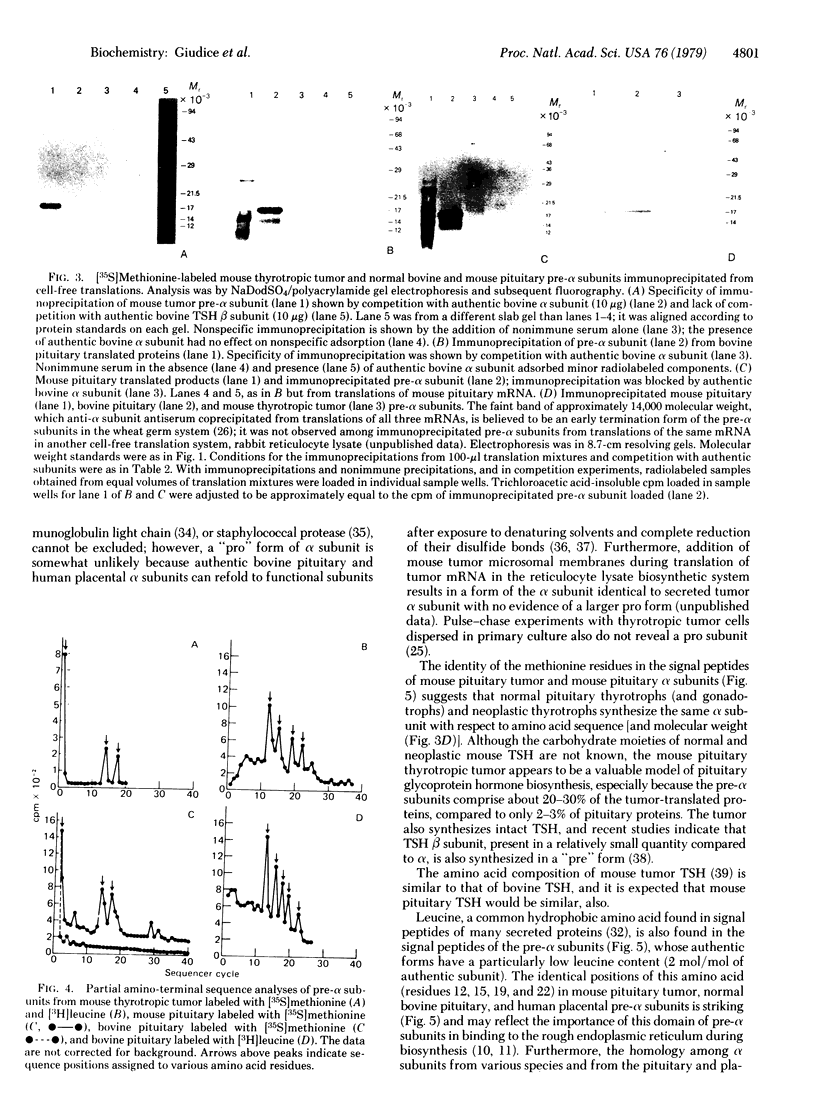
Poly(A)-containing RNA isolated from bovine and mouse pituitaries and a mouse pituitary thyrotropic tumor was translated in a wheat germ cell-free biosynthetic system. A precursor of the glycoprotein hormone α subunit, “pre-α,” was immunoprecipitated from the translation mixtures with antiserum against bovine luteinizing hormone (LH; lutropin) α. The specificity of the immunoprecipitation was shown by competition with authentic bovine LHα and lack of competition with bovine thyroid-stimulating hormone (TSH; thyrotropin) β. Bovine and mouse pre-α subunits migrated identically in sodium dodecyl sulfate gradient polyacrylamide slab gels with an apparent molecular weight of about 17,000. Pre-α comprised 2-3% and 20-30% of the total proteins translated with pituitary and pituitary tumor mRNA, respectively. Microanalysis of amino acid sequence of the pre-α subunits containing various radiolabeled amino acids gave the following partial sequence for mouse tumor pre-α: [Formula: see text] Met was also found in positions 1, 14, and 17 in mouse pituitary pre-α but only in residue 1 of the bovine pituitary pre-α subunit. Leu was found in identical positions in bovine pituitary pre-α, with an additional Leu in position 17. Leu in the common positions (12, 15, 19, and 22) has also been found in human choriogonadotropin pre-α subunit [Birken, S., Fetherston, J., Desmond, J., Canfield, R. & Boime, I. (1978) Biochem. Biophys. Res. Commun. 85, 1247-1253]. The data demonstrate that pituitary as well as placental glycoprotein hormone α subunits are synthesized with an amino-terminal hydrophobic extension, in accord with the “signal hypothesis” for secreted proteins. Furthermore, the positions of the hydrophobic amino acid Leu have been strictly conserved in pre-α subunits from various species and in two different tissues, the pituitary and placenta.
Keywords: cell-free biosynthesis, pituitary mRNA, immunoprecipitation, signal peptide, sequence homology
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahl O. P., Marz L., Kessler M. J. Isolation and characterization of N- and O- glycosidic carbohydrate units of human chorionic gonadotropin. Biochem Biophys Res Commun. 1978 Oct 16;84(3):667–676. doi: 10.1016/0006-291x(78)90757-x. [DOI] [PubMed] [Google Scholar]
- Bielinska M., Boime I. mRNA-dependent synthesis of a glycosylated subunit of human chorionic gonadotropin in cell-free extracts derived from ascites tumor cells. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1768–1772. doi: 10.1073/pnas.75.4.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielinska M., Grant G. A., Boime I. Processing of placental peptide hormones synthesized in lysates containing membranes derived from tunicamycin-treated ascites tumor cells. J Biol Chem. 1978 Oct 25;253(20):7117–7119. [PubMed] [Google Scholar]
- Birken S., Fetherston J., Desmond J., Canfield R., Boime I. Partial amino acid sequence of the preprotein form of the alpha subunit of human choriogonadotropin and identification of the site of subsequent proteolytic cleavage. Biochem Biophys Res Commun. 1978 Dec 29;85(4):1247–1253. doi: 10.1016/0006-291x(78)91137-3. [DOI] [PubMed] [Google Scholar]
- Blackman M. R., Gershengorn M. C., Weintraub B. D. Excess production of free alpha subunits by mouse pituitary thyrotropic tumor cells in vitro. Endocrinology. 1978 Feb;102(2):499–508. doi: 10.1210/endo-102-2-499. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chatterjee M., Baliga B. S., Munro H. N. Synthesis of human placental lactogen and human chorionic gonadotropin by polyribosomes and messenger RNA's from early and full term placentas. J Biol Chem. 1976 May 25;251(10):2945–2951. [PubMed] [Google Scholar]
- Chin W. W., Habener J. F., Kieffer J. D., Maloof F. Cell-free translation of the messenger RNA coding for the alpha subunit of thyroid-stimulating hormone. J Biol Chem. 1978 Nov 25;253(22):7985–7988. [PubMed] [Google Scholar]
- Daniels-McQueen S., McWillians D., Birken S., Canfield R., Landefeld T., Boime I. Identification of mRNAs encoding the alpha and beta subunits of human choriogonadotropin. J Biol Chem. 1978 Oct 10;253(19):7109–7114. [PubMed] [Google Scholar]
- Dannies P. S., Tashjian A. H., Jr Thyrotropin-releasing hormone increases prolactin mRNA activity in the cytoplasm of GH-cells as measured by translation in a wheat germ cell-free system. Biochem Biophys Res Commun. 1976 Jun 21;70(4):1180–1189. doi: 10.1016/0006-291x(76)91027-5. [DOI] [PubMed] [Google Scholar]
- Devillers-Thiery A., Kindt T., Scheele G., Blobel G. Homology in amino-terminal sequence of precursors to pancreatic secretory proteins. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5016–5020. doi: 10.1073/pnas.72.12.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobberstein B., Blobel G. Functional interaction of plant ribosomes with animal microsomal membranes. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1675–1682. doi: 10.1016/0006-291x(77)90637-4. [DOI] [PubMed] [Google Scholar]
- Drapeau G. R. Unusual COOH-terminal structure of staphylococcal protease. J Biol Chem. 1978 Sep 10;253(17):5899–5901. [PubMed] [Google Scholar]
- Giudice L. C., Pierce J. G. Studies on the disulfide bonds of glycoprotein hormones. Complete reduction and reoxidation of the disulfide bonds of the alpha subunit of bovine luteinizing hormone. J Biol Chem. 1976 Oct 25;251(20):6392–6399. [PubMed] [Google Scholar]
- Giudice L. C., Pierce J. G. Studies on the reduction and reoxidation of the disulfide bonds of the alpha and beta subunits of human choriogonadotropin. Biochim Biophys Acta. 1978 Mar 28;533(1):140–146. doi: 10.1016/0005-2795(78)90557-3. [DOI] [PubMed] [Google Scholar]
- Hara K., Rathnam P., Saxena B. B. Structure of the carbohydrate moieties of alpha subunits of human follitropin, lutropin, and thyrotropin. J Biol Chem. 1978 Mar 10;253(5):1582–1591. [PubMed] [Google Scholar]
- Jackson R. C., Blobel G. Post-translational cleavage of presecretory proteins with an extract of rough microsomes from dog pancreas containing signal peptidase activity. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5598–5602. doi: 10.1073/pnas.74.12.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- Kourides I. A., Weintraub B. D. mRNA-directed biosynthesis of alpha subunit of thyrotropin: translation in cell-free and whole-cell systems. Proc Natl Acad Sci U S A. 1979 Jan;76(1):298–302. doi: 10.1073/pnas.76.1.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambin P. Reliability of molecular weight determination of proteins by polyacrylamide gradient gel electrophoresis in the presence of sodium dodecyl sulfate. Anal Biochem. 1978 Mar;85(1):114–125. doi: 10.1016/0003-2697(78)90281-6. [DOI] [PubMed] [Google Scholar]
- Landefeld T. D., McWilliams D. R., Boime I. The isolation of mRNA encoding the alpha subunit of human chorionic gonadotropin. Biochem Biophys Res Commun. 1976 Sep 20;72(2):381–390. doi: 10.1016/s0006-291x(76)80054-x. [DOI] [PubMed] [Google Scholar]
- Landefeld T., Boguslawski S., Corash L., Boime I. The cell-free synthesis of the alpha subunit of human chorionic gonadotropin. Endocrinology. 1976 May;98(5):1220–1227. doi: 10.1210/endo-98-5-1220. [DOI] [PubMed] [Google Scholar]
- Lingappa V. R., Devillers-Thiery A., Blobel G. Nascent prehormones are intermediates in the biosynthesis of authentic bovine pituitary growth hormone and prolactin. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2432–2436. doi: 10.1073/pnas.74.6.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer R. A., Gorski J., McKean D. J. Partial amino acid sequence of rat pre-prolactin. Biochem J. 1977 Jan 1;161(1):189–192. doi: 10.1042/bj1610189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Roberts B. E. Tobacco mosaic virus RNA directs the synthesis of a coat protein peptide in a cell-free system from wheat. J Mol Biol. 1973 Nov 15;80(4):733–742. doi: 10.1016/0022-2836(73)90206-4. [DOI] [PubMed] [Google Scholar]
- Roman R., Brooker J. D., Seal S. N., Marcus A. Inhibition of the transition of a 40 S ribosome-Met-tRNA-i-Met complex to an 80 S ribosome-Met-tRNA-i-Met- complex by 7-Methylguanosine-5'-phosphate. Nature. 1976 Mar 25;260(5549):359–360. doi: 10.1038/260359a0. [DOI] [PubMed] [Google Scholar]
- Schechter I. Partial amino acid sequence of the precursor of immunoglobulin light chain programmed by messenger RNA in vitro. Science. 1975 Apr 11;188(4184):160–162. doi: 10.1126/science.803715. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Shields D., Blobel G. Cell-free synthesis of fish preproinsulin, and processing by heterologous mammalian microsomal membranes. Proc Natl Acad Sci U S A. 1977 May;74(5):2059–2063. doi: 10.1073/pnas.74.5.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchanek G., Kreil G. Translation of melittin messenger RNA in vitro yields a product terminating with glutaminylglycine rather than with glutaminamide. Proc Natl Acad Sci U S A. 1977 Mar;74(3):975–978. doi: 10.1073/pnas.74.3.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager H. S., Steiner D. F. Peptide hormones. Annu Rev Biochem. 1974;43(0):509–538. doi: 10.1146/annurev.bi.43.070174.002453. [DOI] [PubMed] [Google Scholar]
- Thibodeau S. N., Lee D. C., Palmiter R. D. Identical precursors for serum transferrin and egg white conalbumin. J Biol Chem. 1978 Jun 10;253(11):3771–3774. [PubMed] [Google Scholar]
- Waechter C. J., Lennarz W. J. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu Rev Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]
- Weintraub B. D., Stannard B. S. Precursor-product relationships in the biosynthesis and secretion of thyrotropin and its subunits by mouse thyrotropic tumor cells. FEBS Lett. 1978 Aug 15;92(2):303–307. doi: 10.1016/0014-5793(78)80775-3. [DOI] [PubMed] [Google Scholar]






