Abstract
The development of an effective T cell based HIV vaccine would need to elicit cell mediated immune responses with superior magnitude, breadth, and quality. Since blocking the interactions between inhibitory receptors with their associated ligands using soluble PD-1 (sPD-1) and soluble Tim-3 (sTim-3) have been shown to reverse T cell exhaustion and enhance cell mediated immune responses, we tested if co-administration of sPD-1 and sTim-3 with an adenovirus vectored SIV vaccine (rAd5-SIV) can enhance cell mediated immune responses. The frequency of SIV antigen specific IFN-γ spot-forming cells and the secretion of IFN-γ and TNF-α by splenocytes from rAd5-SIV immunized mice was significantly increased when stimulated ex vivo with SIV peptides in the presence of sPD-1 or sTim-3 or both sPD-1 and sTim-3. The magnitude of cell mediated immune responses elicited by rAd5-SIV was enhanced by co-administration of sPD-1 and sTim-3. Co-administration of both sPD-1 and sTim-3 induced higher frequency of SIV antigen specific IFN-γ+ spot-forming cells to poorly immunogenic Vif and Tat. The percentage of cell mediated responses for each SIV antigen became more balanced, with reduction to Gag but induction to non-structural proteins. Furthermore, co-injection of rAd5-sPD1 and rAd5-sTim3 with rAd5-SIV in mice enhanced T cell proliferation capability and generated more antigen specific IFN-γ+ CD4+ and CD8+ T cells. Our study provided a new approach to enhance vaccine induced cell mediated immune responses, which may be applicable to improve the efficacy of vaccines against SIV/HIV.
Keywords: soluble PD-1, soluble Tim-3, adjuvant, immunogenicity, SIV
Introduction
After 30 y of extensive research, a safe and effective HIV vaccine, which is believed to be the ultimate solution for the control HIV/AIDS epidemic, has so far been elusive. Increasing evidence has suggested that HIV-specific CD8+ T cells play an important role in the suppression of viral replication.1 Previously, we developed an SIV vaccine comprising of the nine viral antigens derived from SIVmac239 which could elicit cell mediated immune responses against multiple antigens in immunized mice.2 However, immune responses induced by some antigens, especially the evolutionally more conserved nonstructural proteins, were very weak probably due to antigenic competition and the intrinsic poor immunogenicity of these viral proteins. The elevation of immune responses to evolutionally more conserved nonstructural proteins may improve the vaccine efficacy and minimize the chance of escape of the virus under immune pressure. Therefore, novel adjuvants are needed to boost the magnitude and quality of cell mediated immune responses to target antigens including sub-dominant antigens or epitopes for the development of an effective SIV/HIV vaccine that contains multiple antigens.
Blocking co-inhibitory pathways has been exploited for developing novel vaccines and therapeutic modalities against cancers and infectious diseases.3-10 Among these, programmed death 1 (PD-1) and T-cell immunoglobulin and mucin domain 3 (Tim-3), are dominantly expressed on the surface of activated Th1 T cells, CD8+T cells and other leukocytes.11,12 Cross-linking of PD-1 by its ligands, PD-L1 and PD-L2, can lead to the downregulation of T-cell responses by mediating programmed cell death and inhibiting cell proliferation.13 It has been demonstrated in mice that the interaction of Tim-3 with its ligand, galectin-9, promotes the death of IFN-γ producing Th1 cells and negatively regulates Th1 cell mediated immune responses.14 PD-1 and Tim-3 play a role in inhibiting T cell activation and maintaining peripheral tolerance,13,15-17 and can result in exhaustion of antigen-specific Th1 cells and CTLs in patients with cancer and chronic infections.18-21 The continued antigen stimulation during chronic infections induces high level expression of PD-1 on CD4+ and CD8+ T cells, which plays a major role in the T cell exhaustion, characterized by the loss of cytotoxicity and cytokines production.11 The expression of Tim-3 on T helper 1 (Th1) and CD8+T cells is associated with dysfunction of these cells and disease progression.22 On the other hand, blockade of these inhibitory pathways with antibodies such as anti-PD-1 or anti-PD-L1 or anti-Tim-3 have been shown to restore the functions of the exhausted antigen-specific CD8+ T cells20,23 and vaccine-induced multi-functional CD8+ T cells.24,25 Blocking both PD-1 and Tim-3 pathways have been reported to synergistically restore the functions of exhausted T cells and result in better control of tumor growth and viral infections.26,27 In addition to antibodies, soluble PD-1 (sPD-1) and soluble Tim-3 (sTim-3), which can block the interactions between inhibitory receptors (PD-1 and Tim-3) and their respective ligands (PD-L and Galectin-9), have also attracted interests as molecular adjuvants for vaccines and immunotherapeutics. Incubation of PBMCs from SIV-infected rhesus macaques with SIV peptide pools and sPD-1 has been shown to enhance the proliferation capacity of SIV-specific CD4+ and CD8+ T cells.28 sPD-1 has been shown to enhance tumor-specific CD8+ T cell responses elicited by DNA or adenovirus-vector vaccines.29 Blockade of the Tim-3 pathway via sTim-3 has also been reported to enhance the proliferation of HIV-specific CD4+ and CD8+ T cells from patients.21 However, these two soluble proteins have not yet evaluated as potential molecular adjuvants in the context of an SIV vaccine.
In this study, we evaluated the effects of sPD-1 and sTim-3 on cell mediated immune responses induced by recombinant adenoviruses vectored SIV vaccine (rAd5-SIV) that consists of all SIV proteins, including structure proteins (Gag, Pol, Env) and non-structure proteins (Nef, Vif, Vpx, Vpr, Rev, and Tat). Recombinant adenoviruses expressing sPD-1 and sTim-3 were generated and used in combination with rAd5-SIV. Our study provided insights in the design of new molecular adjuvants that may facilitate the development of an effective HIV/AIDS vaccine.
Results
sPD-1 and sTim-3 increased the frequency and secretion of SIV antigen specific IFN-γ and TNF-α producing cells ex vivo
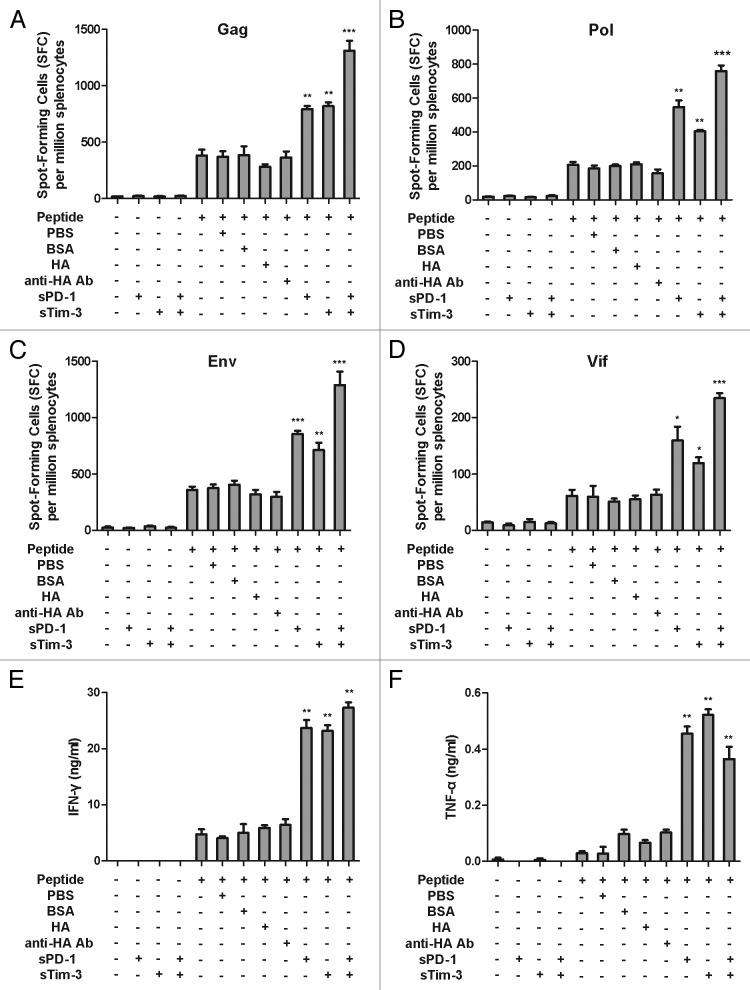
Cell mediated immune responses can be assessed by using a variety of methods. In this study, we applied cytokine enzyme-linked immunospot (ELISPOT) assay, intracellular cytokine staining of CD4+ and CD8+ T cells, T cell proliferation assays, and quantification of cytokines that associated with cell mediated immune response. The frequency of IFN-γ-producing cells has been the most widely used parameter to assess vaccine-induced cell mediated immune responses. TNF-α is another cytokine capable of mediating the killing of a variety of infections.30 To assess if sPD-1 and sTim-3 could block their corresponding inhibitory pathways and exert any effect on cell mediated immune response elicited by an experimental SIV vaccine generated in our lab, we first measured the frequency of SIV antigen specific IFN-γ producing cells and the secretion levels of IFN-γ and TNF-α by splenocytes isolated from the mice immunized with an experimental SIV vaccine rAd5-SIV. Splenocytes were stimulated with either SIV peptides alone or with SIV peptides in combination with sPD-1 or sTim-3 or both sPD-1 and sTim-3. The IFN-γ ELISPOT assay showed that the frequency of SIV antigen specific IFN-γ secreting cells was significantly higher when splenocytes were cultured with SIV peptides, including Gag, Pol, Env, and Vif respectively, in the presence of sPD-1 or sTim-3 (Fig. 1A–D), as compared with splenocytes cultured with SIV peptides alone or with non-relevant soluble proteins such as BSA, HA protein, and an anti-HA monoclonal antibody. Combination of sPD-1 and sTim-3 further increased the frequency of SIV antigen specific IFN-γ secreting cells (Fig. 1A–D). In addition to assessing the frequency of SIV antigen specific IFN-γ spot-forming cells, we also quantified the secretion of IFN-γ and TNF-α by these splenocytes. ELISA analysis showed that production of IFN-γ and TNF-α were significantly higher in splenocytes treated with SIV peptides in the presence of sPD-1 or sTim-3 or both sPD-1 and sTim-3. Splenocytes treated with SIV peptides alone or with non-relevant proteins BSA, HA, and anti-HA monoclonal antibody had similar levels of production of IFN-γ and TNF-α (Fig. 1E and F). Taken together, these results indicated that sPD-1 and sTim-3 could block their respective inhibitory pathways and enhance cell mediated immune responses in splenocytes from mice immunized with an experimental SIV vaccine, upon re-stimulation with SIV antigen ex vivo.
Figure 1. Effects of sPD-1 and sTim-3 on the frequency of IFN-γ spot-forming cells and secretion of IFN-γ and TNF-α of splenocytes from immunized mice ex vivo. (A, B, C, and D). Enhancement of Gag, Pol, Env, and Vif antigen specific IFN-γ spot-forming cells by sPD-1 and sTim-3. Splenocytes were isolated from C57BL/6 mice immunized with the rAd5-SIV and cultured with SIV Gag, Pol, Env, and Vif peptide pools respectively in the presence or absence of sPD-1 or sTim-3, or both sPD-1 and sTim-3. Twenty-four hours later, splenocytes were harvested and subjected to an IFN-γ ELISPOT assay. (E and F). Enhancement of IFN-γ and TNF-α secretion by sPD-1 and sTim-3. Splenocytes were stimulated with SIV peptides in the presence or absence of sPD-1 or sTim-3, or both sPD-1 and sTim-3. Twenty-four hours later, the culture media were measured for the secretion of IFN-γ and TNF-α using ELISA assays. BSA (bovine serum albumin), HA (influenza virus hemagglutinin), and anti-HA Ab (an anti-HA monoclonal antibody) were used as non-related protein controls (the concentration of BSA, HA, and anti-HA Ab in culture media was 16μg/ml). PBS (phosphate-buffered saline) was used as a background control. The data were analyzed by two-way ANOVA. The bars represent the standard errors. *P < 0.05;**P < 0.01; ***P < 0.001. The figure is the representation of the data obtained from two independent experiments.
Expression of sPD-1 and sTim-3 mediated by adenoviral vectors in vitro and in vivo
To enable sPD-1 and sTim-3 to be co-delivered with rAd5-SIV vaccine in mice, we first generated recombinant adenoviral vectors carrying genes encoding sPD-1 and sTim-3 respectively. To assess if rAd5-sPD1 and rAd5-sTim3 could mediate expression of sPD-1 and sTim-3 in cells infected with rAd5-sPD1 and rAd5-sTim3, we first infected 5x106 Vero cells with 5 × 109 viral particles of rAd5-sPD1 and rAd5-sTim3 respectively. At 48 h after infection, the cell culture media were collected for analysis. SDS-PAGE followed by western blot analysis using rabbit antibodies to sPD-1 and Tim-3 respectively confirmed the expression of sPD-1 and sTim-3 proteins (Fig. 2). Using ELISA assays, we detected there are 458ng PD-1 and 531ng sTim-3 in the culture media. We next confirmed that the intramuscular injection of rAd5-sPD1 and rAd5-sTim3 can result in expression of sPD-1 and sTim-3 in mice. Using ELISA assay, we were able to detect the presence of ~2.2 ng/ml sPD-1 in the mouse sera at 3 d after injection with 5 × 109 vp of rAd5-sPD1. However, we were not able to detect a consistent result of sTim-3 level in the mouse sera due to the limitation of the Tim-3 ELISA kit. We were not able to detect the presence of sPD-1 and sTim-3 in the sera of mouse injected with rAd5-empty. These results demonstrated that recombinant adenoviral vectors rAd5-sPD1 and rAd5-sTim3 can mediate the expression of sPD-1 and sTim-3 in vitro and in vivo and thus can be used for co-administration with rAd5-SIV in mice.
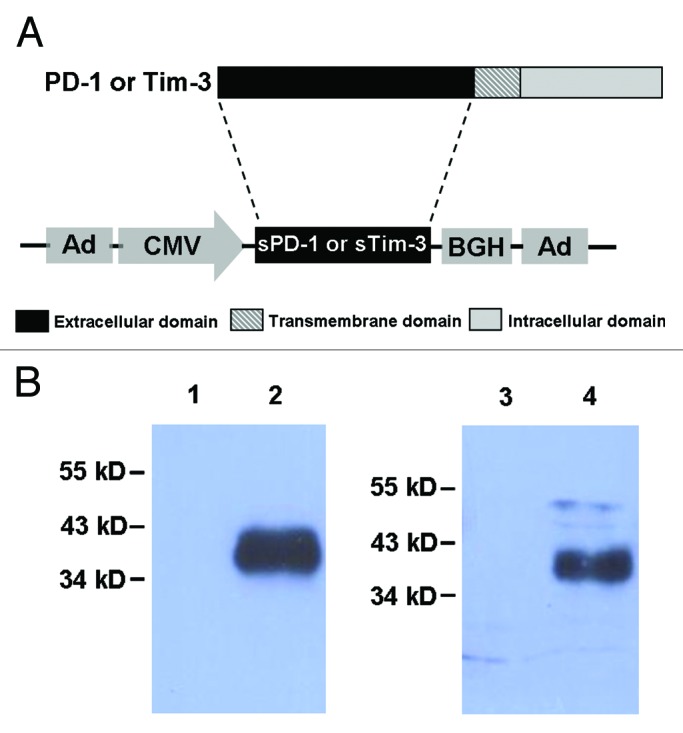
Figure 2. Expression of sPD-1 and sTim-3 mediated by rAd5-sPD1 and rAd5-sTim3. (A) Schematic presentation of rAd5-sPD1 and rAd5-sTim3 carrying genes encoding sPD-1 and sTim-3. The coding sequences of sPD-1 and sTim-3 contain the signal sequence and the extracellular domain of PD-1 and Tim-3 protein. The coding sequences of sPD-1 and sTim-3 were cloned into the E1 region of adenoviral vector to obtain recombinant adenoviruses rAd5-sPD1 and rAd5-sTim3 respectively. (B) Expression of sPD-1 and sTim-3 proteins by rAd5-sPD1 and rAd5-sTim3. Vero cells were infected with rAd5-sPD1, rAd5-sTim3, or rAd5-empty respectively. At 48 h post-infection, the culture media were subjected to SDS-PAGE followed by western blot analysis using rabbit antibodies specific for sPD-1 and sTim-3.
Co-administration of sPD-1 and sTim-3 with SIV vaccine in mice potentiated the magnitude of cell mediated immune responses and broadened the spectrum of antigen recognition
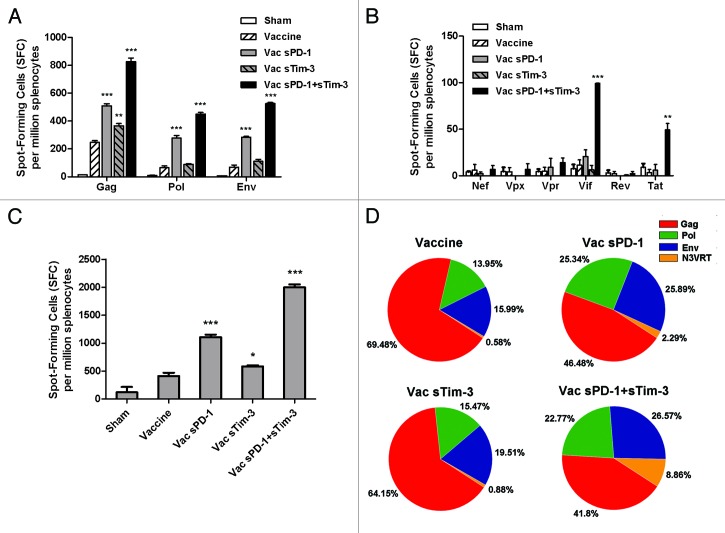
To evaluate if sPD-1 and sTim-3 could exert any potential adjuvant effect on rAd5-SIV vaccine in vivo, we immunized C57BL/6 mice with either rAd5-SIV alone or rAd5-SIV co-administered with rAd5-sPD1 or rAd5-sTim3, or both rAd5-sPD1 and rAd5-sTim3 (Table 1). Splenocytes were isolated from each group at 2 wk after immunization and subjected to an IFN-γ ELISPOT assay. rAd5-sPD1 significantly increased the frequency of IFN-γ spot-forming cells to Gag, Pol, and Env (Fig. 3A). rAd5-sTim3 significantly increased the frequency of IFN-γ spot-forming cells to Gag but the effect on Pol and Env were minimal. The combination of sPD-1 and sTim-3 significantly increased the frequency of IFN-γ spot-forming cells to Gag, Pol, and Env (Fig. 3A). It is of interesting to note that combination of sPD-1 and sTim-3 significantly boosted the frequency of IFN-γ spot-forming cells to nonstructural proteins Vif and Tat, which was negligible when the mice were immunized with rAd5-SIV alone (Fig. 3B). If all SIV structural and non-structural proteins were taken into account as a whole SIV antigen, sPD-1 significantly increased the frequency of IFN-γ spot-forming cells (Fig. 3C). sTim-3 only slightly increased the frequency of IFN-γ spot-forming cells. The most significant enhancement effect was observed when both rAd5-sPD1 and rAd5-sTim3 were co-administered with rAd5-SIV vaccine (Fig. 3C). In contrast, immunization of rAd5-SIV in combination with rAd5 vectors expressing non-relevant proteins, rAd5-EGFP (enhanced green fluorescence protein) or rAd5-HA (influenza hemagglutinin), did not change the frequency of IFN-γ spot-forming cells to SIV peptide pools (Fig. S1). In addition to increasing the frequency of IFN-γ spot-forming cells to SIV antigens, the co-administration of sPD-1 or both sPD-1 and sTim-3 with the SIV vaccine significantly changed the percentage of IFN-γ spot-forming cells that are specific for each SIV antigens, resulting in a more balanced and broader antigen spectrum (Fig. 3D and Table 1). Compared with immunization of rAd5-SIV alone, the percentage of IFN-γ spot-forming cells that are specific for Gag, reduced from 69.5% among the total responses to all SIV proteins to 41.8% when rAd5-SIV was co-administered with both rAd5-sPD1 and rAd5-sTim3 (P < 0.001). Compared with immunization of rAd5-SIV alone, the percentage of Env-specific and Pol-specific IFN-γ spot-forming cells were elevated from 16% to 26.6% and from 14% to 22.8% (P < 0.001) respectively with the co-administration of both rAd5-sPD1 and rAd5-sTim3. The most striking observation was the percentage of IFN-γ spot-forming cells for SIV non-structural proteins, which increased from 0.6% to 8.9% among the responses to all SIV proteins when rAd5-SIV was co-administered with both rAd5-sPD1 and rAd5-sTim3. Co-administration of rAd5-SIV vaccine with rAd5-sTim3 could also achieved the similar results but to a less extend. This result indicated that sPD-1 and sTim-3, especially when used in combination, could enable rAd5-SIV to elicit higher magnitude of cell mediated immune responses with more balanced and broader antigen spectrum in a vaccine that is composed of multiple antigens. The enhanced cell mediated immune response against the more conserved SIV non-structural proteins may provide a unique advantage to control SIV viral infection and replication.
Table 1. Immunization regimen and the frequency of SIV antigen specific IFN-γ spot-forming cells.
| Group | Immunization | Dosagea | Spot-forming cells for structural antigens | Spot-forming cells for non-structural antigens |
|---|---|---|---|---|
| 1 | rAd5-empty | 1.0 × 1010 vp | 53 ± 24 | 21 ± 5 |
| 2 | rAd5-SIV | 0.5 × 1010 vp | 342 ± 38 | 2 ± 1 |
| 3 | rAd5-SIV + | 0.5 × 1010 vp | 1068 ± 72 | 25 ± 4 |
| rAd5-sPD1 | 0.25 × 1010 vp | |||
| 4 | rAd5-SIV + | 0.5 × 1010 vp | 564 ± 31 | 5 ± 2 |
| rAd5-sTim3 | 0.25 × 1010 vp | |||
| 5 | rAd5-SIV + | 0.5 × 1010 vp | 1801 ± 64 | 175 ± 18 |
| rAd5-sPD1 + rAd5-sTim3 |
0.25 × 1010 vp 0.25 × 1010 vp |
a rAd5 vectors at 1 × 1010 viral particles (vp) final dose were used to immunized mice. rAd5-empty was used to make up the final dose (0.5 × 1010 vp for group 2 and 0.25 × 1010 vp for groups 3 and 4).
Figure 3. Effects of sPD-1 and sTim-3 on the frequency of IFN-γ spot-forming cells and the percentage of responses to each antigen in mice immunized with rAd5-SIV vaccine. (A) The frequency of IFN-γ spot-forming cells specific for SIV structural proteins Gag, Pol, and Env. (B) The frequency of IFN-γ spot-forming cells specific for SIV non-structural proteins Nef, Vpx, Vpr, Vif, Rev, and Tat, respectively (C) The frequency of IFN-γ spot-forming cells specific for all SIV antigens. (D) The percentage of IFN-γ spot-forming cells specific for structural antigens Gag, Pol, Env, and nonstructural antigens (N3VRT: Nef, Vpx, Vpr, Vif, Rev, and Tat) were shown in a pie chart. C57BL/6 mice were immunized with either rAd5-SIV alone or rAd5-SIV co-administered with rAd5-sPD1 or rAd5-sTim3, or rAd5-SIV co-administered with both rAd5-sPD1 and rAd5-sTim3. At 2 wk after immunization, splenocytes were harvested and cultured with SIV peptides for each antigen and subjected to an IFN-γ ELISPOT assay. The data were analyzed by two-way ANOVA. The bars represent the standard errors. *P < 0.05; **P < 0.01; ***P < 0.001. The data are the representation of two independent experiments.
Co-administration of sPD-1 and sTim-3 with SIV vaccine in mice increased the number of IFN-γ+ CD4+ and IFN-γ+ CD8+ T cells and enhanced T cell proliferation capability
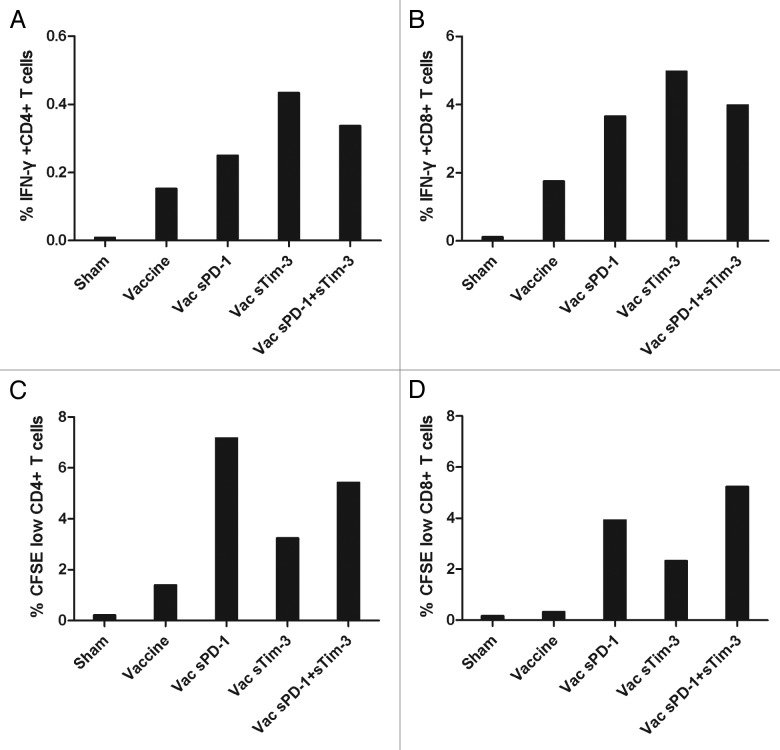
To further investigate whether CD4+ and CD8+ T cell subsets were affected by co-administration of sPD-1 and sTim-3 with rAd5-SIV vaccine, splenocytes were harvested from mice received different immunization regimens (Table 1). Splenocytes were cultured and stimulated with SIV Gag peptides and subjected to flow cytometry analysis for intracellular IFN-γ secretion in CD4+ and CD8+ T cell subsets. Compared with immunization with rAd5-SIV alone, the percentages of Gag-specific IFN-γ+ CD8+ T cells were significantly higher in mice immunized with rAd5-SIV co-administered with rAd5-sPD1 or rAd5-sTim3, or both rAd5-sPD1 and rAd5-sTim3 (Fig. 4B). Gag-specific IFN-γ+ CD4+ T cells were also increased but the magnitude is much lower (Fig. 4A). These results demonstrated that co-administration of sPD-1 and sTim-3 with an experimental SIV vaccine could enhance the quality of T cells in producing IFN-γ, especially CD8+ T cells in responding to antigen stimulation. We next evaluated if sPD-1 and sTim-3 can affect the proliferation capability of antigen specific T cells using a CFSE-based T cell proliferation assay. Splenocytes from each immunization regimens (Table 1) were harvested and stimulated with SIV antigen Gag peptides. The proliferation capability of SIV Gag-specific CD4+ and CD8+ T cells were significantly elevated when mice were immunized with rAd5-SIV in combination with rAd5-sPD1, or rAd5-sTim3 or both rAd5-sPD1 and rAd5-sTim3 (Fig. 4C and D). However, we did not observe a significant additive or synergistic effect with the combination of both sPD-1 and sTim-3. Taken together, these results suggested that co-administration of sPD-1 and sTim-3 with an SIV vaccine could enhance the quality of T cell responses in responding to antigen re-stimulation, especially CD8+ T cells.
Figure 4. Effects of sPD-1 and sTim-3 on CD4+ and CD8+ T cells from mice immunized with rAd5-SIV vaccine in producing IFN-γ and proliferation capability upon stimulation with SIV antigen peptides. (A) The percentage of antigen specific IFN-γ+ CD4+ T cells in total CD4+ T cells. (B) The percentage of antigen specific IFN-γ+ CD8+ T cells in total CD8+ T cells. (C) The percentage of antigen specific proliferating CD4+ T cells. (D) The percentage of antigen specific proliferating CD8+ T cells. C57BL/6 mice were immunized with either rAd5-SIV alone or rAd5-SIV co-administered with rAd5-sPD1 or rAd5-sTim3, or rAd5-SIV co-administered with both rAd5-sPD1 and rAd5-sTim3. At 2 wk after immunization, splenocytes were harvested and cultured with SIV Gag peptides. A flow cytometry assay based on intracellular cytokine staining was used to quantify Gag-specific IFN-γ secreting CD4+ and CD8+ T cells. The percentage of proliferating cells was determined by the ratio of the CFSE-low cells to the total CD4+ T cells or total CD8+ T cells respectively. The data are the representation of two independent experiments.
Discussion
In this study, we demonstrated that two potential molecular adjuvants, sPD-1 and sTim-3, could significantly enhance SIV specific cell mediated immune responses elicited by an adenovirus vectored SIV vaccine that contains all SIV antigens.
The high mutation nature of HIV presents a formidable challenge to current HIV/AIDS vaccine development. Vaccines comprising multiple viral antigens are thought to provide better benefits for controlling viral infection and replication.2,31-34 Although the incorporation of multiple antigens has been considered as a strategy to provide broader spectrum of antigen recognition and to minimize viral escape in the design of HIV/SIV vaccines,2 the immune responses to the sub-dominant antigens or epitopes are usually poor due to antigenic competition and the intrinsic nature of these antigens throughout evolution.35,36 The development of novel molecular adjuvants to enhance cell mediated immune responses to sub-dominant antigens or epitopes is a logical solution to this problem. It is also desirable to elevate the magnitude and improve the quality of T cell responses to every antigen.
We showed that blockade of PD-1 and Tim-3 pathways could enhance the magnitude and improve the quality of cell mediated immune responses to SIV antigens. Importantly, combination of sPD-1 and sTim-3 greatly improved immune responses against SIV nonstructural antigens Vif and Tat. The cellular immune response to Gag decreased from a dominating 69.5% to 41.8%, while the response to SIV non-structural proteins increased from 0.6% to 8.9% among the total responses to all SIV proteins. Pol-specific and Env-specific responses also increased from 14% and 16% to 22.8% and 26.6% respectively. The quantity and quality of antigen specific T cells were enhanced. These results suggested that sPD-1 and sTim-3 could potentiate multiple antigen SIV vaccine to generate cellular immune responses with greater magnitude and broader antigen spectrum. Previously, Onlamoon et al. reported that incubation of PBMCs from SIV-infected rhesus monkeys with SIV peptide pools plus sPD-1 enhanced the proliferation capacity of SIV-specific CD4+ and CD8+ T cells.28 The result we obtained using an experimental SIV vaccine further extend that finding. sTim-3 has also been shown to enhance the proliferation of HIV-specific CD4+ and CD8+ T cells.21 Several studies have demonstrated that the therapeutic effects of a combined blockade of the PD-1 and Tim-3 pathways in tumor-bearing and chronically-infected patients.19,26,27,37,38 However, only a few studies evaluated the effects of sPD-1 and sTim-3 on immune responses elicited by a vaccine.29,39,40 It is of interesting to note that another soluble Tim-3 molecule that lacks the mucin domain was shown to impair T cell responses and facilitate the growth of tumors in mice.41 Consistent with our finding, an sTim-3 containing the IgV portion and the mucin domain, could inhibit the interaction of Tim-3 with its ligands and enhanced cytokine secretion of HIV-specific T cells in humans.21 Future studies will be needed to clarify if the mucin domain has any roles in the function of sTim-3. In this study, we demonstrated that co-administration of sPD-1 or sTim-3 or both could significantly augment the frequency of antigen specific IFN-γ-producing cells ex vivo (Fig. 1A–D), and induce significantly higher magnitude of cell mediated immune responses in vivo (Fig. 3C).
We believed that the usage of sPD-1 and sTim-3 as molecular adjuvants for SIV/HIV vaccines possess advantages over anti-PD-1 and anti-Tim-3 neutralizing antibodies in the context of designing a vaccine. The coding sequences of sPD-1 and sTim-3 are known and are available for further studies. The production of sPD-1 and sTim-3 proteins or construction of plasmid DNA or viral vectors is relative simple and cost-effective compared with generation and production of monoclonal neutralizing antibodies. Tim-3 is expressed on the exhausting T cells at the later stage compared with PD-1.42 Co-expression of different inhibitory receptors including PD-1 and Tim-3 is correlated with more severe T cell exhaustion in HIV infected patients.21 Therefore, combination of sPD-1 and sTim-3 could block the inhibitory pathway at different exhausting stage, and thus may act additively or synergistically to improve the efficacy of a T cell immunity based vaccine, in particular, in the context of HIV vaccine, which has not been able to achieve any promising results in every modality so far. One possible concern associated with the use of sPD-1 and sTim-3 is that they may increase the risk of developing certain autoimmune diseases, given the critical role of PD-1 and Tim-3 receptor played in maintaining tolerance of immune cells.13,17 However, the effect of sPD-1 and sTim-3 when co-administered with a vaccine upon immunization is transient and thus the adverse effects, if any, is likely to be limited.
In summary, we demonstrated that blockade of the PD-1 and Tim-3 pathways with sPD-1 and sTim-3 could improve cell mediated immune responses elicited by an experimental SIV vaccine in mice. Our work provided a new strategy to generate SIV-specific cell mediated immune responses with broader antigen spectrum and greater magnitude. sPD-1 and sTim-3 and their combination have the potential to be used as molecular adjuvants in the design of new HIV/SIV vaccines. This strategy warrants further tests in rhesus macaque models to evaluate the potency of sPD-1 and sTim-3, especially their combination as molecular adjuvants for prophylactic and therapeutic vaccines against SIV infection.
Materials and Methods
Replication-defective recombinant adenoviral vectors
The E1, E3-deleted recombinant adenovirus serotype 5 (rAd5) is used as the vector to deliver target antigens and proteins. rAd5-Gag, rAd5-Pol, rAd5-Env, and rAd5-N3VRT, carrying genes encoding SIV structural proteins (Gag, Pol, Env) and non-structural proteins as a fusion protein N3VRT (Nef, Vpx, Vpr, Vif, Rev, and Tat), were generated previously in our lab.2 rAd5-SIV is referred to as an experimental SIV vaccine that contains all SIV antigens with the equal mixture of rAd5-Gag, rAd5-Pol, rAd5-Env, and rAd5-N3VRT. rAd5-empty is an adenovirus carrying no gene insert and is used as a vector control. rAd5-EGFP and rAd5-HA carrying the genes encoding Enhanced Green Florescence Protein (EGFP) and influenza virus hemagglutinin protein (HA) respectively were used as non-relevant protein controls. The rAd5 vectors carrying murine sPD-1 (amino acids 1–169) and sTim-3 (amino acids 1–191) cDNAs were generated according to the methods described previously.43,44 In brief, genes encoding sPD-1 and sTim-3 were synthesized and sub-cloned into the shuttle vector pGA1 respectively. pGA1-sPD1 and pGA1-sTim3 were subjected to homologous recombination with the linearized Ad5 backbone (deletion of the E1 and E3 genes) in Escherichia coli BJ5183, respectively. The pAd5 plasmids were amplified in Escherichia coli XL-Blue and prepared with Qiagen Plamsid Midi kit (Qiagen, 12145) according to the manufacture’s protocol. pAd5-sPD1 and pAd5-sTim3 were then linearized and transfected into HEK-293 cells to produce respective recombinant adenovirus. To confirm the expression of sPD-1 and sTim-3, Vero cells were infected with purified rAd5-sPD1, rAd5-sTim3, and rAd5-empty respectively. The cell culture media were harvested at 48 h post-infection. The expression of sPD-1 and sTim-3 in the cell culture supernatant was further analyzed by SDS-PAGE and Western-blot by using polyclonal rabbit anti-mouse PD-1 (Sino Biological Inc., 50124-RP02) and anti-mouse Tim-3 (Abcam, ab47069) antibodies, respectively.
Animals and immunization strategies
Female C57BL/6 mice at 6 to 8 wk old were used and housed at the Experimental Animal Care Center of the Guangzhou Institutes of Biomedicine and Health (GIBH). All animal procedures were performed according to the GIBH Institutional Animal Care and Use Committee (IACUC).
The mice were divided into 5 groups with 5 mice in each group (Table 1). Group 1 received intramuscular injection of rAd5-empty vector as control. Group 2 received rAd5-SIV vaccine that includes four adenovirus vectors encoding SIV Gag, Pol, Env, and N3VRT, respectively. Groups 3 received the rAd5-SIV vaccine in combination with rAd5-sPD1. Groups 4 received the rAd5-SIV vaccine in combination with rAd5-sTim3. Group 5 received the rAd5-SIV vaccine plus the rAd5-sPD1 and rAd5-sTim3. rAd5-empty were supplemented to group 2 to 4, to a total dose of 1 × 1010 viral particles in 100μl volume. Half of each dose was injected into quadriceps femoris intramuscularly (i.m.) on the hind legs. Five mice from each group were sacrificed at 2 wk after immunization. Splenocytes were harvested and subjected to subsequent analysis.
IFN-γ enzyme-linked immunospot (ELISPOT) assay
To test the cell mediated immune response to rAd5-SIV vaccine, IFN-γ ELISPOT assays were performed according to the protocol as previously described.2,43,45 Briefly, 96-well microtiter plates containing Immobilon-P membrane (Millipore, MSIPS4510) were coated with monoclonal anti-mouse IFN-γ antibodies (BD PharMingen, 551216) and incubated at 4 °C for overnight. The plates were blocked with RPMI medium 1640 (Gibco, 11875–093) containing 0.05 mM 2-mercaptoethanol, 1 mM sodium pyruvate, 2 mM L-glutamine (Gibco, 25030–081), 10 mM HEPES (Gibco, 15630–080), and 10% fetal bovine serum (FBS, HyClone, SH30070) at 37 °C for 2 h. Splenocytes isolated from animals at 2 wk after immunization with rAd5-SIV were seeded into 96-well plates at 1 × 106 per well. The SIVmac239 antigen peptide pool was added into plates at a final concentration of 2 μg/ml of each peptide to stimulate splenocytes. The SIVmac239 peptide pools contained 15 amino acid peptides (each was overlapped by 11 amino acids), and were gifted from the NIH AIDS Research and Reference Reagent Program. The overlapped SIVmac239 peptides covered the complete amino acid sequences of Gag, Pol, Env, Nef, Vpr, Vpx, Vif, Rev, Tat. Dimethyl sulfoxide (DMSO, Sigma, D2650) was used as the mock stimulation. To test the effects of sPD-1 and sTim-3 on the secretion of cytokines ex vivo, splenocytes were cultured in the presence or absence of purified sPD-1 and/or sTim-3 at 32 μg/ml. Three non-relevant soluble proteins, BSA (Millipore, 126576), HA protein, and an anti-HA monoclonal antibody46 were used as controls. The expression of IFN-γ was detected with the biotinylated polyclonal anti-mouse IFN-γ (BD PharMingen, 551506) and NBT/BCIP reagent (Pierce, 34042) at 24 h after incubation with peptide or DMSO. Finally, the spots were counted with an ELISPOT reader (Bioreader 4000; BIOSYS), and the results were reported as the frequency of spot-forming cells (SFC) per million splenocytes.
Enzyme-linked immunosorbent assay
To test the effects of sPD-1 and sTim-3 on the secretion of cytokines ex vivo, ELISA assay was used to measure the level of IFN-γ, TNF-α in splenocytes culture supernatant. Briefly, splenocytes isolated from animals at 2 wk after immunization with rAd5-SIV were seeded into 96-well plates at 1 × 106 per well and stimulated with the Env peptide pool at 2 μg/ml in the presence or absence of purified sPD-1 and/or sTim-3 at 32 μg/ml. Three non-relevant soluble proteins, BSA (Millipore, 126576), HA protein, and an anti-HA monoclonal antibody were used as controls. The cell culture media were harvested at 24 h later. The levels of IFN-γ, TNF-α in the supernatant were measured by using the mouse ELISA kits (Dakewe Biotech, IFN-γ,.DKW12–2000–096; TNF-α, DKW12–2720–096). Results were analyzed at OD450 using a Synergy HT multimode plate reader (BioTek Instruments, Inc.).
Intracellular cytokine staining and flow cytometry
To specify and quantify the cytokine producing antigen-specific T cells, multicolor flow cytometry was performed to analyze the intracellular cytokine staining (ICS) as previously described.2,43,47 Briefly, freshly isolated splenocytes were seeded into 96-well plates (2 × 106 cells/well) and supplemented with either SIV-specific peptide pools (2 μg/ml of each peptide) or DMSO. Brefeldin A (BD Biosciences, 555029) was added to inhibit the cytokine secretion after 1 h’s incubation at 37 °C. Splenocytes were further incubated at 37 °C for 16 h and proceeded with surface and intracellular staining by using monoclonal antibodies as followings: anti-CD3-PerCP (BD PharMingen, 553067), anti-CD4-FITC (eBioscience, 11–0041–82), anti-CD8-APC (eBioscience, 17–0081–82), and anti-IFN-γ-PE (BD PharMingen, 554412). The samples were analyzed with a BD Accuri™ C6 (BD Biosciences).
CFSE T lymphocyte proliferation assay
To determine the proliferation activity of T cells from animals that were treated with different immunization strategies, CFSE T cell proliferation assay was performed to monitor the dividing cells ex vivo. Briefly, freshly isolated splenocytes were suspended with PBS containing 0.1% FBS at 1 × 106 cells/ml and treated with carboxyfluorescein diacetate succinimidyl ester (CFSE, Molecular Probes, C1157) at 0.25 mM at 37 °C for 10 min. Cells were incubated with ice-cold complete RPMI medium for 5 min to stop the cellular uptake of CFSE. Cells were immediately treated with medium that contained SIV-specific peptide pool (2 μg/ml of each peptide) for 6 d. Finally, cells were stained with anti-CD3-PerCP (BD PharMingen, 553067), anti-CD4-APC (BD PharMingen, 553051) and anti-CD8-PE (BD PharMingen, 553032) and analyzed with a BD Accuri™C6.
Purification and analysis of sPD-1 and sTim-3 proteins
To obtain sPD-1 and sTim-3 proteins, the coding sequences were inserted into pcDNA4 plasmids. 293T cells were transfected with pcDNA4-sPD1 or pcDNA4-sTim3 to express soluble PD-1 or Tim-3 containing a His-tag at the C-terminal. Culture media were concentrated through ultrafiltration and purified using nickel-affinity column chromatography. Quantification of total protein was performed using the bicinchoninic acid assay (BCA, Pierce, 23227). The purity of sPD-1 and sTim-3 protein was estimated using ELISA kits according to the manufacturer's protocol (mouse PD-1 ELISA kit: CSB-E13586 min, Cusabio Biotech Co., Ltd; mouse Tim-3 ELISA kit: HTYR4Y, Huiying Biotech Co., Ltd). The purity of sPD-1 and sTim-3 in the preparation was estimated to be at 40–50% in the total protein. Based on an earlier report in which a recombinant soluble PD-1 at 6.7 μg/ml was able to affect the macaque SIV-specific CD4+ and CD8+ T cells proliferative responses,28 we tested our sPD-1 and sTim-3 at concentration ranging from 4 μg/ml to 64 μg/ml and found that 32 μg/ml could exert the most potent blockade activity on cellular immune response in vitro (Fig. S2).
Data analysis and statistical analysis
Flow cytometry data were analyzed with FlowJo software (version 7.6.2; Tree Star, Inc.). Statistical analysis and graphical presentations were generated with GraphPad Prism software (version 5.01; Graph-Pad Software Inc.). Mean and standard deviation were used to describe the immune response in each group. Comparison between different groups was performed using a two-tailed the Student t test. P < 0.05 was considered statistically significant.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (31200693, 31370923), and the National Key Science and Technology Specific Projects of China (2012ZX10001–009–001). We thank Xuehua ZHENG, Yichu LIU, and Yinfeng ZHANG for their technical assistance throughout the project, Dr Yanhui CAI from Division of Immunology, Tulane National Primate Research Center for editing the manuscript and the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH for kindly provided the SIV Env, Gag, and Pol peptides.
Glossary
Abbreviations:
- Ad5
adenovirus type 5
- AIDS
Acquired Immune Deficiency Syndrome
- BCA
bicinchoninic acid assay
- BSA
Bovine serum albumin
- CFSE
carboxyfluorescein diacetate succinimidyl ester
- DCs
dentritic cells
- DMSO
Dimethyl sulfoxide
- EGFP
Enhanced Green Fluorescent Protein
- ELISA
Enzyme-linked immunosorbent assay
- ELISPOT
Enzyme-linked immunosorbent spot
- FBS
fetal bovine serum
- HA
hemagglutinin protein
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- HIV
Human immunodeficiency virus
- HRP
Horseradish peroxidase
- ICS
Intracellular cytokine staining
- IFN-γ
Interferon gamma
- PBMCs
Peripheral Blood Mononuclear Cells
- PBS
Phosphate Buffered Saline
- NBT/BCIP
Nitro blue tetrazolium/5-Bromo-4-chloro-3-indolyl phosphate
- PD-L1
Programmed cell death 1 ligand 1
- rAd5
recombinant adenovirus type 5
- RLU
relative light units
- RPMI
Roswell Park Memorial Institute
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SEAP
secreted alkaline phosphatase
- SEB
staphylococcus enterotoxin B
- SFC
spot-forming cells
- SIV
Simian immunodeficiency virus
- sPD-1
soluble Programmed Death 1
- sTim-3
soluble T-cell immunoglobulin and mucin domain 3
- TNF-α
Tumor necrosis factor alpha
- vp
viral particles
References
- 1.Thèze J, Chakrabarti LA, Vingert B, Porichis F, Kaufmann DE. HIV controllers: a multifactorial phenotype of spontaneous viral suppression. Clin Immunol. 2011;141:15–30. doi: 10.1016/j.clim.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun C, Zhang L, Zhang M, Liu Y, Zhong M, Ma X, Chen L. Induction of balance and breadth in the immune response is beneficial for the control of SIVmac239 replication in rhesus monkeys. J Infect. 2010;60:371–81. doi: 10.1016/j.jinf.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Assarsson E, Kambayashi T, Persson CM, Chambers BJ, Ljunggren H-G. 2B4/CD48-mediated regulation of lymphocyte activation and function. J Immunol. 2005;175:2045–9. doi: 10.4049/jimmunol.175.4.2045. [DOI] [PubMed] [Google Scholar]
- 4.Bhadra R, Gigley JP, Weiss LM, Khan IA. Control of Toxoplasma reactivation by rescue of dysfunctional CD8+ T-cell response via PD-1-PDL-1 blockade. Proc Natl Acad Sci U S A. 2011;108:9196–201. doi: 10.1073/pnas.1015298108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.del Rio ML, Lucas CL, Buhler L, Rayat G, Rodriguez-Barbosa JI. HVEM/LIGHT/BTLA/CD160 cosignaling pathways as targets for immune regulation. J Leukoc Biol. 2010;87:223–35. doi: 10.1189/jlb.0809590. [DOI] [PubMed] [Google Scholar]
- 6.Finnefrock AC, Tang A, Li F, Freed DC, Feng M, Cox KS, Sykes KJ, Guare JP, Miller MD, Olsen DB, et al. PD-1 blockade in rhesus macaques: impact on chronic infection and prophylactic vaccination. J Immunol. 2009;182:980–7. doi: 10.4049/jimmunol.182.2.980. [DOI] [PubMed] [Google Scholar]
- 7.Fougeray S, Brignone C, Triebel F. A soluble LAG-3 protein as an immunopotentiator for therapeutic vaccines: Preclinical evaluation of IMP321. Vaccine. 2006;24:5426–33. doi: 10.1016/j.vaccine.2006.03.050. [DOI] [PubMed] [Google Scholar]
- 8.Peng B, Lu C, Tang L, Yeh IT, He Z, Wu Y, Zhong G. Enhanced upper genital tract pathologies by blocking Tim-3 and PD-L1 signaling pathways in mice intravaginally infected with Chlamydia muridarum. BMC Infect Dis. 2011;11:347. doi: 10.1186/1471-2334-11-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pilon-Thomas S, Mackay A, Vohra N, Mulé JJ. Blockade of programmed death ligand 1 enhances the therapeutic efficacy of combination immunotherapy against melanoma. J Immunol. 2010;184:3442–9. doi: 10.4049/jimmunol.0904114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Triebel F. LAG-3: a regulator of T-cell and DC responses and its use in therapeutic vaccination. Trends Immunol. 2003;24:619–22. doi: 10.1016/j.it.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Jin HT, Ahmed R, Okazaki T. Role of PD-1 in regulating T-cell immunity. Curr Top Microbiol Immunol. 2011;350:17–37. doi: 10.1007/82_2010_116. [DOI] [PubMed] [Google Scholar]
- 12.Gleason MK, Lenvik TR, McCullar V, Felices M, O’Brien MS, Cooley SA, Verneris MR, Cichocki F, Holman CJ, Panoskaltsis-Mortari A, et al. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood. 2012;119:3064–72. doi: 10.1182/blood-2011-06-360321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–42. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–52. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 15.Sehrawat S, Reddy PB, Rajasagi N, Suryawanshi A, Hirashima M, Rouse BT. Galectin-9/TIM-3 interaction regulates virus-specific primary and memory CD8 T cell response. PLoS Pathog. 2010;6:e1000882. doi: 10.1371/journal.ppat.1000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–45. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 17.Sabatos CA, Chakravarti S, Cha E, Schubart A, Sánchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ, Kuchroo VK. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol. 2003;4:1102–10. doi: 10.1038/ni988. [DOI] [PubMed] [Google Scholar]
- 18.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 19.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175–86. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, Castelblanco N, Kuchroo V, Gretch DR, Rosen HR. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol. 2009;83:9122–30. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–79. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kane LP. T cell Ig and mucin domain proteins and immunity. J Immunol. 2010;184:2743–9. doi: 10.4049/jimmunol.0902937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 24.Ha SJ, Mueller SN, Wherry EJ, Barber DL, Aubert RD, Sharpe AH, Freeman GJ, Ahmed R. Enhancing therapeutic vaccination by blocking PD-1-mediated inhibitory signals during chronic infection. J Exp Med. 2008;205:543–55. doi: 10.1084/jem.20071949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–10. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A. 2010;107:14733–8. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207:2187–94. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onlamoon N, Rogers K, Mayne AE, Pattanapanyasat K, Mori K, Villinger F, Ansari AA. Soluble PD-1 rescues the proliferative response of simian immunodeficiency virus-specific CD4 and CD8 T cells during chronic infection. Immunology. 2008;124:277–93. doi: 10.1111/j.1365-2567.2007.02766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song MY, Park SH, Nam HJ, Choi DH, Sung YC. Enhancement of vaccine-induced primary and memory CD8(+) T-cell responses by soluble PD-1. J Immunother. 2011;34:297–306. doi: 10.1097/CJI.0b013e318210ed0e. [DOI] [PubMed] [Google Scholar]
- 30.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–58. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 31.Amexis G, Young NS. Multiple antigenic peptides as vaccine platform for the induction of humoral responses against dengue-2 virus. Viral Immunol. 2007;20:657–63. doi: 10.1089/vim.2007.0029. [DOI] [PubMed] [Google Scholar]
- 32.Bilello JP, Manrique JM, Shin YC, Lauer W, Li W, Lifson JD, Mansfield KG, Johnson RP, Desrosiers RC. Vaccine protection against simian immunodeficiency virus in monkeys using recombinant gamma-2 herpesvirus. J Virol. 2011;85:12708–20. doi: 10.1128/JVI.00865-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, Whizin N, Oswald K, Shoemaker R, Swanson T, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–7. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holman DH, Wang D, Raja NU, Luo M, Moore KM, Woraratanadharm J, Mytle N, Dong JY. Multi-antigen vaccines based on complex adenovirus vectors induce protective immune responses against H5N1 avian influenza viruses. Vaccine. 2008;26:2627–39. doi: 10.1016/j.vaccine.2008.02.053. [DOI] [PubMed] [Google Scholar]
- 35.Grifantini R, Finco O, Bartolini E, Draghi M, Del Giudice G, Kocken C, Thomas A, Abrignani S, Grandi G. Multi-plasmid DNA vaccination avoids antigenic competition and enhances immunogenicity of a poorly immunogenic plasmid. Eur J Immunol. 1998;28:1225–32. doi: 10.1002/(SICI)1521-4141(199804)28:04<1225::AID-IMMU1225>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 36.Johansson BE, Moran TM, Kilbourne ED. Antigen-presenting B cells and helper T cells cooperatively mediate intravirionic antigenic competition between influenza A virus surface glycoproteins. Proc Natl Acad Sci U S A. 1987;84:6869–73. doi: 10.1073/pnas.84.19.6869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vali B, Jones RB, Sakhdari A, Sheth PM, Clayton K, Yue F-Y, Gyenes G, Wong D, Klein MB, Saeed S, et al. HCV-specific T cells in HCV/HIV co-infection show elevated frequencies of dual Tim-3/PD-1 expression that correlate with liver disease progression. Eur J Immunol. 2010;40:2493–505. doi: 10.1002/eji.201040340. [DOI] [PubMed] [Google Scholar]
- 38.Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, Murphy WJ, Azuma M, Anderson AC, Kuchroo VK, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501–10. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou J, Cheung AKL, Tan Z, Wang H, Yu W, Du Y, Kang Y, Lu X, Liu L, Yuen KY, et al. PD1-based DNA vaccine amplifies HIV-1 GAG-specific CD8+ T cells in mice. J Clin Invest. 2013;123:2629–42. doi: 10.1172/JCI64704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou J, Cheung AKL, Liu H, Tan Z, Tang X, Kang Y, Du Y, Wang H, Liu L, Chen Z. Potentiating functional antigen-specific CD8⁺ T cell immunity by a novel PD1 isoform-based fusion DNA vaccine. Mol Ther. 2013;21:1445–55. doi: 10.1038/mt.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geng H, Zhang G-M, Li D, Zhang H, Yuan Y, Zhu H-G, Xiao H, Han LF, Feng ZH. Soluble form of T cell Ig mucin 3 is an inhibitory molecule in T cell-mediated immune response. J Immunol. 2006;176:1411–20. doi: 10.4049/jimmunol.176.3.1411. [DOI] [PubMed] [Google Scholar]
- 42.Rahman AN, Clayton K, Mujib S, Fong IW, Ostrowski MA. TIM-3 and Its Immunoregulatory Role in HIV Infection. J Clin Cell Immunol. 2012;S7:007. [Google Scholar]
- 43.Casimiro DR, Tang A, Chen L, Fu TM, Evans RK, Davies ME, Freed DC, Hurni W, Aste-Amezaga JM, Guan L, et al. Vaccine-induced immunity in baboons by using DNA and replication-incompetent adenovirus type 5 vectors expressing a human immunodeficiency virus type 1 gag gene. J Virol. 2003;77:7663–8. doi: 10.1128/JVI.77.13.7663-7668.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu X, Meng W, Dong Z, Pan W, Sun C, Chen L. Comparative immunogenicity of recombinant adenovirus-vectored vaccines expressing different forms of hemagglutinin (HA) proteins from the H5 serotype of influenza A viruses in mice. Virus Res. 2011;155:156–62. doi: 10.1016/j.virusres.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Sun C, Feng L, Xiao L, Chen L. Enhancement of Gag-specific but reduction of Env- and Pol-specific CD8+ T cell responses by simian immunodeficiency virus nonstructural proteins in mice. AIDS Res Hum Retroviruses. 2012;28:374–83. doi: 10.1089/aid.2011.0061. [DOI] [PubMed] [Google Scholar]
- 46.Meng W, Pan W, Zhang AJX, Li Z, Wei G, Feng L, Dong Z, Li C, Hu X, Sun C, et al. Rapid Generation of Human-Like Neutralizing Monoclonal Antibodies in Urgent Preparedness for Influenza Pandemics and Virulent Infectious Diseases. PLoS One. 2013;8:e66276. doi: 10.1371/journal.pone.0066276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun C, Feng L, Zhang Y, Xiao L, Pan W, Li C, Zhang L, Chen L. Circumventing antivector immunity by using adenovirus-infected blood cells for repeated application of adenovirus-vectored vaccines: proof of concept in rhesus macaques. J Virol. 2012;86:11031–42. doi: 10.1128/JVI.00783-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.