Abstract
Peptide-based subunit vaccines are of great interest in modern immunotherapy as they are safe, easy to produce and well defined. However, peptide antigens produce a relatively weak immune response, and thus require the use of immunostimulants (adjuvants) for optimal efficacy. Developing a safe and effective adjuvant remains a challenge for peptide-based vaccine design. Recent advances in immunology have allowed researchers to have a better understanding of the immunological implication of related diseases, which facilitates more rational design of adjuvant systems. Understanding the molecular structure of the adjuvants allows the establishment of their structure-activity relationships which is useful for the development of next-generation adjuvants. This review summarizes the current state of adjuvants development in the field of synthetic peptide-based vaccines. The structural, chemical and biological properties of adjuvants associated with their immunomodulatory effects are discussed.
Keywords: peptide vaccine, adjuvant, dendritic cell, toll-like receptor, vaccine delivery
Introduction
Vaccination has emerged as the most effective and economically viable medical discovery to improve public health. The use of vaccines has saved millions of lives globally. Certain diseases such as smallpox, polio and measles have almost been eradicated through vaccination.1 Despite the remarkable success of vaccination against some types of disease, there are still remaining several infectious diseases for which vaccines are not yet available, including malaria, group A streptococcus (GAS), and human immunodeficiency virus (HIV). Traditionally, most vaccine formulations consisted of live attenuated microorganisms or inactivated microorganisms. However, problems such as unwanted host reactions, reversion to virulence (especially in immunocompromised individuals) and difficulty in culturing the pathogenic microorganisms are often associated with the traditional vaccine approach.2 The impracticality of traditional vaccines has resulted in the emergence of subunit vaccine that contains purified antigens instead of the whole organisms. Subunit vaccines are generally safe to use as they do not possess the features of original pathogens. In the field of subunit vaccines, protein-based antigens have been investigated extensively. Nevertheless, the development of protein-based subunit vaccines has been hampered by several issues such as complexity in production, instability, and impurity.3 In addition, the use of proteins as antigens may inadvertently stimulate an autoimmune response as observed in vaccine development for GAS infection.4
Other than using the whole protein, it is feasible to identify individual epitopes (such as short peptides) within these proteins that have the capacity to stimulate protective immunity (Fig. 1). Thus, peptide-based subunit vaccines may diminish the stimulation of an autoimmune response as they contain a specific, well-defined and synthetically produced fragment of a pathogen. Additionally, peptides as antigens are of great interest for development as they are able to induce very specific cell-mediated (T cells) and humoral (antibody) immunity.5 Unfortunately, peptide-based subunit vaccines are poorly immunogenic by themselves. Thus, the inclusion of immunostimulant (adjuvant) in the vaccine formulation is necessary to induce a more potent immune response. The role of adjuvant in vaccine formulation was originally described as ‘substances used in combination with a specific antigen that produced a more robust immune response than the antigen alone’.6 Many efforts have been made toward the development of an effective adjuvant since 1970s, yet very few of them have been licensed for clinical application (alum, MF59, AS03 and AS04). In order to achieve approval for clinical use, adjuvants need to satisfy strict requirements such as: (1) stimulating a strong humoral/T-cell response; (2) govern long-term immunity; (3) being non-toxic; (4) not inducing autoimmune or allergic responses. The use of the correct adjuvant is critical for the development of synthetic peptide vaccines, because peptides undergo enzymatic degradation even more rapidly than folded protein and are unlikely to be deposited at the administration site. In contrast to protein-based vaccines, peptide antigens usually lack of T helper epitopes which need to be incorporated into the delivery system. Protein antigens can be immunogenic on their own or require a weak immunostimmulant to be immunogenic whereas peptide antigens require stronger adjuvants for effective immunization. For example, alum was found to be an effective adjuvant for protein-based vaccine candidates but was too mild to enhance immune stimulation against peptides alone.7
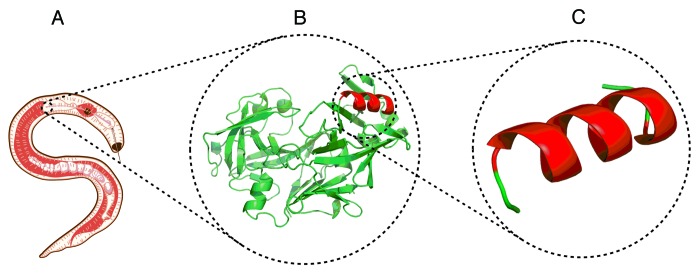
Figure 1. The evolution of vaccines: (A) traditional vaccine utilizing a whole pathogen, (B) protein-based subunit vaccine, and (C) peptide-based subunit vaccine.
Recent advances in immunology allow the structural characterization of different adjuvants and identification of receptors that are concomitant to their biological activities. This information is useful for the establishment of the structure-activity relationship of adjuvants. The identification of pharmacophores by which different adjuvants stimulate the immune system is important for the design of safer and more potent adjuvants. The focus of this review is to elucidate the structural, chemical and biological properties of selected adjuvants in the context of peptide-based vaccine development. Additionally, the key immunological concepts that govern rational vaccine design will be discussed.
Immunostimulant and the Key Regulators of Immune Responses
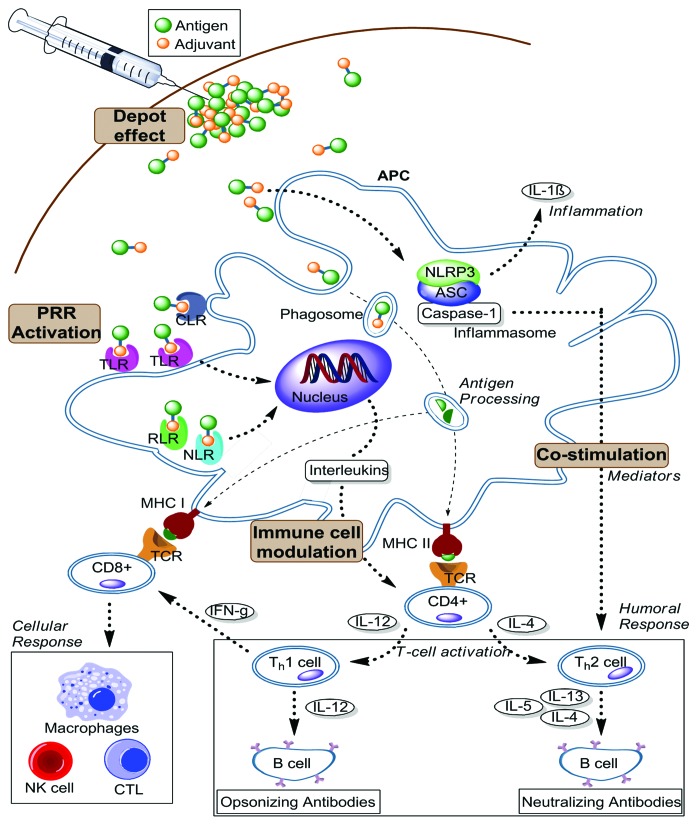
The inclusion of adjuvant along with antigens in vaccine formulation is a well-established practice in experimental immunology and represents one of the best methods for developing the next generation of peptide subunit vaccines. Adjuvants can be grouped by their ability to generate immunological ‘events’ that are necessary to induce the desired immune response (Fig. 2). Since some adjuvants act by stimulating more than one immunological pathway, alternative classification by physical and chemical properties may be more useful. Adjuvants may exhibit their immunostimulatory effects via the following mechanisms: (1) providing antigen depot; (2) activation of innate immunity through pathogen recognition receptors (PRR) engagement; (3) co-stimulation of immune cells; (4) immunomodulation, e.g., maturation of Antigen Presenting Cells (APC).
Figure 2. Schematic overview of the immunological cascade induced by adjuvants. These immunological events are essential for enhancing and directing the adaptive immune response against vaccine antigens. The responses are primarily mediated by two main types of lymphocytes, T and B cells. (APC: Antigen Presenting Cell; CTL: Cytotoxic T Lymphocyte; NK cell: Natural killer cell; PRR: Pattern recognition receptor; TLR: Toll-like receptor; RLR: retinoic acid-inducible gene I (RIG-I)-like receptor; NLR: NOD-like receptor; MHC: Major histocompatibility complex; NLRP3: NOD-like receptor family; ASC: The inflammasome adaptor; TCR: T cell receptor; CLR: C-type lectin receptors)
All of the mechanisms involve direct or indirect stimulation of APC, particularly dendritic cells (DC). DC play a critical role in bridging the innate and the adaptive immune system by non-specific internalization and processing of the antigens which are then presented to the sequence-specific T cells. The encountered antigens may constantly be taken up by DC via pinocytosis and/or phagocytosis while their PRR act as a “detector” for infectious agents. Upon stimulation of PRR, naïve DC release various soluble mediators, such as inflammatory cytokines and type 1 interferon (IFN) as part of the innate immune response. The activated DC initiates an adaptive immune response by processing the antigens and presenting them to naïve CD4+ T cells. At the same time, DC upregulate MHC class II and co-stimulatory molecules, inducing interactions between DC and CD4+ T cells. This immunological cascade promotes optimal CD4+ stimulation. However, this immunological cascade is insufficient for CD8+ T cell priming, which is essential for vaccine efficacy against cancer or intracellular pathogens. Cross-presentation is required for dendritic cells to present MHC class I molecule-antigenic peptide complexes to CD8+ T cells to trigger a CTL response. The detailed immunological activities required to generate this response have been extensively reviewed by Joffre and colleagues.8
Among the PRR, Toll-Like receptors (TLR) are the most studied receptors and are widely recognized as the main interest of modern adjuvant targeting. TLR are type 1 transmembrane proteins with an extracellular domain of interspersed leucine-rich repeat (LRR) motifs that are involved in recognition of pathogen associated molecular patterns (PAMP), such as lipopolysaccharide (LPS).9 Engagement of PAMP with TLR will lead to the activation of mitogen-activated protein kinase pathway and NFκβ which results in regulation of pro-inflammatory cytokines (interleukin-1β and TNF-α, and chemokines). TLR activation also plays a major role in initiating adaptive immune response, for instance by directing the immune system toward Th1- and Th2-biased responses.10 Th1 responses are mediated by the secretion of IFNγ and are thought to be responsible for killing intracellular pathogens. In contrast, Th2 responses are characterized by the secretion of IL-4, IL-5, IL-6, and IL-10, which resulted in the induction of antibodies production. It was shown that vaccine adjuvants that contained TLR ligands were able to induce T cell responses of higher avidity. This prompted extensive research to utilize the TLR ligand as a potential adjuvant. It is believed that adjuvants based on the TLR ligand should be co-delivered with the desire antigen to target the same phagosome cargo at the APC for optimal antigen presentation and subsequent stimulation of antigen-specific T cell responses.11 Thus the ability of TLR to link innate and adaptive immune responses represents a promising approach for the design of new vaccines.
Various adjuvants execute their effect as a delivery platform for the vaccine antigens. Particulate-based adjuvants such as alum, liposomes and emulsion have been observed to enhance antigen uptake by prolongation of the exposure of antigen to the DC.12 Two subpopulations of DC have been identified: tissue-resident (immature) DC and migratory (mature) DC. At the administration site, antigens are presented to resident DC located in non-lymphoid tissues close to the mucosal surfaces. Upon antigen encounter, tissue resident DC undergo a maturation process which initiates their migration to the lymph nodes, and thus are subsequently termed migratory DC.13 Naïve T and B cells do not circulated at the non-lymphoid areas, including most injection sites in the body. They are usually found at secondary lymphoid organs, including the lymph nodes. Therefore, antigens need to reach the lymph nodes via migratory DC in order to be presented to the specific T cells. These types of adjuvants provide a depot formation that trap antigens at injection site, providing a gradual release of antigens to be acquired by migratory DC. Thus, these adjuvants influence the fate of vaccine antigen in time, place and concentration by sustaining the activation of antigen-specific CD4+ T cells and promote uptake of the antigen at injection site. In addition, particulate adjuvants have the ability to bind antigens and form multi-molecular aggregates. The aggregation complex usually exhibits similar dimensions to pathogens, which encourages their uptake into APC via phagocytosis.14 Hence these adjuvants may contribute to the maturation of APC.
Other adjuvants act as immunopotentiators by having a more direct impact to provoke an immune response. To act this way, adjuvants required interaction with co-stimulatory molecules on APC that contributed to the priming of T helper cells (CD4+).15 The stimulation of APC provided information to T cells that the presented antigens are proper subjects to prompt an immune response. This facilitates the recognition of antigen for further B cell proliferation and antibody production. The absence of this induction may lead to the immunological tolerance or anergy. Under physiological conditions, proteins fold into a single stable conformation while short peptides derived from a protein are usually unable to preserve their native conformation. However, retaining this native conformation is essential for the activation of B cell receptors to elicit a humoral immune response.16 Several strategies are available to induce the desired secondary structure of peptide epitopes, including incorporation of additional flanking peptide and hydrophobic moieties such as lipopeptide or polymers.17-19 Unlike humoral immunity, there is no requirement for the peptide epitope to adopt a specific conformation to stimulate a CTL response. However, APC must process the antigenic peptides to produce peptides of a defined length.20 The induction of either CTL or antibody immunity requires additional stimulation of T helper cells. This is typically achieved through the conjugation of the peptide epitope to a carrier protein, such as tetanus toxoid that includes a T-helper epitopes. Alternatively, a universal t-helper such as PADRE (AKFVAAWTLKAAA) can be incorporated into the delivery system.21
Adjuvants play a critical role in determining the quality and magnitude of immune response against the antigens. Different pathogens require different types of immune response to target the disease. Thus, the selection of the appropriate type of adjuvant for peptide-based vaccines is important to induce the desired antigen-specific immune response for effective vaccination.
Aluminum Salt Adjuvant
Aluminum salts are the most extensively used adjuvants in vaccine formulation and are the only licensed adjuvant for routine human vaccination by FDA. These salts, including aluminum hydroxide [Al(OH)3], aluminum phosphate (AlPO4) and alum precipitated materials are often referred as “alum” in the literature. Presently, there are many marketable vaccines containing alum such as diphteria, tetanus and hepatitis A and B vaccines. The adjuvanting effect of Alum was first discovered by Glenny et al. An alum-precipitated vaccine of diphtheria toxoid provided greater immune protection against challenge than a solution of diphtheria toxoid alone.22 The adsorption between alum compounds and antigen in vaccine formulation can be predominantly achieved via electrostatic attraction and ligand exchange. It has been hypothesized that antigens need to be adsorbed to the aluminum compounds for alum to act as an effective adjuvant. However, recent findings indicated that antigen adsorption to alum-containing adjuvant may not be necessary to enhance the immune response.23 Since alum-based adjuvants are composed of very small primary particles they are easily aggregated into a functioning unit in the vaccine formulation and contribute to the uniform distribution of antigen throughout the aluminum-containing vaccine.24 The resulting aggregate features irregular and porous shapes ranging from 1 to 20 µm in diameter. Some in vitro studies reported that DC is more efficient at internalizing alum-adsorbed antigen through phagocytosis than macropinocitosis in a size-dependent manner.25 The aggregation behavior of alum allows the antigen to be presented in a multivalent system which enhanced their uptake by DC and improved antigen presentation to the adaptive immune system.26
Alum is generally known to provoke a strong Th2 response. It has been shown that administration of ovalbumin (OVA) peptide-alum led to the Th2 effector response that generates production of IL-4, IL-5, and IL-10.27 However, the underlying mechanism of action at molecular level is still unclear and remains a subject of ongoing investigation. One of the proposed mechanisms for the stimulation of an immune response by alum is its ability to present antigen efficiently to APC. Alum-based adjuvants may provide a depot formation at the injection site, allowing the slow release of the antigen over time. This event may lead to a longer exposure of the antigen to the APC and lymphocytes. It is proposed that antigens are retained at the injection site due to the adsorption force with aluminum salts or by being trapped in the porous spaces within the aggregation particles.26 Other proposed mechanisms for the adjuvanting activity of alum included the activation of complement cascade,28 generation of granulomas at the injection site, recruitment of eosinophils and neutrophils, and induction of IL-4 secreting cells in the spleen that are responsible for optimal B cell priming. The immunostimulatory effect of alum has been associated with the activation of NLRP3 inflammasome pathway. It was demonstrated that DT/TT or OVA antigen with alum produced significantly lower antibody titers in NLRP3 deficient mice than in wild-type mice.29 Additional in vitro studies support this data; Th2 cell priming and antibody response to OVA or human serum albumin (HSA) co-delivered with alum were also reduced in NLRP3 deficient mice.30 Furthermore, a link between the innate inflammasome pathway and the adaptive humoral response has been found. However, other in vitro data found no difference in antibody response between NLRP3-deficient mice and wild type animals when they were injected with HSA with alum.31 This study suggested that the NLRP3 inflammasome pathway is dispensable for alum adjuvanticity. These conflicting results may have arisen due to the use of different protocols to conduct the experiment; particularly immunization route and the method of measuring antibody titers. Later findings showed that alum provoked the secretion of uric acid, which may contribute to the activation of NLRP3.27 Uric acid concentration increased locally after the administration of antigen mixed with alum in the peritoneum. In response to the uric acid secretion, inflammatory monocytes congregated to the injection site, recruited the antigen and process them for further T cell priming. Recently, alum was found to induce cell death that resulted in subsequent release of host cell DNA, thus inducing innate immunity.32
Alum has a proven record of safety. However, the use of alum as an adjuvant has several limitations. Alum failed to provide satisfactory immune response when used with certain vaccines such as influenza and typhoid fever.33,34 Notably, alum adjuvanticity is biased toward Th2 immunity and consequently ineffective to be used for several life-threatening infections such as cancer and tuberculosis that required cytotoxic T cell response. It has been reported that alum-based adjuvants are incompatible with small peptide antigens. This is because alum may cause the peptide antigens to be proteolytically degraded more efficiently.35 As a result, the peptide antigen may lose its ability to stimulate a protective immune response. Partial denaturation of the peptide antigen due to adsorption with alum may also contribute to this incompatibility. Some studies demonstrated that alum did not induce significant antibody titers when conjugated with peptides antigens derived from Malaria parasite and herpes simplex virus proteins.36,37 Therefore a novel adjuvant needs to be developed to enable the production of vaccines that cannot currently be adjuvanted by alum-based vaccination strategies.
Emulsion
There are two types of emulsion used in vaccine formulation; oil-in-water and water-in-oil systems. Most of the water-in-oil emulsion adjuvants work based on delivery system that stimulates the elongation of antigen presentation. The high content of non-degradable oils in water-in-oil formulations is thought to establish an antigen depot at the injection site. The binding force occurred between the antigen and droplet surface enable them to have a longer retention time at the injection site. Moreover, water-in-oil adjuvants are able to protect peptides against enzymatic degradation as emulsion sequesters peptides from peptidase in the body fluids.38 Complete Freund’s adjuvant (CFA) is water-in-oil emulsion that additionally contains heat-killed mycobacteria. CFA has been considered to be the “gold standard” adjuvant in vaccine research as it is more effective than alum and other commercially available adjuvants. An extensive body of research has used CFA as an adjuvant in peptide-based subunit vaccine development. For example, initial study by Frazer and coworkers in the development of therapeutic HPV peptide-based vaccines has utilized CFA to induce immune response against a tumor-derived peptide.39 Nevertheless, the usage of CFA in vaccine formulation is associated with undesirable side effects such as weight loss, leucocytosis, and granulomatous peritonitis in mice.40 The side-effects were still observed even when the mice were injected with a low dose of CFA. The undesirable toxicity of CFA led to the development of incomplete Freund’s adjuvant (IFA), which excludes the heat-killed mycobacteria. IFA has an improved safety profile but was found to be a relatively poor adjuvant. Modified versions of IFA have been developed: e.g., Montanide ISA 51 (containing mannide monooleate) and Montanide ISA 720 (containing squalene). It has been demonstrated that the use of Montanide ISA 51 in combination with a cocktail of HIV-derived peptide antigen (contain mixture of B and T cell peptide epitopes) enhanced the immunogenicity of the antigenic peptide.41 However, the vaccine candidate failed to meet safety requirement in clinical trials. Healthy adults that developed higher antibody response were also more likely to produce severe systemic or local adverse reactions. When Montanide ISA 720 was delivered with a malaria vaccine candidate (modified hepatitis B virus core particle (HBc) bearing peptide epitopes derived from circumsporozoite protein (CS) of Plasmodium falciparum), it was found to induce a higher humoral response than alum-derived adjuvant, Alhydrogel.42 A phase 1 clinical trial of Montanide ISA 720 formulated with peptide antigen showed its ability to stimulate a potent immune response, but a significant number of local reactions such as granuloma, tenderness and erythema were also observed.43 Recently, a new water-in-oil adjuvant formulation, dubbed “NH2” has been developed and is comprised of mineral oil and sorbitan monooleate. NH2 was extensively used as an adjuvant for peptide-based cancer vaccination and outperformed Montanide ISA 51 in the induction of a cellular immune response in mice.44 The efficacy of the peptide-based vaccine adjuvanted by NH2 was confirmed in phase-1 clinical trials with advanced cancer patients.
Oil-in-water emulsions are generally considered to be safer than water-in-oil emulsion. MF59 is a representative of oil-in-water type of emulsions that are composed of squalene. MF59 is licensed in Europe as the adjuvant component of influenza vaccines.45 In contrast to water-in-oil emulsions, MF59 did not provide a long-lived depot at the injection site. Immunofluorescence analysis showed that only 10% of radiolabeled squalene was present at the injection site 6 h after injection.46 It is proposed that the key element for the adjuvanticity of MF59 is the induction of chemokine secretion at the injection site, resulting in antigen uptake by monocytes.47 This stimulated the differentiation of monocytes into DC, facilitating their migration to the local lymph nodes where the adaptive immune response was induced against the vaccine antigen. MF59 was shown to induce protective immunity for Alzheimer disease when delivered with full-length amyloid-β peptide [Abeta(42)].48 The resultant anti-Abeta42 antibody neutralized the cytotoxicity of Abeta42, and higher antibody titers were produced with multiple immunizations. Another oil-in-water emulsion-based adjuvant is AS03, which composed of α-tocopherol, squalene and polysorbate 80. This adjuvant was shown to trigger an equivalent immune response at a 4-fold lower dose than non-adjuvanted antigen.49 It was reported that AS03 exhibit their adjuvanticity by enhancing antigen migration to lymph node and activating the innate immune system.50 The immunostimulatory activity of AS03 is associated with the presence of α-tocopherol in their composition. α-Tocopherol is a form of Vitamin E that is known to have immunomodulating effects, including counteracting age-related impairment of naïve T cells and neutrophils.51 Both the MF59 and AS03 have been extensively used and licensed in Europe as an adjuvant for protein-based subunit vaccine against pandemic influenza. Unfortunately, AS03 has not been studied as an adjuvant for synthetic peptide-based vaccines; even though, peptide epitope derived from influenza protein such as NP373/NP458 (fragment from nucleoprotein),52 synthetic M2e peptide53 and influenza M1(58-66) peptide54 were recently discovered.
Polymeric Particles
Polymeric nanoparticle- and microparticle-based adjuvant systems have been widely explored in vaccine formulation.3 The most common polymers are polylactic acid (PLA), chitosan, poly (lactide-co-glycolide) acid (PLGA), polypeptides, polysterene, polyethylene glycol and their copolymers. The copolymers can serve as antigen reservoirs, allowing a longer release of the antigen over time. The antigen is usually entrapped or adsorbed on the surface of the polymeric particles to create a vaccine formulation. Among them, PLGA is one of the most widely investigated adjuvant for controlled and effective single-dose delivery of vaccine antigens, including synthetic peptides. It has been observed that PLGA polyesters degrade to produce lactic and glycolic acids in vivo, allowing the gradual and continuous release of encapsulated immunogens that promote constant stimulation of APC, thus eliminate the need of multiple immunizations.55 In addition, PLGA are acquired by DC in culture via phagocytosis which allows further biological processing of the carried antigen.56 A study conducted by Sharp and coworkers showed that PLGA enhanced IL-1β secretion which consequently triggered caspase-1 activation.14 This event led to the activation of NALP3 inflammasome that contributes to the induction of adaptive immune responses. Although the presence of a TLR agonist was required to induce the release of IL-1β in vitro, injection of PLGA particles in the absence of a TLR agonist was still able to promote IL-1β secretion. Schwendeman and coworkers have employed a novel formulation of PLGA with magnesium carbonate to improve the delivery of hCG peptide antigens for contraceptive vaccine.55 Encapsulation of hCG peptide antigen to this new formulation was found to induce a strong immune response after a single-dose immunization in rabbit. PLGA also showed promising result as an adjuvant for peptide-based cancer vaccination. A tumor antigenic peptide encapsulated with PLGA nanoparticles was found to stimulate a strong CTL response in vivo, even at the lower dose of antigen than the IFA-adjuvanted formulation.57 The use of PLGA microspheres as an adjuvant has reached clinical evaluation for a synthetic HIV peptide vaccine that is employed as an oral immunization strategy.58 However, in the phase I clinical study PLGA was unable to induce a substantial immune response when administered orally.
Chitosan (and its derivatives) (Fig. 3) is another polymer that attracts great interest in its potential application as a vaccine adjuvant. Chitosan can be obtained via alkaline deacetylation of chitin, a linear β-(1–4)-linked copolymer of D-glucosamine and N-acetyl-D-glucosamine. The chemical versatility of chitosan is dependent on the reactivity of its amine groups. Chitosan as an adjuvant has been extensively investigated as an adjuvant with the aim of developing an anti-GnRH peptide vaccine. Conventional strategies for anti-GnRH vaccination are predominantly based on bonding of GnRH as a hapten to a highly immunogenic carrier protein such as bovine albumin or tetanus toxin. However, the immune responses were primarily directed against the carrier protein instead of the desired antigen. Thus, a peptide-based vaccine was designed to overcome this problem. Formulations of anti-GnRH peptide antigen with different types of chitosan induced high levels of IgG isotype 1 production in rats compared with immunization with CFA.59 Chitosan with the highest acetylation degree was able to induce an immune response mediated by IgG isotype 2a. Chitosan has also been a popular choice as a delivery platform for mucosal vaccination due to its mucoadhesive properties. Mannosylation of chitosan was reported to further augment its efficacy for mucosal vaccine delivery.60 However, chitosan has poor solubility that may impair its effectiveness in peptide-based immunotherapy. To increase its water-solubility, various chitosan derivatives such as N-trimethylated chitosan (TMC), mono-n-carboxylated chitosan (MCC) and glycated chitosan (GC) were developed as potential adjuvants.61
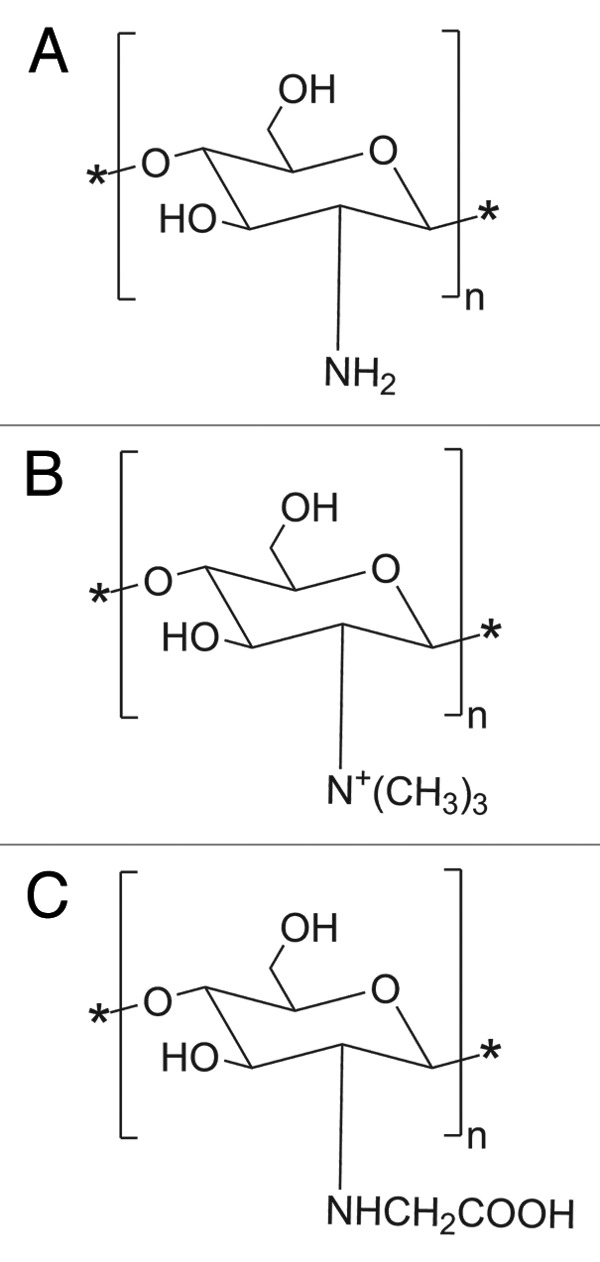
Figure 3. Chemical structure of (a) Chitosan, (b) N-trimethylated chitosan (TMC) and (c) mono-N-carboxymethyl chitosan (MCC).
One of the latest polymer-adjuvant innovations is the development of dendritic polymers and branched polymers with star-like topologies. The dendrimers are composed of repeating units of polymers stemming from a central core. The antigenic molecules can be conjugated at the end of the branching polymers that allows the presentation of multiple epitopes.62 One example of this approach is the development of a dendritic poly (t-butyl)acrylate (PtBA) polymer that acts as an adjuvant for a GAS vaccine (Fig. 4).18,63,64 The peptide epitope, J14 (GAS M protein derived peptide) conjugated to this polymer readily self-assembles in an aqueous environment to form nanoparticles. The PtBA-antigenic-peptide conjugates induced high antibody titers in mice. The application of PtBA-based dendritic polymers is not only promising for the development of prophylactic vaccines but may also be effective for therapeutic vaccination. The conjugation of a peptide antigen derived from human papillomavirus (HPV) E7 protein to this star polymer system has also been investigated to develop a vaccine candidate against HPV-related cancers.65 As a result, this vaccine candidate was found to reduce tumor growth and eradicate E7-expressing TC-1 tumors in mice after a single-dose immunization. Interestingly, when acrylate polymer was administered as physical mixture with peptide antigens it failed to induce any immune response.18
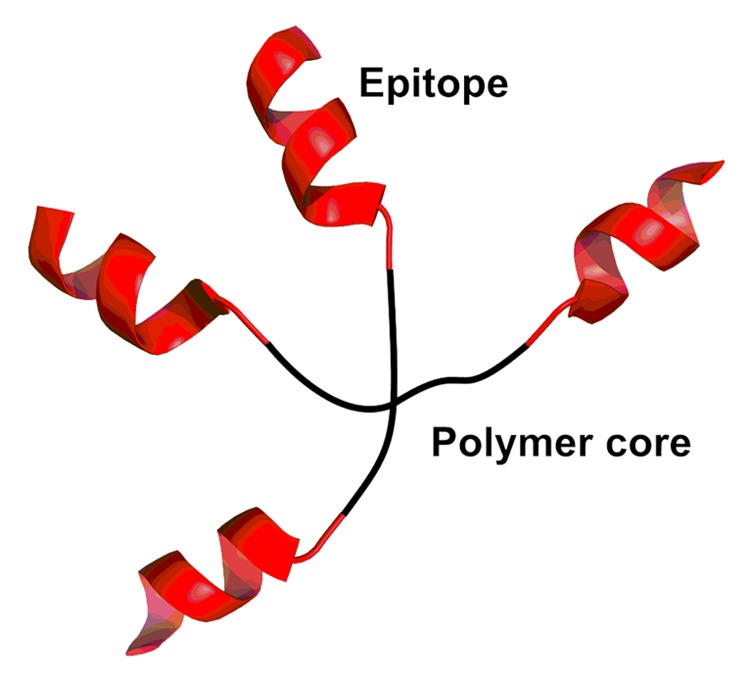
Figure 4. A schematic representation of the four-arm star polymer.
Parameters such as the size and surface charge of the polymeric particles play a crucial role in their interactions with APCs. Kanchan and Panda demonstrated that PLA-HBsAg nanoparticles (200–600 nm) were taken up by macrophages more efficiently than their microparticle (2–8 μm) counterparts.66 Additionally, nanoparticles were found to promote higher levels of IFNγ secretion, upregulation of MHC class I molecules, and were biased toward a Th1 immune response. PLGA nanoparticles (300 nm) incorporating a hepatitis B virus peptide antigen produced a strong cell-mediated immune response with a predominant IFNγ profile.67 Conversely, immunization with microparticles was associated with IL-4 production, upregulation of MHC class II molecules and favored a Th2-biased immune response. In general, charged polymeric particles present antigens more effectively through electrostatic interactions with antigens. A formulation of HIV-1 peptide antigen adsorbed on the surface of anionic PLG microparticles significantly enhanced the immune response.68 The availability of a variety of polymeric structures with a documented safety profile and flexible chemical modification options makes this system particularly attractive for peptide-based vaccine delivery system.
Liposomes
Liposomes, lipid bilayers composed of natural or synthetic phospholipids that surround an aqueous core, have been studied extensively for vaccine delivery. In vaccine formulation, the antigenic peptide determinant can be encapsulated within the aqueous core, integrated within the lipid bilayer, or attached to the outer surface of the liposomes (Fig. 5). Liposomes can efficiently protect the immunogenic peptide from enzymatic degradation, are easily altered to obtain optimum presentation of the antigen, and can be efficiently taken up by APCs due to their particulate nature.69 Liposome-antigen complexes induced a significantly higher cellular immune response compared with the use of alum-antigen complexes following subcutaneous vaccination in mice.70 Liposomal formulation with peptide-based hepatitis C vaccine (HCV) induced a strong CTL response.71 Peptide based antigens are often favorably adsorbed to the surface of liposome in the formulation. CTL peptide epitopes derived from severe acute respiratory syndrome (SARS) coronavirus (SARS-CoV) adsorbed to the liposomal surface induced effective CTL immune responses in mice.72 Alternatively, the peptide antigen could be conjugated to lipid moieties and anchored to the liposome.73 The lipid moieties themselves may also be TLR 2 or TLR 4 agonists and may contribute to the enhancement of the immunogenicity of this liposome-based adjuvant. Although the mechanism of action of liposomes is not completely understood, increased retention time of antigen at the injection site, and enhancing antigen uptake by macrophages are the primary explanations for their adjuvanting role. Cationic liposomes are believed to provide an ideal platform for antigen delivery because they enhance antigen uptake and presentation to the adaptive immune system.74 This may be due to the present of abundant anionic group on the cell membrane of APCs, which allows efficient chemical attraction with the cationic liposomes.
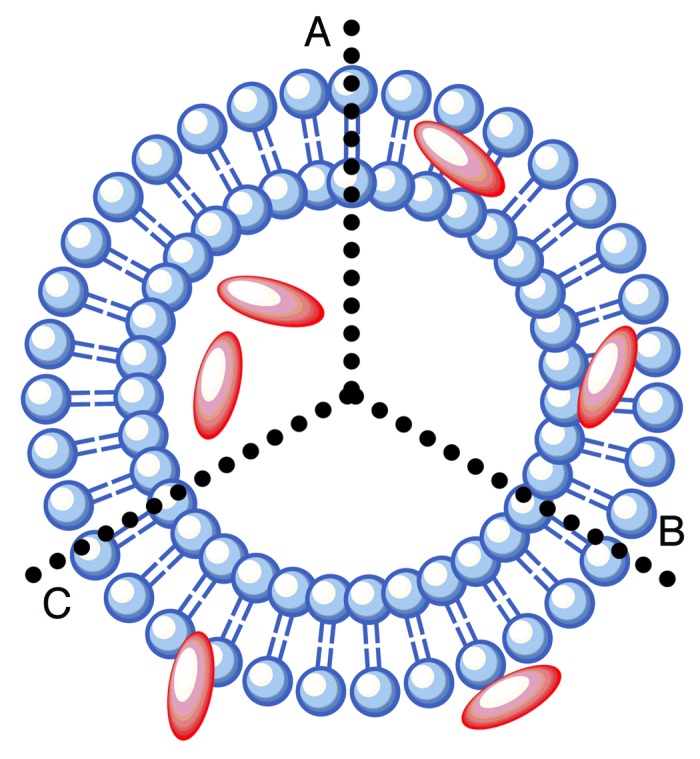
Figure 5. The mode by which antigens are incorporated into liposomes is dependent on their chemical nature. (A) Hydrophilic antigens can be entrapped within the aqueous core of liposomes; (B) hydrophobic antigens can be conjugated at the liposome surface; (C) ampiphilic antigens can be integrated within the phospholipid bilayer.
In addition to charge, liposome composition and size also contribute to the adjuvanticity of liposomes. Inclusion of a fusogenic lipid such as 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) within the lipid bilayers induced a superior IgG2a response against OVA peptide, which was indicative of a generating a Th1-biased response.75 Since liposome uptake occurs via the endocytosis route, size plays a crucial role in determining the immune response. Jamie and colleagues found that larger liposomes (~980 nm) into which the influenza vaccine was incorporated induced a high level of IFN-γ and IgG2a secretion, indicative of a Th1-biased response. In contrast, smaller liposomes (~250 nm) enhanced the Th2-biased response. Thus, larger liposome complexes conferred greater protection against influenza virus challenge in ferrets.76
Recent advances in liposome formulation include the incorporation TLR agonist moieties.69 Inclusion of MPL in cationic dimethyl dioctadecyl ammonium (DDA) liposomes stimulated higher levels of proinflammatory cytokines and chemokines than alum.77 Furthermore, DDA liposomes formulated with monomycoloyl glycerol (MMG, an apolar lipid derived from the mycobacterial cell wall) were also studied. These complexes were shown to induce a cellular immune response in TLR2/4 knockout mice.78 This suggest that the these particular TLRs do not play a role in the immunostimulatory effect of DDA liposome:MMG complexes. Another type of liposomal adjuvant is the DiC14-amidine liposomes. DiC14-amidine cationic liposomes were recently shown to promote Th1 responses when mixed with antigen. The presence of DiC14-amidine in the liposome allows them to stimulate the dendritic cells via TLR4 receptors, which resulted in the activation of MyD88 intracellular pathway.79 Additionally, mannose receptor has been exploited to target APC oriented delivery system. Thomann and coworkers incorporated a mannosylated ligand to the liposome-TLR ligand construct to target the antigen presenting cells.80 As a result, the mannose-targeted liposomes co-delivered with ErbB2 (protein expressed by variety of tumor cell lines) CTL peptide epitopes displayed high therapeutic efficiency against tumor.80 Additionally, cell-penetrating peptides such as trans-activating transcriptional activator (TAT) peptide provide promising inclusion in the liposome system to enhance its immunogenicity as observed by Torchilin.81
These examples showed that liposome-based systems are promising as effective vaccine adjuvants. The versatility of liposomes in their availability for conjugation with different types of antigen or other immunostimulants offer unique advantages in peptide-based vaccine development.
Virus Like-Particles (VLP) and Virosomes
Virus-like particles (VLP) are composed of several recombinant viral structural proteins that are self-assembled to mimic the conformation of native viruses. VLP are theoretically safe as they lack viral genetic material. VLP have the ideal size (20–100 nm in diameter) to be efficiently taken up by key immune cells (e.g., DC or macrophages). VLPs also stimulated an efficient CTL response without the need of carrying any genetic information.82 This is because recombinant VLP may gain access to the cytosol, which allows them to be internalized via endogenous processing pathways within APC, thus activating antigen-specific CD8+ CTL through an event called cross-presentation. This is one of the key factors that make VLP preferable for use as a vector for antigen to stimulate the desired immune response. The particulate nature of VLP allows them to be taken up by APC to provide a long lasting CTL response. Studies of conformation behavior showed that both hepatitis B virus (HBV) core particles and parvovirus VLP exhibit an ordered and repetitive epitope presentation that is useful to overcome B cell tolerance.83 The antigenic peptides can be incorporated with VLP either by recombination (genetically fused the encoded gene to the formed VLP) or chemical coupling.84 For example, Pejadar-Gaddy and colleagues constructed bovine papillomavirus (BPV) VLP that were chemically conjugated with a synthetic derivative of human mucin-1 (MUC1) peptide.85 The subcutaneous administration of this VLP-based vaccine in MUC1 transgenic mice triggered a robust activation of DC and led to the presentation of antigen to both MHC class I and II. This study validated the capabilities of the VLP adjuvant in inducing humoral and cellular immune response. Vaccines-based on VLPs that are currently approved for commercial use are Gardasil and Cervarix. These vaccines are used to provide protection against cervical cancer caused by human papilloma virus (HPV).86,87
Virosomes are composed of reconstituted viral envelopes in the absence of a viral genome. Unlike VLPs, virosomes have liposomal components that have viral envelope protein attached in their lipid membrane (Fig. 6). Virosomes were constructed using a short-chain phospholipid solubilization and reconstitution method.88 Antigens were integrated with the virosome by several methods: (1) direct fusion into the membrane; (2) adsorption to the virosomal surface; (3) chemical conjugation to the lipids; and (4) encapsulation within the vesicle. The majority of virosomes that are currently under investigation are based on the reconstitution of influenza virus envelopes, which are called immunopotentiating reconstituted influenza virosomes (IRIV). IRIV contain influenza virus-derived proteins hemagglutanin (HA) and neuraminidase (NA) intercalated in the surface of the phospholipid bilayer. HA is one of the key features of the IRIV system because they can mediate membrane fusion activity.89 HA binds to monosaccharide sialic acid, which is present on the surface of the APC. This interaction allows IRIV attachment to the APC, which leads to further uptake through endocytosis. The cell begins to digest the content of endosome upon acidification of HA, transforming into endolysosome. NA possesses enzymatic activity that enables the virosome to escape from endolysosome. This process allows the release of the antigen into the cytoplasm. Thus, it is believed that virosomes can induce a strong MHC I-restricted CTL response through the delivery of antigen to the APC cytosol, enabling cross-presentation.90 This phenomenon provides useful insight toward the development of virosome as an adjuvant for cancer immunotherapy.91 In addition, a fraction of the antigen is still degraded within the endosome, allowing them to be presented to MHC class II molecules and resulting in stimulation of the CD4+ immune response.90 Moreover, the particulate forms of IRIV enable them to present the antigen to the immune system in a repetitive array to further enhance the immune response.92 The potential use of virosomes as an adjuvant has been investigated for peptide based vaccines against numerous diseases including malaria,93 hepatitis C,94 and melanoma.95 So far, virosome formulations with synthetic peptides derived from Plasmodium falciparum have undergone stage I clinical trials for anti-malaria vaccination.96 These virosomal malaria vaccines were found to be safe and elicit an appropriate immune response against malaria.
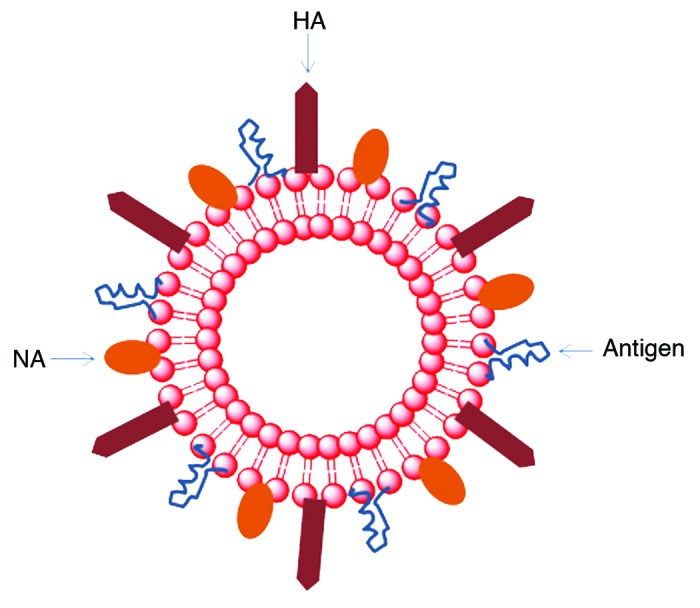
Figure 6. Virosomes are made up of a phospholipid bilayer which is similar to a liposome. This structure provides a platform to hold influenza virus surface protein hemagglutinin (HA) and neuraminidase (NA). Antigens are incorporated into the virosome system.
Iscom, Iscomatrix, and Saponins
Immunostimulatory complex (ISCOM) is antigen-containing cage like structure (~40 nm) that is composed of cholesterol, phospholipids and saponin. In ISCOM vaccines, the antigen is incorporated into the structure during the construction process. ISCOMATRIX is a proprietary preformed adjuvant based on ISCOM that is mixed with antigen prior to delivery.97 ISCOM/ISCOMATRIX adjuvanticity is derived from APC recruitment and activation, prolonged antigen presentation to drain lymph nodes, and inductions of CD8+ cross presentation.98 A study conducted by Ebert and coworkers showed that the synthetic peptide antigen derived from NYESO-1 formulated with ISCOMATRIX was successfully taken up by DC and cross-presented to MHC I cells, producing a potent T cell immunity.99
Quillaja (and its derivatives) are the most common saponins used in ISCOM systems.100 Quillaja saponins possess a triterpene unit with an aldehyde moiety and two oligosaccharide chains (Fig. 7A).101,102 One of the oligosaccharides is acylated by two repeating units of lipophilic aliphatic acids linked by an ether bond to a fucose. The particulate nature of ISCOM allows them to be efficiently taken up by APCs via endocytosis pathways. The oligosaccharide chain of saponins can mediate their uptake by targeting DEC-205 (a macrophage mannose receptor family of c-type lectin endocytic receptors) on the APCs surface that may result in higher uptake and more competent presentation of antigens to T cells.103 The aldehyde group plays a critical role in stimulating a Th1-biased immune response by forming an imine (Schiff base) with amino groups on T cell receptors to provide a co-stimulatory signal for T cell activation.104 The lipophilic acyl side-chain appears to enhance the ability of quillaja saponins to stimulate CTL production, yet also contribute to the quillaja saponins’ toxicity and instability under physiological conditions.101,104 Although deacylated saponins (Fig. 7B) were proven to be less toxic and capable of stimulating a Th2-biased immune response, they failed to induce Th1 or CTL immune responses.105 Thus, degradation of quillaja saponins changed the type of stimulated immune response.
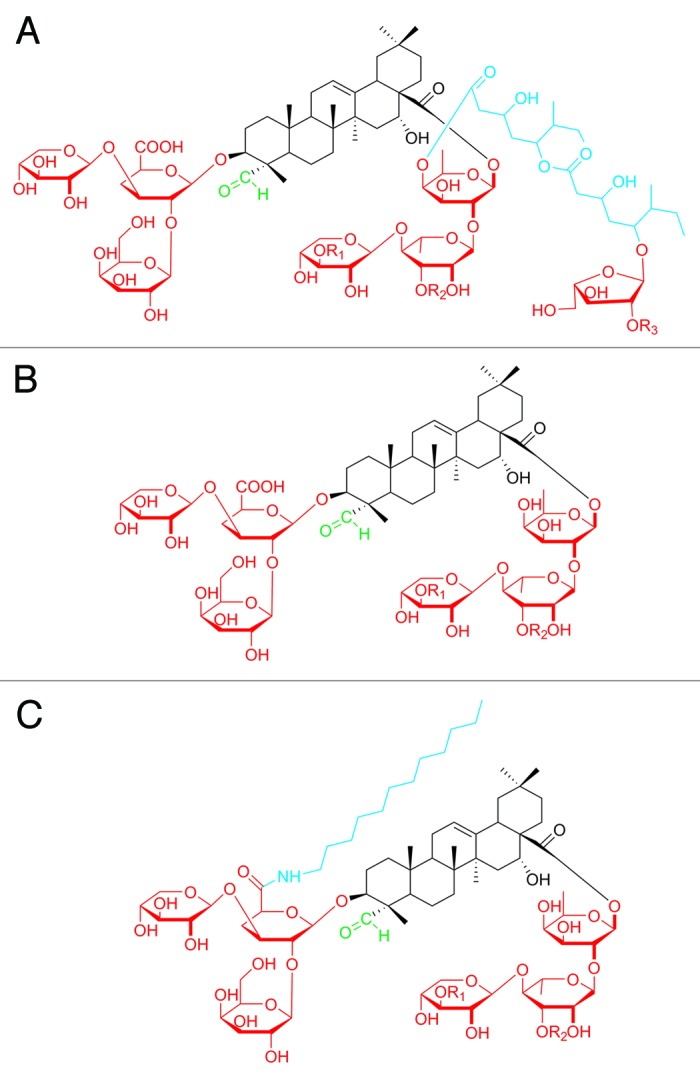
Figure 7. Chemical structure of (A) quillaja saponin, (B) deacylated saponins, and (C) GPI-0100. The lipophilic chain (blue) was mainly responsible for the delivery of antigen, the carbohydrate residues (red) enhanced targeting to the immune cells and the aldehyde group (green) was important for co-stimulation activity.
To overcome the limitation of quillaja saponins, a derivative called GPI-0100, with a lipophilic moiety (dodecylamide) bound to the glucuronic acid residue, has been constructed (Fig. 7C). GPI-0100 was stable, safer than unmodified quillaja saponins, and capable of stimulating a Th1-biased immune response and CTL production.104 It is believed that the stability of GPI-0100 results from resistance of the dodecylamide moiety to hydrolysis.104 It was reported that a formulation of peptide-based cancer antigen (MUC1 peptide) with GPI-0100 was superior to quillaja saponins in producing a T cell response, and IgM and IgG antibodies.106
Self-Assembling Peptides
Biomaterial constructed from self-assembled peptides received great attention as potential vaccine adjuvants. The constructed biomaterials could either be in the form of tubular, fibrillar or spherical nanostructure by utilizing the known aggregation properties (e.g., helical, β-sheet, etc.) of the selected peptides. Self-assembling peptides provided several advantages including multivalency, biological compatibility, multifunctionality, synthetic definition, molecular specificity and allow control over the nanoscale positioning of antigens.107
Burkhard and colleagues have used the pentameric coiled coil oligomerization domain derived from cartilage oligomeric matrix protein (COMP)108 and a de novo minimal trimeric coiled-coil oligomerization domain109 to construct self-assembling peptides as a multiple antigen-display platform. Burkhard’s group have exploited this system to present a B cell peptide epitope (DPPPPNPN)2D derived from malaria parasite Plasmodium berghei circumsporozoite protein.110 It was demonstrated that the vaccine formulation successfully induced high antibody titers and conferred durable immune protection. Most of the mice were protected against the parasite challenge for up to 6 mo. This self-assembled peptide-based adjuvant conjugated to (DPPPPNPN)2D is currently under pre-clinical evaluation in the USA. Additionally, the same research group has also used this system against the severe acute respiratory syndrome (SARS) virus.111 The antigen tested was HCR1, an α-helical coiled-coil B cell epitope derived from the SARS S protein. The antigen-peptide conjugate self-assembled to form 25 nm nanoparticles and successfully displayed multiple copies of the HRC1 epitope on their surface. Additionally, circular dichroism analysis demonstrated that this system was able to maintain the α-helical conformation of the epitope. The conjugate elicited a specific immune response, with antisera exhibiting neutralization activity against SARS virus in an in vitro infection inhibition assay.111
Another self-assembly peptide that is currently under intensive investigation is Q11 (QQKFQFQFEQQ). Q11 is an established fibrilizing peptide that spontaneously self-assembles to form a β-sheet fibrillar network. Rudra and coworkers have recently used Q11 as a platform for antigen delivery.112 They found that Q11 peptide coupled to a model antigen (OVA peptide) stimulated higher IgG titers than a formulation of the antigen with CFA. Recently, they also conjugated the Q11 peptide to a malaria peptide epitope (NANP)3 derived from Plasmodium falciparum circumsporozoite (CS) protein.113 The (NANP)3-Q11 formulation induced a high antibody titer which lasted up to 40 wk in mice without requiring an additional adjuvant. They found that the antibody response is dependent on CD4+ T cells and the MyD88 pathway. Their study validated that the Q11 peptide is not immunogenic by itself, even in the presence of CFA.
It was also reported that the isopeptide derivative could undergo self-assembly to form fibril-like formation. Toth and coworkers developed an isopeptide using the application of an O-N intramolecular acyl migration strategy (Fig. 8).114 The isopeptide construct was conjugated to the antigenic peptide as a single unit to produce a ‘self-adjuvanting vaccine’. They found that the vaccine candidate adopted a β-sheet conformation and aggregated to form fibrils in a pH dependent manner. This discovery provides useful insight into the development of a safe vaccine adjuvant system since it can be produced in a highly controlled manner.
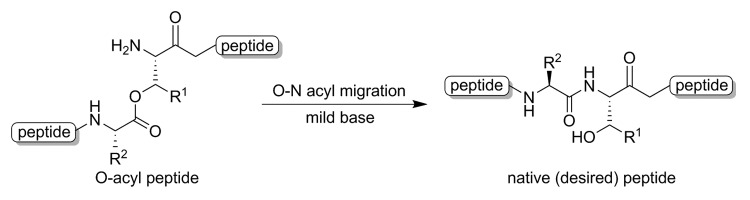
Figure 8. O-N intramolecular acyl migration reactions
In light of the application of self-assembled peptides as vaccine adjuvants, most studies have demonstrated that their adjuvanticity effects are primarily dominated by the presentation of the epitope on their surface while maintaining the epitope native conformation (Fig. 9). Vaccine formulation using self-assembly peptides as adjuvants is a promising approach for the development of next generation peptide-based subunit vaccines as they permit native antigen conformation which is crucial for B cell activation.
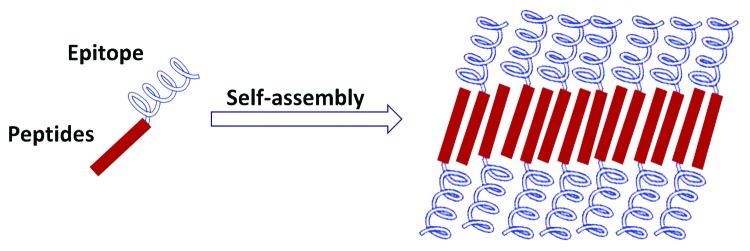
Figure 9. Schematic display of a repetitive antigen in a self-assembling peptide adjuvant system.
Lipopolysaccharides (LPS) and Other Adjuvants Derived from Bacteria
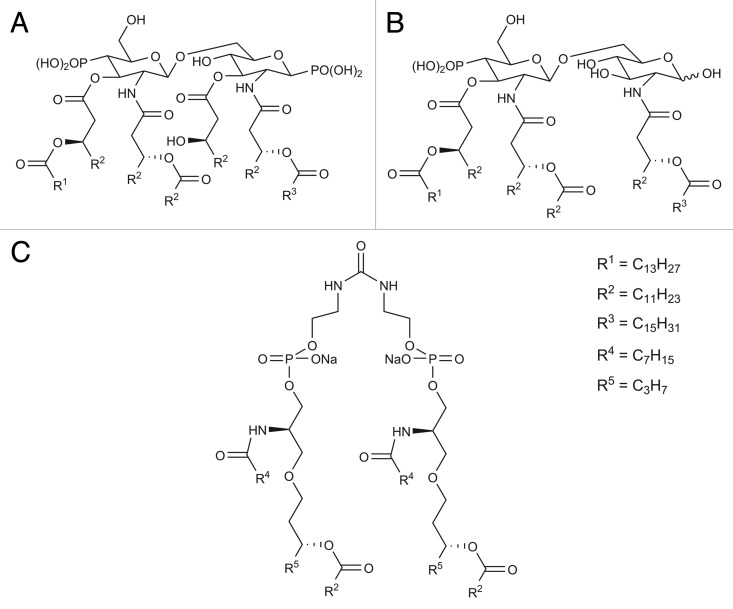
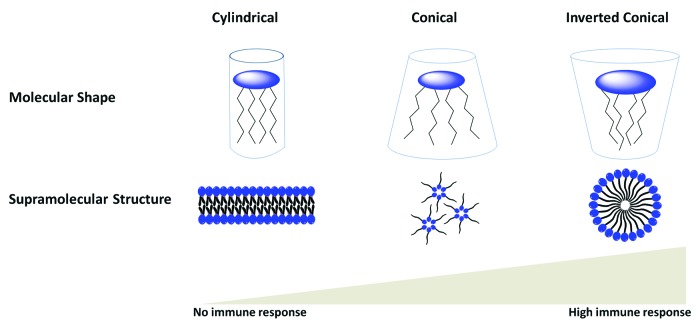
Lipopolysaccharides (LPS) are the outer membrane surface components present in Gram-negative bacteria. They are composed of hydrophilic polysaccharide and a lipophilic phospholipid (lipid A). Lipid A represents the key immunoreactivity components of LPS (Fig. 10A). Lipid A is recognized by TLR4 and directs DC toward Th1 immunity.115 However, the use of lipid A as an adjuvant is often associated with high toxicity. The elimination of the phosphate group and 3-O-deacylation of lipid A yields monophosphoryl lipid A (MPL), which is less toxic yet retains its adjuvant properties (Fig. 10B).116,117 It has been postulated that the β (1–6) diglucosamine backbone and the six hydrophobic chains of MPL are strongly associated with their pharmacophore activity.116 The amphiphillic properties of MPL allow the formation of supramolecular aggregates in an aqueous environment. The aggregated MPL can be transported to the targeted immune cell membranes by LPS-binding protein. The lipidic chain of MPL can diffuse through the cell membrane and promote TLR-4 activation.118 However, only conical lipid molecules that form cubic inverted aggregate structures conferred immunostimulatory activity, while cylindrical lipid molecules that bias toward lamellar-like aggregate structures provided low or no immunostimulatory activity (Fig. 11).119 The lipophilic molecule ER-803022, a synthetic analog of MPL, adopts a conical shape (cubic inverted structure) was found to induce immune response by targeting the same LPS receptors (Fig. 10C).120 This data indicates that structural modifications of lipid A (and its derivatives) may influence aggregation behavior, which would influence their binding affinity as TLR4 agonists. Other synthetic analogs of LPS have been also reported.121 The MPL and its derivative have been studied with the aim of developing peptide-based vaccine. The conjugation of MPL to a peptide epitope enhances the immunogenicity of the vaccine antigen.122 Different vaccination formulations such as emulsification of CD8 T cell peptide epitope with MPL analogs also induced IFN-γ production and conferred protection against the targeted parasite.123 Historically, MPL was the first T-cells-stimulating adjuvant that was licensed for use in the routine vaccination. AS04 adjuvant that contain combination of MPL and alum was used as part of the HPV vaccine and recently applied for hepatitis B virus (HBV) vaccine.115 An adjuvant formulation that includes MPL as the constituents (AS01 and AS02) is currently under clinical evaluation for protein/peptide-based vaccine against malaria and tuberculosis.124,125
Figure 10. (A) Chemical structure of lipid A, (B) chemical structure of MPL, and (C) and its analog: ER-803022.
Figure 11. The correlation between supramolecular structures of lipidic chain of Lipid A (from different origins) and their effect on biological activity.
It is well established that flagellin (structural protein present on bacterial flagellum) is potent activator of broad range of immune cells. Flagellin showed promise as an adjuvant; intramuscular administration of recombinant flagellin with influenza virus peptide epitopes stimulated both humoral and cellular responses against influenza challenge.126 Interestingly, the same study found that this formulation also provided some protection against Influenza A virus subtype (H5N1). The use of flagellin as an adjuvant for cancer vaccination via the intranasal route showed promising results. For example, the intranasal immunization with flagellin-adjuvanted peptide-based HPV antigen elicited a robust cellular immune response that managed to suppress tumor growth in a mouse model.127 The strong immune response stimulated by flagellin was mediated by the activation of TLR5. The flexibility of flagellin structure contributes to the ease of fusion with peptide antigen, making it possible to create a powerful peptide vaccine against a variety of diseases including tumors.
Several other adjuvants based on bacterial components are currently under development. One of these adjuvants is a synthetic oligonucleotide (ODN), CpG-ODN. CpG-ODN contains unmethylated CpG motifs that resemble the structure of bacterial DNA. CpG-ODNs are known to activate TLR9, which located within the APC.128 TLR9 signaling can lead to the maturation of dendritic cells and enhance B cell differentiation into antibody-secreting plasma cells.128 The use of CpG-DNA as an adjuvant was found to enhance the immunogenicity of the immunodominant CD8 T cell peptide epitopes.129 They found that the CD8 T cell peptide epitopes alone were able to be presented by immature DC in lymph nodes, but failed to stimulate a CTL response. However, administration of the same peptide epitope with CpG-DNA induced the maturation of DC, which led to direct stimulation of cytolytic T cells. Thus CpG-DNA was able to promote a CTL response against MHC class 1 restricted T cell epitopes without requiring Th cell activation. It was determined that the CPG-ODNs need to be in close proximity (physical contact) with the antigen to provide maximum immunostimulatory effect.130 These results were supported by the findings of Daftarian et al., where CpG-ODN covalently linked to the peptide epitope stimulated greater epitope recognition than a non-linked formulation of the same molecules.131
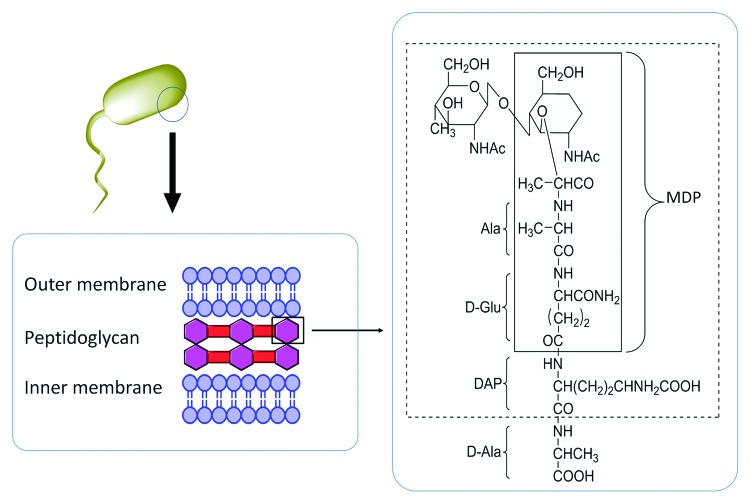
Peptidoglycans, a bacterial cell wall component, are highly investigated as potential immunostimulants. Adjuvants based on peptidoglycan constituents, known as muramyl dipeptide (MDP) (Fig. 12) were found to modulate the immune response. It was reported that MDP and their derivatives confer their adjuvanticity effect by activating the NF-κB pathway through the NOD2 receptor.132 The same group also found that a tetrasachharide that contained MDP promoted additional production of IgG2b subclasses compared with MDP alone, which instead induced IgG1 production. However, MDP does not stimulate strong immune response as other TLR agonists under a variety of immunization conditions.133 Thus, MDP is a less favorable adjuvant for peptide-based antigens which require a relatively strong immune modulator to exhibit potency.
Figure 12. A schematic presentation of muramyl dipeptide (MDP) structure that is derived from a Gram-negative bacterial cell-wall component.
The use of bacterial components as adjuvants is effective because bacteria represent a foreign antigen threat and trigger an immune response. However, if these components are not synthetically produced, it will be challenging to control the level of potential bacterial contaminants. Thus, the purity of the bacterial components must be carefully evaluated during their isolation and production.
Lipopeptides and MAP System
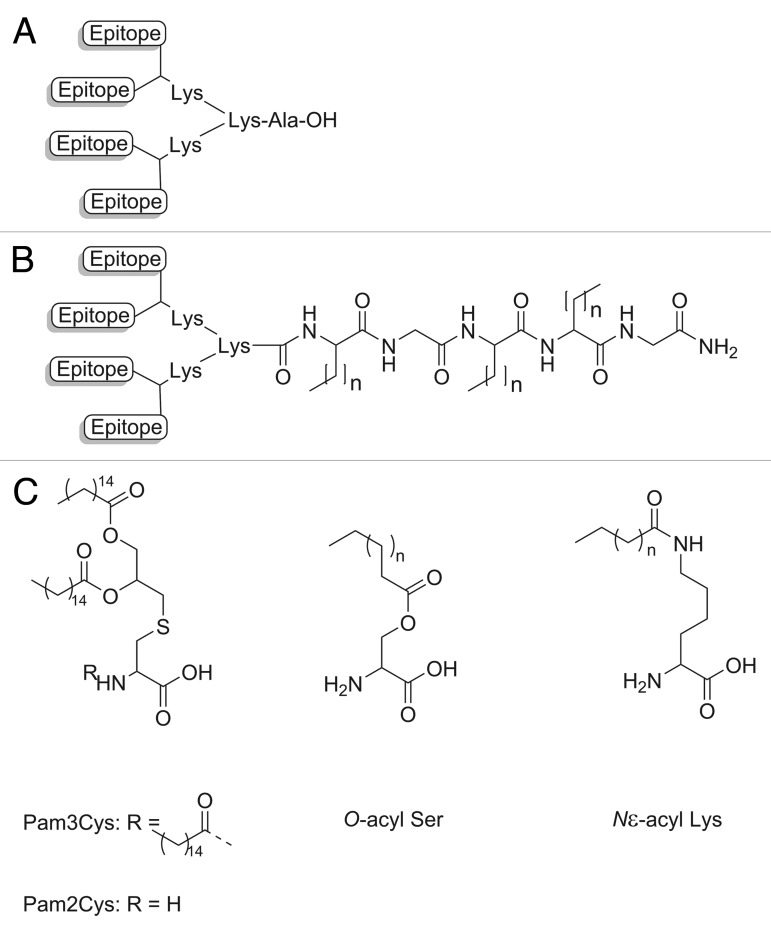
Lipidation of antigenic peptides is a promising strategy to improve their potency for stimulating immune responses. Lipopeptide carrier systems can stimulate TLR2 activation, improve peptide diffusion across epithelial barriers, and enhance peptide stability from enzymatic degradation.134 Furthermore, lipidation may also contribute to the enhancement of peptide secondary structure. Lipopeptides can undergo aggregation and thus exhibit protein analogous micelles properties which enable the short peptides to regain their native conformation.5 Lipidation provides “self-adjuvanting vaccine” properties as the antigen and lipid-based adjuvant are incorporated into a single construct. Thus, the same APCs encounter both the antigen and adjuvant. It was reported that the antigen and TLR agonist should be colocalized in a similar phagosome for effective MHC–II antigen presentation.135 Physical mixture of lipids and peptides failed to stimulate immune response.136 Varieties type of lipid moieties conjugated to peptide antigen have been studied. The simplest designs of lipopeptide vaccine are comprised of a fatty acid moiety conjugated to an antigenic peptide epitope. Jackson and colleagues have demonstrated that a T cell peptide epitope derived from influenza virus when covalently linked to Pam3Cys stimulated a T cell mediated immune response.137 Although proven to enhance the immunogenicity of peptide antigens, lipopeptides comprised of Pam3Cys have usually poor water-solubility. A similar derivative, Pam2Cys, lacking one palmitic acid group, was conjugated to a B cell peptide epitope for contraceptive vaccination138 and T cell peptide epitope for hepatitis C virus vaccine.139 These conjugates upon imminization in mice enhanced the antigen-specific immune responses and shown significantly better solubility than Pam3Cys analogs.136
Lipopeptides have been also used in conjugation with the multiple antigen pepetide system (MAP), which allows incorporation of multiple copies of the peptide antigen in a single construct. The MAP system uses lysine as a core dendrimer because it has two functional side-chains available for branching purposes, the α- and ε-amino groups (Fig. 13A). Kamo and coworkers showed that a linear peptide that was ineffective at raising antibodies could become immunogenic when presented in MAP format.140 Lipid core peptides (LCP) that use the functionalities of lipoamino acids in the MAP system have been extensively studied to develop an efficient vaccine delivery system (Fig. 13B).141 Lipoamino acids are recognized as TLR2 agonists and their lipophilicity can be tailored by varying the length of the alkyl side chain.142 It was demonstrated that an LCP constructed with the peptide epitope (J8) derived from GAS M protein as an antigen induced IgG antibodies, heterologous opsonic antibodies, and conferred complete protection against GAS challenge in mice without any additional adjuvant.143 LCP constructs were reported as vaccine candidates against wide a range of diseases including GAS,144 cancer,145 malaria,146 and hookworm.19 Other lipid moieties such as palmatic acid derivatives (Pam3Cys and Pam2Cys), O-acyl serine, N-acyl lysine, and glycolipid compounds that were found to stimulate innate immunity were also investigated for this purpose (Fig. 13C).121,147
Figure 13. (A) A schematic representation of the origin MAP system, (B) an example of an LCP core based MAP system (n = total number of carbon molecules than can be varied), and (C) other immunostimulating lipid moieties.
Generation of an optimal response to a B cell epitope requires recognition of the antigenic fragments by T helper cells. The activated T helper cells can deliver activating signals to B cells, subsequently stimulating B cell proliferation into antibody-secreting cells. Thus an effective adjuvant to target a humoral response should activate both B cell and T helper cells. The MAP system can meet this requirement because it has the ability to incorporate multiple epitopes in the same construct. MAP that possesses T helper epitopes and the antigenic determinants has been widely investigated. The inclusion of a universal T helper epitope, along with peptide antigen derived from malaria parasite enhanced the immune response against a peptide antigen that was found to be ineffective when used alone.148 Incorporation of T-helper epitope into the LCP and Pam2Cys-MAP constructs allowed induction of a potent immune response in an outbred mouse population.149,150
The incorporation of functional immune components into a MAP system can be exploited to design a more potent peptide-based vaccine. Vaccine efficacy can be optimised by altering the ratio of B cell epitopes and other functional components to develop a safer, more cost-effective vaccine.
Concluding Remarks
Stimulation of a potent and long-term immune response against protective antigens is crucial for the creation of any vaccine. In particular, peptide-based antigens usually target a specific immune response. However, peptides are not able to trigger the recruitment and activation of cells involved in the non-specific immune response (innate immunity), which potentiate the activation of a specific response (adaptive immunity). This issue must be addressed through the development of next generation adjuvants. An adjuvant should not only act as a delivery system but also should stimulate an innate immune response. However, safety needs to be considered in the development of strong immunoadjuvants, as modern adjuvants usually induce inflammatory cytokines such as TNF and IL-1. Excessive proinflammatory responses may lead to undesirable side-effects which have been observed with squalene oil emulsions that triggered autoimmune disease in animal models.
The activation of APCs, especially DCs is paramount to the development of an effective adjuvant. DC activation can result in enhanced antigen uptake, antigen migration to the draining lymph nodes, acquisition of co-stimulatory molecules and enhanced antigen presentation to T cells. DC activation can be stimulated via several mechanisms. One mechanisms of interest is to engage with the TLR that are presented by the DC. TLR activation can lead to the generation of co-stimulatory molecules (CD40, CD80, CD70) and trigger the release of Th1 cytokines such as IL-1, IL-2, IL-6, and TNF. The incorporation of antigen and TLR agonist in the same construct would contribute to a greater immune response. This is due to the fact that some of the endosomal organelles of DC may express TLRs, which would enable antigen processing and DCs activation to occur simultaneously.
Additionally, the construction of adjuvant by mimicking natural pathogens is a promising approach as pathogens are easily recognized by cells of the innate immune system. Constructed adjuvants that have similar properties to pathogens (e.g., particulate behavior, size, and repetitive display of antigenic epitopes) are easily taken up by DC to undergo further biological processing. It has been demonstrated that the particulate nature of certain adjuvant system such as virosomes, liposomes, polymeric nanoparticles, and ISCOM help to boost their uptake by DC and appears to trigger antigen cross-presentation and priming of CD8+ T cells.
The combination of adjuvants in formulations has brought a new era to modern vaccine development. It is clearly shown that adjuvants may confer their adjuvanticity effect via different mechanisms. Thus, combining two or more adjuvants may result in a more potent immune response through a synergystic effect. This strategy has already been proven to work, with recent licensed vaccine formulations such as Gardasil (VLP and alum) and Cervarix (MPL and alum) which incorporate a combination of adjuvants. With increased interest in targeting TLR, it would be a promising option to combine multiple TLR agonists in a vaccine formulation in order to exploit their synergistic effects on cytokine production, ultimately stimulating a superior immune response. In addition, multiple TLR agonists can also be conjugated with a particulate based adjuvant to enhance their uptake by DC and gain the benefit of multiple antigenic displays for the development of a next generation adjuvant system. However, the possibility of stimulating an excessive inflammatory response needs to be considered when combining several adjuvants. Thus, optimizing the balance between an effective immunostimulant and potentially excessive inflammatory responses would be crucial in moving toward this approach.
Growing knowledge about the stimulation of immunological events associated with an adjuvants mode of action, especially the identification of particular signaling pathways that lead to an adaptive immune response, provides hope that effective peptide vaccines can be developed in the near future.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia. We thank Thalia Guerin for her critical review of the manuscript.
Glossary
Abbreviations:
- APC
antigen presenting cells
- ASC
apoptosis-associated speck-like protein containing a CARD (caspase recruitment domain)
- BPV
bovine papillomavirus
- CFA
Complete Freund’s adjuvant
- COMP
cartilage oligomeric matrix protein
- CpG
cytosine–phosphate–guanine
- CTL
cytotoxic T lymphocyte
- DC
dendritic cells
- DDA
dimethyl dioctadecyl ammonium bromide
- DNA
deoxyribonucleic acid
- DOPE
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine
- DT
diphtheria toxoid
- FDA
Food and Drug Administration
- GAS
group A streptococcus
- HA
hemagglutanin
- HBsAg
gepatitis B surface antigen
- HBV
gepatitis B virus
- HIV
human immunodeficiency virus
- HPV
guman papillomavirus
- HSA
guman serum albumin
- IFA
Incomplete Freund’s adjuvant
- IFN
interferon
- IL
interleukin
- IRIV
immunopotentiating reconstituted influenza virosomes
- ISCOM
immunostimulatory complex
- LCP
lipid core peptides
- LPS
lipopolysaccharide
- LRR
leucine-rich repeat
- MAP
multiple antigenic peptide
- MCC
mono-n-carboxylated chitosan
- MDP
muramyl dipeptide
- MHC
major histocompatibility complex
- MMG
monomycoloyl glycerol
- MPL
monophosphoryl lipid A
- NA
neuraminidase
- NK cell
natural killer cell
- NLR
NOD-like receptor
- NLRP3
NOD-like receptor family, pyrin domain containing 3
- OVA
Ovalbumin
- PAMP
pathogen associated molecular patterns
- PLA
polylactic acid
- PLGA
Poly (lactide-co-glycolide) acid
- PRR
pathogen recognition receptors
- PtBA
Poly (t-butyl)acrylate
- RLR
retinoic acid-inducible gene I (RIG-I)-like receptor
- SARS
severe acute respiratory syndrome
- SARS-CoV
severe acute respiratory syndrome (SARS) coronavirus
- TAT
trans-activating transcriptional activator
- TCR
T cell receptor
- Th
T helper
- TLR
toll-like receptors
- TMC
trimethylated chitosan
- TT
tetanus toxoid
- VLP
virus-like particle
References
- 1.Purcell AW, McCluskey J, Rossjohn J. More than one reason to rethink the use of peptides in vaccine design. Nat Rev Drug Discov. 2007;6:404–14. doi: 10.1038/nrd2224. [DOI] [PubMed] [Google Scholar]
- 2.Levine MM, Sztein MB. Vaccine development strategies for improving immunization: the role of modern immunology. Nat Immunol. 2004;5:460–4. doi: 10.1038/ni0504-460. [DOI] [PubMed] [Google Scholar]
- 3.Skwarczynski M, Toth I. Peptide-based subunit nanovaccines. Curr Drug Deliv. 2011;8:282–9. doi: 10.2174/156720111795256192. [DOI] [PubMed] [Google Scholar]
- 4.Pruksakorn S, Currie B, Brandt E, Phornphutkul C, Hunsakunachai S, Manmontri A, Robinson JH, Kehoe MA, Galbraith A, Good MF. Identification of T cell autoepitopes that cross-react with the C-terminal segment of the M protein of group A streptococci. Int Immunol. 1994;6:1235–44. doi: 10.1093/intimm/6.8.1235. [DOI] [PubMed] [Google Scholar]
- 5.Black M, Trent A, Tirrell M, Olive C. Advances in the design and delivery of peptide subunit vaccines with a focus on toll-like receptor agonists. Expert Rev Vaccines. 2010;9:157–73. doi: 10.1586/erv.09.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogel FR, Powell MF. A compendium of vaccine adjuvants and excipients. Pharm Biotechnol. 1995;6:141–228. doi: 10.1007/978-1-4615-1823-5_7. [DOI] [PubMed] [Google Scholar]
- 7.Mata E, Igartua M, Hernández RM, Rosas JE, Patarroyo ME, Pedraz JL. Comparison of the adjuvanticity of two different delivery systems on the induction of humoral and cellular responses to synthetic peptides. Drug Deliv. 2010;17:490–9. doi: 10.3109/10717544.2010.483254. [DOI] [PubMed] [Google Scholar]
- 8.Joffre OP, Segura E, Savina A, Amigorena S. Cross-presentation by dendritic cells. Nat Rev Immunol. 2012;12:557–69. doi: 10.1038/nri3254. [DOI] [PubMed] [Google Scholar]
- 9.McGuinness DH, Dehal PK, Pleass RJ. Pattern recognition molecules and innate immunity to parasites. Trends Parasitol. 2003;19:312–9. doi: 10.1016/S1471-4922(03)00123-5. [DOI] [PubMed] [Google Scholar]
- 10.Agrawal S, Agrawal A, Doughty B, Gerwitz A, Blenis J, Van Dyke T, Pulendran B. Cutting edge: different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J Immunol. 2003;171:4984–9. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 11.Guy B. The perfect mix: recent progress in adjuvant research. Nat Rev Microbiol. 2007;5:505–17. doi: 10.1038/nrmicro1681. [DOI] [PubMed] [Google Scholar]
- 12.Cox JC, Coulter AR. Adjuvants--a classification and review of their modes of action. Vaccine. 1997;15:248–56. doi: 10.1016/S0264-410X(96)00183-1. [DOI] [PubMed] [Google Scholar]
- 13.Foti M, Granucci F, Ricciardi-Castagnoli P. A central role for tissue-resident dendritic cells in innate responses. Trends Immunol. 2004;25:650–4. doi: 10.1016/j.it.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 14.Sharp FA, Ruane D, Claass B, Creagh E, Harris J, Malyala P, Singh M, O’Hagan DT, Pétrilli V, Tschopp J, et al. Uptake of particulate vaccine adjuvants by dendritic cells activates the NALP3 inflammasome. Proc Natl Acad Sci U S A. 2009;106:870–5. doi: 10.1073/pnas.0804897106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Podojil JR, Miller SD. Molecular mechanisms of T-cell receptor and costimulatory molecule ligation/blockade in autoimmune disease therapy. Immunol Rev. 2009;229:337–55. doi: 10.1111/j.1600-065X.2009.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Batzloff MR, Pandey M, Olive C, Good MF. Advances in potential M-protein peptide-based vaccines for preventing rheumatic fever and rheumatic heart disease. Immunol Res. 2006;35:233–48. doi: 10.1385/IR:35:3:233. [DOI] [PubMed] [Google Scholar]
- 17.Skwarczynski M, Kamaruzaman KA, Srinivasan S, Zaman M, Lin IC, Batzloff MR, Good MF, Toth I. M-protein-derived conformational peptide epitope vaccine candidate against Group A Streptococcus. Curr Drug Deliv. 2013;10:39–45. doi: 10.2174/1567201811310010007. [DOI] [PubMed] [Google Scholar]
- 18.Skwarczynski M, Zaman M, Urbani CN, Lin IC, Jia Z, Batzloff MR, Good MF, Monteiro MJ, Toth I. Polyacrylate dendrimer nanoparticles: a self-adjuvanting vaccine delivery system. Angew Chem Int Ed Engl. 2010;49:5742–5. doi: 10.1002/anie.201002221. [DOI] [PubMed] [Google Scholar]
- 19.Skwarczynski M, Dougall AM, Khoshnejad M, Chandrudu S, Pearson MS, Loukas A, Toth I. Peptide-based subunit vaccine against hookworm infection. PLoS One. 2012;7:e46870. doi: 10.1371/journal.pone.0046870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hickman HD, Luis AD, Buchli R, Few SR, Sathiamurthy M, VanGundy RS, Giberson CF, Hildebrand WH. Toward a definition of self: proteomic evaluation of the class I peptide repertoire. J Immunol. 2004;172:2944–52. doi: 10.4049/jimmunol.172.5.2944. [DOI] [PubMed] [Google Scholar]
- 21.Alexander J, del Guercio MF, Maewal A, Qiao L, Fikes J, Chesnut RW, Paulson J, Bundle DR, DeFrees S, Sette A. Linear PADRE T helper epitope and carbohydrate B cell epitope conjugates induce specific high titer IgG antibody responses. J Immunol. 2000;164:1625–33. doi: 10.4049/jimmunol.164.3.1625. [DOI] [PubMed] [Google Scholar]
- 22.Glenny AT. Insoluble Precipitates in Diphtheria and Tetanus Immunization. Br Med J. 1930;2:244–5. doi: 10.1136/bmj.2.3632.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romero Méndez IZ, Shi Y, HogenEsch H, Hem SL. Potentiation of the immune response to non-adsorbed antigens by aluminum-containing adjuvants. Vaccine. 2007;25:825–33. doi: 10.1016/j.vaccine.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 24.Morefield GL, HogenEsch H, Robinson JP, Hem SL. Distribution of adsorbed antigen in mono-valent and combination vaccines. Vaccine. 2004;22:1973–84. doi: 10.1016/j.vaccine.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 25.Morefield GL, Sokolovska A, Jiang D, HogenEsch H, Robinson JP, Hem SL. Role of aluminum-containing adjuvants in antigen internalization by dendritic cells in vitro. Vaccine. 2005;23:1588–95. doi: 10.1016/j.vaccine.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 26.Hem SL, Hogenesch H. Relationship between physical and chemical properties of aluminum-containing adjuvants and immunopotentiation. Expert Rev Vaccines. 2007;6:685–98. doi: 10.1586/14760584.6.5.685. [DOI] [PubMed] [Google Scholar]
- 27.Kool M, Soullié T, van Nimwegen M, Willart MAM, Muskens F, Jung S, Hoogsteden HC, Hammad H, Lambrecht BN. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205:869–82. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramanathan VD, Badenoch-Jones P, Turk JL. Complement activation by aluminium and zirconium compounds. Immunology. 1979;37:881–8. [PMC free article] [PubMed] [Google Scholar]
- 29.Wack A, Baudner BC, Hilbert AK, Manini I, Nuti S, Tavarini S, Scheffczik H, Ugozzoli M, Singh M, Kazzaz J, et al. Combination adjuvants for the induction of potent, long-lasting antibody and T-cell responses to influenza vaccine in mice. Vaccine. 2008;26:552–61. doi: 10.1016/j.vaccine.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 30.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–6. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franchi L, Núñez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol. 2008;38:2085–9. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marichal T, Ohata K, Bedoret D, Mesnil C, Sabatel C, Kobiyama K, Lekeux P, Coban C, Akira S, Ishii KJ, et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med. 2011;17:996–1002. doi: 10.1038/nm.2403. [DOI] [PubMed] [Google Scholar]
- 33.Davenport FM, Hennessy AV, Askin FB. Lack of adjuvant effect of A1PO4 on purified influenza virus hemagglutinins in man. J Immunol. 1968;100:1139–40. [PubMed] [Google Scholar]
- 34.Cvjetanovic B, Uemura K. The Present Status of Field and Laboratory Studies of Typhoid and Paratyphoid Vaccines with Special Reference to Studies Sponsored by World Health Organization. Bull World Health Organ. 1965;32:29–36. [PMC free article] [PubMed] [Google Scholar]
- 35.Lindblad EB. Aluminium adjuvants--in retrospect and prospect. Vaccine. 2004;22:3658–68. doi: 10.1016/j.vaccine.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 36.Lew AM, Anders RF, Edwards SJ, Langford CJ. Comparison of antibody avidity and titre elicited by peptide as a protein conjugate or as expressed in vaccinia. Immunology. 1988;65:311–4. [PMC free article] [PubMed] [Google Scholar]
- 37.Geerligs HJ, Weijer WJ, Welling GW, Welling-Wester S. The influence of different adjuvants on the immune response to a synthetic peptide comprising amino acid residues 9-21 of herpes simplex virus type 1 glycoprotein D. J Immunol Methods. 1989;124:95–102. doi: 10.1016/0022-1759(89)90190-7. [DOI] [PubMed] [Google Scholar]
- 38.Aucouturier J, Dupuis L, Ganne V. Adjuvants designed for veterinary and human vaccines. Vaccine. 2001;19:2666–72. doi: 10.1016/S0264-410X(00)00498-9. [DOI] [PubMed] [Google Scholar]
- 39.Tindle RW, Fernando GJP, Sterling JC, Frazer IH. A “public” T-helper epitope of the E7 transforming protein of human papillomavirus 16 provides cognate help for several E7 B-cell epitopes from cervical cancer-associated human papillomavirus genotypes. Proc Natl Acad Sci U S A. 1991;88:5887–91. doi: 10.1073/pnas.88.13.5887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oscherwitz J, Hankenson FC, Yu F, Cease KB. Low-dose intraperitoneal Freund’s adjuvant: toxicity and immunogenicity in mice using an immunogen targeting amyloid-beta peptide. Vaccine. 2006;24:3018–25. doi: 10.1016/j.vaccine.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 41.Graham BS, McElrath MJ, Keefer MC, Rybczyk K, Berger D, Weinhold KJ, Ottinger J, Ferarri G, Montefiori DC, Stablein D, et al. AIDS Vaccine Evaluation Group Immunization with cocktail of HIV-derived peptides in montanide ISA-51 is immunogenic, but causes sterile abscesses and unacceptable reactogenicity. PLoS One. 2010;5:e11995. doi: 10.1371/journal.pone.0011995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langermans JAM, Schmidt A, Vervenne RAW, Birkett AJ, Calvo-Calle JM, Hensmann M, Thornton GB, Dubovsky F, Weiler H, Nardin E, et al. Effect of adjuvant on reactogenicity and long-term immunogenicity of the malaria Vaccine ICC-1132 in macaques. Vaccine. 2005;23:4935–43. doi: 10.1016/j.vaccine.2005.05.036. [DOI] [PubMed] [Google Scholar]
- 43.Audran R, Cachat M, Lurati F, Soe S, Leroy O, Corradin G, Druilhe P, Spertini F. Phase I malaria vaccine trial with a long synthetic peptide derived from the merozoite surface protein 3 antigen. Infect Immun. 2005;73:8017–26. doi: 10.1128/IAI.73.12.8017-8026.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Iseki K, Matsunaga H, Komatsu N, Suekane S, Noguchi M, Itoh K, Yamada A. Evaluation of a new oil adjuvant for use in peptide-based cancer vaccination. Cancer Sci. 2010;101:2110–4. doi: 10.1111/j.1349-7006.2010.01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Hagan DT. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev Vaccines. 2007;6:699–710. doi: 10.1586/14760584.6.5.699. [DOI] [PubMed] [Google Scholar]
- 46.Ott G, Barchfeld GL, Chernoff D, Radhakrishnan R, van Hoogevest P, Van Nest G. MF59. Design and evaluation of a safe and potent adjuvant for human vaccines. Pharm Biotechnol. 1995;6:277–96. doi: 10.1007/978-1-4615-1823-5_10. [DOI] [PubMed] [Google Scholar]
- 47.O’Hagan DT, Ott GS, De Gregorio E, Seubert A. The mechanism of action of MF59 - an innately attractive adjuvant formulation. Vaccine. 2012;30:4341–8. doi: 10.1016/j.vaccine.2011.09.061. [DOI] [PubMed] [Google Scholar]
- 48.Hu JJ, Wang HQ, Qu HG, Xu J, Yao ZB. [Antibody production and its neutralization of Abeta42's cytoxicity in BALB/c mice induced by Abeta42 and its subunit vaccines]. Xi bao yu fen zi mian yi xue za zhi = Chinese journal of cellular and molecular immunology 2004; 20:178-81. [PubMed]
- 49.Roman F, Vaman T, Gerlach B, Markendorf A, Gillard P, Devaster JM. Immunogenicity and safety in adults of one dose of influenza A H1N1v 2009 vaccine formulated with and without AS03A-adjuvant: preliminary report of an observer-blind, randomised trial. Vaccine. 2010;28:1740–5. doi: 10.1016/j.vaccine.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 50.Garçon N, Vaughn DW, Didierlaurent AM. Development and evaluation of AS03, an Adjuvant System containing α-tocopherol and squalene in an oil-in-water emulsion. Expert Rev Vaccines. 2012;11:349–66. doi: 10.1586/erv.11.192. [DOI] [PubMed] [Google Scholar]
- 51.De la Fuente M, Hernanz A, Guayerbas N, Victor VM, Arnalich F. Vitamin E ingestion improves several immune functions in elderly men and women. Free Radic Res. 2008;42:272–80. doi: 10.1080/10715760801898838. [DOI] [PubMed] [Google Scholar]
- 52.Cheung YK, Cheng SCS, Ke Y, Xie Y. Two novel HLA-A*0201 T-cell epitopes in avian H5N1 viral nucleoprotein induced specific immune responses in HHD mice. Vet Res. 2010;41:24. doi: 10.1051/vetres/2009071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swinkels WJC, Hoeboer J, Sikkema R, Vervelde L, Koets AD. Vaccination induced antibodies to recombinant avian influenza A virus M2 protein or synthetic M2e peptide do not bind to the M2 protein on the virus or virus infected cells. Virol J. 2013;10:206. doi: 10.1186/1743-422X-10-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Almanzar G, Herndler-Brandstetter D, Chaparro SV, Jenewein B, Keller M, Grubeck-Loebenstein B. Immunodominant peptides from conserved influenza proteins--a tool for more efficient vaccination in the elderly? Wien Med Wochenschr. 2007;157:116–21. doi: 10.1007/s10354-007-0393-y. [DOI] [PubMed] [Google Scholar]
- 55.Cui C, Stevens VC, Schwendeman SP. Injectable polymer microspheres enhance immunogenicity of a contraceptive peptide vaccine. Vaccine. 2007;25:500–9. doi: 10.1016/j.vaccine.2006.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lutsiak MEC, Robinson DR, Coester C, Kwon GS, Samuel J. Analysis of poly(D,L-lactic-co-glycolic acid) nanosphere uptake by human dendritic cells and macrophages in vitro. Pharm Res. 2002;19:1480–7. doi: 10.1023/A:1020452531828. [DOI] [PubMed] [Google Scholar]
- 57.Ma W, Chen M, Kaushal S, McElroy M, Zhang Y, Ozkan C, Bouvet M, Kruse C, Grotjahn D, Ichim T, et al. PLGA nanoparticle-mediated delivery of tumor antigenic peptides elicits effective immune responses. Int J Nanomedicine. 2012;7:1475–87. doi: 10.2147/IJN.S29506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lambert JS, Keefer M, Mulligan MJ, Schwartz D, Mestecky J, Weinhold K, Smith C, Hsieh R, Moldoveanu Z, Fast P, et al. A Phase I safety and immunogenicity trial of UBI microparticulate monovalent HIV-1 MN oral peptide immunogen with parenteral boost in HIV-1 seronegative human subjects. Vaccine. 2001;19:3033–42. doi: 10.1016/S0264-410X(01)00051-2. [DOI] [PubMed] [Google Scholar]
- 59.Sáenz L, Neira-Carrillo A, Paredes R, Cortés M, Bucarey S, Arias JL. Chitosan formulations improve the immunogenicity of a GnRH-I peptide-based vaccine. Int J Pharm. 2009;369:64–71. doi: 10.1016/j.ijpharm.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 60.Jiang HL, Kang ML, Quan JS, Kang SG, Akaike T, Yoo HS, Cho CS. The potential of mannosylated chitosan microspheres to target macrophage mannose receptors in an adjuvant-delivery system for intranasal immunization. Biomaterials. 2008;29:1931–9. doi: 10.1016/j.biomaterials.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 61.Li X, Min M, Du N, Gu Y, Hode T, Naylor M, Chen D, Nordquist RE, Chen WR. Chitin, chitosan, and glycated chitosan regulate immune responses: the novel adjuvants for cancer vaccine. Clin Dev Immunol. 2013;2013:387023. doi: 10.1155/2013/387023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boas U, Heegaard PMH. Dendrimers in drug research. Chem Soc Rev. 2004;33:43–63. doi: 10.1039/b309043b. [DOI] [PubMed] [Google Scholar]
- 63.Ahmad Fuaad AA, Jia Z, Hartas J, Ziora ZM, Lin I, Moyle PM, Batzloff MR, Good MF, Monteiro MJ, Istvan T. Polymer–peptide hybrids as a highly immunogenic single-dose nanovaccine. nanomedicine (Lond) 2013. [DOI] [PubMed] [Google Scholar]
- 64.Zaman M, Skwarczynski M, Malcolm JM, Urbani CN, Jia Z, Batzloff MR, Good MF, Monteiro MJ, Toth I. Self-adjuvanting polyacrylic nanoparticulate delivery system for group A streptococcus (GAS) vaccine. Nanomedicine. 2011;7:168–73. doi: 10.1016/j.nano.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 65.Liu TY, Hussein WM, Jia Z, Ziora ZM, McMillan NAJ, Monteiro MJ, Toth I, Skwarczynski M. Self-adjuvanting polymer-peptide conjugates as therapeutic vaccine candidates against cervical cancer. Biomacromolecules. 2013;14:2798–806. doi: 10.1021/bm400626w. [DOI] [PubMed] [Google Scholar]
- 66.Kanchan V, Panda AK. Interactions of antigen-loaded polylactide particles with macrophages and their correlation with the immune response. Biomaterials. 2007;28:5344–57. doi: 10.1016/j.biomaterials.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 67.Chong CSW, Cao M, Wong WW, Fischer KP, Addison WR, Kwon GS, Tyrrell DL, Samuel J. Enhancement of T helper type 1 immune responses against hepatitis B virus core antigen by PLGA nanoparticle vaccine delivery. J Control Release. 2005;102:85–99. doi: 10.1016/j.jconrel.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 68.Kazzaz J, Singh M, Ugozzoli M, Chesko J, Soenawan E, O’Hagan DT. Encapsulation of the immune potentiators MPL and RC529 in PLG microparticles enhances their potency. J Control Release. 2006;110:566–73. doi: 10.1016/j.jconrel.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 69.Giddam AK, Zaman M, Skwarczynski M, Toth I. Liposome-based delivery system for vaccine candidates: constructing an effective formulation. Nanomedicine (Lond) 2012;7:1877–93. doi: 10.2217/nnm.12.157. [DOI] [PubMed] [Google Scholar]
- 70.Lincopan N, Espíndola NM, Vaz AJ, da Costa MHB, Faquim-Mauro E, Carmona-Ribeiro AM. Novel immunoadjuvants based on cationic lipid: Preparation, characterization and activity in vivo. Vaccine. 2009;27:5760–71. doi: 10.1016/j.vaccine.2009.07.066. [DOI] [PubMed] [Google Scholar]
- 71.Schwendener RA, Ludewig B, Cerny A, Engler O. Liposome-Based Vaccines. Liposomes: Methods and Protocols, Vol 1 2010; 605:163-75. [DOI] [PubMed]
- 72.Ohno S, Kohyama S, Taneichi M, Moriya O, Hayashi H, Oda H, Mori M, Kobayashi A, Akatsuka T, Uchida T, et al. Synthetic peptides coupled to the surface of liposomes effectively induce SARS coronavirus-specific cytotoxic T lymphocytes and viral clearance in HLA-A*0201 transgenic mice. Vaccine. 2009;27:3912–20. doi: 10.1016/j.vaccine.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Espuelas S, Roth A, Thumann C, Frisch B, Schuber F. Effect of synthetic lipopeptides formulated in liposomes on the maturation of human dendritic cells (vol 42, pg 721, 2005) Mol Immunol. 2006;43:772. doi: 10.1016/j.molimm.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 74.Korsholm KS, Agger EM, Foged C, Christensen D, Dietrich J, Andersen CS, Geisler C, Andersen P. The adjuvant mechanism of cationic dimethyldioctadecylammonium liposomes. Immunology. 2007;121:216–26. doi: 10.1111/j.1365-2567.2007.02560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brgles M, Habjanec L, Halassy B, Tomasić J. Liposome fusogenicity and entrapment efficiency of antigen determine the Th1/Th2 bias of antigen-specific immune response. Vaccine. 2009;27:5435–42. doi: 10.1016/j.vaccine.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 76.Mann JFS, Shakir E, Carter KC, Mullen AB, Alexander J, Ferro VA. Lipid vesicle size of an oral influenza vaccine delivery vehicle influences the Th1/Th2 bias in the immune response and protection against infection. Vaccine. 2009;27:3643–9. doi: 10.1016/j.vaccine.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 77.Korsholm KS, Petersen RV, Agger EM, Andersen P. T-helper 1 and T-helper 2 adjuvants induce distinct differences in the magnitude, quality and kinetics of the early inflammatory response at the site of injection. Immunology. 2010;129:75–86. doi: 10.1111/j.1365-2567.2009.03164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Andersen CS, Agger EM, Rosenkrands I, Gomes JM, Bhowruth V, Gibson KJC, Petersen RV, Minnikin DE, Besra GS, Andersen P. A simple mycobacterial monomycolated glycerol lipid has potent immunostimulatory activity. J Immunol. 2009;182:424–32. doi: 10.4049/jimmunol.182.1.424. [DOI] [PubMed] [Google Scholar]
- 79.Tanaka T, Legat A, Adam E, Steuve J, Gatot JS, Vandenbranden M, Ulianov L, Lonez C, Ruysschaert JM, Muraille E, et al. DiC14-amidine cationic liposomes stimulate myeloid dendritic cells through Toll-like receptor 4. Eur J Immunol. 2008;38:1351–7. doi: 10.1002/eji.200737998. [DOI] [PubMed] [Google Scholar]
- 80.Thomann JS, Heurtault B, Weidner S, Brayé M, Beyrath J, Fournel S, Schuber F, Frisch B. Antitumor activity of liposomal ErbB2/HER2 epitope peptide-based vaccine constructs incorporating TLR agonists and mannose receptor targeting. Biomaterials. 2011;32:4574–83. doi: 10.1016/j.biomaterials.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 81.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–60. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 82.Ruedl C, Storni T, Lechner F, Bächi T, Bachmann MF. Cross-presentation of virus-like particles by skin-derived CD8(-) dendritic cells: a dispensable role for TAP. Eur J Immunol. 2002;32:818–25. doi: 10.1002/1521-4141(200203)32:3<818::AID-IMMU818>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 83.Lo-Man R, Rueda P, Sedlik C, Deriaud E, Casal I, Leclerc C. A recombinant virus-like particle system derived from parvovirus as an efficient antigen carrier to elicit a polarized Th1 immune response without adjuvant. Eur J Immunol. 1998;28:1401–7. doi: 10.1002/(SICI)1521-4141(199804)28:04<1401::AID-IMMU1401>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 84.Guillen G, Aguilar JC, Duenas S, Hermida L, Guzman MG, Penton E, Iglesias E, Junco J, Torrens I, Lobaina Y, et al. Virus-Like Particles as vaccine antigens and adjuvants: application to chronic disease, cancer immunotherapy and infectious disease preventive strategies. Procedia Vaccinol. 2010;2:128–33. doi: 10.1016/j.provac.2010.07.004. [DOI] [Google Scholar]
- 85.Pejawar-Gaddy S, Rajawat Y, Hilioti Z, Xue J, Gaddy DF, Finn OJ, Viscidi RP, Bossis I. Generation of a tumor vaccine candidate based on conjugation of a MUC1 peptide to polyionic papillomavirus virus-like particles. Cancer Immunol Immunother. 2010;59:1685–96. doi: 10.1007/s00262-010-0895-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.FDA Licensure of Bivalent Human Papillomavirus Vaccine (HPV2, Cervarix) for Use in Females and Updated HPV Vaccination Recommendations From the Advisory Committee on Immunization Practices (ACIP) (Reprinted from MMWR, vol 59, pg 626-629, 2010) Jama-J Am Med Assoc. 2010;304:632–4. [PubMed] [Google Scholar]
- 87.Liu TY, Hussein WM, Toth I, Skwarczynski M. Advances in peptide-based human papillomavirus therapeutic vaccines. Curr Top Med Chem. 2012;12:1581–92. doi: 10.2174/156802612802652402. [DOI] [PubMed] [Google Scholar]
- 88.Homhuan A, Prakongpan S, Poomvises P, Maas RA, Crommelin DJA, Kersten GFA, Jiskoot W. Virosome and ISCOM vaccines against Newcastle disease: preparation, characterization and immunogenicity. Eur J Pharm Sci. 2004;22:459–68. doi: 10.1016/j.ejps.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 89.Felnerova D, Viret JF, Glück R, Moser C. Liposomes and virosomes as delivery systems for antigens, nucleic acids and drugs. Curr Opin Biotechnol. 2004;15:518–29. doi: 10.1016/j.copbio.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 90.Bungener L, Huckriede A, de Mare A, de Vries-Idema J, Wilschut J, Daemen T. Virosome-mediated delivery of protein antigens in vivo: efficient induction of class I MHC-restricted cytotoxic T lymphocyte activity. Vaccine. 2005;23:1232–41. doi: 10.1016/j.vaccine.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 91.Angel J, Chaperot L, Molens JP, Mezin P, Amacker M, Zurbriggen R, Grichine A, Plumas J. Virosome-mediated delivery of tumor antigen to plasmacytoid dendritic cells. Vaccine. 2007;25:3913–21. doi: 10.1016/j.vaccine.2007.01.101. [DOI] [PubMed] [Google Scholar]
- 92.Bachmann MF, Hengartner H, Zinkernagel RM. T helper cell-independent neutralizing B cell response against vesicular stomatitis virus: role of antigen patterns in B cell induction? Eur J Immunol. 1995;25:3445–51. doi: 10.1002/eji.1830251236. [DOI] [PubMed] [Google Scholar]
- 93.Okitsu SL, Silvie O, Westerfeld N, Curcic M, Kammer AR, Mueller MS, Sauerwein RW, Robinson JA, Genton B, Mazier D, et al. A virosomal malaria peptide vaccine elicits a long-lasting sporozoite-inhibitory antibody response in a phase 1a clinical trial. PLoS One. 2007;2:e1278. doi: 10.1371/journal.pone.0001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hunziker IP, Zurbriggen R, Glueck R, Engler OB, Reichen J, Dai WJ, Pichler WJ, Cerny A. Perspectives: towards a peptide-based vaccine against hepatitis C virus. Mol Immunol. 2001;38:475–84. doi: 10.1016/S0161-5890(01)00083-9. [DOI] [PubMed] [Google Scholar]
- 95.Cho JE, Kim HS, Ahn WS, Park YS. Enhanced cytotoxicity of doxorubicin encapsulated in liposomes with reconstituted Sendai F-proteins. J Microencapsul. 2001;18:421–31. doi: 10.1080/02652040010019550. [DOI] [PubMed] [Google Scholar]
- 96.Genton B, Pluschke G, Degen L, Kammer AR, Westerfeld N, Okitsu SL, Schroller S, Vounatsou P, Mueller MM, Tanner M, et al. A randomized placebo-controlled phase Ia malaria vaccine trial of two virosome-formulated synthetic peptides in healthy adult volunteers. PLoS One. 2007;2:e1018. doi: 10.1371/journal.pone.0001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Duewell P, Kisser U, Heckelsmiller K, Hoves S, Stoitzner P, Koernig S, Morelli AB, Clausen BE, Dauer M, Eigler A, et al. ISCOMATRIX adjuvant combines immune activation with antigen delivery to dendritic cells in vivo leading to effective cross-priming of CD8+ T cells. J Immunol. 2011;187:55–63. doi: 10.4049/jimmunol.1004114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Furrie E, Smith RE, Turner MW, Strobel S, Mowat AM. Induction of local innate immune responses and modulation of antigen uptake as mechanisms underlying the mucosal adjuvant properties of immune stimulating complexes (ISCOMS) Vaccine. 2002;20:2254–62. doi: 10.1016/S0264-410X(02)00106-8. [DOI] [PubMed] [Google Scholar]
- 99.Davis ID, Chen W, Jackson H, Parente P, Shackleton M, Hopkins W, Chen Q, Dimopoulos N, Luke T, Murphy R, et al. Recombinant NY-ESO-1 protein with ISCOMATRIX adjuvant induces broad integrated antibody and CD4(+) and CD8(+) T cell responses in humans. Proc Natl Acad Sci U S A. 2004;101:10697–702. doi: 10.1073/pnas.0403572101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kensil CR, Wu JY, Soltysik S. Structural and immunological characterization of the vaccine adjuvant QS-21. Pharm Biotechnol. 1995;6:525–41. doi: 10.1007/978-1-4615-1823-5_22. [DOI] [PubMed] [Google Scholar]
- 101.Liu G, Anderson C, Scaltreto H, Barbon J, Kensil CR. QS-21 structure/function studies: effect of acylation on adjuvant activity. Vaccine. 2002;20:2808–15. doi: 10.1016/S0264-410X(02)00209-8. [DOI] [PubMed] [Google Scholar]
- 102.Marciani DJ. Vaccine adjuvants: role and mechanisms of action in vaccine immunogenicity. Drug Discov Today. 2003;8:934–43. doi: 10.1016/S1359-6446(03)02864-2. [DOI] [PubMed] [Google Scholar]
- 103.Mahnke K, Guo M, Lee S, Sepulveda H, Swain SL, Nussenzweig M, Steinman RM. The dendritic cell receptor for endocytosis, DEC-205, can recycle and enhance antigen presentation via major histocompatibility complex class II-positive lysosomal compartments. J Cell Biol. 2000;151:673–84. doi: 10.1083/jcb.151.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Marciani DJ, Press JB, Reynolds RC, Pathak AK, Pathak V, Gundy LE, Farmer JT, Koratich MS, May RD. Development of semisynthetic triterpenoid saponin derivatives with immune stimulating activity. Vaccine. 2000;18:3141–51. doi: 10.1016/S0264-410X(00)00118-3. [DOI] [PubMed] [Google Scholar]
- 105.Marciani DJ, Pathak AK, Reynolds RC, Seitz L, May RD. Altered immunomodulating and toxicological properties of degraded Quillaja saponaria Molina saponins. Int Immunopharmacol. 2001;1:813–8. doi: 10.1016/S1567-5769(01)00016-9. [DOI] [PubMed] [Google Scholar]
- 106.Kim SK, Ragupathi G, Cappello S, Kagan E, Livingston PO. Effect of immunological adjuvant combinations on the antibody and T-cell response to vaccination with MUC1-KLH and GD3-KLH conjugates. Vaccine. 2000;19:530–7. doi: 10.1016/S0264-410X(00)00195-X. [DOI] [PubMed] [Google Scholar]
- 107.Jung JP, Nagaraj AK, Fox EK, Rudra JS, Devgun JM, Collier JH. Co-assembling peptides as defined matrices for endothelial cells. Biomaterials. 2009;30:2400–10. doi: 10.1016/j.biomaterials.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Malashkevich VN, Kammerer RA, Efimov VP, Schulthess T, Engel J. The crystal structure of a five-stranded coiled coil in COMP: a prototype ion channel? Science. 1996;274:761–5. doi: 10.1126/science.274.5288.761. [DOI] [PubMed] [Google Scholar]
- 109.Burkhard P, Meier M, Lustig A. Design of a minimal protein oligomerization domain by a structural approach. Protein Sci. 2000;9:2294–301. doi: 10.1110/ps.9.12.2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kaba SA, Brando C, Guo Q, Mittelholzer C, Raman S, Tropel D, Aebi U, Burkhard P, Lanar DE. A nonadjuvanted polypeptide nanoparticle vaccine confers long-lasting protection against rodent malaria. J Immunol. 2009;183:7268–77. doi: 10.4049/jimmunol.0901957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pimentel T, Yan Z, Jeffers SA, Holmes KV, Hodges RS, Burkhard P. Peptide nanoparticles as novel immunogens: Design and biophysical analysis of a prototype SARS vaccine. Abstr Pap Am Chem S. 2009;237:443. doi: 10.1111/j.1747-0285.2008.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rudra JS, Tian YF, Jung JP, Collier JH. A self-assembling peptide acting as an immune adjuvant. Proc Natl Acad Sci U S A. 2010;107:622–7. doi: 10.1073/pnas.0912124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rudra JS, Mishra S, Chong AS, Mitchell RA, Nardin EH, Nussenzweig V, Collier JH. Self-assembled peptide nanofibers raising durable antibody responses against a malaria epitope. Biomaterials. 2012;33:6476–84. doi: 10.1016/j.biomaterials.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Skwarczynski M, Kowapradit J, Ziora ZM, Toth I. pH-triggered peptide self-assembly into fibrils: a potential peptide-based subunit vaccine delivery platform. Bio Chem Comp 2013; 1.
- 115.Baldridge JR, Crane RT. Monophosphoryl lipid A (MPL) formulations for the next generation of vaccines. Methods. 1999;19:103–7. doi: 10.1006/meth.1999.0834. [DOI] [PubMed] [Google Scholar]
- 116.Ismaili J, Rennesson J, Aksoy E, Vekemans J, Vincart B, Amraoui Z, Van Laethem F, Goldman M, Dubois PM. Monophosphoryl lipid A activates both human dendritic cells and T cells. J Immunol. 2002;168:926–32. doi: 10.4049/jimmunol.168.2.926. [DOI] [PubMed] [Google Scholar]
- 117.Jiang ZH, Budzynski WA, Qiu D, Yalamati D, Koganty RR. Monophosphoryl lipid A analogues with varying 3-O-substitution: synthesis and potent adjuvant activity. Carbohydr Res. 2007;342:784–96. doi: 10.1016/j.carres.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 118.Blunck R, Scheel O, Müller M, Brandenburg K, Seitzer U, Seydel U. New insights into endotoxin-induced activation of macrophages: involvement of a K+ channel in transmembrane signaling. J Immunol. 2001;166:1009–15. doi: 10.4049/jimmunol.166.2.1009. [DOI] [PubMed] [Google Scholar]
- 119.Sigalov AB. “Monovalent” ligands that trigger TLR-4 and TCR are not necessarily truly monovalent. Mol Immunol. 2012;51:356–62. doi: 10.1016/j.molimm.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 120.Seydel U, Hawkins L, Schromm AB, Heine H, Scheel O, Koch MHJ, Brandenburg K. The generalized endotoxic principle. Eur J Immunol. 2003;33:1586–92. doi: 10.1002/eji.200323649. [DOI] [PubMed] [Google Scholar]
- 121.Fujita Y, Taguchi H. Current status of multiple antigen-presenting peptide vaccine systems: Application of organic and inorganic nanoparticles. Chem Cent J. 2011;5:48. doi: 10.1186/1752-153X-5-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Moyle PM, Toth I. Self-adjuvanting lipopeptide vaccines. Curr Med Chem. 2008;15:506–16. doi: 10.2174/092986708783503249. [DOI] [PubMed] [Google Scholar]
- 123.Tabatabai LB, Pugh GW., Jr. Modulation of immune responses in Balb/c mice vaccinated with Brucella abortus Cu-Zn superoxide dismutase synthetic peptide vaccine. Vaccine. 1994;12:919–24. doi: 10.1016/0264-410X(94)90035-3. [DOI] [PubMed] [Google Scholar]
- 124.Bojang KA, Olodude F, Pinder M, Ofori-Anyinam O, Vigneron L, Fitzpatrick S, Njie F, Kassanga A, Leach A, Milman J, et al. Safety and immunogenicty of RTS,S/AS02A candidate malaria vaccine in Gambian children. Vaccine. 2005;23:4148–57. doi: 10.1016/j.vaccine.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 125.Reed S, Lobet Y. Tuberculosis vaccine development; from mouse to man. Microbes Infect. 2005;7:922–31. doi: 10.1016/j.micinf.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 126.Adar Y, Singer Y, Levi R, Tzehoval E, Perk S, Banet-Noach C, Nagar S, Arnon R, Ben-Yedidia T. A universal epitope-based influenza vaccine and its efficacy against H5N1. Vaccine. 2009;27:2099–107. doi: 10.1016/j.vaccine.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 127.Nguyen CT, Hong SH, Ung TT, Verma V, Kim SY, Rhee JH, Lee SE. Intranasal immunization with a flagellin-adjuvanted peptide anticancer vaccine prevents tumor development by enhancing specific cytotoxic T lymphocyte response in a mouse model. Clin Exp Vaccine Res. 2013;2:128–34. doi: 10.7774/cevr.2013.2.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vollmer J, Krieg AM. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv Drug Deliv Rev. 2009;61:195–204. doi: 10.1016/j.addr.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 129.Vabulas RM, Pircher H, Lipford GB, Häcker H, Wagner H. CpG-DNA activates in vivo T cell epitope presenting dendritic cells to trigger protective antiviral cytotoxic T cell responses. J Immunol. 2000;164:2372–8. doi: 10.4049/jimmunol.164.5.2372. [DOI] [PubMed] [Google Scholar]
- 130.Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. 2011;10:499–511. doi: 10.1586/erv.10.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Daftarian P, Sharan R, Haq W, Ali S, Longmate J, Termini J, Diamond DJ. Novel conjugates of epitope fusion peptides with CpG-ODN display enhanced immunogenicity and HIV recognition. Vaccine. 2005;23:3453–68. doi: 10.1016/j.vaccine.2005.01.093. [DOI] [PubMed] [Google Scholar]
- 132.Meshcheryakova E, Makarov E, Philpott D, Andronova T, Ivanov V. Evidence for correlation between the intensities of adjuvant effects and NOD2 activation by monomeric, dimeric and lipophylic derivatives of N-acetylglucosaminyl-N-acetylmuramyl peptides. Vaccine. 2007;25:4515–20. doi: 10.1016/j.vaccine.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 133.Moreira LO, Smith AM, DeFreitas AA, Qualls JE, El Kasmi KC, Murray PJ. Modulation of adaptive immunity by different adjuvant-antigen combinations in mice lacking Nod2. Vaccine. 2008;26:5808–13. doi: 10.1016/j.vaccine.2008.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zaman M, Skwarczynski M, Toth I. Toll-Like Receptor 2 Mediated Dendritic Cell Activation-Key Target for Lipopeptide Vaccines Design. Cell Bio Res Prog 2010:63-80. [Google Scholar]
- 135.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440:808–12. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 136.Zeng W, Ghosh S, Lau YF, Brown LE, Jackson DC. Highly immunogenic and totally synthetic lipopeptides as self-adjuvanting immunocontraceptive vaccines. J Immunol. 2002;169:4905–12. doi: 10.4049/jimmunol.169.9.4905. [DOI] [PubMed] [Google Scholar]
- 137.Deres K, Schild H, Wiesmüller KH, Jung G, Rammensee HG. In vivo priming of virus-specific cytotoxic T lymphocytes with synthetic lipopeptide vaccine. Nature. 1989;342:561–4. doi: 10.1038/342561a0. [DOI] [PubMed] [Google Scholar]
- 138.Eriksson EMY, Jackson DC. Recent advances with TLR2-targeting lipopeptide-based vaccines. Curr Protein Pept Sci. 2007;8:412–7. doi: 10.2174/138920307781369436. [DOI] [PubMed] [Google Scholar]
- 139.Chua BY, Eriksson EM, Brown LE, Zeng W, Gowans EJ, Torresi J, Jackson DC. A self-adjuvanting lipopeptide-based vaccine candidate for the treatment of hepatitis C virus infection. Vaccine. 2008;26:4866–75. doi: 10.1016/j.vaccine.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kamo K, Jordan R, Hsu H, Hudson D. Development of a monoclonal antibody to the conserved region of p34cdc2 protein kinase. J Immunol Methods. 1992;156:163–70. doi: 10.1016/0022-1759(92)90022-L. [DOI] [PubMed] [Google Scholar]
- 141.Skwarczynski M, Zaman M, Toth I. Lipo-peptides/saccharides in peptide vaccine delivery. In handbook of the biologically active peptides 2013:571-9. [Google Scholar]
- 142.Zaman M, Abdel-Aal AB, Phillipps KSM, Fujita Y, Good MF, Toth I. Structure-activity relationship of lipopeptide Group A streptococcus (GAS) vaccine candidates on toll-like receptor 2. Vaccine. 2010;28:2243–8. doi: 10.1016/j.vaccine.2009.12.046. [DOI] [PubMed] [Google Scholar]
- 143.Olive C, Hsien K, Horváth A, Clair T, Yarwood P, Toth I, Good MF. Protection against group A streptococcal infection by vaccination with self-adjuvanting lipid core M protein peptides. Vaccine. 2005;23:2298–303. doi: 10.1016/j.vaccine.2005.01.041. [DOI] [PubMed] [Google Scholar]
- 144.Zhong W, Skwarczynski M, Toth I. Lipid Core Peptide System for Gene, Drug, and Vaccine Delivery. Aust J Chem. 2009;62:956–67. doi: 10.1071/CH09149. [DOI] [Google Scholar]
- 145.Moyle PM, Olive C, Ho M-F, Pandey M, Dyer J, Suhrbier A, Fujita Y, Toth I. Toward the development of prophylactic and therapeutic human papillomavirus type-16 lipopeptide vaccines. J Med Chem. 2007;50:4721–7. doi: 10.1021/jm070287b. [DOI] [PubMed] [Google Scholar]
- 146.Apte SH, Groves PL, Skwarczynski M, Fujita Y, Chang C, Toth I, Doolan DL. Vaccination with lipid core peptides fails to induce epitope-specific T cell responses but confers non-specific protective immunity in a malaria model. PloS one 2012; 7:e40928-e. [DOI] [PMC free article] [PubMed]
- 147.Lau YF, Deliyannis G, Zeng W, Mansell A, Jackson DC, Brown LE. Lipid-containing mimetics of natural triggers of innate immunity as CTL-inducing influenza vaccines. Int Immunol. 2006;18:1801–13. doi: 10.1093/intimm/dxl114. [DOI] [PubMed] [Google Scholar]
- 148.Kumar A, Arora R, Kaur P, Chauhan VS, Sharma P. “Universal” T helper cell determinants enhance immunogenicity of a Plasmodium falciparum merozoite surface antigen peptide. J Immunol. 1992;148:1499–505. [PubMed] [Google Scholar]
- 149.Zaman M, Abdel-Aal ABM, Fujita Y, Phillipps KSM, Batzloff MR, Good MF, Toth I. Immunological evaluation of lipopeptide group A streptococcus (GAS) vaccine: structure-activity relationship. PLoS One. 2012;7:e30146. doi: 10.1371/journal.pone.0030146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Brown LE, Jackson DC. Lipid-based self-adjuvanting vaccines. Curr Drug Deliv. 2005;2:383–93. doi: 10.2174/156720105774370258. [DOI] [PubMed] [Google Scholar]