Abstract
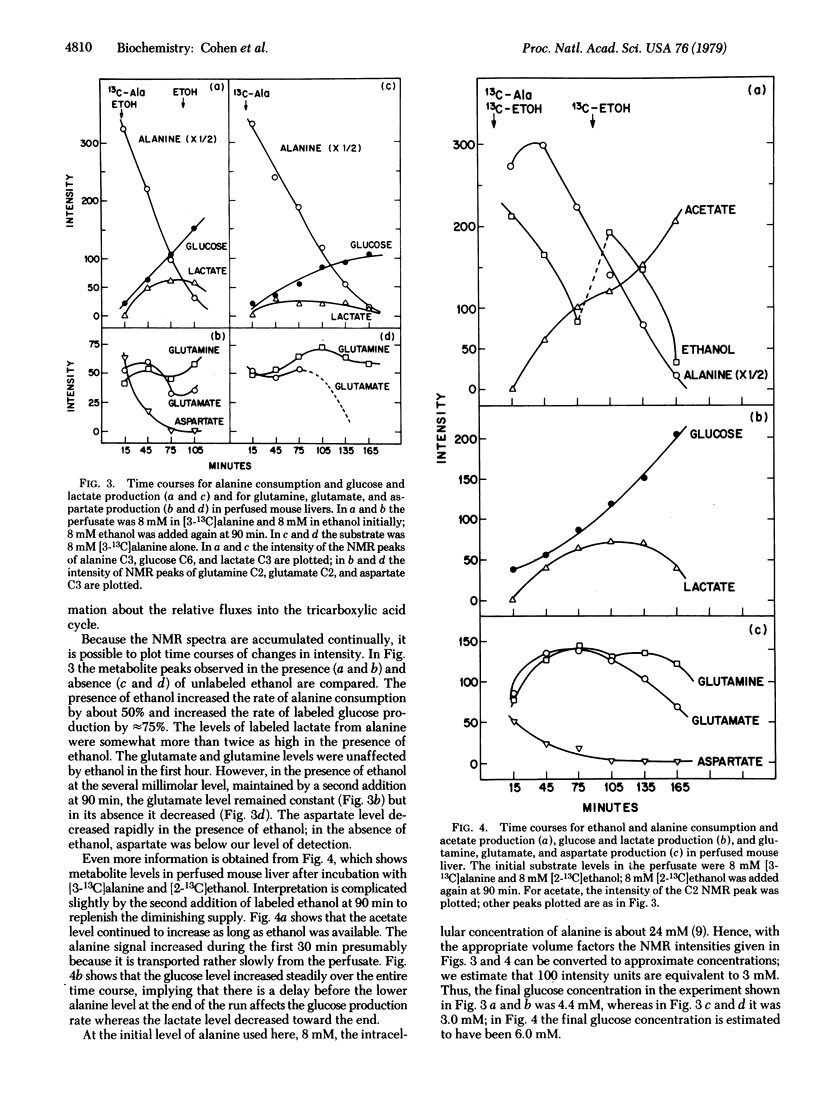
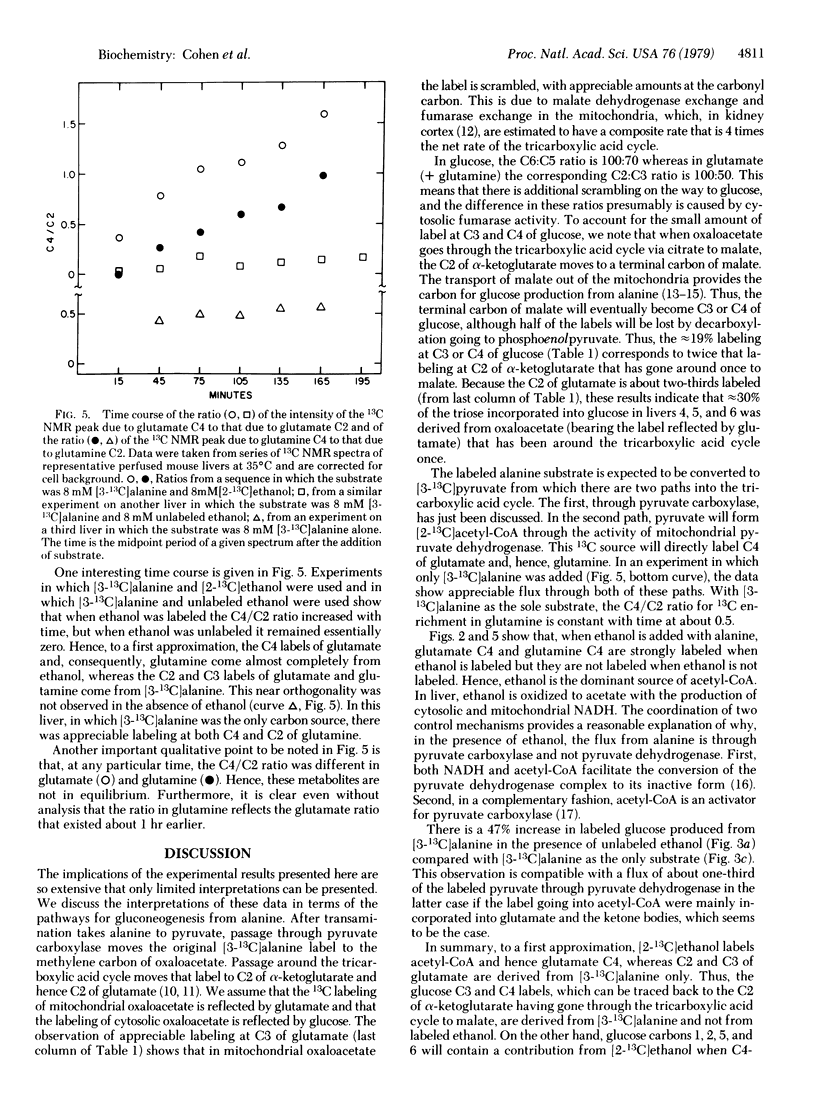
Time courses of 13C labeling from alanine and ethanol in perfused mouse livers have been followed by NMR. The enrichment at specific carbons of glucose, glutamate, glutamine, aspartate, acetate, acetoacetate, beta-hydroxybutyrate, and lactate has been measured. The specific labeling of glutamate in the presence of labeled alanine and labeled or unlabeled ethanol shows that, under these conditions, alanine enters the tricarboxylic acid cycle almost exclusively through pyruvate carboxylation, whereas ethanol is the exclusive source of acetyl-CoA. In the absence of ethanol, the alanine label flows through both paths. By comparing the scrambling of 13C between C3 and C2 of glutamate it is possible to estimate the mitochondrial fumarase activity; the C6-to-C5 ratios in glucose give the additional scrambling by cytosolic fumarase activity. In addition, the C6-to-C1 and C5-to-C2 ratios in glucose show that there is about 15% flux through the pentose cycle. Finally, the C4-to-C2 ratios in glutamine and glutamate are unequal at any time (the glutamine labels reflect the label distribution in glutamate measured 1 hr earlier), providing a method for studying flow through glutamine synthetase in situ.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batenburg J. J., Olson M. S. Regulation of pyruvate dehydrogenase by fatty acid in isolated rat liver mitochondria. J Biol Chem. 1976 Mar 10;251(5):1364–1370. [PubMed] [Google Scholar]
- Cohen S. M., Ogawa S., Shulman R. G. 13C NMR studies of gluconeogenesis in rat liver cells: utilization of labeled glycerol by cells from euthyroid and hyperthyroid rats. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1603–1609. doi: 10.1073/pnas.76.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek J. B., Njogu R. M. Glutamate transport and the trans-membrane pH gradient in isolated rat-liver mitochondria. FEBS Lett. 1976 Dec 1;71(2):341–346. doi: 10.1016/0014-5793(76)80966-0. [DOI] [PubMed] [Google Scholar]
- Katz J., Rognstad R. The labeling of pentose phosphate from glucose-14C and estimation of the rates of transaldolase, transketolase, the contribution of the pentose cycle, and ribose phosphate synthesis. Biochemistry. 1967 Jul;6(7):2227–2247. doi: 10.1021/bi00859a046. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Hems R., Lund P. Accumulation of amino acids by the perfused rat liver in the presence of ethanol. Biochem J. 1973 Jul;134(3):697–705. doi: 10.1042/bj1340697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallette L. E., Exton J. H., Park Effects of glucagon on amino acid transport and utilization in the perfused rat liver. J Biol Chem. 1969 Oct 25;244(20):5724–5728. [PubMed] [Google Scholar]
- Mendes-Mourāo J., Halestrap A. P., Crisp D. M., Pogson C. I. The involvement of mitochondrial pyruvate transport in the pathways of gluconeogenesis from serine and alanine in isolated rat and mouse liver cells. FEBS Lett. 1975 Apr 15;53(1):29–32. doi: 10.1016/0014-5793(75)80674-0. [DOI] [PubMed] [Google Scholar]
- Parrilla R., Goodman M. N. Nitrogen metabolism in the isolated perfused rat liver. Nitrogen balance, redox state and rates of proteolysis. Biochem J. 1974 Mar;138(3):341–348. doi: 10.1042/bj1380341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rognstad R., Katz J. Gluconeogenesis in the kidney cortex. Quantitative estimation of carbon flow. J Biol Chem. 1972 Oct 10;247(19):6047–6054. [PubMed] [Google Scholar]
- Tischler M. E., Friedrichs D., Coll K., Williamson J. R. Pyridine nucleotide distributions and enzyme mass action ratios in hepatocytes from fed and starved rats. Arch Biochem Biophys. 1977 Nov;184(1):222–236. doi: 10.1016/0003-9861(77)90346-0. [DOI] [PubMed] [Google Scholar]
- Veech R. L., Raijman L., Dalziel K., Krebs H. A. Disequilibrium in the triose phosphate isomerase system in rat liver. Biochem J. 1969 Dec;115(4):837–842. doi: 10.1042/bj1150837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R., Anderson J., Browning E. T. Inhibition of gluconeogenesis by butylmalonate in perfused rat liver. J Biol Chem. 1970 Apr 10;245(7):1717–1726. [PubMed] [Google Scholar]
- Williamson J. R., Browning E. T., Scholz R. Control mechanisms of gluconeogenesis and ketogenesis. I. Effects of oleate on gluconeogenesis in perfused rat liver. J Biol Chem. 1969 Sep 10;244(17):4607–4616. [PubMed] [Google Scholar]
- Williamson J. R., Scholz R., Browning E. T., Thurman R. G., Fukami M. H. Metabolic effects of ethanol in perfused rat liver. J Biol Chem. 1969 Sep 25;244(18):5044–5054. [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Perfusion in situ with tritium oxide to measure hepatic lipogenesis and lipid secretion. Normal and orotic acid-fed rats. J Biol Chem. 1966 Jun 25;241(12):2891–2899. [PubMed] [Google Scholar]


