Abstract
The nasal route is attractive for the delivery of vaccines in that it not only offers an easy to use, non-invasive, needle-free alternative to more conventional parenteral injection, but it also creates an opportunity to elicit both systemic and (crucially) mucosal immune responses which may increase the capability of controlling pathogens at the site of entry. Immune responses to “naked” antigens are often modest and it is widely accepted that incorporation of an adjuvant is a prerequisite for the achievement of clinically effective nasal vaccines. Many existing adjuvants are sub-optimal or unsuitable because of local toxicity or poor enhancement of immunogenicity. Chitosan, particularly chitosan salts, have now been used in several preclinical and clinical studies with good tolerability, excellent immune stimulation and positive clinical results across a number of infections. Particularly significant evidence supporting chitosan as an adjuvant for nasal vaccination comes from clinical investigations on a norovirus vaccine; this demonstrated the ability of chitosan (ChiSys®), when combined with monophosphoryl lipid, to evoke robust immunological responses and confer protective immunity following (enteral) norovirus challenge. This article summarizes the totality of the meaningful information (including key unpublished data) supporting the development of chitosan-adjuvanted vaccines.
Keywords: adjuvant, avian influenza, chitosan, diphtheria, intranasal, mucosa, norovirus, safety, vaccine
Introduction
The use of vaccines has transformed public health through the prevention of many infectious diseases. It has been estimated that more than 2.5 million deaths have been averted annually from diseases such as diphtheria, measles, pertussis, and tetanus. But, as many as 5 million annual deaths could be prevented if vaccines were used across wider geographies and demographies.1
Vaccines contain live-attenuated or inactivated pathogens or their subunits which can be composed of natural or recombinant protein or polysaccharide2 and are usually given by subcutaneous or intramuscular injection, routes that are likely to remain important for vaccine delivery for years to come.3 However, inconvenience, fear, and dislike of injection remain obstacles to uptake; furthermore, the injection route creates a risk of needle-stick injuries.4,5 For these reasons, a significant research effort has focused on the development of alternative, needle-free administration routes.
Mucosal and particularly nasal delivery is widely regarded as the most acceptable alternative to the parenteral route.6 The nasal cavity represents an easy to use, highly accessible, and highly vascularised surface that would allow for non-invasive/needle-free administration of vaccines. Although some individuals may have an aversion to nasal sprays their use is likely to encourage greater public acceptance when compared with injection, as has already been found for children. In addition, the intra-nasal approach offers the added advantage of the induction of local immunity, which can augment protection against organisms invading the body via the respiratory tract.
Attempts to develop nasal vaccines have often resulted in poor immunogenic responses, most commonly in response to inactivated pathogens, which has been overcome by the use of live pathogen (as in the case of FluMist nasal influenza virus vaccine, MedImmune) and/or inclusion of an adjuvant.7 There are two key obstacles to the development of an adjuvant. First, it is critical that the adjuvant not only increases any immunological response, but also that this immunological response is associated with greater clinical benefit. Second, safety has been a major obstacle for the development of new vaccine adjuvants;8 the challenge being to offer a sufficient degree of incremental efficacy while avoiding or minimizing reactogenicity or toxicity.9 Unfortunately, the majority of adjuvants used in human clinical trials appear to have been associated with local and occasionally systemic toxicity.10 The most widely accepted vaccine adjuvants are in the form of aluminum salts (alum), first used as vaccine adjuvants about 80 y ago and a component of several licensed parenteral vaccines. However, alum is limited by its inability to enhance cell-mediated Th1 or CTL responses, which are important in controlling most intracellular pathogens.11 Furthermore, alum is not a good inducer of mucosal immunity.12 Despite continued research effort and the recent introduction of several ‘new-generation’ adjuvants, lack of adjuvants has created a bottleneck in the development of new parenteral and mucosal vaccine products.13
Safety concerns raised in recent years have retarded the development of adjuvanted nasal vaccine products in general. First came reports of brain uptake/reactogenicity of nasally applied adjuvants based on the bacterial enterotoxins Cholera toxin (CT) and heat-labile enterotoxin from Escherichia coli (LT) in certain strains of mice (e.g., Balb/c and C57BL/6) apparently as a result of transit via the olfactory nerve; interestingly these adjuvants were not detected in the brains of other strains of mice (e.g., CD-1 and other outbred strains) nor in rats, rabbits, and baboons.14-16 Second came concerns over use of an LT-based nasal influenza virus vaccine (Nasalflu, Berna Biotech) due to association with Bell’s palsy (facial nerve paralysis) which subsequently led to the product being withdrawn from the market.17,18 In view of these events, more comprehensive information on the safety of mucosal adjuvants is a prerequisite for development and there is an increasing need for route-specific adjuvants and vaccines.19
Archimedes Development Ltd (Archimedes) has developed a novel delivery technology based on chitosan (ChiSys®), which has been shown to enhance the intranasal delivery of conventional drugs as well as peptides and proteins.20,21 This technology has shown promise for the nasal delivery of vaccine antigens, with the added benefit that chitosan displays positive adjuvant properties, with a potential for clinical benefit. With respect to the safety issues raised above, a substantial amount of preclinical and clinical safety data has been generated on chitosan and its safe use for intranasal drug delivery and as a vaccine adjuvant has been widely reported in the literature.22-33
Chitosan: Chemical and Physicochemical Characteristics
Chitosan, the generic term for a family of linear polysaccharides which exist as copolymers of β-(1-4)-linked glucosamine and N-acetylglucosamine, is commercially obtained by partial de-acetylation of α-chitin produced from the exoskeletons of crustacea or the cell walls of fungi.34-36
The molecular weight, degree of de-acetylation (charge density) and distribution of acetyl groups strongly influence the physicochemical and biological properties of chitosan and directly affect its utility.20,37,38 Natural chitosan salts tend to be largely insoluble above pH 6 which could be problematic for the delivery of vaccine antigens that are soluble and stable at neutral pH or above.6
Improved aqueous solubility of chitosan has been achieved by molecular modification largely associated with primary amine groups although hydroxyl groups have also been modified.6,20
In solution, amino groups of chitosan are protonated and the resultant soluble polysaccharide is positively charged (cationic) conferring chitosan with mucoadhesive properties which are a critical component of its use for nasal drug and vaccine delivery applications.39,40
Safety of Chitosan
General safety of chitosan
As described above, the term ‘chitosan’ can represent a range of polymers individually characterized by their molecular weight, degree of de-acetylation and derivatisation, so published safety data need to be interpreted with some caution. Nevertheless, chitosan is widely regarded as a biocompatible, nontoxic, and non-allergenic material that is, therefore, highly suitable for use in medical and pharmaceutical applications.41
Chitosan salts, especially those derived from shellfish, have been tested for safety and toxicity in a number of animal species, and by various routes of administration.22,23 Kitozyme24 and Primex Corporations25,26 have compiled comprehensive information as part of self-certifications to support a “generally recognized as safe” (GRAS) status. The safety of chitosan has been recently and comprehensively reviewed by, among others, Baldrick42 and Kean and Thanou.43
In vivo, the stability of chitosan is affected by molecular weight, degree of de-acetylation, and chemical modification;43 nevertheless, it is generally accepted that chitosan is not degraded within the human intestine and thus effectively functions as an inert dietary fiber which is excreted via faeces.44-46 The oral LD50 in mice is reported to be 16 g/kg, which is similar to that of sugar.46,47
The use of chitosan for pharmaceutical and medical applications requires highly purified GMP-grade material comprising carbohydrate containing little or no residual protein; chitosan-based products should comply with appropriate pharmacopoeial tests. Numerous researchers have demonstrated the absence of any allergic response in subjects with shellfish allergy following oral challenge with shellfish-derived glucosamine.48,49 Further evidence of safety comes from the absence of allergic reactions, or any other adverse event, following the use of chitosan dressings, even in people with shellfish allergies.50
Chitosan is thus considered to be a nontoxic and non-allergenic material that is suitable for use in medical and pharmaceutical applications.41,43
Safety of intranasal chitosan
General considerations
The local pH (5.5–6.5) in the nasal cavity combined with the high degree of de-acetylation of chitosans typically used in drug/vaccine delivery, and the relatively short time course for transit, prevents significant degradation ensuring that nasally administered chitosan is cleared by mucociliary mechanisms and swallowed intact. Thus, the potential for toxicological responses is reduced and largely limited to transient local effects; systemic effects are not apparent in any studies conducted on this intra-nasally delivered adjuvant.
Preclinical studies conducted with chitosan
Archimedes has conducted more than ten preclinical toxicology studies to assess the safety of shellfish-derived (ex FMC Biopolymer) chitosan glutamate (ChiSys®) when given via the nasal route. Nine of these studies have included control groups that received drug-free chitosan vehicle; these data have not been previously reported (Table 1). All studies were conducted according to Good Laboratory Practice (GLP) and they included administration for 28 d in rats, for 3 mo in dogs and for 6 mo in rabbits.
Table 1. Summary of findings from animal safety studies on drug-free intranasal chitosan formulations†.
| Study ref. | Species/strain [no. animals dosed] |
Dosing regimen | Details of chitosan glutamate formulation | Nominal daily chitosan dose | Chitosan treatment-related nasal findings‡ | |
|---|---|---|---|---|---|---|
| Mg | Mg/kg | |||||
| #A1 | Rat/Sprague Dawley [10M/10F] |
Once daily x 14 d | 5 mg/ml | — | 1.7 | None reported |
| #A2 | Rat/Sprague Dawley [10M/10F] |
Once daily x14 d | 5 mg/ml | 0.5 | 2.0 | None reported |
| #A3 | Rat/Sprague Dawley [10M/10F] [5M/5F in recovery group] |
Twice daily x 28 d | 5 mg/ml | 0.125 | 0.5 | None reported |
| #A4 | Rabbit/NZW [5M/5F] |
Once daily x 10 d | 20 mg/ml | 2 | 0.67 | None reported |
| #A5 | Rabbit/NZW [5M/5F] |
Once daily x14 d | Powder | 40 | 20 | Increased incidence/severity of: epithelial inflammatory cell infiltrates, inflammatory exudate overlying epithelium, epithelial hyperplasia. |
| #A6 | Rabbit/NZW [Interim: 3M/3F Main: 6M/6F] |
Twice daily x3 or 6 mo |
5 mg/ml | 2 | 0.6 | Mild hyperplasia, minimal erosion and minimal inflammation of mucosa and minimum or mild inflammation of mucosa in 2/6 rabbits dosed at 8 mg/day for 3 mo. At 6 mo: minimal or mild inflammation of mucosa observed in 3/12 rabbits dosed at 2 mg/day; 3/12 rabbits dosed at 4 mg/day; and 1/12 rabbits dosed at 8 mg/kg. |
| 10 mg/ml | 4 | 1.2 | ||||
| 20 mg/ml | 8 | 2.4 | ||||
| #A7 | Dog/Beagle [3M/3F] |
Once daily x 14 d | 5 mg/ml | 4 | 0.5 | None reported |
| #A8 | Dog/Beagle [3M/3F] |
Once daily x 14 d | 5 mg/ml | 2.5 | 0.25 | Tracheal squamous metaplasia observed in 2M/1F animals. |
| #A9 | Dog/Beagle [3M/3F + 2M/2F in recovery group] |
Once/twice daily x 3 mo | 5 mg/ml | 4 | 0.4 | None reported |
Note: †The data shown in the table above are the unpublished data of Archimedes. ‡Significant systemic effects are not typically seen in intranasal studies on adjuvant-only formulations. F, female; M, male; NZW, New Zealand White (rabbit).
Chitosan produced either no treatment-related findings, or events which were generally of mild severity such as epithelial hyperplasia, inflammation, or increased secretions; there were no findings that would preclude the use of chitosan in a human nasal vaccine (Table 1).
Clinical studies conducted with chitosan
On the basis of robust preclinical safety data, Archimedes has administered approximately 3000 doses of shellfish-derived chitosan glutamate to more than 1000 human subjects and patients.
Archimedes has conducted three studies that specifically evaluated drug-free chitosan in man (Table 2). In a 10 d nasal tolerability study in healthy human subjects (Table 2 Study #H1) there were 4 groups of subjects; 2 groups received chitosan glutamate as solution or powder, and 2 groups received placebo solution or powder. Both chitosan formulations were well tolerated with a good safety profile across a range of nasal and throat symptom scales with no difference between formulations; the most common symptoms (found in up to 10% of instances) were dry or runny nose and all symptoms were mild and transient. Based on nasal examinations the most frequent findings were erythema and rhinorrhoea, both of which were present in around 50% of examinations; all findings were classed as mild or moderate and were easily tolerated.
Table 2. Summary details of some human clinical trials conducted on chitosan-based intranasal formulations.
| A. Studies conducted on drug-free solution or powder formulations of chitosan | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study title [publication details] | CSN form | CSN conc. | CSN dose | Dosing regimen | No. subjects |
No. doses |
Conclusions on local adverse events | |
| #H1 | Tolerability of intranasal administration of a new excipient, chitosan glutamate [Archimedes, unpublished data] |
Solution Powder |
5mg/mL 100% |
4.0mg/day for 10 d 40mg/day for 10 d |
Multiple doses (0.1mL to both nostrils four times on Days 1–10) Multiple doses(10mg to both nostrils two times on Days 1–10) |
8 9 |
320 180 |
Safety was satisfactory and tolerability acceptable. |
| #H2 | Deposition, clearance and tolerability of chitosan solution [Newman et al. 2004] |
Solution | 5mg/mL | 0.5mg/day for 5 d | Multiple doses (0.1mL to one nostril on Days 1–5) Radiolabelled on Days 1, 3, 5 |
14 | 70 | Chitosan was well tolerated nasally |
| #H3 | In vivo mucociliary transport study using human volunteers [Aspden, et al. 1997] |
Solution | Approx. 6mg/mL† | ~0.6mg/day for 7 d |
Multiple doses (0.1mL to one nostril on Days 1–7) | 10 | 70 | Chitosan was well tolerated nasally and the nasal membrane appeared healthy and normal in all volunteers following endoscopic examination |
| B. Studies conducted on solution formulations of chitosan containing a drug | ||||||||
| #H4 | Pharmacokinetic profile of alniditan nasal spray during and outside migraine attacks [Roon, et al. 1999] |
Solution containing 20mg/mL alniditan | Not reported | Not reported | Two doses (0.1mL to one nostril on each of two days) | 13 | 53 | Intranasal alniditan administration was generally well tolerated. |
| Solution containing 40mg/mL alniditan | Not reported | Two doses (0.1mL to one nostril on each of two days) | 14 | |||||
| #H5 | Analgesic efficacy and safety of morphine-chitosan nasal solution in patients with moderate to severe pain following orthopedic surgery [Stoker et al. 2008] |
Solution containing 75mg/mL morphine | Not reported | Not reported | Single dose (0.05mL to one nostril) | 24 | 24 | Local adverse events associated with intranasal administration were transient and mainly of mild severity |
| Not reported | Single dose (0.1mL to one nostril) | 24 | 24 | |||||
| Not reported | Single dose (2 x 0.1mL) | 24 | 24 | |||||
| Not reported | Single dose (4 x 0.1mL) | 23 | 23 | |||||
| Not reported | Multiple doses (0.1mL to one nostril per dose)‡ | 90‡ | >90 | |||||
| Not reported | Multiple doses (2 x 0.1mL per dose) ‡ |
>87 | ||||||
| #H6 | The analgesic efficacy and safety of a novel intranasal morphine formulation (morphine plus chitosan), immediate release oral morphine, intravenous morphine, and placebo in a postsurgical dental pain model [Christensen et al. 2008] |
Solution#1 containing 75 mg/mL morphine |
Not reported | Not reported | Single dose (0.1mL to one nostril) | 45 | 45 | Study medications were generally well tolerated. |
| Solution#2 containing150 mg/mL morphine | 5mg/mL | Not reported | Single dose (0.1mL to one nostril) | 45 | 45 | |||
Note: †reported as 0.25% w/v as chitosan base; salt content = 42% w/w. ‡177 subjects received multiple doses of morphine-chitosan as follows: 7.5mg morphine (n = 90) or 15mg morphine (n = 87). Of these 177 subjects, 87 had already received single intranasal doses whereas the remaining 90 subjects received intranasal morphine-chitosan for the first time. CSN, chitosan glutamate.
In a separate study (Table 2 Study #H2), deposition and clearance were assessed in healthy human subjects following 5 d of intranasal administration of a chitosan glutamate solution.51 Four subjects reported a total of 5 adverse effects (blood shot eye, headache, itchy nose, sore throat, and watery eyes) which were all mild in severity (data not previously reported), again indicating that chitosan was well tolerated.
Aspden et al.52 (Table 2 Study #H3) investigated the effect of chitosan glutamate solution (once daily administration via one nostril for 7 d) on mucociliary transport rate in outpatients undergoing surgery to correct a deviated nasal septum. Chitosan had no effect on saccharin clearance times, nor did it affect nasal histology based on examination of tissue removed from treated and untreated sides of the nasal cavity.
The literature cites numerous non-Archimedes sponsored clinical studies that have been conducted to investigate the efficacy and safety of chitosan as an intranasal drug delivery system. Roon et al.32 (Table 2 Study #H4) investigated the pharmacokinetic profile of alniditan-chitosan nasal spray both during and outside individual migraine attacks in 27 patients. Intranasal administration was well tolerated; nose and mild throat irritation and taste disturbance were reported as the main local adverse events.
Stoker et al.33 (Table 2 Study #H5) published details of a two-stage study undertaken in 187 post-bunionectomy patients. In the first stage, patients were randomized to 5 groups for an initial single-dose: intranasal morphine-chitosan at 3 dose levels; intravenous (IV) morphine or placebo; in the second stage, patients were randomized to 2 dose levels of intranasal morphine-chitosan and received up to 6 doses over 24 h. Local adverse events associated with intranasal administration (primarily nasal congestion, rhinorrea, sneezing, throat irritation, and taste disturbance) were mostly mild and transient even during the multiple-dose stage. The frequency of events was seen to decrease over time; regardless of route of administration, systemic adverse events, were dose-related and consistent with expected opioid effects.
Christensen et al.28 (Table 2 Study #H6) reported details of a study undertaken in 225 patients with moderate to severe pain after third molar extraction. The patients were randomized to 5 single-dose groups: intranasal morphine-chitosan at two dose levels, IV morphine, oral morphine, or placebo. Study medications were generally well tolerated, with no withdrawals due to adverse events nor other safety concerns; no serious events were reported. The most frequently reported adverse events were typical systemic opioid effects. In the 90 patients who received intranasal morphine-chitosan, only limited local adverse events were observed: erythema in 6 patients, nasal congestion in 4 patients, nasal passage irritation in 6 patients, and rhinorrhea in 5 patients.
Effectiveness of Chitosan as a Vaccine Adjuvant
The efficacy of chitosan as an adjuvant has been assessed in a series of studies covering numerous disease areas. Some of these data have appeared in individual study reports, but the following summary provides the first review of the totality of the data.
Diphtheria
Published data from studies in mice and guinea pigs have demonstrated that intranasal chitosan glutamate significantly enhances both local and systemic antibody responses to a diphtheria antigen (a non-toxic cross reacting material of diphtheria toxin, CRM197).53 Mills et al.30 have explored the impact of a chitosan adjuvant in man.
Study outline
Three groups of healthy subjects participated in a proof-of-principle trial; two groups (n = 10) received two doses (on days 0 and 28) of intranasal diphtheria vaccine (Group 1 with chitosan; Group 2 without chitosan); while a third group (n = 5) received a single intramuscular injection (on day 0) of alum-adsorbed diphtheria toxoid vaccine. Efficacy, was assessed by changes in serum (systemic) toxin-neutralising antibody and nasal wash (mucosal) anti-DT secretory IgA on Days 27 and 42.
Results
Safety/local tolerability and immunogenicity findings obtained in the clinical trial are summarized in Table 3. The intranasal administrations were well tolerated with only transient mild to moderate nasal effects observed (nasal discharge, blockage, and discomfort); IM vaccination was associated with mild pain at the injection site.
Table 3. Summary of safety/local tolerabilty and immunogenicity data after intranasal or intramuscular administration of diphtheria vaccines30.
| Safety/local tolerability | |||||
|---|---|---|---|---|---|
| Adverse event | Number of subjects reporting adverse events | ||||
| Intranasal dose groups (n = 10/group) | IM DT dose group (n = 5) (Day 0 only) |
||||
| First dose (Day 0) | Second dose (Day 28) | ||||
| CRM197 + CSN | CRM197 + mannitol | CRM197 + CSN | CRM197 + mannitol | ||
| Nasal discharge | |||||
| Mild | 5 | 4 | 5 | 5 | - |
| Moderate | 1 | 1 | 0 | 0 | - |
| Nasal blockage | |||||
| Mild | 4 | 0 | 2 | 5 | - |
| Moderate | 1 | 1 | 1 | 0 | - |
| Nasal discomfort | |||||
| Mild | 4 | 2 | 1 | 3 | - |
| Moderate | 2 | 2 | 2 | 1 | - |
| Fever | |||||
| Mild | 0 | 0 | 0 | 1 | - |
| Severe | 0 | 0 | 1 | 0 | - |
| Headache | |||||
| Mild | 3 | 4 | 0 | 3 | - |
| Moderate | 2 | 0 | 1 | 0 | - |
| Severe | 0 | 0 | 1 | 0 | - |
| Myalgia | |||||
| Mild | 0 | 1 | 0 | 1 | 1 |
| Pain at injection site | |||||
| Mild | - | - | - | - | 2 |
| Moderate | - | - | - | - | 1 |
| Redness at injection site | |||||
| Moderate | - | - | - | - | 1 |
| Immunogenicity | |||||
| Day 27 | Day 42 | Day 27 and Day 42 | |||
|
Serum anti-diphtheria toxin neutralising antibody levels |
14.8 | 5.4 | 20 | ~7 | 6.3 and ~7 |
CRM197, cross reacting material of diphtheria toxin; CSN, chitosan glutamate; DT, diphtheria toxoid.
After the first dose (assessed on day 27), the induction of toxin-neutralising antibody were similar for nasal CRM197 without chitosan and IM vaccination (5.4 IU/ml vs 6.3 IU/ml respectively); the inclusion of chitosan increased the toxin-neutralising antibody substantially (14.8 IU/ml). This response exceeds the well accepted protective levels of 0.1 IU/ml.54
After the second dose (assessed on day 42) neutralizing antibody levels remained similar at about 7 IU/ml in subjects receiving nasal CRM197 without chitosan or the IM vaccination; but in the group receiving CRM197 + chitosan, levels were significantly boosted (20 IU/ml).
No secretory anti-DT IgA was found in subjects at recruitment or after IM injection. A highly significant IgA response was observed in nasal washings from the dosed nostril after nasal administration, but only after the second dose (on day 42); the presence of chitosan increased this response more than 10-fold compared with nasal CRM197 without chitosan.
Avian influenza
Mann et al.55 conducted a live-virus challenge study in ferrets, a recognized preclinical model for human influenza. Both chitosan glutamate (CSN) and N,N,N-trimethylated chitosan (TM-CSN) were investigated as adjuvants for an inactivated NIBRG-14 (H5N1) subunit antigen (derived from influenza antigen A/Vietnam/1194).
Study outline
Ferrets received doses of intranasal vaccine or placebo (0.1ml per nostril) on days 0 and 21. There were 6 groups (each of 6 animals): antigen alone, two with chitosan, one with TM-CSN- (all 15 µg HA per dose) and two of placebo control (phosphate buffered saline [PBS]). On Day 49 animals were given a viral challenge with 105 times the median dose required to infect a tissue culture (105 TCID50) with highly pathogenic avian influenza (HPAI) Influenza A/Vietnam/1194/2004 (H5N1) virus (3 ml intra-tracheal [IT] for all representative groups or 0.3ml intra-nasal [IN] for placebo and CSN groups only).
Animals were monitored for mortality (survival), morbidity, clinical signs, and changes to body weight and temperature. Blood samples were collected before and after the viral challenge for assessment of homologous and cross-clade antibody responses by haemagglutination inhibition (HAI), virus neutralization (VN), and single radial hemolysis (SRH) assays. Viral load was assessed daily for 5 d (quantitative PCR and cell culture methods) in the upper respiratory tract (URT), lower respiratory tract (LRT), and central nervous system (CNS) by analysis of nasal and throat swabs plus representative tissue samples at termination (lung, nasal turbinates, brain, and olfactory bulb etc.). Histopathological examination of tissue samples was also performed.
Results
Results are summarized in Table 4. Both CSN- and TM-CSN-vaccines produced significant antibody responses; serologically protective levels of HAI (≥40) and SRH (≥25 mm2) were achieved after administration of the TM-CSN adjuvanted vaccine. Neither the placebo nor the vaccine without adjuvant elicited any antibody response.
Table 4. Summary of treatment groups and key findings in intranasal study of H5N1 vaccine candidates in ferrets55.
| Treatment group (abbreviated description) [n = 6/group]] |
Challenge route | Key findings | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-challenge | Post-challenge | ||||||||
| Immunology – serological responses to homologous Clade 1 virus after 2 doses GMT (number of animal demonstrating seroconversion) |
Mortality | Number of animals demonstrating a detectable viral load | |||||||
| HAI | VN | SRH | URT | LRT | CNS | ||||
| 1 | 15 μg HA + CSN (CSN adjuvanted) | IN | 22 (7/12 = 58%) |
29 (8/12 = 75%) |
23 (7/12 = 58%) |
0/6 | 6/6 (100%) | 0/6 (0%) | 2/6 (33%) |
| 2 | 15 μg HA + CSN (CSN adjuvanted) | IT | 0/6 | 3/6 (50%) | 2/6 (33%) | 0/6 0%) | |||
| 3 | 15 μg HA alone (Unadjuvanted) | IT | <5 (0/6 = 0%) |
<5 (0/6 = 0%) |
<4 (0/6 = 0%) |
2/6 | 6/6 (100%) | 6/6 (100%) | 4/6 (67%) |
| 4 | 15 μg HA + TM-CSN (TM-CSN adjuvanted) | IT | 259 (6/6 = 100%) |
71 (6/6 = 100%) |
57 (6/6 = 100%) |
0/6 | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) |
| 5 | 0 μg HA (PBS) (Placebo control) | IN | <5 (0/12 = 0%) |
<5 (0/12 = 0%) |
5 (1/12 = 8%) |
0/6 | 6/6 (100%) | 0/6 (0%) | 5/6 (83%) |
| 6 | 0 μg HA (PBS) (Placebo control) | IT | 5/6 | 6/6 (100%) | 6/6 (100%) | 5/6 (83% | |||
CNS, central nervous system; CSN, chitosan glutamate; GMT, geometric mean titer; HA, haemagglutinin; HAI, haemagglutinin inhibition; IN, intranasal; IT, intratracheal, LRT, lower respiratory tract; PBS, phosphate buffered saline; SRH, serial radial hemolysis; TM-CSN, trimethylated chitosan; URT, upper respiratory tract; VN, virus neutralization.
After viral challenge the key findings (Table 4) were highly consistent with immunological results. All animals receiving CSN- or TM-CSN-vaccines showed significant protection against H5N1 infection and all survived the IT challenge (Fig. 1). TM-CSN-vaccine was particularly effective in that no animal showed any symptoms nor any detectable virus in the respiratory tract or brain. Animals receiving the CSN-vaccine had no infection in the brain, but some animals showed signs of infection in the respiratory tract. By contrast, IT challenge in animals receiving placebo or vaccine without adjuvant was associated with death (83% and 33% respectively, Fig. 1) and more obvious infection (fever, weight loss, and also histopathological damage associated with viral replication in the LRT).
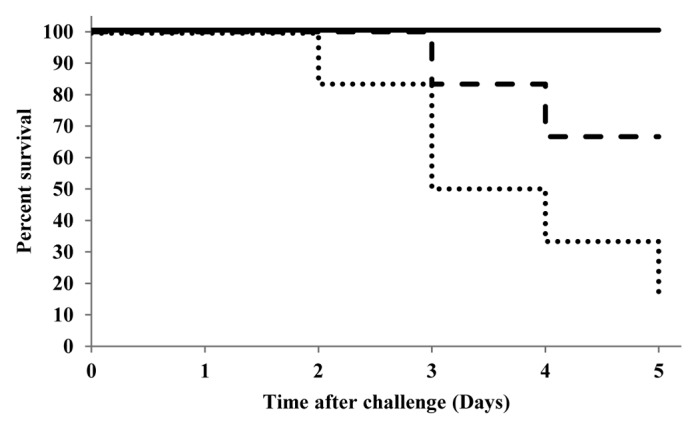
Figure 1. Kaplan-Meier plot showing mortality (percent survival) of ferrets (n=6/group) following IT challenge with HPAI H5N1 virus (replotted from55). Bold line = CSN and TM-CSN adjuvanted vaccines, dashed line = unadjuvanted vaccine (HA only), dotted line = placebo control. Abbreviations: CSN, chitosan glutamate; highly pathogenic avian influenza (HPAI), IT, intratracheal, TM-CSN, trimethylated chitosan.
Predictably, IN challenge produced less severe infection and was not lethal in any animal in either of the groups tested (placebo and CSN-vaccine). However, there were still demonstrable benefits associated with the addition of the CSN adjuvant: most notably reduced fever, fewer animals demonstrating viral load in the CNS (33% vs 83%) as well as reduction in the viral load in both the CNS and URT; viral load associated with the URT was also shown to abate over the experimental period
Overall it was concluded that both CSN and TM-CSN were clinically effective intranasal adjuvants with the potential to protect against mortality and morbidity arising from avian influenza infection.
Norovirus
Norovirus infection is the most common cause of viral gastroenteritis in humans56-58 and would benefit from a safe and convenient means of mass vaccination, which is not presently available. The strategy for development of a nasal vaccine product as opposed to one given by parenteral injection was founded on the potential to stimulate not only systemic immune responses but significantly mucosal immunity. Moreover, there is potential to elicit a mucosal immune response at both local (nasal) and distal sites which could provide protective immunity against enteric pathogens. In contrast, orally administered norovirus VLPs are reported to be only modestly immunogenic in human subjects.29
The potential of an intranasal norovirus vaccine was first demonstrated in the rabbit.59,60 Norwalk Virus Like Particles (VLP), a specific class of sub-unit antigen, derived from norovirus GI.1 genotype, were successfully administered as a powder in combination with chitosan (ChiSys®,) and a monophosphoryl lipid (MPL) adjuvant/immunoenhancer.61
Study outline
Definitive clinical studies involving the MPL/chitosan-VLP vaccines have been reported.27,29 El-Kamary et al.29 conducted a preliminary dose-escalation study before investigating safety and immunological responses in 4 groups of healthy subjects who received two doses (3 wk apart) of either MPL/chitosan (50 or 100 μg VLP per dose, as powder), chitosan only or true placebo (puff of air).
Atmar et al.27 assessed the efficacy of intranasal MPL/chitosan-VLP vaccine (as powder). Subjects received two doses 3 wk apart of either the vaccine (100 μg VLP per dose) or placebo; after a further three weeks, subjects were challenged by oral administration of virus. Serum antibody data were collected at baseline and 3 wk after each dose and clinical efficacy was assessed by the presence of infection, onset, severity, and duration of illness.
Results
Safety/tolerability
Safety/local tolerability findings obtained in the clinical trials are summarized in Table 5. Across the two studies, intranasal administration was well tolerated; local adverse events were typically mild and transient with a frequency and severity that was similar in VLP-treated and control- or placebo-treated subjects. The most common adverse events were nasal stuffiness, discharge, and itching.
Table 5. Summary of local tolerability findings after intranasal administration of chitosan-based norovirus (Norwalk VLP) vaccines and associated control formulations27,29.
| As reported by El-Kamary et al. (2010)29 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Local adverse event | Percentage of subjects reporting adverse event | |||||||
| 50 μg vaccine (n = 20) | 100 μg vaccine (n = 20) | MPL/chitosan control (n = 10) | Placebo control (n = 11) | |||||
| First dose | Second dose | First dose | Second dose | First dose | Second dose | First dose | Second dose | |
| Nasal pain | 45.0 | 38.9 | 40.0 | 5.3 | 10.0 | 11.1 | 18.2 | 0 |
| Nasal stuffiness | 75.0 | 83.3 | 80.0 | 78.9 | 80.0 | 66.7 | 54.5 | 27.3 |
| Nasal discharge | 75.0 | 77.8 | 55.0 | 57.9 | 90.0 | 66.7 | 45.5 | 18.2 |
| Nasal itching | 45.0 | 27.8 | 65.0 | 47.4 | 40.0 | 44.0 | 9.1 | 0 |
| Sneezing | 60.0 | 66.7 | 75.0 | 57.9 | 40.0 | 22.0 | 27.3 | 0 |
| Blood-tinged nasal mucus | 10.0 | 5.6 | 20.0 | 15.8 | 0 | 0 | 9.1 | 0 |
| Nasal bleeding | 0 | 5.6 | 5.0 | 0 | 0 | 0 | 0 | 0 |
| As reported by Atmar et al. (2011) 27 | ||||||||
| Local adverse event | Percentage of subjects reporting adverse event | |||||||
| 100 μg vaccine | Placebo | |||||||
| First dose (n = 50) | Second dose (n = 47) | First dose (n = 47) | Second dose (n = 43) | |||||
| Nasal discomfort | 14 | 11 | 4 | 5 | ||||
| Nasal discharge | 30 | 32 | 34 | 23 | ||||
| Nasal stuffiness | 30 | 23 | 26 | 19 | ||||
| Nasal itching | 14 | 21 | 21 | 12 | ||||
| Sneezing | 22 | 34 | 23 | 21 | ||||
| Blood-tinged mucus | 2 | 0 | 2 | 0 | ||||
| Nasal bleeding | 0 | 0 | 0 | 0 | ||||
MPL, monophosphoryl lipid; VLP, virus-like particles.
Immunological responses
Immunogenicity findings obtained in the clinical trials are summarized in Table 6. In the El-Kamary29 study VLP-specific systemic IgG and IgA antibodies increased 4.6-fold and 7.6-fold respectively at the 50μg dose of VLP and slightly more (4.8-fold and 9.1-fold) at the 100 μg dose. Immunological responses were absent in subjects receiving MPL/chitosan or placebo. It was concluded that the vaccine with adjuvants was “highly immunogenic” and “a candidate for additional study”.
Table 6. Summary of immunogenicity findings (Norwalk VLP-specific antibody titers) after intranasal administration of chitosan-based norovirus (Norwalk VLP) vaccines and associated control formulations27,29.
| As reported by El-Kamary et al. (2010)29 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vaccine group | Geometric mean titer | Geometric mean fold increase vs. pre-vaccination | ||||||||
| Serum HAI | Serum IgG | Serum IgA | ||||||||
| Pre-vaccination | After dose 1 (Day 21) | After dose 2 (Day 56) | After dose 2 (Day 56) | After dose 2 (Day 56) | ||||||
| 50 μg vaccine (n = 18) | 13.2 | 32.0 | 52.8 | 4.6 | 7.6 | |||||
| 100 μg vaccine (n = 19) | 25.7 | 111.9 | 234.9 | 4.8 | 9.1 | |||||
| MPL/chitosan control (n = 9) | 6.9 | 9.3 | 9.3 | 1.1 | 1.0 | |||||
| Placebo control (n = 11) | 12.4 | 19.3 | 21.9 | 0.9 | 1.2 | |||||
| As reported by Atmar et al. (2011) 27 | ||||||||||
| Vaccine group | Geometric mean titer | |||||||||
| Serum IgG | Serum IgA | |||||||||
| Pre-vaccination | After dose 1 (Day 21) | After dose 2 (Day 42) | Pre-vaccination | After dose 1 (Day 21) | After dose 2 (Day 42) | |||||
| 100 μg vaccine | 3.2 | 6.5 | 14.1 | 1.0 | 3.4 | 7.4 | ||||
| Placebo control | 4.6 | 4.5 | 4.8 | 1.2 | 1.1 | 1.2 | ||||
HAI, haemagglutinin inhibition; IgA, immunoglobulin A; IgG, imuunoglobulin G; MPL, monophosphoryl lipid; VLP, virus-like particles.
In the Atmar27 study there were similar responses to the MPL/chitosan-VLP (4.4 and 7.4-fold increases respectively at the 100 μg dose), with no immunogenic responses to the placebo.
Morbidity after Challenge
Atmar27 reported that the incidence of acute gastroenteritis due to norovirus was significantly decreased compared with placebo (37% vs 69%; P = 0.006). Similarly, the incidence of infection (defined as one of: evidence of faecal virus shedding; antigen detection; a 4-fold, or greater, increase in norovirus-specific antibodies in serum from pre-challenge to 30 d post-challenge), decreased from 82% to 61% (P = 0.05). The duration, and severity, of any illness was also significantly reduced in those receiving MPL/chitosan-VLP (P = 0.011).
These data demonstrate that an intranasal norovirus vaccine provides protection against illness and infection following oral norovirus challenge. Significantly they represent the first demonstration in man that a chitosan-based vaccine can be effective for preventing disease and underpin the comprehensive immunological data that have been generated using a variety of antigens.
Concluding Remarks
Chitosan is a biocompatible (non-toxic) and biodegradable mucoadhesive adjuvant that has been shown to augment immunological responses to a variety of nasally administered antigens in preclinical models and, more crucially, in human subjects. The efficacy of chitosan-based vaccines, in terms of their ability to elicit protective immunity, has also been clearly demonstrated by means of live virus challenge studies.
The mechanism of action of chitosan as an adjuvant for nasally administered vaccine antigens is not fully understood. The mucoadhesive properties of chitosan would appear to play a crucial role in reducing clearance of administered formulations from the nasal cavity.62 Chitosan may also facilitate uptake of antigen into nasal associated lymphoid tissue (NALT) through its transient effect on epithelial tight junctions. Chitosan is also reported to exhibit immunomodulatory properties such as stimulation of natural killer (NK) cell and macrophage populations.63 The viscous properties of chitosan, thereby creating an environment which can protect the antigen, are important for its role as an experimental adjuvant for subcutaneously administered vaccines63,64 and it is possible that chitosan also protects nasally applied antigens prior to uptake into NALT.
Depending on the particular vaccine antigen employed, chitosan can function as a standalone adjuvant or as a co-adjuvant. The excellent safety profile of chitosan is particularly significant, given that the majority of alternative vaccine adjuvants have been associated with some reactogenicity and/or toxicity.
Encouragingly, there is growing evidence for a number of antigens that positive preclinical findings are translating into good immunological responses in humans and, in some instances, actual clinical benefits.6 Over the last five years, more than 25 preclinical and clinical studies have been conducted on intranasal chitosan-based vaccines that are being developed to combat at least ten separate diseases, with Archimedes being actively involved in most. The most compelling data to date come from a clinical investigation on an intranasal dry-powder norovirus vaccine which demonstrates the ability of chitosan (ChiSys®), when combined with monophosphoryl lipid to evoke robust immunological responses and confer protective immunity following (enteral) norovirus challenge.
In view of the tolerability and safety data already available, chitosan salts probably represent the greatest opportunity for developing nasal vaccines in the imminent future with chitosan derivatives and nanoparticles representing longer-term promise.6
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- CNS
central nervous system
- CRM197
non-toxic cross reacting material of diphtheria toxin
- CSN
chitosan glutamate
- CT
cholera enterotoxin, CTL, cytotoxic T lymohpocyte
- DT
diphtheria toxoid
- GMP
good manufacturing practice
- GRAS
generally recognized as safe
- HA
haemagglutinin
- HAI
haemagglutination inhibition
- HPAI
highly pathogenic avian influenza
- IgA
immunoglobulin-A
- IgG
immunoglobulin-G
- IM
intramuscular
- IN
intranasal
- IT
intratracheal
- IU
international units
- IV
intravenous
- MPL
monophosphoryl lipid
- LD50
median lethal dose
- LRT
lower respiratory tract
- PBS
phosphate buffered saline
- PCR
polymer chain reaction
- sIgA
secretory immunoglobulin-A, SRH, single radial haemolysis
- TCID50
median tissue culture infective dose
- Th1
T helper cell type 1
- TM-CSN
N,N,N-trimethylated chitosan
- URT
upper respiratory tract
- VLP
virus-like particles
- VN
virus neutralization
References
- 1.WHO. Department of Immunization, Vaccines and Biologicals, IVB Strategic plan 2010-15. Draft 24 March 2010. Obtained from: www.who.int/entity/immunization/.../IVB_SP_2010-15_final_Ver.pdf [Accessed 23 July 2012]
- 2.Schijns VEJC. Immunological concepts of vaccine adjuvant activity - commentary. Curr Opin Immunol. 2000;12:56–463. doi: 10.1016/S0952-7915(00)00120-5. [DOI] [PubMed] [Google Scholar]
- 3.Amorij J-P, Kersten GFA, Saluja V, Tonnis WF, Hinrichs WLJ, Slütter B, Bal SM, Bouwstra JA, Huckriede A, Jiskoot W. Towards tailored vaccine delivery: needs, challenges and perspectives. J Control Release. 2012;161:363–76. doi: 10.1016/j.jconrel.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 4.ASDReports. Stakeholder opinions: vaccine administration technologies–beyond needles. Report code: ASDR‐1755. 2009. Published by Datamonitor Healthcare.
- 5.Miller MA, Pisani E. The cost of unsafe injections. Bull World Health Organ. 1999;77:808–11. [PMC free article] [PubMed] [Google Scholar]
- 6.Jabbal-Gill I, Watts P, Smith A. Chitosan-based delivery systems for mucosal vaccines. Expert Opin Drug Deliv. 2012;9:1051–67. doi: 10.1517/17425247.2012.697455. [DOI] [PubMed] [Google Scholar]
- 7.Jabbal-Gill I. Nasal vaccine innovation. J Drug Target. 2010;18:771–86. doi: 10.3109/1061186X.2010.523790. [DOI] [PubMed] [Google Scholar]
- 8.O'Hagan DT. New generation vaccine adjuvants. In: Encyclopedia of Life Sciences (eLS): John Wiley & Sons Ltd, 2007:[doi: 10.1002/9780470015902.a0020177]. [Google Scholar]
- 9.Gupta RK, Relyveld EH, Lindblad EB, Bizzini B, Ben-Efraim S, Gupta CK. Adjuvants--a balance between toxicity and adjuvanticity. Vaccine. 1993;11:293–306. doi: 10.1016/0264-410X(93)90190-9. [DOI] [PubMed] [Google Scholar]
- 10.Dekker CL, Gordon L, Klein J. Dose optimization strategies for vaccines: the role of adjuvants and new technologies. Report approved at NVAC Meeting February 2008. Obtained from: www.hhs.gov/nvpo/nvac/doseoptimizationstrategies_200802.doc [Accessed 3 September 2012]
- 11.Reed SG, Bertholet S, Coler RN, Friede M. New horizons in adjuvants for vaccine development. Trends Immunol. 2009;30:23–32. doi: 10.1016/j.it.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Rhee JH, Lee SE, Kim SY. Mucosal vaccine adjuvants update. Clin Exp Vaccine Res. 2012;1:50–63. doi: 10.7774/cevr.2012.1.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petrovsky N, Heinzel S, Honda Y, Lyons AB. New-age vaccine adjuvants, friend or foe? BioPharm Int 2 2007, Obtained from:http://www.biopharminternational.com/biopharm/Downstream+Processing/New-Age-Vaccine-Adjuvants-Friend-or-Foe/ArticleStandard/Article/detail/444996 [Accessed 3 September 2012].
- 14.Fujihashi K, Koga T, van Ginkel FW, Hagiwara Y, McGhee JR. A dilemma for mucosal vaccination: efficacy versus toxicity using enterotoxin-based adjuvants. Vaccine. 2002;20:2431–8. doi: 10.1016/S0264-410X(02)00155-X. [DOI] [PubMed] [Google Scholar]
- 15.NIAID (U.S. National Institute of Allergy and Infectious Diseases). Safety evaluation of toxin adjuvants delivered intranasally 2001, Obtained from: www.niaid.nih.gov/topics/entericdiseases/Documents/intranasal.pdf [Accessed 13 November 2013].
- 16.van Ginkel FW, Jackson RJ, Yuki Y, McGhee JR. Cutting edge: the mucosal adjuvant cholera toxin redirects vaccine proteins into olfactory tissues. J Immunol. 2000;165:4778–82. doi: 10.4049/jimmunol.165.9.4778. [DOI] [PubMed] [Google Scholar]
- 17.Mutsch M, Zhou W, Rhodes P, Bopp M, Chen RT, Linder T, Spyr C, Steffen R. Use of the inactivated intranasal influenza vaccine and the risk of Bell’s palsy in Switzerland. N Engl J Med. 2004;350:896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 18.Stephenson I, Zambon MC, Rudin A, Colegate A, Podda A, Bugarini R, Del Giudice G, Minutello A, Bonnington S, Holmgren J, et al. Phase I evaluation of intranasal trivalent inactivated influenza vaccine with nontoxigenic Escherichia coli enterotoxin and novel biovector as mucosal adjuvants, using adult volunteers. J Virol. 2006;80:4962–70. doi: 10.1128/JVI.80.10.4962-4970.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pedersen G, Cox R. The mucosal vaccine quandary: intranasal vs. sublingual immunization against influenza. Hum Vaccin Immunother. 2012;8:689–93. doi: 10.4161/hv.19568. [DOI] [PubMed] [Google Scholar]
- 20.Amidi M, Mastrobattista E, Jiskoot W, Hennink WE. Chitosan-based delivery systems for protein therapeutics and antigens. Adv Drug Deliv Rev. 2010;62:59–82. doi: 10.1016/j.addr.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Illum L. Nasal drug delivery - recent developments and future prospects. J Control Release. 2012;161:254–63. doi: 10.1016/j.jconrel.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 22.Dornish M, Hagen A, Hansson E, Pecheur C, Verdier F, Skaugrud Ø. Safety of Protasan™: ultrapure chitosan salts for biomedical and pharmaceutical use. In: Domard A, Roberts GAF, Vårum KM, eds. Advances in Chitin Science, Vol II, Andre Publisher, Lyon, France, 1997: 664-670. [Google Scholar]
- 23.Novamatrix. Safety and toxicology of Protasan™ UP chitosans. Product Information Bulletin, 2002. Obtained from: http://www.novamatrix.biz/Portals/novamatrix/Content/Docs/Technology/PIB-Safety%20and%20Tox%20of%20Protasan%20UP%20chitosan022007.pdf [Accessed: 11 September 2012].
- 24.Kitozyme SA. (2011). Chitosan GRAS notice. Obtained from: http://www.accessdata.fda.gov/scripts/fcn/gras_notices/GRN000397.pdf [Accessed 11 September 2012]
- 25.Primex Ingredients ASA. GRAS claim notification: Primex chitosan, 2001 Obtained from: http://www.accessdata.fda.gov/scripts/fcn/gras_notices/grn000073.pdf [Accessed 11 September 2012].
- 26.Primex EHF. (2005). Shrimp-derived chitosan – GRAS notification, 2005. Obtained from: http://www.accessdata.fda.gov/scripts/fcn/gras_notices/grn000170.pdf [Accessed 11 September 2012].Raafat D, Sahl HG. Chitosan and its antimicrobial potential – a critical literature survey. Microb Biotech 2009, 2:186-201. [DOI] [PMC free article] [PubMed]
- 27.Atmar RL, Bernstein DI, Harro CD, Al-Ibrahim MS, Chen WH, Ferreira J, Estes MK, Graham DY, Opekun AR, Richardson C, et al. Norovirus vaccine against experimental human Norwalk Virus illness. N Engl J Med. 2011;365:2178–87. doi: 10.1056/NEJMoa1101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christensen KS, Cohen AE, Mermelstein FH, Hamilton DA, McNicol E, Babul N, Carr DB. The analgesic efficacy and safety of a novel intranasal morphine formulation (morphine plus chitosan), immediate release oral morphine, intravenous morphine, and placebo in a postsurgical dental pain model. Anesth Analg. 2008;107:2018–24. doi: 10.1213/ane.0b013e318187b952. [DOI] [PubMed] [Google Scholar]
- 29.El-Kamary SS, Pasetti MF, Mendelman PM, Frey SE, Bernstein DI, Treanor JJ, Ferreira J, Chen WH, Sublett R, Richardson C, et al. Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J Infect Dis. 2010;202:1649–58. doi: 10.1086/657087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mills KH, Cosgrove C, McNeela EA, Sexton A, Giemza R, Jabbal-Gill I, Church A, Lin W, Illum L, Podda A, et al. Protective levels of diphtheria-neutralizing antibody induced in healthy volunteers by unilateral priming-boosting intranasal immunization associated with restricted ipsilateral mucosal secretory immunoglobulin a. Infect Immun. 2003;71:726–32. doi: 10.1128/IAI.71.2.726-732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Read RC, Naylor SC, Potter CW, Bond J, Jabbal-Gill I, Fisher A, Illum L, Jennings R. Effective nasal influenza vaccine delivery using chitosan. Vaccine. 2005;23:4367–74. doi: 10.1016/j.vaccine.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 32.Roon KI, Soons PA, Uitendaal MP, de Beukelaar F, Ferrari MD. Pharmacokinetic profile of alniditan nasal spray during and outside migraine attacks. Br J Clin Pharmacol. 1999;47:285–90. doi: 10.1046/j.1365-2125.1999.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoker DG, Reber KR, Waltzman LS, Ernst C, Hamilton D, Gawarecki D, Mermelstein F, McNicol E, Wright C, Carr DB. Analgesic efficacy and safety of morphine-chitosan nasal solution in patients with moderate to severe pain following orthopedic surgery. Pain Med. 2008;9:3–12. doi: 10.1111/j.1526-4637.2007.00300.x. [DOI] [PubMed] [Google Scholar]
- 34.Brück WM, Slater JW, Carney BF. Chitin and chitosan from marine organisms. In: Kim S.-K, ed. Chitin, Chitosan, Oligosaccharides and Their Derivatives, CRC Press New York, 2011:11-23. [Google Scholar]
- 35.Knezevic-Jugovic Z, Petronijevic Z, Smelcerovic A. Chitin and chitosan from microorganisms. In: Kim S.-K, ed. Chitin, Chitosan, Oligosaccharides and Their Derivatives, CRC Press New York, 2011:25-36. [Google Scholar]
- 36.Nwe N, Furuike T, Tamura H. Chitin and chitosan from terrestrial organisms. In: Kim S.-K, ed. Chitin, Chitosan, Oligosaccharides and Their Derivatives, CRC Press New York, 2011:3-10. [Google Scholar]
- 37.Rinaudo M, Domard A. Solution properties of chitosan. In: Skjak-Bræk G, Anthonsen T, Sandford P eds. Chitin and Chitosan, Sources, Chemistry, Biochemistry, Physical Properties and Applications: Elsevier, 1989:71-86. [Google Scholar]
- 38.Roberts GAF. Chitin Chemistry: The Macmillan Press Ltd, London, 1992. [Google Scholar]
- 39.Harding S. Characterisation of chitosan-mucin complexes by sedimentation velocity analytical ultracentrifugation. In: Muzzarelli RAA, Peter MG eds. Chitin Handbook. European Chitin Society, 1997:457-466. [Google Scholar]
- 40.Morris G, Kök S, Harding S, Adams G. Polysaccharide drug delivery systems based on pectin and chitosan. Biotechnol Genet Eng Rev. 2010;27:257–84. doi: 10.1080/02648725.2010.10648153. [DOI] [PubMed] [Google Scholar]
- 41.Arca HC, Günbeyaz M, Senel S. Chitosan-based systems for the delivery of vaccine antigens. Expert Rev Vaccines. 2009;8:937–53. doi: 10.1586/erv.09.47. [DOI] [PubMed] [Google Scholar]
- 42.Baldrick P. The safety of chitosan as a pharmaceutical excipient. Regul Toxicol Pharmacol. 2010;56:290–9. doi: 10.1016/j.yrtph.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 43.Kean T, Thanou M. Biodegradation, biodistribution and toxicity of chitosan. Adv Drug Deliv Rev. 2010;62:3–11. doi: 10.1016/j.addr.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Ebihara K, Schneeman BO. Interaction of bile acids, phospholipids, cholesterol and triglyceride with dietary fibers in the small intestine of rats. J Nutr. 1989;119:1100–6. doi: 10.1093/jn/119.8.1100. [DOI] [PubMed] [Google Scholar]
- 45.Ormrod DJ, Holmes CC, Miller TE. Dietary chitosan inhibits hypercholesterolaemia and atherogenesis in the apolipoprotein E-deficient mouse model of atherosclerosis. Atherosclerosis. 1998;138:329–34. doi: 10.1016/S0021-9150(98)00045-8. [DOI] [PubMed] [Google Scholar]
- 46.Furda I. Chitin and chitosan – a special class of dietary fiber. In: Spiller GA, ed. CRC Handbook of Dietary Fiber in Human Nutrition, Third Edition, CRC Press, 2001:45–47. [Google Scholar]
- 47.Arai K, Kinumaki T, Fujitta T. Toxicity of chitosan. Bull Tokai Reg Fish Res Lab. 1968;56:89–94. [Google Scholar]
- 48.Gray HC, Hutcheson PS, Slavin RG. Is glucosamine safe in patients with seafood allergy? J Allergy Clin Immunol. 2004;114:459–60. doi: 10.1016/j.jaci.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 49.Villacis J, Rice TR, Bucci LR, El-Dahr JM, Wild L, Demerell D, Soteres D, Lehrer SB. Do shrimp-allergic individuals tolerate shrimp-derived glucosamine? Clin Exp Allergy. 2006;36:1457–61. doi: 10.1111/j.1365-2222.2006.02590.x. http:// [DOI] [PubMed] [Google Scholar]
- 50.Waibel KH, Haney B, Moore M, Whisman B, Gomez R. Safety of chitosan bandages in shellfish allergic patients. Mil Med. 2011;176:1153–6. doi: 10.7205/MILMED-D-11-00150. [DOI] [PubMed] [Google Scholar]
- 51.Newman SP, Simpson M, Fisher T, Iqbal K. Quantification of lung deposition and nasal mucociliary clearance for a nasally administered drug formulation containing chitosan. In: Dalby RN, Byron PR, Peart J, Suman JD and Farr SJ eds. Respiratory Drug Delivery IX, Virginia Commonwealth University, Virginia, 2004:617-19. [Google Scholar]
- 52.Aspden TJ, Mason JDT, Jones NS, Lowe J, Skaugrud O, Illum L. Chitosan as a nasal delivery system: the effect of chitosan solutions on in vitro and in vivo mucociliary transport rates in human turbinates and volunteers. J Pharm Sci. 1997;86:509–13. doi: 10.1021/js960182o. [DOI] [PubMed] [Google Scholar]
- 53.McNeela EA, O’Connor D, Jabbal-Gill I, Illum L, Davis SS, Pizza M, Peppoloni S, Rappuoli R, Mills KH. A mucosal vaccine against diphtheria: formulation of cross reacting material (CRM(197)) of diphtheria toxin with chitosan enhances local and systemic antibody and Th2 responses following nasal delivery. Vaccine. 2000;19:1188–98. doi: 10.1016/S0264-410X(00)00309-1. [DOI] [PubMed] [Google Scholar]
- 54.WHO (World Health Organization). The Immunological Basis for Immunization Series: Module 2 – diphtheria update 2009. Obtained from: http://whqlibdoc.who.int/publications/2009/9789241597869_eng.pdf [Accessed 4 July 2013].
- 55.Mann AJ, Noulin N, Catchpole A, Stittelaar KJ, de Waal L, Veldhuis Kroeze EJB, Hinchcliffe M, Smith A, Montomoli E, Piccirella S, et al. Intranasal H5N1 vaccination, adjuvanted with chitosan derivatives, protects ferrets against highly pathogenic avian influenza intranasal and intratracheal challenge. PLoS ONE. doi: 10.1371/journal.pone.0093761. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allen DJ, Noad R, Samuel D, Gray JJ, Roy P, Iturriza-Gómara M. Characterisation of a GII-4 norovirus variant-specific surface-exposed site involved in antibody binding. Virol J. 2009;6:150. doi: 10.1186/1743-422X-6-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lindesmith L, Moe C, Marionneau S, Ruvoen N, Jiang X, Lindblad L, Stewart P, LePendu J, Baric R. Human susceptibility and resistance to Norwalk virus infection. Nat Med. 2003;9:548–53. doi: 10.1038/nm860. [DOI] [PubMed] [Google Scholar]
- 58.Richardson C. Development of vaccines to prevent norovirus acute gastroenteritis. Presented at Phacilitate Vaccine Forum, Barcelona, Spain, June 24, 2009. [Google Scholar]
- 59.Richardson C. Product development and clinical plan for a mucosally-delivered Norwalk vaccine. Presented at 4th International Conference on Vaccines for Enteric Diseases (VED), Lisbon, Portugal, April 27, 2007. [Google Scholar]
- 60.Richardson C, Tino WT, Foubert T, Sublett R, Steadman BL, Hinchcliffe M, et al. Design and preclinical evaluation of a dry powder intranasal norovirus vaccine. Poster presented at Third International Calicivirus Conference, Cancun, Mexico, November 12, 2007. [Google Scholar]
- 61.Wilson-Welder JH, Torres MP, Kipper MJ, Mallapragada SK, Wannemuehler MJ, Narasimhan B. Vaccine adjuvants: current challenges and future approaches. J Pharm Sci. 2009;98:1278–316. doi: 10.1002/jps.21523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Illum L, Jabbal-Gill I, Hinchcliffe M, Fisher AN, Davis SS. Chitosan as a novel nasal delivery system for vaccines. Adv Drug Deliv Rev. 2001;51:81–96. doi: 10.1016/S0169-409X(01)00171-5. [DOI] [PubMed] [Google Scholar]
- 63.Zaharoff DA, Rogers CJ, Hance KW, Schlom J, Greiner JW. Chitosan solution enhances both humoral and cell-mediated immune responses to subcutaneous vaccination. Vaccine. 2007;25:2085–94. doi: 10.1016/j.vaccine.2006.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heffernan MJ, Zaharoff DA, Fallon JK, Schlom J, Greiner JW. In vivo efficacy of a chitosan/IL-12 adjuvant system for protein-based vaccines. Biomaterials. 2011;32:926–32. doi: 10.1016/j.biomaterials.2010.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]


