Abstract
Two live, attenuated hepatitis A vaccines, H2 and LA-1 virus strains, were developed through serial passages of the viruses in cell cultures at 32 °C and 35 °C respectively. Both vaccines were safe and immunogenic, providing protection against clinical hepatitis A in 95% of the vaccinees, with a single dose by subcutaneous injection. The vaccine recipients were not protected from asymptomatic, subclinical hepatitis A virus (HAV) infection, which induced a similar antibody response as for unvaccinated subjects. A second dose caused anamnestic response and can be used for boosting. Oral immunization of human with H2 vaccine or of marmoset with LA-1 vaccine failed, and no evidence was found for person-to-person transmission of H2 strain or for marmoset-to-marmoset transmission of LA-1 strain by close contact. H2 strain was genetically stable when passaged in marmosets, humans or cell cultures at 37 °C; 3 consecutive passages of the virus in marmosets did not cause virulence mutation. The live vaccines offer the benefits of low cost, single dose injection, long- term protection, and increased duration of immunity through subclinical infection. Improved sanitation and administration of 150 million doses of the live vaccines to children had led to a 90% reduction in the annual national incidence rate of hepatitis A in China during the 16-year period, from 1991 to 2006. Hepatitis A (HA) immunization with both live and inactivated HA vaccines was implemented in the national routine childhood immunization program in 2008 and around 92% of the 16 million annual births received the affordable live, attenuated vaccines at 18 months of age. Near elimination of the disease was achieved in a county of China for 14 years following introduction of the H2 live vaccine into the Expanded Immunization Program (EPI) in 1992.
Keywords: hepatitis A, live attenuated, vaccine, immunization, China
Introduction
Hepatitis A (HA), transmitted through fecal-oral route from human to human, is a significant health problem worldwide and hepatitis A virus (HAV) vaccines have been recommended for routine childhood immunization.1 Adults are more likely to contract symptomatic HA with acute infection and less likely to get subclinical HAV infection than young children.2,3 Improved sanitation in many nations has decreased the risk of HAV infection in children thereby increasing the proportion of susceptible adults.2-4 HA vaccines have thus also been recommended for adults at high risk.5 Both inactivated and live, attenuated HA vaccines were developed.6-11 This is a review of previous publications on live, attenuated HA vaccines. It includes the following 4 parts: (1) preclinical studies on the vaccine development; (2) clinical studies on the vaccine safety and immunogenicity; (3) field evaluation of vaccine protection; (4) HA immunization program and possibility of HAV elimination with the live vaccines in China.
Preclinical Studies
Attenuation of the virus strains
Both H2 and LA-1 strains were derived from fecal specimens of HA patients in China and were isolated in cell cultures.10,11 For preparation of the H2 vaccine, the original wild virus strain was passaged in new monkey kidney (NMK) cells 15 times at 35 °C, 5 times at 32 °C, and passaged in human diploid fibroblasts (KMB17) 4 times at 32 °C.10 The LA-1 strain was obtained after 4 serial passages of the virus in human fibroblast cell cultures (2BS) at 32 °C , followed by 17–23 passages in the same cells at 35 °C.11
Animal studies
The attenuated H2 virus (106.5 TCID50), administrated to 45 anti-HAV negative Rhesus monkeys by intravenous (iv) injection, did not cause elevated liver enzyme activity or any pathologic change in the liver, yet it induced immune responses in 43 animals (96%).12 When injected iv into 11 marmosets (Callithrix jacchus) for 2 serial passages, H2 caused a mild elevation in the levels of liver enzyme activity in 1 of the 6 marmosets after second passage; but no hepatic pathological change was found and all marmosets sero-converted to anti-HAV13; also, there was no evidence for elevated alanine-aminotransferase (ALT) in humans who had received H2 virus.13 LA-1 (104.5 - 5.5 TCID50) injected by iv into marmosets (Saguinus mystax), caused a mild and transient elevation of liver enzyme activity in 3 of 6 animals, but not in the 30 human volunteers who had received the same virus strain by intramuscular injection11; similarly, no hepatic pathologic change was found in the marmosets and all animals seroconverted to neutralizing antibody.11 Oral transmission of LA-1 to a marmoset did not cause sero-conversion to anti-HAV.14 A marmoset kept in the same cage with another marmoset that had received LA-1 strain and was shedding the virus in feces, did not sero-convert to anti-HAV, nor did it show elevated liver enzyme activities.11 It appeared that LA-1 strain was not transmitted from marmoset-to-marmoset by oral route or close contact.
Provost developed F’, an HAV strain which was attenuated in cell cultures at 35 °C.15 The vaccine candidate was safe and immunogenic in human volunteers, yet it caused slight inflammation in the liver biopsy of infected chimpanzees. Karron RA reported another live HAV vaccine, prepared by serial passages of HM175 strain in African Green Monkey kidneys and MRC-5 fibroblast cells at 35 °C.16 This vaccine candidate was also safe and immunogenic in humans, yet caused elevation in levels of liver enzyme activity and hepatic pathologic changes in both marmosets and chimpanzees.17 Hence, compared with the wild HAV, all the 4 attenuated HAV strains appeared to be less virulent in marmosets and avirulent in humans.
Genetic stability of H2 strain
H2w, the progenitor of H2 strain, was serially propagated in KMB17 cells up to 30 passages at 35 °C or 32 °C (for the passages from 15 to 20). Pair-wise comparisons of the nearly full-length genomic sequences were performed between H2w and its 6 progenies collected at passage 5, 10, 15, 20, 25, and 30 respectively.18 Most mutations occurred by passage 5 and all mutations were observed by passage 15. These mutations remained stable throughout the prolonged 15–30 passages in KMB17 cells.18
To assess the genetic stability in mammalian hosts and cell cultures at higher temperature, the H2 strain was passaged 1–2 times in marmosets (M1 and M2), or 7 times in KMB 17 cells at 37 °C plus once in marmosets (K15), or once in human volunteers plus once in marmosets (V). The nucleotide sequence identity between these four derivative virus strains and the H2 virus was 99.4% for M1; 99.4% for M2; 98.8% for V, and 99.4% for K15 compared with 90.5% for the wild type of HAV1 and 89.9% for the wild type of HAV2.13 Hence, H2 virus is genetically stable when grown in animals or cell cultures at 37 °C.
Potential reverse mutation of H2 strain through serial passages in marmosets
H2 strain was passaged three times consecutively in marmosets by iv in order to check reverse mutation of H2 strain. The vaccine strain at a titer of 106.82 - 7.33 TCID50, was used for the first passage in the marmosets.19 Fecal specimens collected from the marmosets after the 1st or 2nd passage of the virus, were inoculated in cell cultures for virus replication respectively. The viral materials from the cell cultures as well as the fecal specimens in 20% suspensions, were used for the second and third passages. All 13 marmosets for the passage study seroconverted, the Geometric Mean Titer (GMT) of anti-HAV after each of the passages, ranged between 1:128 and 1:256 compared with 1:3,043 for the 7 control animals that had been infected with wild HAV.19 Confirmed hepatic pathological changes were observed only in the 7 control animals, not in the 13 marmosets for the passage study.19 Hence, no reverse mutation of H2 strain could be found after the three passages of the strain in marmosets. Similar report was given by Provost who failed to find virulence mutations for the live, attenuated hepatitis A virus, CR326F strain, which had been passaged three times in marmosets.20
Clinical Studies
Vaccine safety
Twelve anti-HAV sero-negative adult volunteers received the experimental H2 vaccine (106.5 TCID 50) subcutaneously and were followed for 20 wk.10 Neither elevation of ALT, nor severe local or systematic reaction was detected by weekly or biweekly examinations during the first 12 wk, and then every 4 wk afterwards; all developed anti-HAV. Fecal viral shedding could be detected only in cell cultures of concentrated fecal specimens. Subsequently, 234 anti-HAV sero-negative children aged 7–8 y and 579 sero-negative subjects aged 4–27 y were immunized with H2 vaccine of the same titer,21,22 and none reported any severe adverse event.21 In a placebo-controlled trial, mild ALT elevation was found in 0.62% of H2 recipients (5/812) compared with 0.57% (1/174) in the placebo group.13 In the safety study of LA-1 strain, no severe adverse event was noted in 277 sero-negative vaccinees.22 Excretion of the attenuated virus, LA-1, could be found only by cell cultures of fecal specimens after a second blind passage.11 Fecal specimens collected from humans and marmosets, both had received H2 strain (human by im, marmosets by iv), were inoculated into 2 groups of susceptible marmosets by iv.13 Group 1, which had received fecal specimens from the marmosets, had much higher anti-HAV GMT (1:256) than group 2, which had received the specimens from human volunteers (GMT = 1:2.69). ALT elevation was found in 2 of 8 animals in group 2 and none from the 6 animals in group 1. Judging from the GMTs of sero-conversion to anti-HAV, we believe that the attenuated virus was excreted in lower amounts from the humans than from the marmosets.
Oral immunization of 16 children aged 3–4 y and 22 children aged 8–9 y with H2 vaccine, failed to induce antibody response at 8 wk after the immunization. In the control group, all 22 8–9-y-old children who had been injected with H2 vaccine subcutaneously, developed anti-HAV.23 Possibility of human-to-human transmission of H2 virus was studied in 228 anti-HAV negative pupils of the same school classes; 141 of them had received H2 vaccine subcutaneously and sero-converted to anti-HAV. None of the 87 unvaccinated children who had been in close contact with the vaccinees for 5–11 mo, sero-converted. Unlike oral polio vaccine, H2 vaccine was not transmitted from person to person through close contact.24
Vaccine immunogenicity
Immunogenicity of a “low-dose” of the H2 vaccine
H2 vaccine at a titer of 105.50 TCID50 which had been approved for our studies in the first 3 y, was given to sero-negative school children for immunization. Sero-conversion to anti-HAV was observed in 29.6% vaccinees (16/54) at 3 mo and declined to 14.8% (8/54) at 24 mo. The rate of sero-conversion was so low that we decided to give a booster dose of the same titer (105.50 TCID50) at 24 mo, which increased the anti-HAV sero-positivity to 58.5% (31/53) in 2 wk. In the concurrent control group who got the vaccine of the same titer for primary vaccination, the same 29.6% (21/71) of the vaccinees developed anti-HAV at 3 mo post-immunization. The immune responses to the booster at 2 wk indicated immunological memory after the primary immunization. The observed sero-conversion of 29.6% (16/54) after the first dose in the booster group, as measured by the modified HAVAB,15,16 underestimated the immune response to the primary immunization.25
A second dose of H2 at higher titer(106.5TCID50) given at 12 mo after a low dose of the vaccine (105.50TCID50), induced a better booster response, which was demonstrated by an increase in anti-HAV sero-positivity from 39.3% at 1 mo to 100% (27/27) at 13 mo with GMT of 2036 mIU/mL.26
Dose escalating immunogenicity trial
The low dose (105.50TCID50) vaccine could not be recommended for routine immunization program. To find an optimal dose of the vaccine, we conducted a dose-escalating immunogenicity trial. A single dose of 106.17TCID50, or 106.52TCID50, or 106.83TCID50 of H2 vaccine was injected subcutaneously into sero-negative volunteers. The Abbott IMx Microparticle Enzyme Immunoassay (MEIA) system (Abbott Laboratories) was employed to quantify anti-HAV antibodies. The system automatically calculates a signal to cutoff (S/CO). The concentrations of anti-HAV were calculated in mIU/mL in reference to W1041 anti-HAV immunoglobulin (CLB) using a nonlinear regression standard curve. Sero-conversion increased with the escalation of vaccine titer (Table 1). To ensure a sero-conversion of >85% at 3 mo of the vaccination, the vaccine titer was thus required to be ≥106.5TCID50/mL. At a dose of 106.83TCID50 and inactivated at 60 °C for 3 h (Group D), the attenuated virus induced sero-conversion in 73.9% of vaccinees, compared with 95.7% in those receiving the vaccine of the same lot and at the same titer (106.83TCID50) but not inactivated (Group C) (Table 1)27. HAV is not completely inactivated at 60 °C for 3 h.28 The higher sero-conversion in Group C than Group D pointed to the importance of viral replication for the immunogenicity; on the other hand, the moderate immune responses observed in Group D indicated that the HAV antigen mass included in a single dose of the vaccine were immunogenic as well. Therefore, the vaccine immunogenicity might be associated with limited virus replication in humans, in addition to the immunologic stimuli from the HAV antigen mass. Sero-conversion in 106.52TCID50 dose recipients was 91.5% for adults compared with 90.4% for children.29 Thus, the same vaccine dose was recommended for both children and adults.
Table 1. Dose-escalating immunogenicity of H2 vaccine in single dose among children aged 6 to 8 y, 3 mo after vaccination@.
| Group | Dose (TCID50) | n | Anti-HAV# Rate | Anti-HAV GMT (mIU/mL) (95% CI^) |
||
| (+) | % (95% CI^) | |||||
| A | 106.17 | 38 | 32 | 84.2 (72.6–95.8) | 46.3 | 34.7–61.9 |
| B | 106.52 | 47 | 43 | 91.5 (83.5–99.5) | 75.0 | 53.5–105.3 |
| C | 106.83 | 46 | 44 | 95.7 (89.7–100) | 197.9 | 141.8–276.3 |
| D* | 106.83 | 23 | 17 | 73.9 (55.9–91.8) | 41.3 | 26.6–64.1 |
+ This table was adopted with permission from Wang XY, et al. Immunogenicity and long-term persistence of anti-HAV induced by the live attenuated or inactivated hepatitis A vaccine. Zhong Hua Liu Xing Bing Xue Za Zhi 2001; 22:111–3. *Group D vaccine was inactivated at 60 °C for 3 h. ^95% Confidence Interval. #anti-HAV IgG. @ Dose-response for Group A, B, and C: Cochran–Armitage test statistic = 2.78, P < 0.01 ; comparison of anti-HAV positivity between Group C and D: χ2 = 5.10, P < 0.05.
The immune responses to a single vaccine dose were similar between H2 (106.82TCID50) and LA-1 strain (106.75TCID50) recipients (Table 2). The anti-HAV sero-positive rate in H2 vaccine group dropped from 92% with GMT of 126 mili-international unit per mililiter (mIU/mL) at 3 mo to 75% with GMT of 81 mIU/mL at 36 mo as measured by Abbott IMx system, the detection level of which was around 30 mIU/mL 30 (Table 2). The rate was 71.6% with a GMT of 89 mIU/mL at 8 y.30 Thus, the antibody decline was quite slow over the 8-y period. The Chinese inactivated HAV vaccine (Healive) in 2 doses caused much higher antibody level; it induced 100% sero-conversion with a GMT of 3237–3814 mIU/mL at 7 mo after the first dose or 1 mo after the second dose.31 On the other hand, cellular immune responses were observed in humans immunized with the live attenuated vaccine.32 Therefore, persistence of the immune responses to the live vaccines, in comparison with those to the inactivated HA vaccine, requires further studies. The lower limit of anti-HAV needed for protection of hepatitis A, has not been determined. Studies of an inactivated HAV vaccine, VAQTA, have used anti-HAV concentration of 10 mIU/mL as protective level.33 Obviously, Merck Company that sponsored the VAQTA project, developed more sensitive anti-HAV assay for their vaccine studies.
Table 2. Anti-HAV# in recipients of LA-1 and H2 vaccines during 3 y follow-up+.
| Month* | LA-1 Vaccine Group | H2 Vaccine Group | ||||||
| n | (+) | % | Anti-HAV GMT (95%CI^ ) | n | (+) | % | Anti-HAV GMT (95%CI^ ) | |
| 3 | 119 | 88 | 74.0 | 122.7 (84.6–178.0) | 102 | 94 | 92.2 | 126.2 (99.9–159.1) |
| 6 | 121 | 104 | 86.0 | 99.4 (78.5–125.7) | 101 | 88 | 87.1 | 90.7 (71.9–114.5) |
| 12 | 51 | 41 | 80.4 | 91.7 (68.5–122.4) | 101 | 81 | 80.2 | 81.0 (63.9–102.8) |
| 24 | 92 | 73 | 79.4 | 90.5 (71.4–114.8) | 74 | 56 | 75.7 | 82.1 (61.7–109.3) |
| 36 | 63 | 50 | 79.4 | 91.0 (72.5–114.3) | 64 | 48 | 75.0 | 80.8 (60.1–108.6) |
+ This table was adopted with permission from Xu ZY, et al. Immunogenicity and efficacy of two live attenuated hepatitis A vaccines (H2 Strains and LA-1 strains). Zhong Hua Yi Xue Za Zhi 2002; 82:678–81. *Month after vaccination. ^95% Confidence Interval. #anti-HAV IgG.
(3) Use of H2 vaccine in combination with immunoglobulin
Children who had received H2 vaccine and immunoglobulin simultaneously, had a lower sero- prevalence of anti-HAV than those receiving H2 vaccine alone (P = 0.07).26
Field Evaluation of Protective Efficacy of the Vaccines
Efficacy trial of the low-dose vaccine in a single dose
In the first efficacy trial, a single dose of 105.50TCID50 given subcutaneously, was evaluated. A total of 54 746 children, 6–9 y old, were randomized to H2 vaccine, LA-1 vaccine or the control group.34 Month of birth was used for the randomization. The control group did not receive the live HA vaccine until the follow-up study was completed. The children were not tested for susceptibility before the vaccination. Sero-prevalence of anti-HAV in a sample of the study population before the trial was 22.3% (1079/4837). The existing surveillance system for infectious diseases was used for follow up. Local clinicians were trained to report suspected acute hepatitis cases, fill out the forms of case report, and collect serum specimens for laboratory diagnosis. Local CDC people visited the hospitals for monitoring case-finding and specimens collection. They also visited schools regularly, found suspected patients among absentees, and collected serum specimens from them. All specimens were tested for IgM anti- HAV at the central laboratory; those with positive test, elevated ALT and clinical symptoms of hepatitis were defined as HA cases.
No severe adverse event was reported. Sero-conversion at 3 mo of vaccination, measured with modified HAVAB,15,16 was 36.3% for H2 vaccine group and 35.3% for LA-1 vaccine group. During the 17-mo follow-up period after the 18 d of the vaccination, 5 hepatitis A cases were reported in 17 733 subjects of H2 vaccine group (2.82 per 10 000), 6 in 18 397 of LA-1 vaccine group (3.26 per 10 000) vs. 22 in 18 616 of control group (11.82 per 10 000). The differences in the incidence rates between each of the 2 vaccine groups and the control group, were all statistically significant and the protective efficacy of the vaccines was estimated to be 76.14% for H2 vaccine and 72.42% for LA-1 vaccine respectively.34 Interestingly, of the 4026 children who had been allocated to H2 or LA-1 vaccine group and had missed the vaccination, 3 were reported as hepatitis A, a rate of 7.45 per 10 000, which was not significantly different from the rate in the control group (P > 0.05). A total of 716 children allocated to the control group, had mistakenly received either of the 2 vaccines and none of them was reported as having clinical hepatitis A. Thus, we failed to identify the bias in the detection of hepatitis A cases toward the vaccine group or control group. The sero-conversion of the 2 vaccine groups were much lower than the protective efficacy because of insensitivity of the antibody assay.
Efficacy trial of the vaccines in an optimal dose
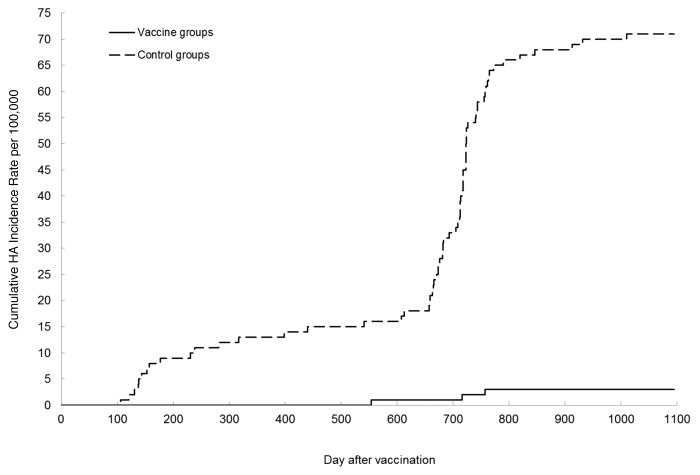
To test whether the vaccine efficacy could be increased, the 2 vaccines were tested at a higher dose (106.75–6.82 TCID50).35 Study subjects were enrolled and randomized by month of birth to vaccine or control group separately for the 2 vaccines. Both hepatitis A and typhoid fever were endemic in the area for the trial of LA-1 vaccine, and the study subjects agreed to receive either HA or typhoid Vi vaccine blindly. The control group for LA-1 vaccine thus received typhoid Vi vaccine. Both the study subjects and the clinicians who collected specimens and reported acute hepatitis, were blinded to which group the study subjects and the suspected hepatitis A patients were allocated to. All specimens from the patients were tested in the central laboratory for final diagnosis. In the trial of H2 vaccine, the control group did not receive any vaccine. Detection bias that was likely introduced into the open labeled trials, was measured by comparing the incidence rate of acute hepatitis B between the vaccine and the control groups. The final diagnosis of Hepatitis A or B was known only to the laboratory workers at the central laboratory, who did not know whether the suspected hepatitis A patients had received the live HA vaccine. The surveillance system was the same as described for the low- dose vaccine trial except that all specimens sent to the laboratory, were tested for both IgM anti- HAV and IgM anti-HBc. A total of 58 892 children, 1–12 y of age, participated in the individually randomized trial of H2 vaccine, and they were followed for 3 y; 183 666 high school students, aged 12–18 y, participated in the cluster (school class) randomized trial of H2 vaccine and followed for 2 y. A total of 114 588 children aged 1–10 y were enrolled in the double-blind trial of LA-1 vaccine. Confirmed hepatitis A and acute hepatitis B cases detected at 18 d after the vaccination were counted for calculation of incidence rate of hepatitis A and B in the vaccine and control groups. Age distributions were similar between the vaccine and control groups. The peak sero-conversion in anti-HAV negative subjects after the vaccination, was 92.2% for the H2 vaccine group and 86% for the LA-1 vaccine group (Table 2). In the individually randomized trial, one hepatitis A case was found in H2 vaccine recipients (3.6 per 100 000) vs. 22 cases in the control group (70.4 per 100 000), a reduction of 94.9% (Table 3), while hepatitis B was reported in 1 vs 2 cases for the 2 groups respectively. In the LA-1 vaccine trial, 2 hepatitis A cases were observed in vaccine recipients (3.4 per 100 000) compared to 38 in the control group (68.3 per 100 000) with an efficacy estimate of 95.0%. Pooled analysis of the 2 study results showed a combined vaccine efficacy of 95% with a lower 95% confidence limit of 86.5%. These estimates of protective efficacy are similar to those of inactivated HAV vaccines produced by GSK36 or Merck.37 Again, the sero-positivity of anti-HAV in vaccinees (Table 2 and 3) was lower than the vaccine protection. When the sero-positivity declined from 86–92% in the first year to 75–79% in the third year (Table 2), protective efficacy of the 2 vaccines remained unchanged (Fig. 1).
Table 3. Protective efficacy of H2 and LA-1 vaccines among children aged 1 to 9 y during 3-y follow- up+.
| Vaccine | Vaccine group | Control group | VE^ (%) | 95% lower confidence limit | ||||
| n* | No. of cases# | Incidence Rate (1/100 000/year) | n* | No. of cases# | Incidence Rate (1/100 000) | |||
| H2 Strain | 27 668 | 1 | 3.6 | 31 224 | 22 | 70.4 | 94.9 | 73.6 |
| LA-1 Strain | 58 955 | 2 | 3.4 | 55 633 | 38 | 68.3 | 95.0 | 87.1 |
| Pooled | 86 623 | 3 | 3.5 | 86 857 | 60 | 69.1 | 95.0 | 86.5 |
+ Per protocol analysis. This table was adopted with permission from Xu ZY, et al. Immunogenicity and efficacy of two live attenuated hepatitis A vaccines (H2 Strains and LA-1 strains). Zhong Hua Yi Xue Za Zhi 2002; 82:678–81. ^VE: Vaccine Efficacy. *Number of subjects assigned randomly to vaccine or control group. #Number of hepatitis A cases reported for vaccine and control groups respectively.
Figure 1. Cumulative HA incidence rate per 100 000 in vaccine and control groups.
In the cluster randomized trial of H2 vaccine, no hepatitis A case was reported in the vaccine group while 22 cases were reported in the control group. This difference was statistically significant (P < 0.001). However, the numbers of reported acute hepatitis B cases were similar in the 2 groups: 9 in the vaccine group (10.7 per 100 000) vs. 10 in the control group (12.6 per 100 000) during the 2 y follow-up.35 Therefore we were unable to detect the bias in case finding. We selected month of birth for randomization because one cannot choose the month of birth and these data had been recorded at the Government Registry Offices.
Vaccine efficacy for preventing subclinical HAV infection
In countries with poor sanitation and high risk of HAV infection, most children acquire immunity through subclinical HAV infection and thus escape the risk of symptomatic hepatitis A when they become adults. Subclinical HAV infection can be a natural booster and induce a stronger immunity than vaccine. During the second efficacy trial of the H2 and LA-1 vaccines, 3870 sero-negative children, aged 1–12 y, were randomly allocated to the vaccine or control groups, and were followed for subclinical HAV infection for 3 y.35 Anti-HAV titers in the recipients of either of the 2 vaccines ranged between 1:1 and 1:16. Thus, an anti-HAV titer of ≥1:100 without clinical symptoms and ALT elevation was defined as subclinical HAV infection, the rates of which in the vaccine and control groups were 3.1% (25/802) vs 3.6% (27/758) for H2 strain (P > 0.05) and 2.3% (27/1168) vs 4.2% (48/1142) for LA-1 strain (P < 0.05), a reduction in 13.9% and 45.2% respectively. No hepatitis symptom was found among the subclinically infected persons. Interestingly, the H2 vaccine did not protect the vaccinees from a subsequent subclinical infection, which turned out to be a natural booster for vaccinees, whereas the LA-1 might provide low protection against subclinical infection.
Vaccine efficacy against clinical hepatitis A and subclinical HAV infection during a hepatitis A outbreak
Hepatitis A outbreak was reported at a school of Hebei Province where a part of 6–9-y-old children had been immunized with H2 strain at 8 mo previously.38 One HA case was found in 271 children vaccinated with H2 vaccine (0.4%) compared with 21 cases in 314 unvaccinated children of the same age group (6.7%) (P < 0.001), a reduction in 94.5%. Subclinical HAV infection that was defined by positive IgM anti-HAV, without clinical symptoms of acute hepatitis and ALT elevation, was observed in 4.1% (11/271) of vaccinees vs. 6.7% (21/314) of unvaccinated children (P > 0.05). The ratio of clinical to subclinical infection was 1:11 for the vaccinees vs. 1:1 for the unvaccinated children.38 Though the rate of clinical hepatitis A was lower in the vaccinees by 94.5%, the chance of subclinical HAV infection was not significantly lower in the vaccinees than in the unvaccinated children (P > 0.05).38
In the randomized trials, anti-HAV GMT of 1:348 (95% CI: 1:242–501) was found for the sub-clinically infected H2 vaccine recipients vs. 1:565 (95% CI: 1:411–777) for the controls.39 The difference was not statistically significant (P > 0.05). The recipients of the live vaccine thus might have acquired longer- term immunity through subclinical infection. Fecal shedding of HAV in large amounts has been proven in subclinically infected individuals without history of HA vaccination.40 However, this is unlikely to occur for persons who were immunized with H2 vaccine and acquired subclinical HAV infection subsequently, because in the population covered with this vaccine, both the rates of clinical HA and subclinical HAV infection dropped dramatically.41
Hepatitis A immunization Programs and Possibility of HAV Elimination with Live HAV Vaccines in China
National HA immunization program in China
The live attenuated HA vaccines had been available for Chinese children at user’s fee and a total of 150 million doses were distributed in the country from 1992 through 2007. The domestic inactivated HA vaccine (Healive) was licensed in 2002 and accounted for a moderate proportion in the market. Hepatitis A immunization with either one dose of live, attenuated vaccine for babies at 18 mo of age, or 2 doses of the inactivated vaccine for babies at 18 and 24 mo of age respectively, was recommended for the national routine childhood immunization program in 2008. From 2008 through 2012, around 23–30 million vaccine doses were manufactured in the country annually; the live attenuated vaccines held 80% of market share. For the national childhood routine immunization program, however, 92% of the 16 million annual births were immunized with the affordable live vaccines, without which the national HA immunization program would be impossible. In general, the inactivated HA vaccine is consumed privately for non-EPI program.
Impact of hepatitis A immunization program on the disease
The annual national incidence rate of hepatitis A was reported to be 52.6/100 000 in 1990, and declined to 5.1/100 000 in 2006.42 This decline was made possible primarily by rapid urbanization and improved sanitation in Chinese populations.42 Immunization with HA vaccines played a secondary role.42 The reported annual incidence rate of hepatitis A kept falling afterward: from 5.91 per 100 000 in 2007 to 1.82 per 100 000 in 2012, a reduction in 69%. This reduction cannot be associated with the current HA immunization program that has been run for only 5 y, while the peak incidence of hepatitis A fell on children between 5–14 y of age in the less developed western provinces and on adults in the well developed eastern provinces. The impact of the national routine childhood hepatitis A immunization program on the risk of Hepatitis A may not be seen clearly within 5–10 y until the vaccinated babies grow up. However, the new immunization policy together with the nationwide continuing urbanization, will result in further reduction of HAV transmission in China in the future.
Advantages and disadvantages of the live attenuated vaccines
The live attenuated vaccines offer the benefits of low cost, a single dose of injection, high protection, and increased duration of immunity through natural boosting of subclinical infection. Hence, 27 of the 31 provinces and cities of China chose the live vaccines for their routine childhood immunization program. The major disadvantage is the theoretically possible reverse mutation of the live vaccine strains. In addition, no effort has been made to study the possibility of mutational shifts of the live vaccine strains in field conditions. Hence, for the safety concerns, the health authorities of Shanghai, Beijing, Tianjin, and Jiangsu, the 3 rich cities and a rich province, procured the more expensive, inactivated HA vaccines for the routine childhood immunization program. The live vaccines did not provide post-exposure protection because of slow immune response to the vaccines43 and cannot be used for emergency immunization to control outbreaks. The inactivated vaccine may possibly abort hepatitis A outbreaks due to rapid induction of anti-HAV after the immunization. Compared with the inactivated vaccine given in 2 doses, a single dose of the live vaccines induced lower sero-conversion and antibody GMT. Whether it would cause a shorter duration of protection is not known. Anti-HAV at protective level, caused by the inactivated vaccine, persists at least for 20–25 y.44 The duration of protection has not been determined for the live vaccines. One demonstration study on H2 vaccine reported that the protection lasted at least for 14 y, though the rate of sero-conversion declined from 98.6% at 2 mo to 81.3% at 14 y of the vaccination.41 Declining immunity level over years could result in an increasing proportion of susceptibles in those adults who were vaccinated in childhood and thus being at risk of clinical hepatitis A when infected. In poor countries or regions 90% of children get immune due to high risk of HAV infection. The live attenuated vaccine is affordable for poor people and the vaccinated children would not miss the chance of boosting by subclinical HAV infection when possible. On the other hand, with decreasing circulation of HAV in population as a result of mass HA vaccination and improvement of sanitation, the chance of subclinical HAV infection is getting rarer and one cannot rely on booster effect of subclinical infection for longer term of vaccine protection.
Possibility of HAV elimination with the live vaccine in China
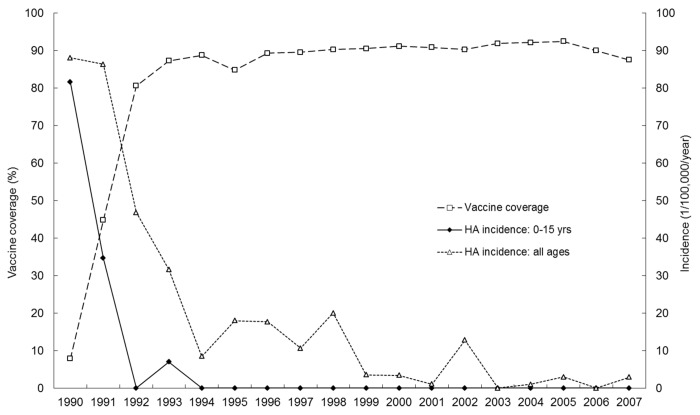
In Jiaojiang, Zhejiang Province, a catch-up campaign for immunizing 1–15-y-old children with H2 vaccine in a single dose of 106.50TCID50 ,was conducted in 1992–1993 and the vaccine was subsequently integrated into the routine childhood immunization program.41 Annual vaccine coverage in the target age groups ranged from 84.75% to 94.22%. Annual hepatitis A incidence dropped rapidly with increasing vaccine coverage (Fig. 2). No single hepatitis A case was found among the 20 661 subjects vaccinated under 15 y of age during the 14 y from 1994 through 2007. The annual incidence of hepatitis A also dropped rapidly in the adult population (Fig. 2), probably due to reduced HAV transmission in the population41 as reported in Israel.45 This example demonstrates that elimination of hepatitis A with live vaccines is theoretically possible. On the other hand, at the vaccine coverage level of 84.75–94.22%, 15 of the 134 subjects (11.19%) who had been immunized and successfully followed for 15 y, showed subclinical HAV infection with an annual rate of 0.74%. More and longer studies are needed to test whether sustained elimination of HAV transmission is feasible in the population of Jiaojiang County in which most adults had been infected with HAV during the pre- immunization era.46 However, for the developed countries where only a small proportion of the entire population has experienced HAV infection, HAV elimination would take much longer unless both adults and children would be targeted for HAV vaccination.
Figure 2. Coverage with HAV immunization and HA incidence rates in population, Jiaojiang, 1990–2007: demonstration of long-term effectiveness of live attenuated vaccine (H2 strain).
Acknowledgments
The financial support of this study was provided by the Chinese Ministry of Science and Technology and the China Medical Board in New York. We thank the dedicated staff of Centers for Disease Control in Hebei, Guangxi, Shanghai and Suzhou, who made this study possible. We acknowledge Dr Chen Ying for technical assistance for the paper.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Fiore AE, Wasley A, Bell BP, Advisory Committee on Immunization Practices (ACIP) Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55(RR-7):1–23. [PubMed] [Google Scholar]
- 2.Jacobsen KH, Koopman JS. Declining hepatitis A seroprevalence: a global review and analysis. Epidemiol Infect. 2004;132:1005–22. doi: 10.1017/S0950268804002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobsen KH, Koopman JS. The effects of socioeconomic development on worldwide hepatitis A virus seroprevalence patterns. Int J Epidemiol. 2005;34:600–9. doi: 10.1093/ije/dyi062. [DOI] [PubMed] [Google Scholar]
- 4.Kunasol P, Cooksley G, Chan VF, Isahak I, John J, Loleka S, Villar EP, Poovorawan Y, Seong NH, Sulaiman HA, et al. Hepatitis A virus: declining seroprevalence in children and adolescents in Southeast Asia. Southeast Asian J Trop Med Public Health. 1998;29:255–62. [PubMed] [Google Scholar]
- 5.Rein DB, Fiore AE, Bell BP. What’s next for the hepatitis A vaccine? Lancet. 2006;367:546–8. doi: 10.1016/S0140-6736(06)68196-8. [DOI] [PubMed] [Google Scholar]
- 6.Peetermans J. Production, quality control and characterization of an inactivated hepatitis A vaccine. Vaccine. 1992;10(Suppl 1):S99–101. doi: 10.1016/0264-410X(92)90557-Z. [DOI] [PubMed] [Google Scholar]
- 7.Armstrong ME, Giesa PA, Davide JP, Redner F, Waterbury JA, Rhoad AE, Keys RD, Provost PJ, Lewis JA. Development of the formalin-inactivated hepatitis A vaccine, VAQTA from the live attenuated virus strain CR326F. J Hepatol. 1993;18(Suppl 2):S20–6. doi: 10.1016/S0168-8278(05)80373-3. [DOI] [PubMed] [Google Scholar]
- 8.Vidor E, Fritzell B, Plotkin S. Clinical development of a new inactivated hepatitis A vaccine. Infection. 1996;24:447–58. doi: 10.1007/BF01713047. [DOI] [PubMed] [Google Scholar]
- 9.Glück R, Mischler R, Brantschen S, Just M, Althaus B, Cryz SJ., Jr. Immunopotentiating reconstituted influenza virus virosome vaccine delivery system for immunization against hepatitis A. J Clin Invest. 1992;90:2491–5. doi: 10.1172/JCI116141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mao JS, Dong DX, Zhang HY, Chen NL, Zhang XY, Huang HY, Xie RY, Zhou TJ, Wan ZJ, Wang YZ, et al. Primary study of attenuated live hepatitis A vaccine (H2 strain) in humans. J Infect Dis. 1989;159:621–4. doi: 10.1093/infdis/159.4.621. [DOI] [PubMed] [Google Scholar]
- 11.Hu MD, Zhang X, Sun BF, He JF, Yin PP, Lu XX, Wu WH, Wang PF, Zhao KJ, Wang W, et al. Observation of live attenuated hepatitis A vaccine in human. Zhong Hua Yu Fang Yi Xue Za Zhi. 1993;27:65–8. [PubMed] [Google Scholar]
- 12.Qian W, Chen J, Cai MJ. Safety and immunogenicity of live, attenuated hepatitis A (H2 strain) vaccine in Rhysus monkeys and humans. Shang Hai Shi Yan Dong Wu Ke Xue. 1998;18:227. [Google Scholar]
- 13.Dong DX, Cao YY, Lian YH, Zhou DJ, Huang XQ, Tan SG, Chen TQ, Luo QS, Bai HZ, Yang JS, et al. Residual virulence and its reversion study of live attenuated hepatitis A vaccine (H2 strian) Chin Med Sci J. 1999;14:11–6. [Google Scholar]
- 14.Hu MD, Sun BF, Qiu ZF. Study on the live attenuated hepatitis A vaccine candidate: attenuation and monky test. Shang Hai Yi Xue 1988; 11. [Google Scholar]
- 15.Provost PJ, Bishop RP, Gerety RJ, Hilleman MR, McAleer WJ, Scolnick EM, Stevens CE. New findings in live, attenuated hepatitis A vaccine development. J Med Virol. 1986;20:165–75. doi: 10.1002/jmv.1890200208. [DOI] [PubMed] [Google Scholar]
- 16.Karron RA, Daemer R, Ticehurst J, D’Hondt E, Popper H, Mihalik K, Phillips J, Feinstone S, Purcell RH. Studies of prototype live hepatitis A virus vaccines in primate models. J Infect Dis. 1988;157:338–45. doi: 10.1093/infdis/157.2.338. [DOI] [PubMed] [Google Scholar]
- 17.Sjogren MH, Purcell RH, McKee K, Binn L, Macarthy P, Ticehurst J, Feinstone S, Caudill J, See A, Hoke C, et al. Clinical and laboratory observations following oral or intramuscular administration of a live attenuated hepatitis A vaccine candidate. Vaccine. 1992;10(Suppl 1):S135–7. doi: 10.1016/0264-410X(92)90568-5. [DOI] [PubMed] [Google Scholar]
- 18.Tang CH, Mao JS, Chai SA, Chen Y, Zhuang FC. Molecular evolution of hepatitis A virus in a human diploid cell line. World J Gastroenterol. 2007;13:4630–5. doi: 10.3748/wjg.v13.i34.4630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang XQ, Lian YH, Wang QL, Wang X, Yang JS, Bai HZ. YY C. Experimental in vivo passage of H2 strain of live attenuated vaccine of hepatitis A in common marmosets. Zhong Hua Yu Fang Yi Xue Za Zhi. 1997;31:260–2. [PubMed] [Google Scholar]
- 20.Provost PJ, Banker FS, Wadsworth CW, Krah DL. Further evaluation of a live hepatitis A vaccine in marmosets. J Med Virol. 1991;34:227–31. doi: 10.1002/jmv.1890340406. [DOI] [PubMed] [Google Scholar]
- 21.Qian W, Chen J, Liu CJ, Lin CX, Cai MJ. Observation on 22 batches of live, attenuated hepatitis A vaccine (H2 strain) in human. Zhe Jiang Shen Yi Xue Ke Xue Yuan Xue Bao. 1998;9:13–4. [Google Scholar]
- 22.Chen YZ, Cui YJ, Tang Q, Zhou ZQ, Liu LJ, Wu JF, Liu XL, Dong M, Liu ZH, Liu JY. Adverse Reaction and Immunogenicity Induced by Freeze-dried Live Attenuated Hepatitis A Vaccine. Zhong Guo Sheng Wu Zhi Pin Xue Za Zhi. 2009;22:914–6. [PubMed] [Google Scholar]
- 23.Cai SA, Zhang HC, Zhang SY, Chen NL, Huang HY, Liu CJ, Xie RY, Mao JS. Several genetic characteristics of live, attenuated hepatitis A vaccine, H2 strain. Zhonghua Yi Xue Za Zhi (Taipei) 1993;73:335–7. [Google Scholar]
- 24.Mao JS, Chai SA, Xie RY, Chen NL, Jiang Q, Zhu XZ, Zhang SY, Huang HY, Mao HW, Bao XN, et al. Further evaluation of the safety and protective efficacy of live attenuated hepatitis A vaccine (H2-strain) in humans. Vaccine. 1997;15:944–7. doi: 10.1016/S0264-410X(96)00304-0. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y, Xu ZY, Ouyang PY, Zhao SJ, Wu WZ. [Studies on re-immunization with live attenuated hepatitis A vaccine] Zhonghua Yu Fang Yi Xue Za Zhi. 1998;32:162–4. [PubMed] [Google Scholar]
- 26.Wang XY, Meng ZD, Li RC, Ma JC, Luo D, Liu HB, Gong J, Xu ZY, Zhang YL, Han CQ, et al. Comparison of immunogenicity between live attenuated hepatitis A vaccine and inactivated hepatitis A vaccine. Zhonghua Yi Xue Za Zhi (Taipei) 2000;80:422–4. [PubMed] [Google Scholar]
- 27.Wang XY, Ma JC, Zhang Y, Zhang YL, Zhang YW, Han CQ, Xing ZC, Chen JC, Zhao SJ, Xu ZY. [Immunogenicity and long-term persistence of anti-HAV in groups with different attenuated and inactived hepatitis A vaccine dosage] Zhonghua Liu Xing Bing Xue Za Zhi. 2001;22:111–3. [PubMed] [Google Scholar]
- 28.Murphy P, Nowak T, Lemon SM, Hilfenhaus J. Inactivation of hepatitis A virus by heat treatment in aqueous solution. J Med Virol. 1993;41:61–4. doi: 10.1002/jmv.1890410113. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Lin XM, Zhao SJ, Ouyang PY, Xu ZY. Imunogenicity of a live attenuated hepatitis A vaccine in humans. He Bei Yi Ke Da Xue Xue Bao. 1997;18:209–10. [Google Scholar]
- 30.Wang XY, Xu ZY, Ma JC, von Seidlein L, Zhang Y, Hao ZY, Han OP, Zhang YL, Tian MY, Ouyang PY, et al. Long-term immunogenicity after single and booster dose of a live attenuated hepatitis A vaccine: results from 8-year follow-up. Vaccine. 2007;25:446–9. doi: 10.1016/j.vaccine.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Jiang WP, Chen JT, Wang X, Wang YL, Liu Y, Chen WY, Xu WG, Qiu YZ, Yin WD. Immunogenicity and safety of three consecutive lots of a new preservative-free inactivated hepatitis A vaccine (Healive): a double-blind, randomized and controlled trial. Vaccine. 2008;26:2297–301. doi: 10.1016/j.vaccine.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 32.Xin YJ, He YH, Zhuang FC, Gao LM, Qian W, Tang CH, Chen YQ, Chai SA, Mao JS. Cellular immune responses in humans immunized with the lyophlized, live, attenuated hepatitis A vaccine (H2 strain) Zhong Guo Yi Miao He Mian Yi. 2008;14:246–9. [Google Scholar]
- 33.Nalin DR, Kuter BJ, Brown L, Patterson C, Calandra GB, Werzberger A, Shouval D, Ellerbeck E, Block SL, Bishop R, et al. Worldwide experience with the CR326F-derived inactivated hepatitis A virus vaccine in pediatric and adult populations: an overview. J Hepatol. 1993;18(Suppl 2):S51–5. doi: 10.1016/S0168-8278(05)80379-4. [DOI] [PubMed] [Google Scholar]
- 34.Wu WZ, Xu ZY, Xia JP, Ouyang PY, Min XJ, Lin XM, Liu Y. Field evaluation on the efficacy of live attenuated hepatitis A vaccine. Zhong Guo Gong Gong Wei Sheng. 1996;12:535–6. [Google Scholar]
- 35.Xu ZY, Wang XY, Li RC, Meng ZD, Zhang Y, Gong J, Ma JC, Li YT, Zhao SJ, Li YP, et al. Immunogenicity and efficacy of two live attenuated hepatitis A vaccines (H2 Strains and LA-1 strains) Zhonghua Yi Xue Za Zhi (Taipei) 2002;82:678–81. [PubMed] [Google Scholar]
- 36.Innis BL, Snitbhan R, Kunasol P, Laorakpongse T, Poopatanakool W, Kozik CA, Suntayakorn S, Suknuntapong T, Safary A, Tang DB, et al. Protection against hepatitis A by an inactivated vaccine. JAMA. 1994;271:1328–34. doi: 10.1001/jama.1994.03510410040030. [DOI] [PubMed] [Google Scholar]
- 37.Werzberger A, Mensch B, Kuter B, Brown L, Lewis J, Sitrin R, Miller W, Shouval D, Wiens B, Calandra G, et al. A controlled trial of a formalin-inactivated hepatitis A vaccine in healthy children. N Engl J Med. 1992;327:453–7. doi: 10.1056/NEJM199208133270702. [DOI] [PubMed] [Google Scholar]
- 38.Meng ZD, Yao JF, Zhao YL, Guo JJ, Wang XY, Cai SA, Liu HB, Duo CG, Xu YG, Mu WD, et al. Further study on efficacy of attenuated live hepatitis A vaccine. Zhonghua Yi Xue Za Zhi (Taipei) 2000;80:9–11. [PubMed] [Google Scholar]
- 39.Xu ZY, Wang XY, Zhao SJ. Protective efficacy of and strategy for immunization with live, attenuated hepatitis A vaccines. Zhonghua Liu Xing Bing Xue Za Zhi. 2002;23:394–6. [Google Scholar]
- 40.Xu ZY, Wang JX, Jiang SP, Fang YM, Yu YF. Detection of HAV in stool of a subclinical infected child. Chung Hua Chuan Jan Ping Tsa Chih. 1984;2:45–6. [Google Scholar]
- 41.Zhuang FC, Mao ZA, Jiang LM, Wu J, Chen YQ, Jiang Q, Chen NL, Chai SA, Mao JS. [Long-term immunogenicity and effectiveness of live attenuated hepatitis A vaccine (H2-strain)-a study on the result of 15 years’ follow up] Zhonghua Liu Xing Bing Xue Za Zhi. 2010;31:1332–5. [PubMed] [Google Scholar]
- 42.Xu ZY, Wang XY, Liu CQ, Li YT, Zhuang FC. Decline in the risk of hepatitis A virus infection in China, a country with booming economy and changing lifestyles. J Viral Hepat. 2008;15(Suppl 2):33–7. doi: 10.1111/j.1365-2893.2008.01026.x. [DOI] [PubMed] [Google Scholar]
- 43.Wang XY, Ma JC, Xu ZY, Liu HB, Zhang YL, Han CQ, Zhang YW, Xing ZC, Chen JC, Meng ZD, et al. Effectiveness of Post-exposure Prophylaxis Using Live Attenuated Hepatitis A Vaccine(H2 Strain) among Schoolchildren. Zhonghua Yi Xue Za Zhi (Taipei) 2002;82:955–7. [PubMed] [Google Scholar]
- 44.Van Damme P, Banatvala J, Fay O, Iwarson S, McMahon B, Van Herck K, Shouval D, Bonanni P, Connor B, Cooksley G, et al. International Consensus Group on Hepatitis A Virus Immunity Hepatitis A booster vaccination: is there a need? Lancet. 2003;362:1065–71. doi: 10.1016/S0140-6736(03)14418-2. [DOI] [PubMed] [Google Scholar]
- 45.Mor Z, Srur S, Dagan R, Rishpon S. Hepatitis A disease following the implementation of universal vaccination: who is at risk? J Viral Hepat. 2010;17:293–7. doi: 10.1111/j.1365-2893.2009.01176.x. [DOI] [PubMed] [Google Scholar]
- 46.Zhuang FC, Jiang Q, Gong YP, Mo SH, Xi YJ, Qian W, Chen NL, Zhang SY, Cai SA, Mao JS. [Epidemiological effects of live attenuated hepatitis A vaccine (H(2)-strain): results of A 10-year observation] Zhonghua Liu Xing Bing Xue Za Zhi. 2001;22:188–90. [PubMed] [Google Scholar]