Abstract
A pseudovirion-based neutralisation assay (PBNA) has been considered the gold standard for measuring specific antibody responses against human papillomavirus (HPV). However, this assay is labor intensive and therefore very difficult to implement in large-scale studies. Previous studies have evaluated the agreement between virus-like particle (VLP)-based ELISA and PBNA for measuring HPV vaccine-induced antibodies. However, the concordance of these assays to detect antibodies induced by natural infection has not yet been fully elucidated. In this study, the results of an Escherichia coli (E. coli)-expressed VLP-based ELISA were found to be highly concordant with those of a baculovirus-expressed VLP-based ELISA (r = 0.96 and 0.97 for HPV-16 and HPV-18) when detecing HPV vaccine induced antibodies and the concordance was medium (r = 0.68 and 0.68 for HPV-16 and HPV-18) when assessing natural infection induced antibodies. The results of the E. coli expressed VLP-based ELISA correlated well with those of the PBNA when testing 1020 post-vaccination human sera collected at one month after vaccination with the E. coli expressed VLP-based bivalent HPV vaccine (r = 0.83 and 0.81 for HPV-16 and HPV-18). The agreement and correlation were moderate (kappa < 0.3 for both HPV types 16 and 18, r = 0.59 and 0.68 for HPV-16 and HPV-18, respectively) when assessing 1600 serum samples from unvaccinated women of age 18–25 years. In conclusion, the VLP-based ELISA is an acceptable surrogate for the neutralizing antibody assay in measuring vaccine responses. However, the use of the VLP-based ELISA in epidemiological studies should be carefully considered.
Keywords: human papillomavirus, pseudovirion-based neutralisation assay, antibody, ELISA, vaccine
Introduction
Human papillomavirus (HPV) infection is the most common sexually transmitted infection worldwide and causes approximately 530 000 new cases of cervical cancer per year, of which more than 80% occur in developing countries.1 HPV types 16 and 18 account for approximately 70% of all cervical cancers. However, a prophylactic HPV vaccine containing type-specific L1 virus-like particles (VLPs) could prevent most, if not all, of these cases.2,3 Two HPV vaccines have been licensed, one quadrivalent vaccine (for HPV types 6, 11, 16, and 18) (Gardasil, Merck and Co.) and one bivalent vaccine (for HPV types 16 and 18) (Cervarix, GlaxoSmithKline Biologicals). The World Health Organization (WHO) has recommended HPV vaccination since 2009.4
HPV serological testing is essential for both HPV vaccinology and HPV epidemiology. Seronegativity is a necessary criterion for defining an HPV-naïve subject eligible for per protocol analysis in HPV vaccine efficacy trials. The antibody level induced by HPV vaccination has been used to bridge the results obtained in efficacy trials conducted in women to children and to optimise vaccination strategies. HPV serology is also useful for understanding the epidemiology of HPV infections.5 Several assays have been developed for measuring type-specific HPV antibody levels. A pseudovirion-based neutralisation assay (PBNA) detects all neutralising antibodies that can arrest the infection of pseudovirus and thus have the potential for providing protection against the virus.6,7 Because this assay is an unbiased assessment that is conducted independently of the vaccine, it is thought of as the gold standard for assessing the presence of protective antibodies induced by a prophylactic HPV vaccine.8 However, the use of the PBNA in large clinical trials is challenging because it is a complex and labor-intensive assay. Generally, enzyme-linked immunosorbent assays (ELISAs) are performed because they are a fast and highly reproducible method for quantifying antibodies against viral antigens. The direct ELISAs based on HPV L1 VLPs yield a value that reflects all of the IgG antibodies that bind to the L1 VLP antigen fixed to a solid surface,9,10 which is independent of their neutralising activity.
The available data10-13 suggest that neutralizing and VLP ELISA antibody titers are usually highly correlated for measuring vaccine induced anti-HPV responses. Therefore, the use of VLP-based ELISAs in serological studies seems ideal. However, there are few published studies that directly correlate the ability of VLP-based ELISAs and PBNAs to detect anti-HPV antibodies induced by natural infection. Moreover, it is unclear whether agreements of these assays are similar when measuring antibody responses induced by both infection and vaccination. Therefore, we conducted a study to analyze the agreement of these two assays to detect antibodies against HPV-16 or HPV-18 using serum samples collected pre- and post-vaccination in an HPV vaccine trial.
Results
A comparison of ELISAs using VLP antigen expressed by E. coli or baculovirus
ELISAs using E. coli-expressed VLP antigen (eVLP-based ELISA) or baculovirus-expressed VLP antigen (bVLP-based ELISA) were performed and compared using the evaluation panel containing serum samples from 60 unvaccinated donors and 30 volunteers who had received 3 doses of Cervarix®. PBNA results were set as the gold standards to discriminate true positive from true negative samples. All samples containing vaccine-induced antibodies were all successfully detected by both eVLP-based and bVLP-based ELISAs (Table 1). According to the PBNA results, the sensitivity and specificity of both tests were comparable for measuring naturally acquired antibodies (Table 1). For detecting unvaccinated samples, the agreements between the two assays were 0.87 and 0.83 for HPV-16 and HPV-18, respectively. The high kappa scores (approximately 0.7) between the two assays suggest a high level of agreement (Table 2).
Table 1. A comparison of the sensitivity and specificity of the eVLP-based ELISA and the bVLP-based ELISA using an evaluation panel.
| HPV-16 | HPV-18 | |||
|---|---|---|---|---|
| eVLP-based ELISA * | bVLP-based ELISA# | eVLP-based ELISA * | bVLP-based ELISA# | |
| Post-vaccination sera (n = 30) | ||||
| Sensitivity (%) | 100 | 100 | 100 | 100 |
| Specificity (%) | N/A | N/A | N/A | N/A |
| Unvaccinated sera (n = 60) | ||||
| Sensitivity (%)(95% CI) | 85.7 (70.8–100.0) | 76.2 (58.0–94.4) | 90.5 (77.9–100.0) | 95.2 (86.1–100.0) |
| Specificity (%)(95% CI) | 59.0 (43.5–74.4) | 53.8 (38.2–69.5) | 87.2 (76.7–97.7) | 64.1 (49.1–79.2) |
eVLP-based ELISA: Escherichia coli (E. coli)-expressed human papillomavirus L1 virus-like particle (VLP)-based ELISA; #bVLP-based ELISA: Baculovirus-expressed human papillomavirus L1 virus-like particle (VLP)-based ELISA.
Table 2. The level of agreement between the eVLP-based ELISA and the bVLP-based ELISA results in unvaccinated samples from the evaluation panel.
| HPV-16 | HPV-18 | |||
|---|---|---|---|---|
| eVLP-based ELISA * (+) | eVLP-based ELISA * (–) | eVLP-based ELISA * (+) | eVLP-based ELISA * (–) | |
| bVLP-based ELISA# (+) | 30 | 4 | 24 | 10 |
| bVLP-based ELISA# (–) | 4 | 22 | 0 | 26 |
| Agreement | 0.87 (0.78–0.95) | 0.83 (0.74–0.93) | ||
| Kappa (95% CI) | 0.73 (0.55–0.90) | 0.68 (0.50–0.85) | ||
eVLP-based ELISA: Escherichia coli (E. coli)-expressed human papillomavirus L1 virus-like particle (VLP)-based ELISA; #bVLP-based ELISA: Baculovirus-expressed human papillomavirus L1 virus-like particle (VLP)-based ELISA.
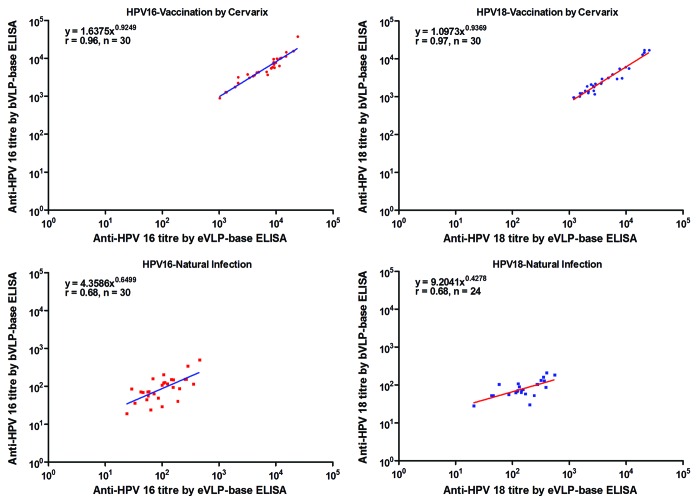
The correlation analysis between testing methodologies was calculated from sera that were determined to be positive by both eVLP-based ELISA and bVLP-based ELISA (double positives). Scatter plots and the results of correlation analysis after logarithmic transformation are shown in Figure 1. For testing Cervarix® induced antibodies, the Pearson correlation coefficients are very high (r = 0.96 and 0.97 for HPV-16 and HPV-18, respectively). While assessing naturally acquired antibodies, the Pearson correlation coefficients decreased (r = 0.68 and 0.68 for HPV-16 and HPV-18, respectively). Therefore, when testing vaccine induced anti-HPV antibody titers, these two assays can be directly compared, and for natural infection induced antibody, the correlation between the two assays are medium.
Figure 1. The correlation between antibody titers measured by the eVLP-based ELISA and the bVLP-based ELISA in double positive samples from the evaluation panel. An evaluation panel containing serum samples collected from 60 unvaccinated donors and 30 volunteers who had received 3 doses of Cervarix® was assessed by both the eVLP-based ELISA and bVLP-based ELISA. Data of pre-vaccination sera and post-vaccination sera were showed in separate pictures. All the 30 post-vaccination sera were double positive by both two methods for both HPV types, while only 30 and 24 pre-vaccination sera were double positive for HPV 16 and HPV 18 separately. eVLP-based ELISA: Escherichia coli (E. coli)-expressed human papillomavirus L1 virus-like particle (VLP)-based ELISA; bVLP-based ELISA: Baculovirus-expressed human papillomavirus L1 virus-like particle (VLP)-based ELISA.
eVLP-based ELISA and PBNA agreement and correlation for measuring naturally acquired anti-HPV antibodies
Baseline serum samples from 1600 women were used to compare the results of the eVLP-based ELISA and the PBNA, with the latter being used as the standard. For HPV-16 antibody testing, both the sensitivity and specificity of the eVLP-based ELISA were less than 80% (Table 3). The specificity increased to 92.1% (95% CI, 90.8–93.4) when measuring antibodies against HPV-18, whereas the sensitivity was lower, at 53.6%. The agreements between the assays were 79.5% and 90.8% for HPV-16 and HPV-18, respectively. The low kappa scores (<0.3) between the two assays, for both HPV types, suggest that there was only a moderate level of agreement (Table 3). In sera that tested positive by both the eVLP-based ELISA and the PBNA (double-positives), the Pearson correlation coefficients between the two sets of assay results were moderate (r = 0.59 [95% CI: 0.45–0.70] and 0.68 [95% CI: 0.50–0.80]) (Fig. 2).
Table 3. The observed agreement between the eVLP-based ELISA and the PBNA for measuring naturally acquired HPV antibodies in the general population.
| PBNA | HPV16 eVLP-based ELISA * |
HPV18 eVLP-based ELISA * |
||||
|---|---|---|---|---|---|---|
| + | - | Total | + | - | Total | |
| + | 86 | 26 | 112 | 30 | 26 | 56 |
| – | 302 | 1186 | 1488 | 122 | 1422 | 1544 |
| Total | 388 | 1212 | 1600 | 152 | 1448 | 1600 |
| Sensitivity (%) | 76.8 (69.0–84.6) | 53.6 (40.5–66.6) | ||||
| Specificity (%) | 79.7 (77.7–81.8) | 92.1 (90.8–93.4) | ||||
| Agreement (%) | 79.5 (77.5–81.5) | 90.8 (89.3–92.2) | ||||
| Positive Predictive Value (%) | 22.2 (18.0–26.3) | 19.7 (13.4–26.1) | ||||
| Negative Predictive Value (%) | 97.9 (97.0–98.7) | 98.2 (97.5–98.9) | ||||
| Kappa score | 0.26 (0.21–0.32) | 0.25 (0.17–0.33) | ||||
eVLP-based ELISA: Escherichia coli (E. coli)-expressed human papillomavirus L1 virus-like particle (VLP)-based ELISA; PBNA, pseudovirion-based neutralisation assay.
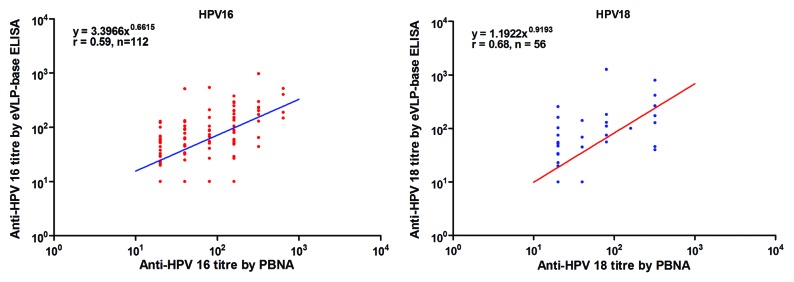
Figure 2. The relationship between antibody titers measured by the eVLP-based ELISA and the PBNA for naturally acquired HPV antibodies. In total, 1600 serum samples from unvaccinated women between the ages of 18 and 25 y were tested by the eVLP-based ELISA and the PBNA. Each dot in the figure represents a pair of results for one serum sample. Lots of samples had similar antibody titers, so the dots might lap over to each other. eVLP-based ELISA: Escherichia coli (E. coli)-expressed human papillomavirus L1 virus-like particle (VLP)-based ELISA; PBNA: pseudovirion-based neutralisation assay.
eVLP-based ELISA and PBNA agreement and correlation for measuring vaccine induced anti-HPV antibodies
The agreement between the results of the eVLP-based ELISA and the PBNA were analyzed using serum samples collected from 1020 women who had received three doses of the bivalent HPV vaccine Cecolin®, at one month following the third vaccination. Almost all of the samples were positive for antibodies against HPV-16 and HPV-18 by both the PBNA and ELISA, except for one sample that was negative for antibodies against HPV-18 alone by the PBNA. The Pearson correlation coefficients were much higher than for the naturally acquired antibodies [r = 0.83 (95% CI: 0.81–0.85) and 0.81 (95% CI: 0.79–0.83) for HPV-16 and HPV-18, respectively] (Fig. 3). When measuring antibodies against HPV 16, the mean ratios of logarithmic titers between ELISA and PBNA are 1.06 (95% CI: 1.01–1.11) and 0.99 (95% CI: 0.99–0.99) for natural antibodies and vaccine antibodies, respectively; When measuring antibodies against HPV 18, the mean ratios of logarithmic titers between ELISA and PBNA are 1.15 (95% CI: 1.04–1.25) and 0.99 (95% CI: 0.99–0.99) for natural antibodies and vaccine antibodies, respectively (Table S1; Fig. S1).
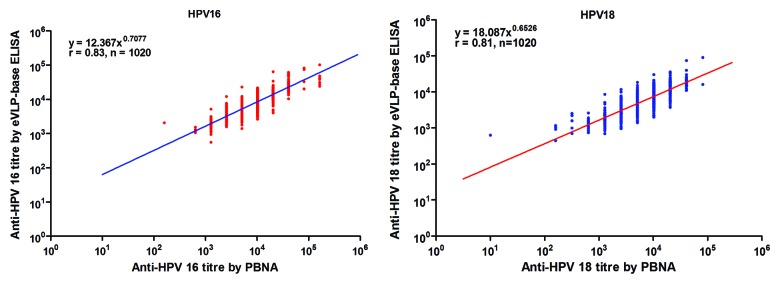
Figure 3. The relationship between antibody titers measured by the eVLP-based ELISA and the PBNA for HPV vaccine-induced antibodies. In total, 1020 post-vaccination sera collected one month after vaccination with 3 doses of the bivalent HPV vaccine was evaluated by the eVLP-based ELISA and the PBNA. Each dot in the figure represents a pair of results for one serum sample. Lots of samples had similar antibody titers, so the dots might lap over to each other. eVLP-based ELISA: Escherichia coli (E. coli)-expressed human papillomavirus.
Discussion
Antibody testing plays an essential role in both HPV vaccinology and HPV epidemiology studies. The PBNA measures the functional ability of neutralising antibodies to prevent pseudovirion infection of a cell line. Therefore, this assay is considered the gold standard in HPV serology. Unfortunately, this assay is highly labor intensive and requires considerable expertise and experience to standardise and is therefore difficult to use for large-scale studies. Previous reports10-13 demonstrated that the simple and reproducible VLP ELISA correlates well with the PBNA for measuring HPV vaccine-induced antibodies. Therefore, this assay has been used in clinical trials as a surrogate for the PBNA. However, the results reported here show that the agreement between the VLP ELISA and the PBNA for detecting naturally acquired anti-HPV antibodies is only moderate, whereas the correlation between these two assays remains high when measuring vaccine-induced antibodies. The in vitro neutralisation assay was found to be considerably more type specific than the VLP ELISA.6 Hence, the use of VLP ELISAs in epidemiological studies should be carefully considered.
In line with previous reports, the results of VLP ELISAs and PBNAs are highly correlated when measuring HPV vaccine-induced antibodies. The ELISA detects IgG antibodies that recognize neutralising or non-neutralising epitopes, whereas the PBNA detects only neutralising antibodies. Nonetheless, vaccination with three doses of L1 VLPs generated high levels of antibodies, mainly induced by conformational neutralising epitopes and mostly with IgG isotype. Hence, the discrepancy between the two assays might be overwhelmed by the common neutralising epitopes when assessing vaccine responses. It should be noted that downstream treatment of VLPs might destroy the conformation of the neutralising epitopes. In the initial development of the HPV vaccine by GlaxoSmithKline, thiomersal was added to the vaccine. Most women receiving this vaccine did not develop neutralising antibody responses despite having a significant increase in antibody titers as determined by ELISA. A further study showed that the incubation of L1 VLPs with thiomersal had resulted in the destruction of the conformational epitope recognized by the V5 virus-neutralising monoclonal antibody.14 Therefore, the VLP ELISA should only be used as a surrogate for the PBNA after it has been verified that vaccine-induced neutralising antibodies are present and the coating VLPs remain in a conformation in which the dominant neutralising epitopes are present.
In contrast, the agreement between the VLP-based ELISA and the PBNA for measuring naturally acquired anti-HPV antibodies is only moderate. Several factors might contribute to the discrepancy observed between these two assays. The inadequate sensitivity of the VLP ELISA might be due to the cross-reactive neutralising antibodies generated by infection with other HPV types but not recognized by vaccine L1 VLPs.15 The specificity of the VLP ELISA might be reduced by the presence of cross-reactive non-neutralising antibodies generated by infection with other HPV types. As a result of several elaborate immune escape mechanisms, such as the infectious cycle being exclusively intraepithelial (and therefore the absence of viraemia) and the production of virus particles in superficial epithelial cells (which are distant from antigen presenting cells),16 the serum antibody levels following natural HPV infections are very low, even at peak titers, and the epitope profiles varies greatly.17 Hence, other plausible explanations are that the VLP ELISA detects additional antibodies raised against non-neutralising epitopes during natural infections and that the minimal quantity of antibody needed for detecting a positive signal by ELISA is less than that needed for showing a neutralising effect by the PBNA.
The observed seropositivities of females aged 18–25 y old by ELISA were 24.3% for HPV-16 and 9.5% for HPV-18, whereas the rates of seropositivity were 7% (HPV-16) and 3.5% (HPV-18) by the PBNA. The seroprevalences of HPV-16 and -18 tested by the PBNA were similar to those observed in other studies.2 If the ELISA were used as one of the criteria for determining the baseline HPV infection level in a vaccine efficacy trial, approximately 20% of subjects would be excluded from the per protocol analysis set in the efficacy study because of false-positive serology, which might have a considerable impact on the balance between the arms established by randomization and lower the power of the study. Conversely, approximately 2% of subjects exposed to HPV might be erroneously included in per protocol analysis set because of false-negative serology. A seropositive individual is more likely to have a persistent HPV infection18 and would therefore underestimate the vaccine efficacy because of the “dilution effect.”19
Vaccination with HPV L1 VLPs provides an opportunity to reduce the incidence of HPV-associated cancers globally.3,17,20-22 Unfortunately, the current cost of the licensed HPV vaccines produced by yeast or baculovirus systems is a significant barrier to their sustained global implementation. Therefore, there was a need for the development of a low cost HPV vaccine using a cheaper E. coli system; thus Cecolin® (Xiamen Innovax), a bivalent HPV type 16 and 18 vaccine candidate, was developed and recently underwent a phase 3 efficacy trial in China (NCT01735006). The eVLP antigens used in this study are the same as the antigen contained in the Cecolin® vaccine. This antigen shows similar particulate appearance to eukaryotically expressed VLPs and can induce similar vigorous neutralising antibody responses in animals and humans. Hence, it is not unexpected that the results of the ELISA with the eVLP antigen are highly correlated with the results of the ELISA using the baculovirus produced bVLP antigen (r = 0.96 and 0.97 for HPV-16 and HPV-18, respectively) and the PBNA (r = 0.83 and 0.81 for HPV-16 and HPV-18, respectively) when measuring vaccine responses. The mean logarithmic ratios of the titers of the eVLP-based ELISA to those of the bVLP-based ELISA were close to 1.0, which suggests that titers determined by the two assays can be directly compared when assessing vaccine induced antibodies (Fig. 1).
Another important measure for evaluating HPV-type-specific antibodies is the competitive luminex immunoassay (cLIA), which only detects the subset of neutralising antibodies that compete with the specific monoclonal antibody for VLP surface binding.23,24 The neutralising antibodies that do not compete with the monoclonal antibody also have protective potential; thus, cLIA might have a lower sensitivity and under-represent the extent of protective antibody responses. This method has been shown to be a practical surrogate test in clinical trials of Gardasil®, a quadrivalent, licensed HPV vaccine. As the HPV-type-specific competitive neutralising monoclonal antibodies were unavailable, we did not assess the specificity and sensitivity of the cLIA, which might be a limitation of our research.
The strength of this study is the relative large number of serum samples containing naturally acquired or vaccines induced HPV antibodies. The limitation is that all the tested post-vaccination samples were collected at one month after vaccination when the antibody levels peaked, more sera collected at more timepoints are necessary to further evaluate the correlation of the eVLP-based ELISA and PBNA for measuring different levels of vaccine induced HPV antibodies. Another limitation is that when assessing the correlation between the eVLP-based ELISA and bVLP-based ELISA, it would be better to also include post-Cecolin® vaccination sera in the serum panel.
In conclusion, the data reported here support previous findings that demonstrated the use of the VLP-based ELISA as an acceptable surrogate to measure vaccine responses after the production of neutralising antibodies had been established and the coating VLP had been validated. However, the use of the VLP-based ELISA in epidemiological studies should be carefully considered.
Materials and Methods
HPV-16 and HPV-18 PBNA
The PBNA was performed as previously described, with minor modifications.25 Briefly, human embryonic kidney cells (293FT cells) were seeded in 96-well flat-bottom plates at 15 000 cells per well. The plates were incubated at 37 °C and 5% CO2 for approximately 4 h until the cells adhered to the bottom of the wells. HPV-16 and HPV-18 pseudovirions were produced by co-transfecting 293FT cells with 3 plasmids that encoded HPV L1, HPV L2, or green fluorescent protein (GFP). Serial 2-fold-diluted serum samples started from dilution of 1:20, negative control and quality control samples were cultured with HPV pseudovirions (at 0.2 multiplicity of infection) at room temperature for 1 h, and then the mixtures were transferred to the monolayer of 239FT cells and cultured at 37 °C and 5% CO2 for 72 h. The positive sample was defined as the sample that caused a 50% reduction in GFP expression compared with the negative control and the neutralisation titers were defined as the highest dilution of positive samples. Samples that caused less than 50% reduction in GFP expression were diagnosed as negative, the neutralisation titers of negative samples were set as 1:10, which is half of the start serum dilution.
Direct ELISA
HPV L1 VLPs for types 16 and 18 expressed by baculovirus in insect cells (bVLPs) were supplied by GlaxoSmithKline Biologicals. HPV L1 VLPs for types 16 and 18 expressed in E. coli (eVLP) were supplied by Xiamen Innovax.26,27 Each well of microtiter plate was coated with 0.27 μg HPV-16 bVLPs, 0.27 μg HPV-18 bVLPs, 0.5 μg HPV-16 eVLPs, or 0.4 μg HPV-18 eVLP resuspended in phosphate buffer at 4 °C overnight, and then the plates were blocked with blocking solution. After washing, diluted serum samples, diluted reference serum samples and controls were added and incubated for 45 min at 37 °C. The plates were then washed before the addition of horse-radish peroxidase-conjugated goat anti-human IgG and further incubated for 45 min at 37 °C. After a second wash, tetramethylbenzidine was added to the samples, and the samples were incubated for 15 min at 37 °C. Next, the reactions were stopped with the addition of 0.36 moL/L H2SO4, and the optical density (OD) was read at 450/620 nm. The reference double logarithm curve was generated from the serially diluted reference serum pool from HPV vaccine recipients. Titers were calculated using the diluted sample with an OD that fell within the working range of the standard curve. Serum samples from 192 healthy children of ages 6 mo to 36 mo were considered true negative samples with which to optimise the cut off value (COV). The COV calculation is based on the average OD value plus three times the standard deviation (SD) of the negative samples.
Evaluation panel
The evaluation panel contained serum samples collected from 60 unvaccinated donors and 30 volunteers who had received 3 doses of Cervarix®, the bivalent vaccine for HPV types 16 and 18. PBNAs were conducted as gold standards to determine true positive or true negative results for these samples. All 30 post-vaccination samples and 11 samples from unvaccinated individuals were positive for both anti-HPV-16 and anti-HPV-18 antibodies; 29 samples from unvaccinated individuals were negative for both anti-HPV-16 and anti-HPV-18 antibodies; 10 samples from unvaccinated individuals were positive for anti-HPV-16 antibodies but not anti-HPV-18 antibodies; and the other 10 samples from unvaccinated individuals were positive for anti-HPV-18 antibodies but not anti-HPV-16 antibodies.
Serum samples
Serum samples were collected from 1600 women of ages 18 to 25 y who participated in a Phase 2 clinical trial of a bivalent L1 VLP vaccine for HPV types 16 and 18 expressed in E. coli (Cecolin®, developed by Xiamen University and produced by Xiamen Innovax). The trial was registered with ClinicalTrials.gov, and the clinical trial number was NCT01356823. The participants were randomized into 4 groups and received different dosages (30 μg, 60 μg, or 90 μg) of Cecolin® vaccine or the control hepatitis B vaccine at day 0, month 1, and month 6. Serum samples were collected before the vaccination and one month after the vaccination. The accuracy of the ELISAs in measuring naturally acquired antibodies and vaccine-induced antibodies was assessed using baseline samples and post-vaccination samples, respectively, with PBNAs used as the gold standard.
Statistical analysis
A qualitative comparison between two methodologies was assessed by the observed agreement (which was defined as the number of double-positive results plus the number of double-negative results divided by the total number of samples analyzed) and by the Cohen’s kappa score calculation. A quantitative comparison between the two methodologies was assessed using Pearson correlation analysis after logarithmic transformation of the titers.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by National Major Scientific and Technological Special Project (2012ZX09101316), the National High-tech R&D Program (863 Program) (2012AA02A408), the International Science and Technology Cooperation Program of China (2011DFG33050), and the Xiamen Scientific Project (3502Z20127027).
Glossary
Abbreviations:
- cLIA
competitive luminex immunoassay
- COV
cut off value
- ELISA
enzyme-linked immunosorbent assay
- GFP
green fluorescent protein
- HPV
human papillomavirus
- OD
optical density
- PBNA
pseudovirion-based neutralisation assay
- SD
standard deviation
- VLP
virus-like particle
- WHO
World Health Organization
References
- 1.Crosbie EJ, Einstein MH, Franceschi S, Kitchener HC. Human papillomavirus and cervical cancer. Lancet. 2013;382:889–99. doi: 10.1016/S0140-6736(13)60022-7. [DOI] [PubMed] [Google Scholar]
- 2.FUTURE II Study Group Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 3.Paavonen J, Naud P, Salmerón J, Wheeler CM, Chow SN, Apter D, Kitchener H, Castellsague X, Teixeira JC, Skinner SR, et al. HPV PATRICIA Study Group Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–14. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 4.Who Human papillomavirus vaccines: WHO position paper. Biologicals. 2009;37:338–44. doi: 10.1016/j.biologicals.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Guidelines to assure the quality, safety and efficacy of recombinant human papilloma virus-like particle vaccines: WHO Press [Internet]. Geneva; 2006. Available from: http://www.who.int/biologicals/publications/trs/areas/vaccines/human_papillomavirus/HPVg%20Final%20BS%202050%20.pdf
- 6.Pastrana DV, Buck CB, Pang YY, Thompson CD, Castle PE, FitzGerald PC, Krüger Kjaer S, Lowy DR, Schiller JT. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004;321:205–16. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 7.Yeager MD, Aste-Amezaga M, Brown DR, Martin MM, Shah MJ, Cook JC, Christensen ND, Ackerson C, Lowe RS, Smith JF, et al. Neutralization of human papillomavirus (HPV) pseudovirions: a novel and efficient approach to detect and characterize HPV neutralizing antibodies. Virology. 2000;278:570–7. doi: 10.1006/viro.2000.0674. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson M, Wilkinson DE, Zhou T. WHO meeting on the standardization of HPV assays and the role of the WHO HPV Laboratory Network in supporting vaccine introduction held on 24-25 January 2008, Geneva, Switzerland. Vaccine. 2009;27:337–47. doi: 10.1016/j.vaccine.2008.10.062. [DOI] [PubMed] [Google Scholar]
- 9.Karem KL, Poon AC, Bierl C, Nisenbaum R, Unger E. Optimization of a human papillomavirus-specific enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 2002;9:577–82. doi: 10.1128/CDLI.9.3.577-582.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dessy FJ, Giannini SL, Bougelet CA, Kemp TJ, David MP, Poncelet SM, Pinto LA, Wettendorff MA. Correlation between direct ELISA, single epitope-based inhibition ELISA and pseudovirion-based neutralization assay for measuring anti-HPV-16 and anti-HPV-18 antibody response after vaccination with the AS04-adjuvanted HPV-16/18 cervical cancer vaccine. Hum Vaccin. 2008;4:425–34. doi: 10.4161/hv.4.6.6912. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson M, Heath A, Johnes S, Pagliusi S, Dillner J, Collaborative Study Participants Results of the first WHO international collaborative study on the standardization of the detection of antibodies to human papillomaviruses. Int J Cancer. 2006;118:1508–14. doi: 10.1002/ijc.21515. [DOI] [PubMed] [Google Scholar]
- 12.Harro CD, Pang YY, Roden RB, Hildesheim A, Wang Z, Reynolds MJ, Mast TC, Robinson R, Murphy BR, Karron RA, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst. 2001;93:284–92. doi: 10.1093/jnci/93.4.284. [DOI] [PubMed] [Google Scholar]
- 13.Einstein MH, Baron M, Levin MJ, Chatterjee A, Edwards RP, Zepp F, Carletti I, Dessy FJ, Trofa AF, Schuind A, et al. HPV-010 Study Group Comparison of the immunogenicity and safety of Cervarix and Gardasil human papillomavirus (HPV) cervical cancer vaccines in healthy women aged 18-45 years. Hum Vaccin. 2009;5:705–19. doi: 10.4161/hv.5.10.9518. [DOI] [PubMed] [Google Scholar]
- 14.Inglis S, Shaw A, Koenig S. Chapter 11: HPV vaccines: commercial research & development. Vaccine 2006; 24 Suppl 3:S3/99-105. [DOI] [PubMed] [Google Scholar]
- 15.Combita AL, Touzé A, Bousarghin L, Christensen ND, Coursaget P. Identification of two cross-neutralizing linear epitopes within the L1 major capsid protein of human papillomaviruses. J Virol. 2002;76:6480–6. doi: 10.1128/JVI.76.13.6480-6486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanley M. Immune responses to human papillomavirus. Vaccine. 2006;24(Suppl 1):S16–22. doi: 10.1016/j.vaccine.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, Wheeler CM, Koutsky LA, Malm C, Lehtinen M, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6:271–8. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 18.Mollers M, Vossen JM, Scherpenisse M, van der Klis FR, Meijer CJ, de Melker HE. Review: current knowledge on the role of HPV antibodies after natural infection and vaccination: implications for monitoring an HPV vaccination programme. J Med Virol. 2013;85:1379–85. doi: 10.1002/jmv.23616. [DOI] [PubMed] [Google Scholar]
- 19.Simondon F, Khodja H. Capture-recapture method for estimating misclassification errors: application to the measurement of vaccine efficacy in randomized controlled trials. Int J Epidemiol. 1999;28:113–6. doi: 10.1093/ije/28.1.113. [DOI] [PubMed] [Google Scholar]
- 20.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–50. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 21.Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, Iversen OE, Olsson SE, Høye J, Steinwall M, Riis-Johannessen G, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer. 2006;95:1459–66. doi: 10.1038/sj.bjc.6603469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paavonen J, Jenkins D, Bosch FX, Naud P, Salmerón J, Wheeler CM, Chow SN, Apter DL, Kitchener HC, Castellsague X, et al. HPV PATRICIA study group Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–70. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- 23.Opalka D, Lachman CE, MacMullen SA, Jansen KU, Smith JF, Chirmule N, Esser MT. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16, and 18 by a multiplexed luminex assay. Clin Diagn Lab Immunol. 2003;10:108–15. doi: 10.1128/CDLI.10.1.108-115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dias D, Van Doren J, Schlottmann S, Kelly S, Puchalski D, Ruiz W, Boerckel P, Kessler J, Antonello JM, Green T, et al. Optimization and validation of a multiplexed luminex assay to quantify antibodies to neutralizing epitopes on human papillomaviruses 6, 11, 16, and 18. Clin Diagn Lab Immunol. 2005;12:959–69. doi: 10.1128/CDLI.12.8.959-969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu WX, Cheng T, Li SW, Pan HR, Shen WT, Chen YX, Zhang T, Zheng Z, Zhang J, Xia NS. [Establishment and application of human papillomavirus type 16 pseudovirions neutralization assay] Sheng Wu Gong Cheng Xue Bao. 2006;22:990–5. [PubMed] [Google Scholar]
- 26.Wei MX, Li SW, Huang B, Shen WT, Su YZ, Zhang CH, Gu Y, Du HL, Zhang J, Xia NS. [Production of human papillomavirus type 16 virus-like particles and its immunogenicity] Bing Du Xue Bao. 2009;25:245–50. [PubMed] [Google Scholar]
- 27.Xie M, Li S, Shen W, Li Z, Zhuang Y, Mo X, Gu Y, Wu T, Zhang J, Xia N. [Expression, purification and immunogenicity analysis of HPV type 18 virus-like particles from Escherichia coli] Sheng Wu Gong Cheng Xue Bao. 2009;25:1082–7. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.