Abstract
Sm-p80, the large subunit of Schistosoma masoni calpain, is a leading antigen candidate for a schistosome vaccine. Prophylactic and antifecundity efficacy of Sm-p80 has been tested using a variety of vaccine approaches. However, the mechanism of Sm-p80-mediated killing is still unknown. In this study, potential role of complement in Sm-p80-mediated protection was studied using both in vitro (cobra venom factor inhibition) and in vivo using mice deficient in C3 (C3 −/−; B6.129S4-C3tm1Crr/J). In the absence of C3, Sm-p80-based vaccine was able to provide significant reduction in adult worm burden following challenge with schistosome cercariae in mice suggesting the effector functions of complement may be limited in this vaccine-induced protection.
Keywords: Schistosoma, Sm-p80, calpain, complement, schistosomiasis, vaccine
Introduction
Development of an effective prophylactic as well as morbidity and transmission reducing vaccine against schistosomiasis would be considered an important milestone in the control of this major neglected tropical disease. Schistosomiasis is a serious public health concern to one billion people (200 million currently infected; additional 779 million at risk to acquire the infection) in 74 countries.1-5 In spite of advances in control via snail eradication, improved sanitation, and Mass Drug Administration (MDA) using praziquantel; this disease continues to be sub-optimally controlled.6-9 Presently, there is no vaccine for this disease. An effective vaccine that reduces pathology and transmission rates coupled with the existing MDA programs would be extremely helpful in reducing the burden of schistosomiasis and will save millions of lives.10-12 The discovery of Schistosoma mansoni calpain by our group13 and later by others,14 has led to the understanding of the role of this protein in surface membrane biogenesis, a mechanism by which schistosomes evade the hostile host immune response.15,16 Reversing the immune evasion mechanisms by vaccination with calpain is therefore an excellent target for a new schistosome vaccine. The large subunit of calpain, Sm-p80, is now a first tier vaccine candidate for S. mansoni,17-31 S. japonicum,32,33 and possibly for S. hematobium34 infections. In addition, Sm-p80 has been found to be expressed in all of the different schistosome life cycle stages15,35,36 and, importantly, is localized in the surface syncytium,15,37 making this host interactive protein a potential target of innate and adaptive immune responses.
The mechanism by which Sm-p80-mediated protection is conferred has still not been fully elucidated. It has previously been shown that schistosomula are susceptible to immune killing in vitro by anti-Sm-p80 antibodies in the presence of exogenous complement.38 Recently, an important role for antibodies was observed in Sm-p80-mediated protection.39,40 In toto, these studies appear to suggest that serum components play a significant role in Sm-p80 mediated protection. In this study we have determined the potential requirement of complement in conferring the Sm-p80 mediated protective immunity with in vitro inactivation of complement by cobra venom factor and via the use of mice deficient in C3 (C3 −/−; B6.129S4-C3tm1Crr/J).
Results
Role of complement in killing of schistosomula in vitro
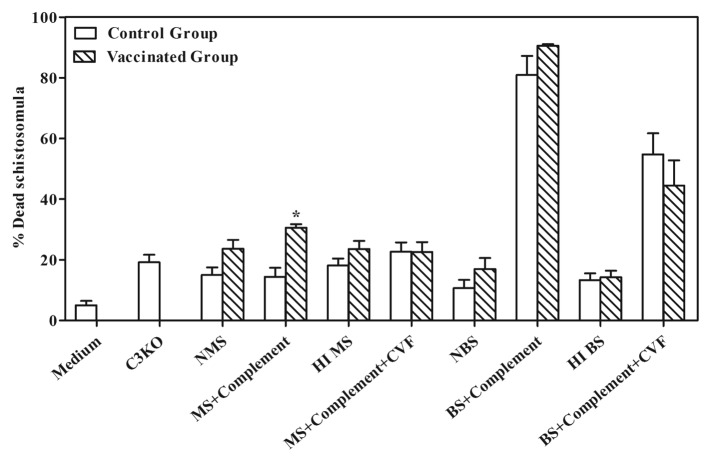
The efficiency of killing in vitro was assessed by determining and comparing the percentages of dead schistosomula maintained in Roswell Park Memorial Institute (RPMI) 1640 medium, serum alone, heat inactive serum, or serum plus exogenous complement with and without cobra venom factor (CVF) (Fig. 1). The antibodies to Sm-p80, generated in mouse and baboons, following vaccination with a Sm-p80-based vaccine formulation,17,21 were able to kill schistosomula in vitro in the presence of exogenous complement. The complement-mediated killing effect on schistosomula was partially reversed when CVF was used; this effect was more pronounced when used with the mouse sera but not as remarkable with the baboon sera (Fig. 1).
Figure 1. In vitro killing of schistosomula in the presence or absence of cobra venom factor (CVF). Normal Mouse and baboon sera were collected from rSm-p80 immunized mice and baboons [for details, please see Ahmad et al. (Reference 17 and 21)] and serially diluted from 1:4 to 1:64. Schistosomula were separately co-cultured with medium, serum alone, heat inactive serum, or serum plus exogenous complement with or without CVF for 24 h; viability of schistosomula was checked under a microscope. The results from optimally diluted sera are shown in the histogram. NMS = normal mice serum; HIMS = heat inactive mouse serum; MS + Complement = mouse serum plus complement; MS + Complement + CVF = mice serum plus complement with CVF; NBS = normal baboon serum; HIBS = heat inactive baboon serum; BS + Complement = baboon serum plus complement; BS + Complement + CVF = baboon serum plus complement with CVF.
Role of complement in Sm-p80-mediated protection in vivo
A distinct role of complement in Sm-p80-mediated protection was confirmed via the use of mice deficient in complement. In two separate experiments, vaccination of wild type (C57BL/6) mice with Sm-p80 plus CpG-ODNs resulted in a reduction of 52.86% in adult worm burden. However, the adult worm reduction was decreased to 34.33% when complement C3 deficient mice (B6.129S4-C3tm1crr/ J mice) were immunized with the same vaccine formulation (Sm-p80 + CpG ODNs) as the wild type mice were. More importantly, only 13.58% reduction in worm burden was observed between vaccinated groups of wild type and C3 deficient mice (Table 1). In the same experiments, a much lower levels (15–25-fold) of trapped eggs in tissues (liver and intestine) of C3 knockout mice was observed compared with the wild type animals (Table 2).
Table 1. Anti-Worm effect in C57BL/6 and B6.129S4-C3tm1crr/ J mice after immunization.
| 2013HV0390R _T1.tif | |
Table 2. Anti-Egg effect in C57BL/6 and B6.129S4-C3tm1crr/ J mice after immunization.
| 2013HV0390R _T2.tif | |
Antibody response to Sm-p80 in immunized mice
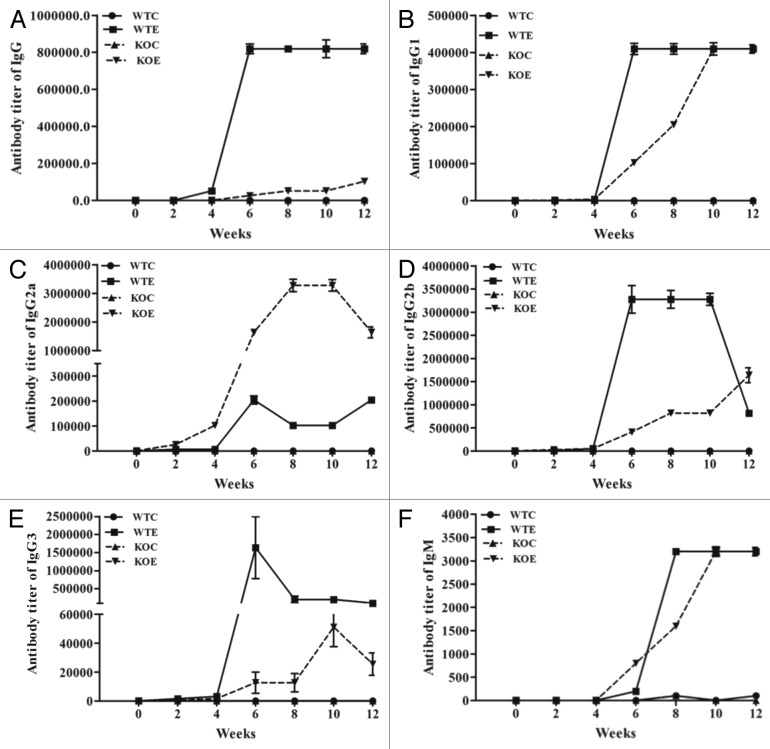
Distinct antibody titers were obtained for the total IgG and its subtypes (IgG1, IgG2a, IgG2b, and IgG3) and IgM in both the wild type and C3 knockout mice (Fig. 2). Furthermore, in the sera obtained from both the wild type and knockout mice, titers started to rise between 4–6 wk and remained steady for several weeks for total IgG (Fig. 2A), IgG1 (Fig. 2B), IgG2a (Fig. 2C), IgG2b (Fig. 2D), IgG3 (Fig. 2E), and IgM (Fig. 2F). Robust titers for IgG2a were detected in C3 knockout mice vaccinated with Sm-p80 vaccine (Fig. 2C). However, the titers of IgG, IgG2b, and IgG3 were lower in C3 knockout group compared with the wild type group (Fig. 2A, D, and E).
Figure 2. Sm-p80 specific antibody titers in immunized mice. The experimental groups (immunized with rSm-p80 formulated in ODN) elicited strong humoral immune responses. The titers of IgG (A), IgG1(B), IgG2a (C), IgG2b (D), IgG3 (E), and IgM (F) in experimental groups were found to be higher compared with their respective control groups. All of the values represent as mean of three experiments ± standard deviation. WTC, wild type control group; WTE, wild type experimental group; KOC, C3 knockout control group; KOE, C3 knockout experimental group.
Expression of cytokine message levels
mRNA expression of 21 cytokines was estimated via the Real-time PCR. The profiles of mRNA expression are summarized in Figure 3. Briefly, expression levels of IL-2 were upregulated at 24 h and steadily increased at 48 h in rSm-p80 plus CpG-ODNs vaccinated groups (Fig. 3). The mRNA levels of IL-21 were also upregulated in rSm-p80 plus CpG-ODNs vaccinated groups at 24 h (Fig. 3A) and at 48h the expression of IFN-γ was strikingly pronounced in rSm-p80 plus CpG-ODNs vaccinated groups (Fig. 3B). IL-17 was another cytokine which was upregulated in vaccinated wild type mice at 48h (Fig. 3B). Some other cytokines that were differentially expressed include: Th1 cytokines (TNFα) and Th2 cytokines (IL-5) in vaccinated wild type group at 24 h; Th2 cell cytokines (IL-4) and inflammatory cytokines (IL-8) in knockout vaccinated group at 48 h (Fig. 3B).
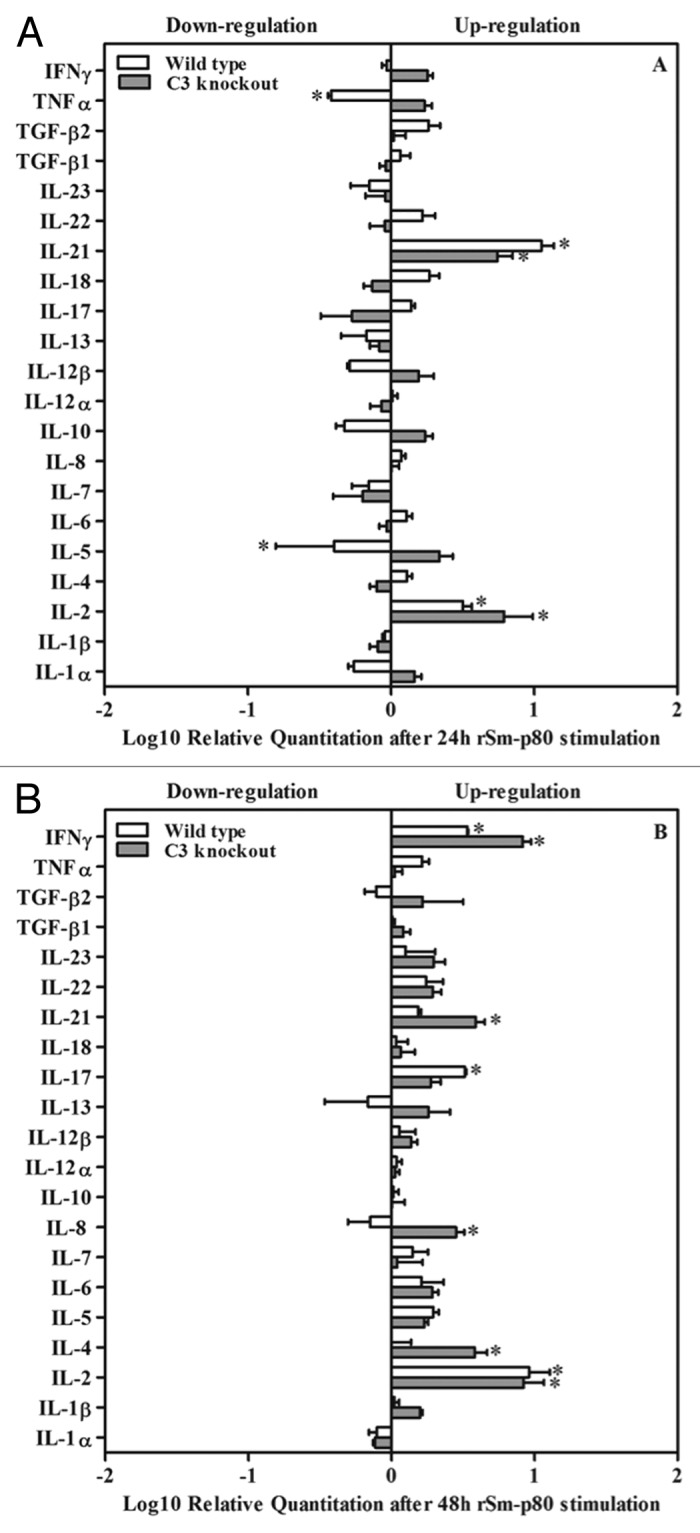
Figure 3. Cytokine mRNA expression assay by RT-PCR. After 24 h or 48 h incubation with rSm-p80, RNA was extracted from stimulated splenocytes and real-time RT-PCR was performed. Relative ratio of mRNA expression was demonstrated in comparison between wild type control group and wild type experimental group after 24 h (A) and 48 h (B) stimulation by rSm-p80. Relative ratios were demonstrated in comparison between complement knockout control group and complement knockout experimental group after 24 h (A) and 48 h (B) stimulation by rSm-p80. The relative cytokine mRNA expression levels were calculated by comparing the differences in the message levels of the control group with the respective experimental group after standardization using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) through DataAssist software V3.0.
Th1, Th2, and Th17 subset assay via Fluorescence-activated cell sorting (FACS)
To explore the potential role of Th1, Th2, and Th17 responses, flow cytometry was performed to observe differences in splenocytes following rSm-p80 re-stimulation in vitro (Fig. 4A–C). Briefly, the splenocytes from rSm-p80 plus CpG-ODNs groups secreted 1.08 to 2.79% more IFN-γ than their respective control groups (Fig. 4C). Interestingly, IFN-γ secretion from splenocytes obtained from the knockout group was much higher than any other group (Fig. 4C). Moreover, all of rSm-p80 plus CpG-ODNs vaccine groups also had slight increase of IL-17A (0.26% to 2.56% in the wild type group; 0.08% to 1.39% in the knockout group) than their respective group after rSm-p80 stimulation (Fig. 4B). After 48 h co-incubation with rSm-p80, the signals of IL-4 exhibited no significant changes among the different groups (Fig. 4A).
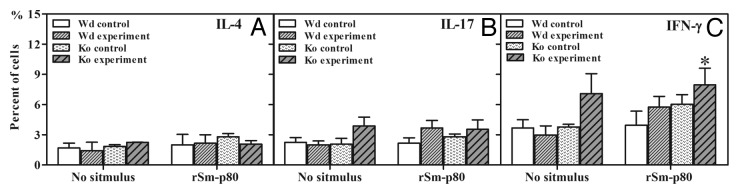
Figure 4. Intracellular interferon-γ (IFN-γ), interleukin 4 (IL-4), and interleukin 17 (IL-17) staining of CD3+ CD4+ cells isolated from spleens. Initial gating was performed using the CD4 marker. The percentage of IL-4 secreted cells in control and experimental groups are shown in panel (A). The percentage of IL-17 secreted cells in control and experimental groups are shown in panel (B). The percentage of IFN-γ secreted cells in control and experimental groups are shown in panel (C). Wd, wild type; KO, C3 knockout.
Discussion
Results presented here indicate that complement plays a minimal role in Sm-p80-mediated protection but the extent of its involvement or absolute requirement for vaccine-mediated killing in vivo still remains unclear. In vitro experimentation using sera obtained from both mice17 and baboons21 that received Sm-p80 vaccine showed killing of schistosomula in the presence of complement. The extent of larval killing was reduced when sera were heat inactivated or CVF was introduced in the incubation media. These findings are consistent with earlier studies by our group using baboon sera38 and by others using mouse sera.41,42 However, when the worm reduction between wild type and C3 knockout groups that received Sm-p80 vaccine was compared, no significant effect (13.58% reduction) was observed; suggesting that complement depletion had minimal effect on the Sm-p80 vaccine-induced protection. Interestingly, in C3 knockout mice, compared with the wild type, 15- and 25-fold lesser number of eggs were recovered both in the control and vaccine groups, respectively. These egg data appear to suggest that complement may be involved in the development of egg-induced pathology.43 It has previously been reported that C3 does not play a direct anti-schistosome effector role but it may have some function in anti-schistosome immune responses.44
In our study, IgG, IgG2b, and IgG3 in the C3 knockout experimental group were significantly lower than the wild type group and also IgG1 and IgM in the C3 knockout experimental group took 2 to 4 wk longer to reach the peak levels. However, IgG2a was surprisingly higher in the C3 knockout experimental group compared with the wild type group. This is indicative that the complement plays a role in IgG class-switching. Similarly, previous studies have shown that protein vaccines formulated with adjuvants could produce higher titers of IgG2a in mice and IgG1 in non-human primates.43,45
Cytokine secretion is another distinct characteristic in innate and adaptive immune response. IL-2 is a growth, survival, and differentiation factor for T lymphocytes. In our studies, after rSm-p80 co-culture, in both experimental groups the production of IL-2 was increased. It indicates that rSm-p80 could be involved in triggering the release of IL-2 from the activated T cells. Apparently the T cell activation is not influenced by C3 deletion. This phenomenon may be related to the dose of antigen. After 3 times vaccination, high dose of antigen may overcome the inhibition on T cells caused by C3 absence, as has been reported in other systems.46,47 Moreover, our results show higher expression levels of IFN-γ synthesis in C3 knockout experimental group compared with the wild type experimental group which is consistent with the previous findings.44,48 In our study, the absence of C3 resulted in the reduction of Th2 cytokines and inflammatory cytokines, such as IL-4, which indicate that C3 is useful in the Th2 differentiation. However, Th17 responses in our study were not significantly altered due to the C3 deficiency.
In summary Sm-p80 is an effective protein vaccine candidate for schistosomiasis and over 30% protection in C3 deficient mice (C3 −/−; B6.129S4-C3tm1Crr/J) is indicative of minimal effector functions of complement in Sm-p80 mediated protection. Nevertheless, the lack of C3 lessened to some extent the robust protection efficacy of Sm-p80 mediated protection this may perhaps be due to the disturbance in the networking of innate and adaptive immune responses.
Methods
Parasites
Biomphalaria glabrata snails infected with Schistosoma mansoni (NMRI strain) were obtained from the Schistosomiasis Resource Center, Biomedical Research Institute, Rockville, MD, USA.
Viability of schistosomula in the presence or absence of Cobra Venom Factor (CVF)
Snails were directly exposed to light for 1 h to stimulate the release of cercariae. Following centrifugation, schistosomula were separated from the tails of mechanically transformed cercariae. Schistosomula were then prepared in mouse or baboon sera. Optimum dilutions of sera were selected to perform the experiment from a range of 1:4 to 1:64. Heat inactive sera were prepared after 30 min incubation at 56 °C. Twenty schistosomula were seeded into each well and maintained with medium, serum alone, heat inactivated serum or serum plus fresh complement (1 CH50 unit per well) (sera, complement guinea pig, Sigma) with or without CVF (62.5ng/well) (Sigma) for culturing in 96 well plates overnight at 37 °C and 5% CO2. After incubation, numbers of live and dead parasites were counted, and efficiency of killing was assessed by comparing the percentage of dead parasites. Statistical analysis was done between the corresponding control and experimental group.
Animals and immunization groups
Normal C57BL/6 mice and complement C3 deficient mice (B6.129S4-C3tm1crr/J mice) were purchased from the Jackson Laboratory. A total of 20 mice (four groups with five mice per group) were immunized intramuscularly with CpG-ODN and recombinant Sm-p80 as follows: Group 1 (without protein normal control) animals were immunized with 50 µg Control CpG-ODN #2137 (TGCTGCTTTT GTGCTTTTGT GCTT; Coley Pharmaceutical Group) at week 0 and boosted with an equal amount of Control ODN #2137 alone at weeks 4 and 8. Group 2 (normal experimental) mice were immunized with 50 µg ODN #10104 (TCGTCGTTTC GTCGTTTTGT CGTT; Coley Pharmaceutical Group) combined with 25 µg recombinant Sm-p80 at week 0 and boosted at weeks 4 and 8 with the same combinations. Similarly, Group 3 (knockout control) and Group 4 (knockout experimental) mice were injected with the same vaccine formulation as Groups 1 and 2. This experiment was repeated with an additional 20 mice.
Challenge infection, necropsy and estimation of worm and egg burdens in the animal tissue
Four weeks after the second boost, mice from the four groups were challenged with 150 cercariae of S. mansoni through tail exposure. The animals were sacrificed 6 wk after challenge; adult worms were perfused from the hepatic portal system and also manually removed from the mesenteric veins. The number of worms recovered from each mouse (worm burden) was recorded, and percent reduction of worm burden between control and vaccinated animals was calculated.17,18,30 After the mice were sacrificed, liver and intestine samples were collected from each animal and digested in 4% KOH. The number of eggs present in the tissue was determined and percent reduction in egg production was calculated.17,18,30
Enzyme-linked immunosorbent assays
Sera obtained following bleeding of all animals were pooled in their respective groups and ultimately used to determine antibody response. Briefly, 96-well microtiter plates were coated with 1.2 µg of recombinant Sm-p80 per well. The antigen-coated wells were sequentially incubated with serial dilutions of test sera and optimally diluted horseradish peroxidase-labeled secondary antibody. To estimate the levels of IgM and IgG and its subtypes, HRP-conjugated anti-mouse IgG, IgG3, and IgM (Alpha Diagnostic International, Inc.) were used at a 1:4000 dilution, and HRP-conjugated anti-mouse IgG1, IgG2a, and IgG2b (Alpha Diagnostic International, Inc.) were used at a 1:10,000 dilution. The enzyme-linked immunosorbent assays (ELISAs) were performed as described previously.17,30 All of the samples were assayed in triplicate. Results are expressed as end-point titers calculated from a curve of optical density versus serum dilution, to a cut-off of two standard deviations above background control values. Results are expressed as mean ± SD.
RNA extraction, cDNA synthesis, and real-time PCR
After sacrificing the animals, spleens were removed aseptically and then meshed through a fine screen in Roswell Park Memorial Institute (RPMI) 1640 medium (HyClone) containing 10% fetal bovine serum (FBS). The splenocytes were collected via centrifugation and the pellet was stored in Opti-Freeze DMSO Cryopreservation Medium (Fisher Scientific), at −85 °C until used. The day of assay, cells were thawed and plated at 1–2 × 107 cells/mL in a final volume of 3mL per well in 6-well flat-bottom plates containing RPMI 1640 medium (HyClone) supplemented with 10% FBS and 100 μg/mL each of penicillin and streptomycin and 10 μg/mL gentamicin. Cells were cultured in the presence or absence of recombinant Sm-p80 antigen, and were maintained at 37 °C in 5% CO2 for 24 and 48 h, respectively. Total RNA was extracted by the TRIzol method (Invitrogen Corp.). Reverse transcription reactions for first strand cDNA synthesis were performed as described previously. For these 24 and 48 h batches, relative quantities of cytokines, including IL-1α, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8 ( = MIP-2), IL-10, IL-12α, IL-12β, IL-13, IL-17, IL-18, IL-21, IL-22, IL-23, TNF-α, IFN-γ, TGF-β1, and TGF-β2, were determined by the Applied Biosystem StepOnePlusTM Real-time PCR System, and levels of cytokine gene expression for each sample were normalized to GADPH expression, and analyzed by Comparative CT method, to determine their relative differences by using DataAssistTMsoftware computer program (Applied Biosystem).
Flow cytometry analysis
The relative percentage of Th1, Th2, and Th17-type CD4+ T-cells was determined by flow cytometry. The harvested splenocytes, pooled from each group, were seeded into 24 well plates (2 × 106 cells per well) for the assay. The cells were incubated with or without rSm-p80 (12 μg/mL) overnight and 4 h before the end of incubation, Brefeldin A at a 1:1000 dilution was added. For the immune phenotyping of Th1-, Th2-, and Th17-CD4+ T-cells, isolated splenocytes were stained with PerCP-CY5.5-conjugated anti-CD4+ to identify CD4+ T-cells. Intracellular staining was performed with PE-conjugated anti-IFN-γ, APC-conjugated anti-IL-4 and FITC-conjugated IL-17 to detect IFN-γ, IL-4, and IL-17-secreting CD4+ T-cells, respectively. The data were obtained using BD CellQuest™ Prosoftware (BD Biosciences) and analyzed in FlowJo (Tree Star, Inc.).
Statistical Analysis
Significance between groups was calculated via one-way ANOVA and within groups by paired t tests, using the GraphPad Prism 5.04 computer program. Bonferroni adjustments were included for multiple comparisons to reduce the risk of reaching false conclusions by chance. P values obtained by these methods were considered significant if they were <0.05.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported in part by a grant from National Institute of Allergy and Infectious Diseases (R01A171223) to A.A.S.
References
- 1.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–18. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 2.Gryseels B. Schistosomiasis. Infect Dis Clin North Am. 2012;26:383–97. doi: 10.1016/j.idc.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Hotez PJ, Fenwick A. Schistosomiasis in Africa: an emerging tragedy in our new global health decade. PLoS Negl Trop Dis. 2009;3:e485. doi: 10.1371/journal.pntd.0000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rollinson D, Knopp S, Levitz S, Stothard JR, Tchuem Tchuenté LA, Garba A, Mohammed KA, Schur N, Person B, Colley DG, et al. Time to set the agenda for schistosomiasis elimination. Acta Trop. 2013;128:423–40. doi: 10.1016/j.actatropica.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Ross AG, Bartley PB, Sleigh AC, Olds GR, Li Y, Williams GM, McManus DP. Schistosomiasis. N Engl J Med. 2002;346:1212–20. doi: 10.1056/NEJMra012396. [DOI] [PubMed] [Google Scholar]
- 6.King CH. Parasites and poverty: the case of schistosomiasis. Acta Trop. 2010;113:95–104. doi: 10.1016/j.actatropica.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King CH, Olbrych SK, Soon M, Singer ME, Carter J, Colley DG. Utility of repeated praziquantel dosing in the treatment of schistosomiasis in high-risk communities in Africa: a systematic review. PLoS Negl Trop Dis. 2011;5:e1321. doi: 10.1371/journal.pntd.0001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parker M, Allen T. Does mass drug administration for the integrated treatment of neglected tropical diseases really work? Assessing evidence for the control of schistosomiasis and soil-transmitted helminths in Uganda. Health Res Policy Syst. 2011;9:3. doi: 10.1186/1478-4505-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prichard RK, Basáñez MG, Boatin BA, McCarthy JS, García HH, Yang GJ, Sripa B, Lustigman S. A research agenda for helminth diseases of humans: intervention for control and elimination. PLoS Negl Trop Dis. 2012;6:e1549. doi: 10.1371/journal.pntd.0001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergquist NR, Leonardo LR, Mitchell GF. Vaccine-linked chemotherapy: can schistosomiasis control benefit from an integrated approach? Trends Parasitol. 2005;21:112–7. doi: 10.1016/j.pt.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 11.Bergquist R, Al-Sherbiny M, Barakat R, Olds R. Blueprint for schistosomiasis vaccine development. Acta Trop. 2002;82:183–92. doi: 10.1016/S0001-706X(02)00048-7. [DOI] [PubMed] [Google Scholar]
- 12.Bergquist R, Utzinger J, McManus DP. Trick or treat: the role of vaccines in integrated schistosomiasis control. PLoS Negl Trop Dis. 2008;2:e244. doi: 10.1371/journal.pntd.0000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karcz SR, Podesta RB, Siddiqui AA, Dekaban GA, Strejan GH, Clarke MW. Molecular cloning and sequence analysis of a calcium-activated neutral protease (calpain) from Schistosoma mansoni. Mol Biochem Parasitol. 1991;49:333–6. doi: 10.1016/0166-6851(91)90078-K. [DOI] [PubMed] [Google Scholar]
- 14.Andresen K, Tom TD, Strand M. Characterization of cDNA clones encoding a novel calcium-activated neutral proteinase from Schistosoma mansoni. J Biol Chem. 1991;266:15085–90. [PubMed] [Google Scholar]
- 15.Siddiqui AA, Zhou Y, Podesta RB, Karcz SR, Tognon CE, Strejan GH, Dekaban GA, Clarke MW. Characterization of Ca(2+)-dependent neutral protease (calpain) from human blood flukes, Schistosoma mansoni. Biochim Biophys Acta. 1993;1181:37–44. doi: 10.1016/0925-4439(93)90087-H. [DOI] [PubMed] [Google Scholar]
- 16.Silva EE, Clarke MW, Podesta RB. Characterization of a C3 receptor on the envelope of Schistosoma mansoni. J Immunol. 1993;151:7057–66. [PubMed] [Google Scholar]
- 17.Ahmad G, Zhang W, Torben W, Haskins C, Diggs S, Noor Z, Le L, Siddiqui AA. Prime-boost and recombinant protein vaccination strategies using Sm-p80 protects against Schistosoma mansoni infection in the mouse model to levels previously attainable only by the irradiated cercarial vaccine. Parasitol Res. 2009;105:1767–77. doi: 10.1007/s00436-009-1646-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmad G, Torben W, Zhang W, Wyatt M, Siddiqui AA. Sm-p80-based DNA vaccine formulation induces potent protective immunity against Schistosoma mansoni. Parasite Immunol. 2009;31:156–61. doi: 10.1111/j.1365-3024.2008.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmad G, Zhang W, Torben W, Damian RT, Wolf RF, White GL, Chavez-Suarez M, Kennedy RC, Siddiqui AA. Protective and antifecundity effects of Sm-p80-based DNA vaccine formulation against Schistosoma mansoni in a nonhuman primate model. Vaccine. 2009;27:2830–7. doi: 10.1016/j.vaccine.2009.02.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahmad G, Zhang W, Torben W, Noor Z, Siddiqui AA. Protective effects of Sm-p80 in the presence of resiquimod as an adjuvant against challenge infection with Schistosoma mansoni in mice. Int J Infect Dis. 2010;14:e781–7. doi: 10.1016/j.ijid.2010.02.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmad G, Zhang W, Torben W, Ahrorov A, Damian RT, Wolf RF, White GL, Carey DW, Mwinzi PN, Ganley-Leal L, et al. Preclinical prophylactic efficacy testing of Sm-p80-based vaccine in a nonhuman primate model of Schistosoma mansoni infection and immunoglobulin G and E responses to Sm-p80 in human serum samples from an area where schistosomiasis is endemic. J Infect Dis. 2011;204:1437–49. doi: 10.1093/infdis/jir545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Ridi R, Tallima H. Schistosoma mansoni ex vivo lung-stage larvae excretory-secretory antigens as vaccine candidates against schistosomiasis. Vaccine. 2009;27:666–73. doi: 10.1016/j.vaccine.2008.11.039. [DOI] [PubMed] [Google Scholar]
- 23.El Ridi R, Tallima H, Mahana N, Dalton JP. Innate immunogenicity and in vitro protective potential of Schistosoma mansoni lung schistosomula excretory--secretory candidate vaccine antigens. Microbes Infect. 2010;12:700–9. doi: 10.1016/j.micinf.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Hota-Mitchell S, Siddiqui AA, Dekaban GA, Smith J, Tognon C, Podesta RB. Protection against Schistosoma mansoni infection with a recombinant baculovirus-expressed subunit of calpain. Vaccine. 1997;15:1631–40. doi: 10.1016/S0264-410X(97)00081-9. [DOI] [PubMed] [Google Scholar]
- 25.Hota-Mitchell S, Clarke MW, Podesta RB, Dekaban GA. Recombinant vaccinia viruses and gene gun vectors expressing the large subunit of Schistosoma mansoni calpain used in a murine immunization-challenge model. Vaccine. 1999;17:1338–54. doi: 10.1016/S0264-410X(98)00391-0. [DOI] [PubMed] [Google Scholar]
- 26.Siddiqui AA, Phillips T, Charest H, Podesta RB, Quinlin ML, Pinkston JR, Lloyd JD, Pompa J, Villalovos RM, Paz M. Enhancement of Sm-p80 (large subunit of calpain) induced protective immunity against Schistosoma mansoni through co-delivery of interleukin-2 and interleukin-12 in a DNA vaccine formulation. Vaccine. 2003;21:2882–9. doi: 10.1016/S0264-410X(03)00159-2. [DOI] [PubMed] [Google Scholar]
- 27.Siddiqui AA, Phillips T, Charest H, Podesta RB, Quinlin ML, Pinkston JR, Lloyd JD, Paz M, Villalovos RM, Pompa J. Induction of protective immunity against Schistosoma mansoni via DNA priming and boosting with the large subunit of calpain (Sm-p80): adjuvant effects of granulocyte-macrophage colony-stimulating factor and interleukin-4. Infect Immun. 2003;71:3844–51. doi: 10.1128/IAI.71.7.3844-3851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siddiqui AA, Pinkston JR, Quinlin ML, Kavikondala V, Rewers-Felkins KA, Phillips T, Pompa J. Characterization of protective immunity induced against Schistosoma mansoni via DNA priming with the large subunit of calpain (Sm-p80) in the presence of genetic adjuvants. Parasite. 2005;12:3–8. doi: 10.1051/parasite/2005121003. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W, Ahmad G, Torben W, Noor Z, Le L, Damian RT, Wolf RF, White GL, Chavez-Suarez M, Podesta RB, et al. Sm-p80-based DNA vaccine provides baboons with levels of protection against Schistosoma mansoni infection comparable to those achieved by the irradiated cercarial vaccine. J Infect Dis. 2010;201:1105–12. doi: 10.1086/651147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W, Ahmad G, Torben W, Siddiqui AA. Sm-p80-based DNA vaccine made in a human use approved vector VR1020 protects against challenge infection with Schistosoma mansoni in mouse. Parasite Immunol. 2010;32:252–8. doi: 10.1111/j.1365-3024.2009.01181.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang W, Ahmad G, Torben W, Siddiqui AA. Schistosoma mansoni antigen Sm-p80: Prophylactic efficacy of a vaccine formulated in human approved plasmid vector and adjuvant (VR 1020 and alum) Acta Trop. 2011;118:142–51. doi: 10.1016/j.actatropica.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ohta N, Kumagai T, Maruyama H, Yoshida A, He Y, Zhang R. Research on calpain of Schistosoma japonicum as a vaccine candidate. Parasitol Int. 2004;53:175–81. doi: 10.1016/j.parint.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 33.Zhang R, Yoshida A, Kumagai T, Kawaguchi H, Maruyama H, Suzuki T, Itoh M, El-Malky M, Ohta N. Vaccination with calpain induces a Th1-biased protective immune response against Schistosoma japonicum. Infect Immun. 2001;69:386–91. doi: 10.1128/IAI.69.1.386-391.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siddiqui AA, Siddiqui BA, Ganley-Leal L. Schistosomiasis vaccines. Hum Vaccin. 2011;7:1192–7. doi: 10.4161/hv.7.11.17017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumagai T, Maruyama H, Hato M, Ohmae H, Osada Y, Kanazawa T, Ohta N. Schistosoma japonicum: localization of calpain in the penetration glands and secretions of cercariae. Exp Parasitol. 2005;109:53–7. doi: 10.1016/j.exppara.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Mourão MM, Dinguirard N, Franco GR, Yoshino TP. Phenotypic screen of early-developing larvae of the blood fluke, schistosoma mansoni, using RNA interference. PLoS Negl Trop Dis. 2009;3:e502. doi: 10.1371/journal.pntd.0000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Braschi S, Wilson RA. Proteins exposed at the adult schistosome surface revealed by biotinylation. Mol Cell Proteomics. 2006;5:347–56. doi: 10.1074/mcp.M500287-MCP200. [DOI] [PubMed] [Google Scholar]
- 38.Siddiqui AA, Pinkston JR, Quinlin ML, Saeed Q, White GL, Shearer MH, Kennedy RC. Characterization of the immune response to DNA vaccination strategies for schistosomiasis candidate antigen, Sm-p80 in the baboon. Vaccine. 2005;23:1451–6. doi: 10.1016/j.vaccine.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 39.Torben W, Ahmad G, Zhang W, Siddiqui AA. Role of antibodies in Sm-p80-mediated protection against Schistosoma mansoni challenge infection in murine and nonhuman primate models. Vaccine. 2011;29:2262–71. doi: 10.1016/j.vaccine.2011.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torben W, Ahmad G, Zhang W, Nash S, Le L, Karmakar S, Siddiqui AA. Role of antibody dependent cell mediated cytotoxicity (ADCC) in Sm-p80-mediated protection against Schistosoma mansoni. Vaccine. 2012;30:6753–8. doi: 10.1016/j.vaccine.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arnon R, Tarrab-Hazdai R, Steward M. A mimotope peptide-based vaccine against Schistosoma mansoni: synthesis and characterization. Immunology. 2000;101:555–62. doi: 10.1046/j.1365-2567.2000.00139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng J, Gold D, LoVerde PT, Fishelson Z. Inhibition of the complement membrane attack complex by Schistosoma mansoni paramyosin. Infect Immun. 2003;71:6402–10. doi: 10.1128/IAI.71.11.6402-6410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Brito T, Hoshino-Shimizu S, da Silva LC, Kanamura H, Costa CM, Pinto PS. Immunopathology of experimental schistosome (S. Mansoni) egg granulomas in mice--possible defence mechanisms mediated by local immune complexes. J Pathol. 1983;140:17–28. doi: 10.1002/path.1711400104. [DOI] [PubMed] [Google Scholar]
- 44.La Flamme AC, MacDonald AS, Huxtable CR, Carroll M, Pearce EJ. Lack of C3 affects Th2 response development and the sequelae of chemotherapy in schistosomiasis. J Immunol. 2003;170:470–6. doi: 10.4049/jimmunol.170.1.470. [DOI] [PubMed] [Google Scholar]
- 45.Buchman GW, Cohen ME, Xiao Y, Richardson-Harman N, Silvera P, DeTolla LJ, Davis HL, Eisenberg RJ, Cohen GH, Isaacs SN. A protein-based smallpox vaccine protects non-human primates from a lethal monkeypox virus challenge. Vaccine. 2010;28:6627–36. doi: 10.1016/j.vaccine.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Böttger EC, Metzger S, Bitter-Suermann D, Stevenson G, Kleindienst S, Burger R. Impaired humoral immune response in complement C3-deficient guinea pigs: absence of secondary antibody response. Eur J Immunol. 1986;16:1231–5. doi: 10.1002/eji.1830161008. [DOI] [PubMed] [Google Scholar]
- 47.Fischer MB, Ma M, Goerg S, Zhou X, Xia J, Finco O, Han S, Kelsoe G, Howard RG, Rothstein TL, et al. Regulation of the B cell response to T-dependent antigens by classical pathway complement. J Immunol. 1996;157:549–56. [PubMed] [Google Scholar]
- 48.Purwar R, Bäumer W, Niebuhr M, Tschernig T, Kietzmann M, Werfel T. A protective role of complement component 3 in T cell-mediated skin inflammation. Exp Dermatol. 2011;20:709–14. doi: 10.1111/j.1600-0625.2011.01295.x. [DOI] [PubMed] [Google Scholar]