Abstract
Epidemiological data from several European countries suggested an increased risk of the chronic sleep disorder narcolepsy following vaccination with Pandemrix™, an AS03-adjuvanted, pandemic A(H1N1)pdm09 influenza vaccine. Further research to investigate potential associations between Pandemrix™ vaccination, A(H1N1)pdm09 influenza infection and narcolepsy is required. Narcolepsy is most commonly caused by a reduction or absence of hypocretin produced by hypocretin-secreting neurons in the hypothalamus, and is tightly associated with HLA-II DQB1*06:02. Consequently, research focusing on CD4+ T-cell responses, building on the hypothesis that for disease development, T cells specific for antigen(s) from hypocretin neurons must be activated or reactivated, is considered essential. Therefore, the following key areas of research can be identified, (1) characterization of hypothetical narcolepsy-specific auto-immune CD4+ T cells, (2) mapping epitopes of such T cells, and (3) evaluating potential mechanisms that would enable such cells to gain access to the hypothalamus. Addressing these questions could further our understanding of the potential links between narcolepsy and A(H1N1)pdm09 vaccination and/or infection. Of particular interest is that any evidence of a mimicry-based mechanism could also explain the association between narcolepsy and A(H1N1)pdm09 influenza infection.
Keywords: narcolepsy, A(H1N1)pdm09 influenza, pandemic, vaccine, Pandemrix, CD4+ T cell, hypocretin
Introduction
An increased risk of narcolepsy (a chronic sleep disorder) following vaccination with Pandemrix™ was initially described in Finland, and then in several other European countries.1-5 Pandemrix™ is an AS03-adjuvanted, inactivated, detergent split-virion, pandemic A/California/7/2009 (A(H1N1)pdm09) influenza vaccine manufactured by GlaxoSmithKline Biologicals SA, Dresden, Germany.6,7 Further research is needed to determine whether the observed risk is related to the vaccine, environmental effects, genetic or other factors, or a combination thereof, and to evaluate whether there are biologically plausible mechanisms by which vaccination with Pandemrix™ could potentially have triggered narcolepsy. As A(H1N1)pdm09 influenza infections may also be linked to increased narcolepsy incidence,8 investigation of potential interactions between infections and Pandemrix™ vaccination should also be part of research efforts.
Narcolepsy as an Auto-Immune Disorder
Narcolepsy is characterized by excessive daytime sleepiness, often accompanied by cataplexy. The reduction or absence of the wake-promoting neuropeptide hypocretin, produced by hypocretin-secreting neurons in the hypothalamus, is the most common cause for narcolepsy-cataplexy.9 An auto-immune etiology for narcolepsy is likely, considering its tight association with distinct Human Leukocyte Antigen (HLA) II haplotypes, in particular for those carrying DQA1*01:02-DQB1*06:02 (denoted DQ0602). DQ0602 is commonly expressed in the general population, and present in over 98% of patients with low cerebrospinal fluid (CSF) hypocretin.9 Other HLA subtypes, particularly DQB1*03:01, increase predisposition in trans of DQB1*06:02, while the presence of DQ1 subtypes other than DQA1*01:02-DQB1*06:02 is protective.10,11 Other polymorphisms besides HLA have been found to modulate disease predisposition, and in almost all cases these also involve immune-related genes implicated in other autoimmune diseases. A significant association with single nucleotide polymorphisms (SNPs) found in the J region (which determines diversity) of the locus encoding the α-chain of the T-cell receptor (TCR) was found.12 As the TCR-α/β heterodimer interacts with peptide-carrying HLA, specific HLA II–TCR interactions likely play a key role in disease. Further supporting a role of the immune system are associations with SNPs in genes involved in antigen presentation and T-cell activation (cathepsin H and TNFSF4) and in the purinergic receptor P2Y (P2RY11) gene.9 P2RY11, which is expressed in NK and CD8+ T cells, is involved in immune cell viability.
The search for auto-antibodies has not yielded conclusive results. Antibodies against Tribbles homolog 2 (TRIB2) were found to be elevated in some narcolepsy patients when compared with controls.9 TRIB2 is expressed, albeit not exclusively, in hypocretin-secreting neurons. The role of putative auto-antibodies is unclear, and anti-TRIB2 antibody responses could also be the result of neuronal damage rather than having any causative role.13 Importantly, irrespective of the role of antibodies, the associations with DQ0602 and with SNPs in the TCR indicate that interactions between CD4+ T cells and DQ0602-restricted antigen presentation are instrumental in pathogenicity.
Interacting genetic and environmental factors are typically involved in autoimmune disease. In narcolepsy, the latter likely include upper respiratory tract infections such as influenza infections.8,9 Indeed, it was observed in an epidemiological study in China that the onset of new narcolepsy cases was seasonal and increased in frequency following the A(H1N1)pdm09 pandemic, with frequencies returning to baseline in the years thereafter.8 Interestingly, genome-wide analysis of narcolepsy cases in China revealed differences in genetic associations before and after 2009, most notably for two different HLA-region SNPs. In addition, a lower proportion of DQB1*06:02 homozygotes was observed among cases with disease onset after, relative to before, the A(H1N1)pdm09 pandemic.14 Influenza infections may, however, not be a sole trigger for narcolepsy, since recent data seem to suggest that children diagnosed with narcolepsy in 2010 in Finland might not have had antibodies against the A(H1N1)pdm09 NS1 protein, which might suggest that these children were not exposed to the virus.15 Streptococcal infection could also play a role, as antibodies against streptolysin O are elevated in patients with recent onset of narcolepsy.16 These infections can lead to induction of Th17 responses, which may affect blood-brain barrier (BBB) integrity.17
Narcolepsy and A(H1N1)pdm09 Vaccination: Avenues for Research
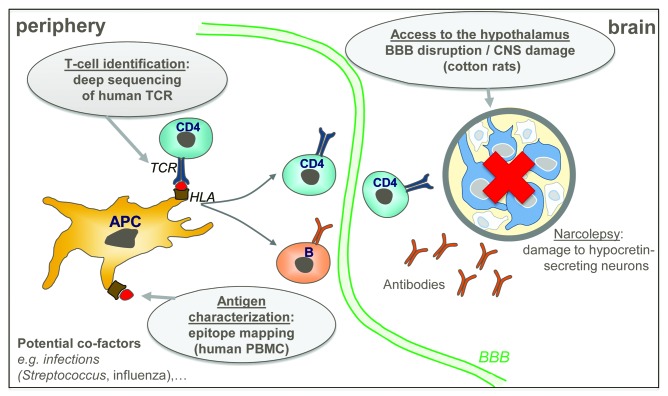
Research approaches should be focused on CD4+ T-cell responses, considering the strong association of narcolepsy with a specific HLA class II molecule, DQ0602. The fact that the lag time between A(H1N1)pdm09 antigenic exposure and the onset of narcolepsy symptoms can be as short as a few days4,18 suggests that pre-existing CD4+ T cells are re-activated in some cases. We therefore hypothesized that two conditions must be met for narcolepsy to develop: T cells specific for antigen(s) from hypocretin neurons must be activated or reactivated, and certain events must allow access of such aberrant responses to the hypothalamus, where hypocretin neurons reside. This hypothesis was translated into three research objectives (Fig. 1) with the aim to identify mechanisms by which Pandemrix™ A(H1N1)pdm09 antigens might have had a role in triggering narcolepsy: (1) Which are the hypothetical T cells that are linked to disease, and how can they be detected? (2) What are the antigenic targets of these T cells? And (3) how could any such auto-immune responses gain access to the CNS?
Figure 1. Narcolepsy pathogenesis model underpinning the research approach. The hypothesized model for narcolepsy pathogenesis is shown at the peripheral level (left panel) and in the brain (right panel). The antigen (indicated by red dots) is presented by antigen-presenting cells to CD4+ T cells through HLA-TCR interaction (left panel). Narcolepsy results from damage to hypocretin-secreting neurons, which could be triggered by auto-immune CD4+ T cells and/or antibodies (right panel). Grey balloons indicate the research objectives addressing the three key questions. TCR, T-cell receptor; HLA, human leucocyte antigen; PBMC, peripheral blood mononuclear cells; BBB, blood-brain barrier; CNS, central nervous system; APC, antigen-presenting cells.
Addressing the first two questions involves characterization of the hypothetical auto-immune CD4+ T cells and the epitopes recognized by these cells, by sequence analysis of the TCRs and mapping the antigenic targets of such T cells, respectively. Addressing the third question involves studying potential changes in BBB integrity and immune cell migration into the CNS following vaccination with Pandemrix™ and/or infection with A(H1N1)pdm09 virus using animal models.
Molecular mimicry vs. bystander activation
A potential link between narcolepsy-specific, auto-immune CD4+ T cells and A(H1N1)pdm09 influenza could be explained by two mechanisms: molecular mimicry or bystander activation. Molecular mimicry implies that functional similarity exists between epitopes of antigens derived from hypocretin neurons, and the vaccine or virus-derived A(H1N1)pdm09 peptides binding to the same alleles. The similarity could lead to cross-reactive responses following antigenic exposure. Bioinformatics analyses revealed no significant sequence homology between the vaccine A(H1N1)pdm09 haemagglutinin (HA) and neuraminidase (NA), and hypocretin and TRIB2 from hypocretin neurons (unpublished results). However, it has been shown that foreign antigens sharing only key sequences and/or structural features with self-antigens could activate auto-reactive T cells, as observed for an Epstein-Barr virus epitope which stimulated T cells specific for a myelin basic protein epitope.19 This suggests that in the case of functional mimicry, bioinformatics analysis alone might not be able to identify potentially cross-reactive epitopes: functional cross-reactivity might exist that is not obviously detectable by sequence comparison, using current tools. Interestingly, recent results from Davis and colleagues indicate that TCR cross-reactivity in CD4+ T-cell responses could be an important driver shaping the repertoire and could be a more widespread phenomenon.20
In contrast, bystander activation would involve the non-specific activation of pre-primed auto-reactive T cells that are not antigenically linked to the presented epitope(s), with stimulation occurring through immune system activation induced by infection and/or vaccination. In this case, T cells “seeing” something completely different are activated anyway. An argument against bystander activation is that the observed safety signal following Pandemrix™ vaccination was limited to narcolepsy, with no increased risks of other immune-mediated diseases such as type 1 diabetes or celiac disease in children,2,21 or of exacerbation of inflammatory rheumatic disease.22
Considering the HLA association and the specificity of the TCR-α repertoire responses required for pathogenicity,9 as well as the unknown effects of genetic background on disease, there is no animal model that can accurately reproduce ‘acquired’ narcolepsy.23 Analysis of human peripheral blood mononuclear cells (PBMC) from DQB1*06:02+ narcolepsy patients, and from HLA-matched, healthy recipients of Pandemrix™ as controls, is therefore considered to be essential.
Finding the hypothetical T cells that are linked to narcolepsy: TCR sequencing
The association with HLA and the presence of SNPs in the TCR α-chain suggest that specific CD4+ T-cell clone(s) could play a key role in disease. The hallmark of T-cell specificity is its TCR α/β sequence. Therefore, disease-specific CD4+ T cells could be identified through their unique TCR sequences. The current ideal tool for TCR identification is the novel technology of massive parallel (‘deep’) sequencing,24 which allows a comparison between TCR repertoires. By comparing TCR repertoires from patients and healthy, HLA-matched controls, a unique, narcolepsy-specific TCR, present in patients but not in controls, could potentially be identified and this would be an important biomarker for disease.
If a narcolepsy-specific TCR signature were found in patients, it would be of considerable interest to determine whether the CD4+ T cells carrying the signature are specific for A(H1N1)pdm09 influenza antigens in the vaccine and/or the virus itself. Should the latter be the case, then this would support a mechanism based on molecular mimicry: narcolepsy-associated TCR signature-positive CD4+ T cells could respond to influenza antigens. The specificity for A(H1N1)pdm09 vaccine antigens can be studied by expanding CD4+ T cells from patients and healthy controls with these antigens, followed by evaluating whether this specific TCR signature can be detected in the influenza-specific CD4+ T cells.
Finding the antigenic targets: Epitope mapping
The importance of the DQ0602 association suggests that epitopes presented by this DQ subtype could play a critical role. As functional molecular mimicry requires only limited sequence identity and can be based on structural similarities,19,25 it would be important to map experimentally any DQ0602-restricted epitopes from A(H1N1)pdm09 influenza proteins and selected proteins from hypocretin neurons.
Identification of putative cross-reactive peptides involves: (1) in vitro mapping of peptides binding to DQ0602, (2) confirmation of CD4+ T-cell activation by these peptides and (3) evaluation of any cross-reactivity between A(H1N1)pdm09 influenza peptides and peptides from hypocretin neurons. Proteins of interest are A(H1N1)pdm09 HA, NA, and PB1, and proteins from hypocretin neurons26 including hypocretin itself.
The potential identification of DQ0602-restricted epitopes in proteins from hypocretin neurons would allow for further cross-reactivity analysis, which could be performed by in vitro T-cell stimulation experiments or by using HLA class II tetramers (synthetic, tetrameric HLA II-peptide complexes that are used to visualize antigen-specific T cells by flow cytometry).20 This method would also allow phenotypic characterization of any auto-immune CD4+ T cells. Of specific interest is the cytokine profile of such T cells, as Th17 cells (a CD4+ T-cell lineage characterized by IL-17, IL-22 and GM-CSF expression) have been linked to changes in the BBB integrity and auto-immune diseases.17,27
Successful identification of cross-reactive, ‘mimicry-type’ CD4+ T-cell responses will make CD4+ T cells the research focal point and could also elucidate a potential role of the AS03 adjuvant. AS03 has been shown to enhance CD4+ T-cell responses to A(H1N1)pdm09 vaccine antigens.6 If mimicry is a reality, then potential cross-reactive CD4+ T cells could potentially be boosted as well.
If responses against proteins from hypocretin neurons are detected but cannot be linked to HA, NA or PB1 epitopes, then other proteins from influenza virus or from hypocretin neurons may be involved (either instead of, or in addition to the molecules assessed) or, alternatively, a bystander effect could be considered.
Immune response access to the hypothalamus
Even if infection and/or vaccination would stimulate auto-immune T-cell responses through mimicry or bystander activation, the question how any aberrant immune responses might gain access to the hypothalamus remains unanswered. Potential research approaches to this question are limited by the lack of a suitable animal model for narcolepsy. Nevertheless, the question of how this access might be enabled can, in part, be addressed by evaluating whether vaccination and/or infection leads to any changes in BBB integrity and/or any inflammatory and/or cellular changes in the CNS. A model of particular interest to be used for these experiments is the cotton rat, as this species can be infected by A(H1N1)pdm09 influenza virus28 while allowing practical CNS histopathological investigations. Thus, immune cell infiltration, microglial cell activation, BBB integrity (by investigating the potential vascular leakage of albumin using immunohistochemistry on brain sections), potential loss of hypocretin-secreting neurons and potential decrease of the CSF hypocretin level can be assessed in these animals following vaccination and/or infection with A(H1N1)pdm09 influenza virus.
In conclusion, completing the research outlined here could further our understanding of the possible mechanistic links between narcolepsy and A(H1N1)pdm09 vaccination and/or infection. If demonstrated, evidence of a mimicry-based mechanism could also explain the association between narcolepsy and A(H1N1)pdm09 influenza infection.
Key Messages
• Epidemiological studies have observed an increased risk of narcolepsy following vaccination with the AS03-adjuvanted A(H1N1)pdm09 influenza vaccine Pandemrix™.
• Research is being supported and conducted to elucidate the putative association between Pandemrix™ vaccination, A(H1N1)pdm09 influenza infection, and narcolepsy.
• Research approaches follow a multi-tiered approach, and include experiments that utilize human peripheral blood mononuclear cells (PBMC) as well as an animal model.
• Research objectives focus on (1) isolation and characterization of hypothetical CD4+ T cells that are linked to the development of narcolepsy, using TCR deep sequencing, (2) mapping antigenic targets (epitopes) of these T cells, and (3) evaluating potential mechanisms that would enable such cells to gain access to the hypothalamus, where hypocretin neurons reside.
Disclosure of Potential Conflicts of Interest
We declare the following interests: R.V.D.M., M.V.M., E.D., M.W., and E.H. are employees of the GlaxoSmithKline group of companies and own shares in GlaxoSmithKline. R.V.D.M., M.V.M., M.W., and E.H. are listed as inventors of patents owned by GlaxoSmithKline Biologicals SA. Writing assistance was utilized in the production of this manuscript and was funded by GlaxoSmithKline Biologicals SA.
Acknowledgments
The authors would like to thank their colleagues Anne Schuind, Corey Mallett, Isabelle Martin, Vincent Bauchau, Ventzislav Vassilev, Camille Planty, and W Ripley Ballou, as well as Prof. Emmanuel Mignot at the Department of Psychiatry, Stanford University Center for Narcolepsy, Palo Alto, CA, for helpful discussions. Finally, they thank Ellen Oe (XPE Pharma & Science on behalf of GlaxoSmithKline) for writing assistance and Ulrike Krause for publication management.
Trademarks
Pandemrix is a trademark of the GlaxoSmithKline group of companies.
Author Contributions
All authors were involved in the initial discussions leading to the design of the research approaches. R.V.D.M., M.V.M., and E.D. further developed the research objectives based on published data and discussions with experts in the field of narcolepsy research. R.V.D.M. wrote the initial draft of the manuscript and is the guarantor of the article. All authors were involved in the further drafting of the manuscript or revising it critically for important intellectual content. All authors approved the manuscript before it was submitted by the corresponding author, had full access to the information used for the manuscript, and had final responsibility to submit for publication.
References
- 1.Nohynek H, Jokinen J, Partinen M, Vaarala O, Kirjavainen T, Sundman J, Himanen SL, Hublin C, Julkunen I, Olsén P, et al. AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS One. 2012;7:e33536. doi: 10.1371/journal.pone.0033536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Centre for Disease Prevention and Control. Narcolepsy in association with pandemic influenza vaccination-a multi-country European epidemiological investigation. Stockholm, ECDC, September. 2012.
- 3.Miller E, Andrews N, Stellitano L, Stowe J, Winstone AM, Shneerson J, Verity C. Risk of narcolepsy in children and young people receiving AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine: retrospective analysis. BMJ. 2013;346:f794. doi: 10.1136/bmj.f794. [DOI] [PubMed] [Google Scholar]
- 4.Heier MS, Gautvik KM, Wannag E, Bronder KH, Midtlyng E, Kamaleri Y, Storsaeter J. Incidence of narcolepsy in Norwegian children and adolescents after vaccination against H1N1 influenza A. Sleep Med. 2013;14:867–71. doi: 10.1016/j.sleep.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Szakács A, Darin N, Hallböök T. Increased childhood incidence of narcolepsy in western Sweden after H1N1 influenza vaccination. Neurology. 2013;80:1315–21. doi: 10.1212/WNL.0b013e31828ab26f. [DOI] [PubMed] [Google Scholar]
- 6.Roman F, Clément F, Dewé W, Walravens K, Maes C, Willekens J, De Boever F, Hanon E, Leroux-Roels G. Effect on cellular and humoral immune responses of the AS03 adjuvant system in an A/H1N1/2009 influenza virus vaccine administered to adults during two randomized controlled trials. Clin Vaccine Immunol. 2011;18:835–43. doi: 10.1128/CVI.00480-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morel S, Didierlaurent A, Bourguignon P, Delhaye S, Baras B, Jacob V, Planty C, Elouahabi A, Harvengt P, Carlsen H, et al. Adjuvant System AS03 containing α-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine. 2011;29:2461–73. doi: 10.1016/j.vaccine.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Han F, Lin L, Warby SC, Faraco J, Li J, Dong SX, An P, Zhao L, Wang LH, Li QY, et al. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Ann Neurol. 2011;70:410–7. doi: 10.1002/ana.22587. [DOI] [PubMed] [Google Scholar]
- 9.Mahlios J, De la Herrán-Arita AK, Mignot E. The autoimmune basis of narcolepsy. Curr Opin Neurobiol. 2013;23:767–73. doi: 10.1016/j.conb.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kornum BR, Faraco J, Mignot E. Narcolepsy with hypocretin/orexin deficiency, infections and autoimmunity of the brain. Curr Opin Neurobiol. 2011;21:897–903. doi: 10.1016/j.conb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Hor H, Kutalik Z, Dauvilliers Y, Valsesia A, Lammers GJ, Donjacour CE, Iranzo A, Santamaria J, Peraita Adrados R, Vicario JL, et al. Genome-wide association study identifies new HLA class II haplotypes strongly protective against narcolepsy. Nat Genet. 2010;42:786–9. doi: 10.1038/ng.647. [DOI] [PubMed] [Google Scholar]
- 12.Hallmayer J, Faraco J, Lin L, Hesselson S, Winkelmann J, Kawashima M, Mayer G, Plazzi G, Nevsimalova S, Bourgin P, et al. Narcolepsy is strongly associated with the T-cell receptor alpha locus. Nat Genet. 2009;41:708–11. doi: 10.1038/ng.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim AS, Scammell TE. The trouble with Tribbles: do antibodies against TRIB2 cause narcolepsy? Sleep. 2010;33:857–8. doi: 10.1093/sleep/33.7.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han F, Faraco J, Dong XS, Ollila HM, Lin L, Li J, An P, Wang S, Jiang KW, Gao ZC, et al. Genome wide analysis of narcolepsy in China implicates novel immune loci and reveals changes in association prior to versus after the 2009 H1N1 influenza pandemic. PLoS Genet. 2013;9:e1003880. doi: 10.1371/journal.pgen.1003880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melén K, Partinen M, Tynell J, Sillanpää M, Himanen S-L, Saarenpää-Heikkilä O, Hublin C, Olsen P, Ilonen J, Nohynek H, et al. No serological evidence of influenza A H1N1pdm09 virus infection as a contributing factor in childhood narcolepsy after Pandemrix vaccination campaign in Finland. PLoS One. 2013;8:e68402. doi: 10.1371/journal.pone.0068402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aran A, Lin L, Nevsimalova S, Plazzi G, Hong SC, Weiner K, Zeitzer J, Mignot E. Elevated anti-streptococcal antibodies in patients with recent narcolepsy onset. Sleep. 2009;32:979–83. doi: 10.1093/sleep/32.8.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–5. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dauvilliers Y, Montplaisir J, Cochen V, Desautels A, Einen M, Lin L, Kawashima M, Bayard S, Monaca C, Tiberge M, et al. Post-H1N1 narcolepsy-cataplexy. Sleep. 2010;33:1428–30. doi: 10.1093/sleep/33.11.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lang HL, Jacobsen H, Ikemizu S, Andersson C, Harlos K, Madsen L, Hjorth P, Sondergaard L, Svejgaard A, Wucherpfennig K, et al. A functional and structural basis for TCR cross-reactivity in multiple sclerosis. Nat Immunol. 2002;3:940–3. doi: 10.1038/ni835. [DOI] [PubMed] [Google Scholar]
- 20.Su LF, Kidd BA, Han A, Kotzin JJ, Davis MM. Virus-specific CD4(+) memory-phenotype T cells are abundant in unexposed adults. Immunity. 2013;38:373–83. doi: 10.1016/j.immuni.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bardage C, Persson I, Örtqvist A, Bergman U, Ludvigsson JF, Granath F. Neurological and autoimmune disorders after vaccination against pandemic influenza A (H1N1) with a monovalent adjuvanted vaccine: population based cohort study in Stockholm, Sweden. BMJ. 2011;343:d5956. doi: 10.1136/bmj.d5956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabay C, Bel M, Combescure C, Ribi C, Meier S, Posfay-Barbe K, Grillet S, Seebach JD, Kaiser L, Wunderli W, et al. H1N1 Study Group Impact of synthetic and biologic disease-modifying antirheumatic drugs on antibody responses to the AS03-adjuvanted pandemic influenza vaccine: a prospective, open-label, parallel-cohort, single-center study. Arthritis Rheum. 2011;63:1486–96. doi: 10.1002/art.30325. [DOI] [PubMed] [Google Scholar]
- 23.Chen L, Brown RE, McKenna JT, McCarley RW. Animal models of narcolepsy. CNS Neurol Disord Drug Targets. 2009;8:296–308. doi: 10.2174/187152709788921717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Metzker ML. Sequencing technologies - the next generation. Nat Rev Genet. 2010;11:31–46. doi: 10.1038/nrg2626. [DOI] [PubMed] [Google Scholar]
- 25.Borbulevych OY, Piepenbrink KH, Baker BM. Conformational melding permits a conserved binding geometry in TCR recognition of foreign and self molecular mimics. J Immunol. 2011;186:2950–8. doi: 10.4049/jimmunol.1003150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cvetkovic-Lopes V, Bayer L, Dorsaz S, Maret S, Pradervand S, Dauvilliers Y, Lecendreux M, Lammers GJ, Donjacour CE, Du Pasquier RA, et al. Elevated Tribbles homolog 2-specific antibody levels in narcolepsy patients. J Clin Invest. 2010;120:713–9. doi: 10.1172/JCI41366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becher B, Segal BMT. T(H)17 cytokines in autoimmune neuro-inflammation. Curr Opin Immunol. 2011;23:707–12. doi: 10.1016/j.coi.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eichelberger MC. The cotton rat as a model to study influenza pathogenesis and immunity. Viral Immunol. 2007;20:243–9. doi: 10.1089/vim.2007.0017. [DOI] [PubMed] [Google Scholar]