Abstract
Adenovirus Types 4 and 7 (Ad4 and Ad7) are associated with acute respiratory distress (ARD). In order to prevent widespread Ad-associated ARD (Ad-ARD) the United States military immunizes new recruits using a safe and effective lyophilized wildtype Ad4 and Ad7 delivered orally in an enteric-coated capsule. We cloned Ad4 and Ad7 and modified them to express either a GFP-Luciferase (GFPLuc) fusion gene or a centralized influenza H1 hemagglutinin (HA1-con). BALB/c mice were injected with GFPLuc expressing viruses intramuscularly (i.m.) and intranasally (i.n.). Ad4 induced significantly higher luciferase expression levels as compared with Ad7 by both routes. Ad7 transduction was restored using a human CD46+ transgenic mouse model. Mice immunized with serial dilutions of viruses expressing the HA1-con influenza vaccine gene were challenged with 100 MLD50 of influenza virus. Ad4 protected BALB/c mice at a lower dose by i.m. immunization as compared with Ad7. Unexpectedly, there was no difference in protection by i.n. immunization. Although Ad7 i.m. transduction was restored in CD46+ transgenic mice, protection against influenza challenge required even higher doses as compared with the BALB/c mice. However, Ad7 i.n. immunized CD46+ transgenic mice were better protected as compared with Ad4. Interestingly, the restoration of Ad7 transduction in CD46+ mice did not increase vaccine efficacy and indicates that Ad7 may transduce a different subset of cells through alternative receptors in the absence of CD46. These data indicate that both Ad4 and Ad7 can effectively induce anti-H1N1 immunity against a heterologous challenge using a centralized H1 gene. Future studies in non-human primates or human clinical trials will determine the overall effectiveness of Ad4 and Ad7 as vaccines for influenza.
Keywords: adenovirus, vaccine, viral vector, centralized gene, hemagglutinin, influenza, adenovirus type 4, adenovirus type 7
Introduction
Adenovirus types 4 and 7 (Ad4 and Ad7) are the primary causative infectious agents in acute respiratory distress (ARD) in military recruits during basic training.1 Typically, Ad-ARD is associated with fever, rhinorrhea, pharyngitis, headache, and lethargy that is self-limiting and usually resolved in 3 to 10 d. While Ad-ARD rarely results in death and about 10% of patients develop pneumonia, the major impact of Ad-ARD is the loss of training time. Ad-ARD spreads rapidly and has been shown to affect up to 80% of recruits within a confined military training facility resulting in 20% of patients requiring hospitalization.2-4 Since 1971, millions of military recruits have been vaccinated against Ad4 and Ad7 using an orally delivered lyophilized wildtype virus.5 This vaccine has been found to be safe and effective.6,7 The vaccine used by the military was so effective at reducing the rates of respiratory febrile illness that officials opted to suspend the vaccine program. Once the vaccine program was halted, cases of Ad4 and Ad7 associated respiratory illnesses began to increase and, in 2001, efforts to restore the vaccine program were initiated. Over the past 10 y, the US military has invested ~$100 million to fully restore vaccine production and vaccination of military recruits in 2011.7 Ad-ARD cases declined rapidly upon the restoration of this vaccine program.
Adenovirus Type 5 (Ad5) has been shown to be a very effective viral vector for use in vaccine studies.8-13 However, There are very high levels of pre-existing immunity to Ad5 in all populations.14-16 In many populations the pre-existing immunity is 100% by the age of 2.14 Therefore, Ad5 seroprevalence is a fundamental problem that is inhibitory to the use of Ad5 as a vaccine vector in adult humans. A study of 303 military basic trainees indicated that Ad4 and Ad7 levels of seroprevalence were 34% and 27%, respectively. Therefore, 89% of these recruits would be susceptible to vaccination with at least 1 of the 2 Ad4 or Ad7 vaccines.17 Ad4 and Ad7 have been studied as vaccine vectors for several infectious diseases such as HIV, Hepatitis B, respiratory syncytial virus (RSV) and, most recently, bird flu (H5N1).6,18-22 As Ad4 and Ad7 are the only 2 adenoviruses ever used as vaccines in humans to prevent Ad4 and Ad7 ARD, they are very attractive as vaccine vectors for the inclusion of secondary pathogen genes. Military recruits are vaccinated or boosted against several other pathogens with traditional vaccines.23 However, some recruits will be vaccinated against pathogens such as smallpox and anthrax. Vaccines for these pathogens are considered dangerous or require multiple immunizations with annual boosters.23 In this study we show that these viral vectors are capable of inducing anti-H1N1 influenza immune responses. We hope that these data, in combination with vaccine studies from other groups, will result in the design of a multivalent vaccine platform.
Results
In vivo transduction of viral vectors
BALB/c mice were immunized with Ad4 and Ad7 viral vectors expressing the GFPLuc gene and imaged 24 h. later. By intramuscular immunization, the Ad4 viral vector induced significantly higher levels of luciferase activity as compared with the Ad7 viral vector (P = 0.02) (Fig. 1). By the intranasal route, the Ad4 viral vector also induced significantly higher levels of luciferase activity as compared with Ad7 (P = 0.01) (Fig. 1). However, when CD46+ transgenic mice were immunized intramuscularly with the Ad7 viral vector, luciferase activity was restored to levels equivalent to that of Ad4 in BALB/c mice (Fig. 1B and C). A commonly used Ad5 replication-defective viral vector was used as a comparator. Ad4 and Ad7 also induced higher luciferase levels as compared with the Ad5 vector (P = 0.04 and P = 0.06, respectively) (Fig. 1B and C). Interestingly, the Ad4-GFPLuc viral vector appeared to migrate from the intramuscular injection site to the liver as detected by imaging. This may be an indicator of the liver tropic properties of adenoviruses that use the coxsackie and adenovirus receptor (CAR) for transduction.
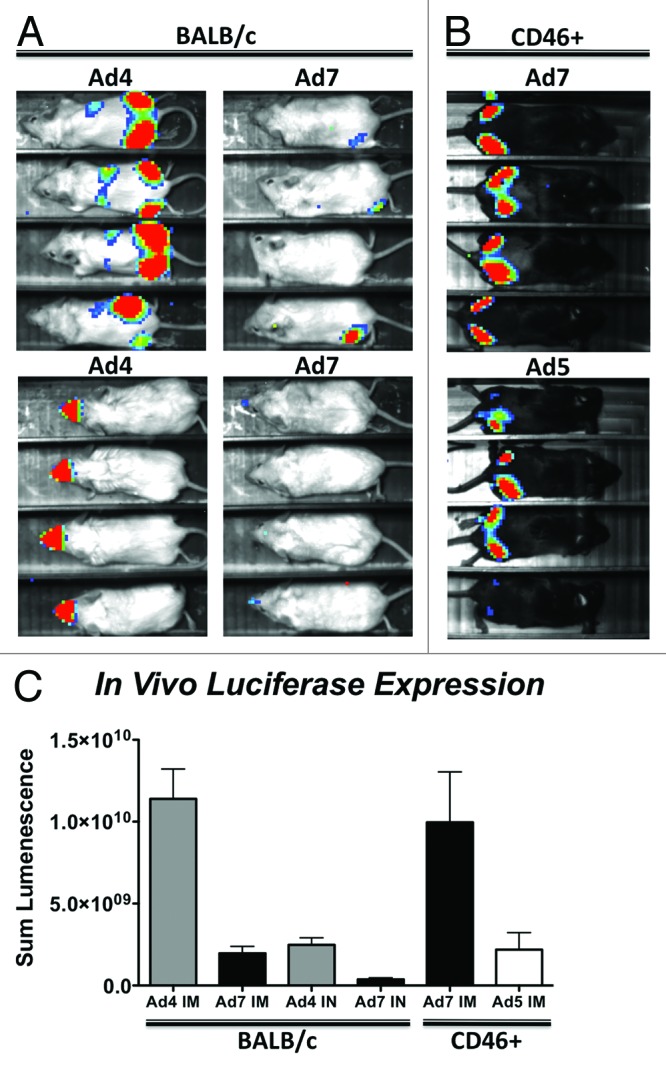
Figure 1. In vivo transduction of Ad4 and Ad7 vectors. Ad4 and Ad7 viruses expressing a GFPLuc fusion gene were used to immunize mice by intramuscular and intranasal routes and imaged for luciferase activity 24 h later. In vivo transduction and luciferase expression in BALB/c mice is shown (A). Intramuscular transduction of Ad7 was restored in CD46+ transgenic mice and compared with a standard replication-defective Ad5 vector expressing the same transgene (B). The sum lumenescence was determined using the lumazone analysis software and levels were compared using a 2-tailed T-Test (C). Error bars indicate standard error.
Protection by systemic intramuscular immunization in BALB/c mice
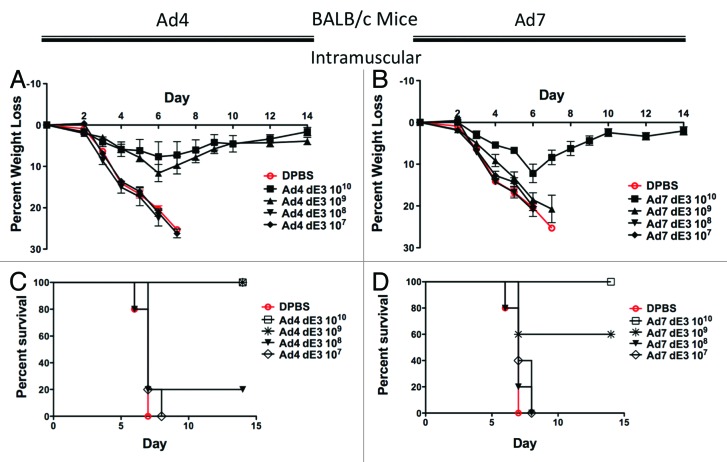
Groups of 5 female BALB/c mice were immunized intramuscularly with serial 10-fold dilutions of Ad4 and Ad7 vaccine vectors expressing a centralized H1 hemagglutinin gene, HA1-con. Three weeks post-immunization, the mice were challenged with 100 MLD50 of influenza A/PR/8/34. Mice immunized with Ad4 at a dose as low as 109 vp/mouse showed reduced disease and weight loss (Fig. 2A). Immunization with 109 vp/mouse of Ad4 vaccine also protected 100% of mice from death due to a lethal challenge of influenza virus (Fig. 2B). In contrast, 1010 vp/mouse of Ad7 vaccine was required to reduce weight loss in influenza virus challenged BALB/c mice (Fig. 2C). Intramuscular vaccination with Ad7 required a 10-fold higher concentration (1010 vp/mouse) to completely protect mice from death as compared with the Ad4 vaccine (Fig. 2D).
Figure 2. Protection by systemic intramuscular immunization in BALB/c mice. Groups of 5 female BALB/c mice were immunized intramuscularly with 10-fold serial dilutions of Ad4 or Ad7 expressing the centralized HA1-con hemagglutinin gene. Three weeks after immunization the mice were challenged with 100 MLD50 of mouse-adapted influenza A/PR/8/34. Protection against weight loss and survival induced by the Ad4 vaccine is shown in A and B, respectively. Protection against weight loss and survival induced by the Ad7 vaccine is shown in C and D, respectively. Mice that lost 25% of baseline body weight were humanely euthanized.
Protection by mucosal intranasal immunization in BALB/c mice
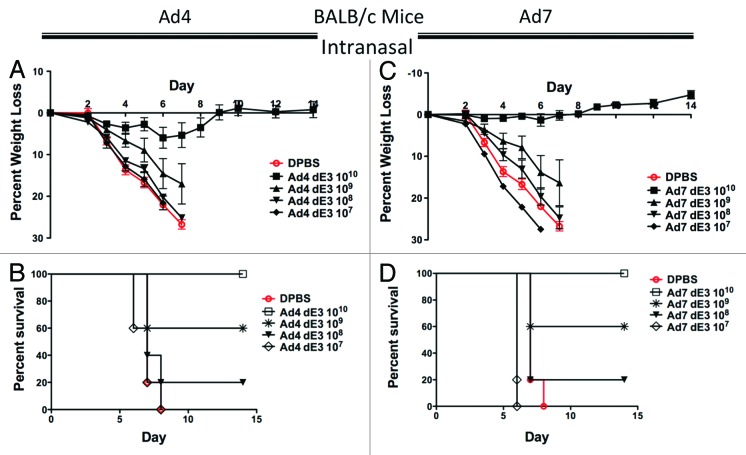
Groups of 5 female BALB/c mice were immunized intranasally with serial 10-fold dilutions of Ad4 and Ad7 vaccine vectors expressing HA1-con. Three weeks post-immunization, the mice were challenged with 100 MLD50 of influenza A/PR/8/34. By this route and in this mouse model, there were no differences in vaccine efficacy by either Ad4 or Ad7 (Fig. 3). However, the dose required to completely protect mice from death was 10-fold higher by the intranasal route as compared with the intramuscular route (Fig. 3A and B). Interestingly, the Ad7 mice immunized with 1010 vp/mouse did not lose any weight and showed no signs of disease or death (Fig. 3C and D). In fact, mice immunized with this dose by the i.m. route showed > 10% weight loss, whereas, mice immunized i.n. showed an overall weight gain over the 2 wk after influenza challenge (Fig. 3C).
Figure 3. Protection by mucosal intranasal immunization in BALB/c mice. Groups of 5 female BALB/c mice were immunized intranasally with 10-fold serial dilutions of Ad4 or Ad7 expressing the centralized HA1-con hemagglutinin gene. Three weeks after immunization the mice were challenged with 100 MLD50 of mouse-adapted influenza A/PR/8/34. Protection against weight loss and survival induced by the Ad4 vaccine is shown in A and B, respectively. Protection against weight loss and survival induced by the Ad7 vaccine is shown in C and D, respectively. Mice that lost 25% of baseline body weight were humanely euthanized.
Protection by systemic intramuscular immunization in CD46+ transgenic mice
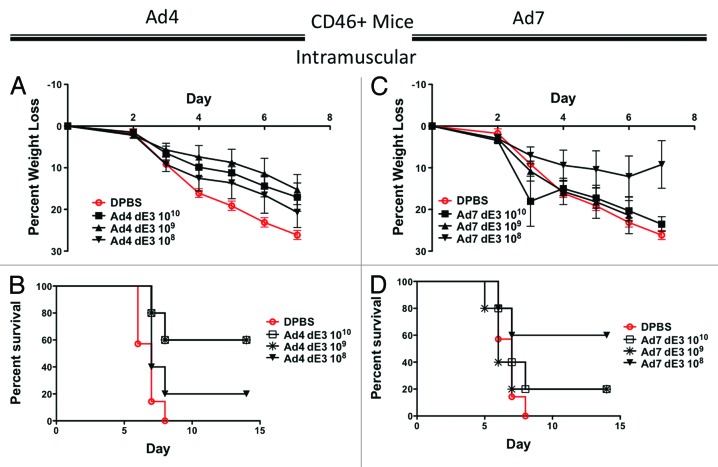
Since CD46 appeared to restore Ad7 transduction, we immunized groups of 5 female CD46+ transgenic mice intramuscularly with serial 10-fold dilutions of Ad4 and Ad7 vaccine vectors expressing HA1-con. The mice were challenged 3 wk post-immunization with 100 MLD50 of influenza A/PR/8/34. In this mouse model, Ad4 was even less effective at protecting mice from weight loss (Fig. 4A) and even groups of mice receiving the highest dose of 1010 vp/mouse of Ad4 were not completely protected from death upon influenza challenge (Fig. 4B). In contrast to the in vivo luciferase data, the Ad7 vaccine was even less effective at protecting mice from weight loss and death after a lethal influenza challenge (Fig. C and D). Interestingly, the lowest does of Ad7 vaccine (108 vp/mouse) resulted in the highest survival rate of 60% as compared with 20% for the higher doses (Fig. 4D).
Figure 4. Protection by systemic intramuscular immunization in human CD46+ transgenic mice. Groups of 5 female CD46+ mice were immunized intramuscularly with 10-fold serial dilutions of Ad4 or Ad7 expressing the centralized HA1-con hemagglutinin gene. Three weeks after immunization the mice were challenged with 100 MLD50 of mouse-adapted influenza A/PR/8/34. Protection against weight loss and survival induced by the Ad4 vaccine is shown in A and B, respectively. Protection against weight loss and survival induced by the Ad7 vaccine is shown in C and D, respectively. Mice that lost 25% of baseline body weight were humanely euthanized.
Protection by mucosal intranasal immunization in CD46+ transgenic mice
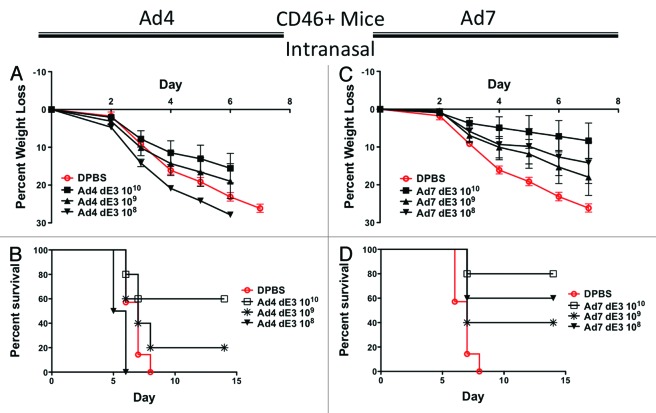
Groups of 5 female CD46+ transgenic mice were immunized intranasally with serial 10-fold dilutions of Ad4 and Ad7 vaccine vectors expressing HA1-con and challenged 3 wk post-immunization with 100 MLD50 of influenza A/PR/8/34. Ad4 induced lower levels of protection by this route as compared with the i.m. route. Even the highest i.n. dose of Ad4 vaccine was unable to completely protect CD46+ mice from weight loss and death (Fig. 5A and B). Vaccination of mice with 1010 vp/mouse of Ad4 vaccine resulted in only 60% survival (Fig. 5B). Ad7 immunized CD46+ mice had a delayed weight loss as compared with the Ad4 immunized mice (Fig. 5C). However, all doses of Ad7 resulted in death after influenza challenge (Fig. 5D). Interestingly, the intermediate (109) and low (108) doses of Ad7 vaccine induced greater levels of survival as compared with the Ad4 vaccine.
Figure 5. Protection by mucosal intranasal immunization in human CD46+ transgenic mice. Groups of 5 female CD46+ mice were immunized intranasally with 10-fold serial dilutions of Ad4 or Ad7 expressing the centralized HA1-con hemagglutinin gene. Three weeks after immunization the mice were challenged with 100 MLD50 of mouse-adapted influenza A/PR/8/34. Protection against weight loss and survival induced by the Ad4 vaccine is shown in A and B, respectively. Protection against weight loss and survival induced by the Ad7 vaccine is shown in C and D, respectively. Mice that lost 25% of baseline body weight were humanely euthanized.
Discussion
There is a definite need to improve our current vaccine technologies. Here we report the cloning of Ad4 and Ad7 into single low copy plasmids. These plasmids allow for the ability to rapidly create and modify the genomes to act as vaccine vectors. We deleted the E3 genes of both Ad4 and Ad7 and replaced them with CMV-transgene cassettes that expressed either a GFPLuc or a centralized H1 hemagglutinin (HA1-con). We then tested these viral vectors to transduce BALB/c and CD46+ transgenic mice and to induce anti-influenza immunity. While both vectors were capable of inducing protection against a heterologous influenza A/PR/8/34 lethal virus challenge, the dose required to induce protection was higher than previously observed using a replication-defective Ad5 (RD-Ad) vectored vaccine.11 In order to determine if the levels of protein expression were equivalent in both Ad4 and Ad7 vaccines, we evaluated GFP and luciferase expression in vitro. We found that Ad4 and Ad7 viruses had similar virus particle to infectious unit ratios of 103.6 and 95.1, respectively (Fig. S1A). In addition, there were no significant differences in luciferase activity expressed by either Ad4 or Ad7 (Fig. S1B). While these data do not represent the actual vaccine gene (HA1-con) expression, they do indicate that, at least in the context of these expression cassettes, it may be inferred that HA1-con expression levels are equivalent.
Since the Ad4 and Ad7 viral vaccines were replication-competent (RC-Ad) they may have induced changes to the Th1/Th2 balance, T-regulatory responses, or inflammatory cytokine profiles as compared the RD-Ad vector. The E3 genes play a significant role in immunoevasion and immunomodulation during Ad infections.24-26 The deletion of the E3 genes without the deletion of the E1 genes may have impaired this vector as a vaccine, at least in mice. The anti-Ad4 and Ad7 immune responses generated in the mice by the E3 deleted vectors may have weakened the anti-influenza immune responses. Vaccination with RD-Ad4 and Ad7 viruses may ameliorate this effect. A RD-Ad4 and Ad7 vector that is delivered to military recruits in a manner similar to the FluMist vaccine may induce stronger anti-vector and anti-transgene immune responses. In addition, studies have shown that Ad vectors can be re-administered when delivered intranasally.27-32 Therefore, boosting with RD-Ad4 and Ad7 carrying other pathogen genes could be used to make the vaccine even more polyvalent.
Even though Ad7 transduction was restored in the CD46+ transgenic mice, the vaccine efficacy was actually reduced as compared with the BALB/c vaccinated mice. The primary receptor for Ad7 has been described to be Desmoglein 2 (DSG2) and mouse DSG2 was found to be refractory to Ad3 transduction.33 Therefore, the restored transduction in the CD46+ transgenic mice may not be representative of Ad7 receptor usage in humans. More interestingly, the Ad7 vaccine in the CD46+ mice appeared to work equally well or better at lower doses, in contrast to the Ad4 vaccine. Also of interest is the fact that Ad4 and Ad7 were equally effective at inducing anti-H1NI immunity in BALB/c mice when vaccinated intranasally. Since, the imaging data indicated that Ad7 did not transduce BALB/c mice very efficiently, as compared with Ad4 and Ad5, it is likely that the Ad7 vaccine is transducing a smaller subset of cells. This subset of cells may be better antigen presenting cells than those transduced by the CAR binding Ad4 vaccine. In addition, it is possible that the higher levels of Ad7 vaccine in CD46+ mice were inducing higher levels of damage to the lungs counteracting the vaccine efficacy. Human CD46 is a complement regulatory protein and, in the setting of a mouse model, may not accurately represent its function in natural immune responses.34,35 It is possible that while serving as one of the receptors for Ad7, CD46 may also induce immune dysfunction in both Ad4 and Ad7 vaccinated mice as indicated by contradictions in our imaging and vaccine data. The CD46+ transgenic mice do provide valuable information into the complications inherent to small animal models of human disease. The fact that increased transgene expression does not correlate with increased vaccine efficacy contradicts logical vaccine ideology. Recently, a human DSG2 transgenic has been established for studying adenoviruses types 3, 7, 11, and 14. As DSG2 has been found to be the primary high affinity receptor for Ad7 this newly derived mouse model may better serve to evaluate human adenoviruses as vaccine vectors.36
While our data indicate that Ad4 and Ad7 may be suitable vaccine vectors for translation to human use, there were several limitations involved with our in vitro analyses. Our Ad4 vaccine, when delivered intramuscularly, induced 100% survival in mice against a lethal heterologous influenza challenge at a dose of 109 vp/mouse. The human vaccine dose (~105 infectious units) used to provide protection against Ad4 and Ad7 ARD is small compared with the dose required to protect mice from influenza infection.37 The differences in the vaccine doses needed to protect against influenza virus and Ad-ARD can be explained by fundamental differences in the viruses and associated disease. First, adenoviral genomes are very stable and have an extremely low mutation rate as compared with influenza virus.38 The stability of the adenovirus genome ensures that the challenges are always homologous. This is not the case for influenza virus and is the central idea behind the use of a centralized HA gene as a broadly reactive influenza vaccine. In this study the influenza challenge is heterologous and the degree of mismatch between the vaccine HA and the challenge flu HA is 8.5%. In this light, we feel that our data indicate that protection against endemic or even pandemic influenza, would be achieved at much lower doses of vaccine, especially in the case of a homologous matched challenge influenza. Second, vaccinated mice are challenged with a dose of 100 MLD50 of mouse-adapted influenza virus whereas Ad-ARD infections are generally not lethal The only measure of vaccine efficacy in this study was a lethal influenza challenge. A significant shortcoming is the lack of hemagglutination inhibition or influenza virus neutralization data. These immune correlates would provide valuable insight into the degree and breadth of the induced immune responses and will be included in future studies. Although we did not do any in vitro anti-influenza analyses a previous study evaluated the centralized HA1-con gene to induce protection against 2009 pandemic swine flu in vivo. When vaccines were mismatched the centralized HA1-con gene was a better vaccine gene as compared with wildtype heterologous HA genes.11
In 2010, 1.24 million people, mostly children, died from malaria worldwide.39 Malaria infections remain a significant health hazard to our military personnel.40,41 In a recent study by the US Military Malaria Vaccine Program, vaccination using a DNA prime and Ad5 boost expressing circumsporozoite protein (CSP) and apical membrane antigen-1 (AMA1) from Plasmodium falciparum protected 27% of vaccinees against a live malaria challenge. All of the vaccinees that had moderate to high titers of anti-Ad5 pre-existing immunity were susceptible to malaria infection.42 The authors suggest that the use of alternative lower seroprevalent Ad vectors and the inclusion of a third antigen should be considered a priority for future studies. While Ad4 and Ad7 have been shown to have moderate levels of pre-existing immunity, it is estimated that nearly 90% of unimmunized US Army trainees would be susceptible to at least 1 of these Ad types. Studies have shown that pre-existing immunity to Ad5 is as high as 70% in the USA and 90–100% in Asia and Africa, the prime sites for the implementation of a malaria vaccine.14,16,43 We speculate that recombinant Ad4 and Ad7 expressing P. falciparum genes may be optimal Ad vectors to create a multivalent malaria vaccine. We feel that this data supports the future exploration of Ad4 and Ad7 as vaccine vectors against alternative pathogens.
Materials and Methods
Ethics statement
Female, 6–8-wk-old, BALB/c mice were obtained from Charles River. Human CD46+ transgenic mice on a C57/BL6 genetic background were obtained from the Mayo Clinic toxicology core and a breeding colony was established under the IACUC, protocol A61312. Mice were housed in the Mayo Clinic Animal Facility under the Association for Assessment and Accreditation of Laboratory Animal Care (AALAC) guidelines with animal use protocols approved by the Mayo Clinic Animal Use and Care Committee, protocol A24111). All animal experiments were performed according to the provisions of the Animal Welfare Act, PHS Animal Welfare Policy, the principles of the NIH Guide for the Care and Use of Laboratory Animals, and the policies and procedures of Mayo Clinic.
Virus and DNA purification
Adenovirus type 4 strain RI-67 (ATCC VR-1572) and type 7 strain Gomen (ATCC VR-7) were purchased from ATCC. The Ad4 and Ad7 strains were obtained from throat swabs of California military recruits with pharyngitis in 1954.44 Final amplification of the virus was performed in a Corning 10-cell stack (~6300 cm2). Virus was amplified in 293 cells and purified on 2 sequential CsCl ultracentrifuge gradients. Purified viruses were quantitated by OD260. Viral genomic DNA (gDNA) was purified using high-titer Ad4 and Ad7 purified virus and the PureLink Viral RNA/DNA mini kit (Invitrogen).
Cloning of the Ad4 and Ad7 Genomes
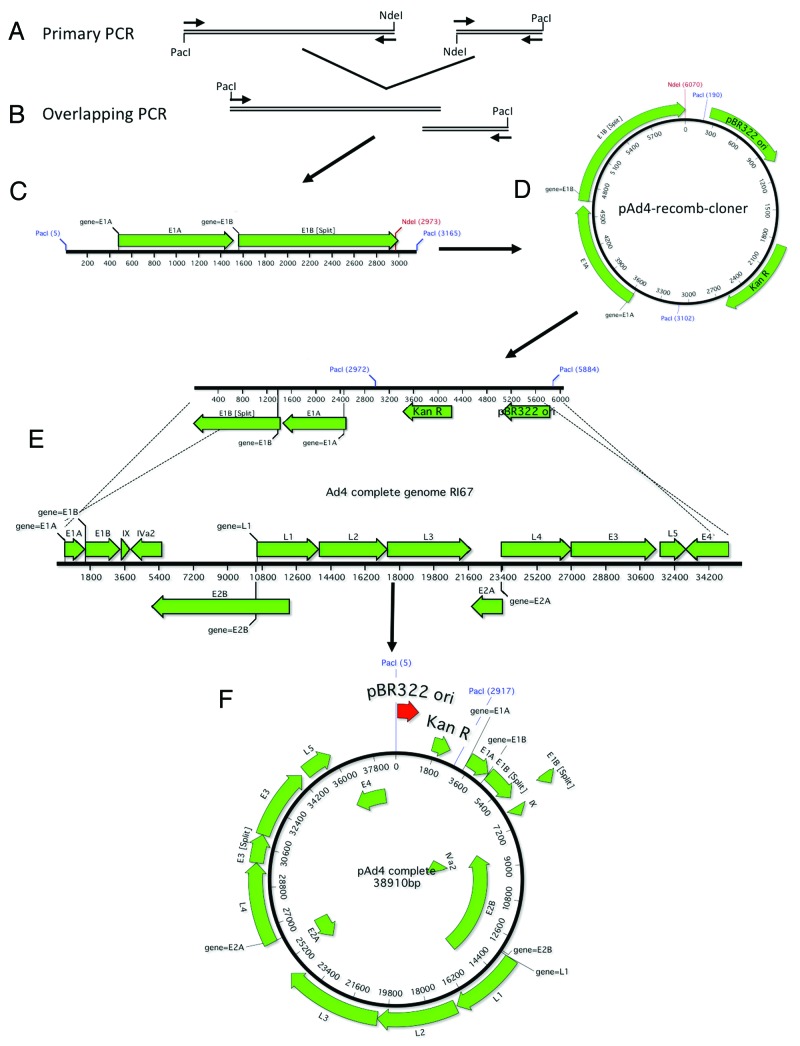
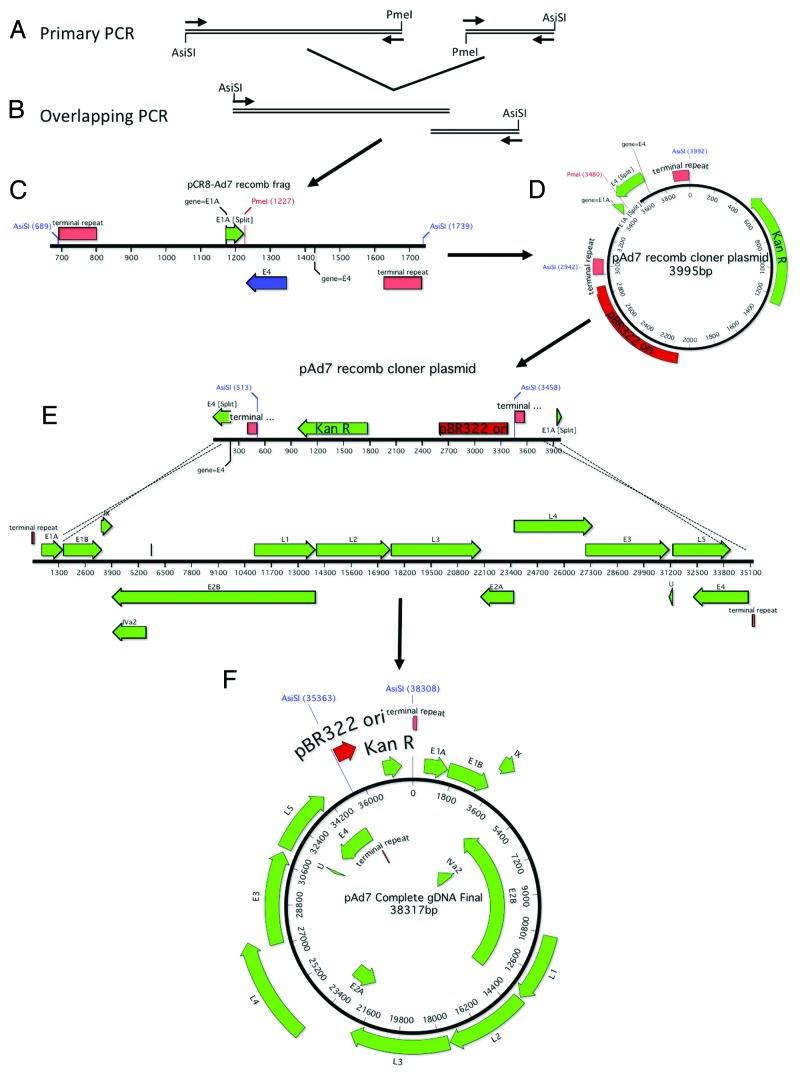
An overlapping PCR product was amplified in order to clone the Ad4 gDNA by homologous recombination. First, the left and right arms of the Ad4 gDNA were amplified by PCR using Platinum Taq Supermix High Fidelity (Invitrogen) (Fig. 6A). Unique PacI restriction enzyme site were added just outside of the left and right ITRs. The primers were designed to overlap and contained a unique NdeI site in between the left and right arms of the genome (Fig. 6A). Table 1 shows the primers used to clone the Ad4 gDNA. The Ad4-PacI-ITR acts as both a forward and reverse primer since the sequence is an inverted repeat. All PCR reactions were performed using Platinum Taq Supermix High Fidelity (Invitrogen). In order to amplify the left arm of Ad4, primers Ad4-PacI-ITR and Ad4-Recomb-R (200 mM final) were combined with 0.5 µg of Ad4 gDNA in 45 µl of Platinum Supermix. In order to amplify the right arm of Ad4, primers Ad4-PacI-ITR and Ad4-Recomb-F (200 mM final) were combined with 0.5 µg of Ad7 gDNA in 45 µl of Platinum Supermix. The following parameters were used for PCR: 95 °C (2 min); 20 cycles of 95 °C (30s): 55 °C (30s): 68 °C (3.5 min); 68 °C (10 min); 4 °C (∞). One microliter of each PCR reaction was combined in 45 µl of Platinum Supermix and the primer Ad4-PacI-ITR was added to a final concentration of 400 mM. The following parameters were used for PCR: 95 °C (2 min); 20 cycles of 95 °C (30s): 55 °C (30s): 68 °C (3.5 min); 68 °C (10 min); 4 °C (∞). The overlapping PCR product was cloned into TOPO pCR8 (Invitrogen) and confirmed by sequencing (Fig. One B and C). The Kanamycin resistance gene (KANR) and the pBR322 origin of replication (pBR322 ori) from pAd-Shuttle (Stratagene) was amplified using the primers Kan ori PacI-F and Kan ori PacI-R and the PCR product was cloned into TOPO-XL (Invitrogen) (Table 1). The KanR and pBR322 ori were ligated to the overlapping PCR using the PacI sites to form the cloning plasmid, pAd4-recomb-cloner (Fig. 6D). The pAd4-recomb-cloner plasmid was digested overnight with NdeI and dephosphorylated with Antarctic Alkaline Phosphatase (New England Biolabs). Digested cloner plasmid and 1.0 μg of purified Ad4 gDNA were cotransformed into BJ5183 electrocompetent cells, incubated at 37 °C with shaking for 1 h and plated on LB Kanamycin (Fig. 6E). The plates were incubated at 37 °C overnight and small pinpoint colonies were picked for minipreps. The miniprep DNA was confirmed by restriction enzyme digestion and transformed into XL-1 blue electrocompetent cells. The DNA was maxiprepped using Qiagen HI Speed Maxi kits. The Ad7 genome was cloned in a similar manner with the exception that AsiSI unique restriction sites flanked the Kanr and pBR322 oriP regions and the pAd7 recomb cloner plasmid was linearized with PmeI (Fig. 7).
Figure 6. Schematic of Ad4 gDNA cloning. The right and left arms of Ad4 were amplified with primers designed to incorporate a unique NdeI restriction site, an overlap of ~27 nucleotides and flanking PacI restriction sites (A). The 2 PCR products were fused together in a second round of amplification (B). A schematic representation of the cloning fragment is shown (C). The cloning PCR fragment is ligated to the Kanamycin resistance gene and the pBR322 origin of replication (D). The recomb cloner plasmid was digested with NdeI and recombined with the Ad4 genomic DNA in vitro using BJ5183 electrocompetent cells (E). The complete Ad4 genomic DNA plasmid is shown (F).
Table 1. Primers for cloning Ad4 and Ad7 gDNA.
| Ad4-PacI-ITR | TTAATTAACT ATCTATATAA TATACCTTA TTTTT |
| Ad4-Recomb-F | CAGTCATATG CTGGCCGCCG TGCATGGTCA CCTCTCAACG ACTTTCAAAT TCC |
| Ad4-Recomb-R | GGAATTTGAA AGTCGTTGAG AGGTGACCAT GCACGGCGGC CAGCATATGA CTG |
| Kan ori PacI-F | TTAATTAACG GTGTGAAATA CCGCACAGAT GCG |
| Kan ori PacI-R | TTAATTAAGA ATTAATTCGA TCCTGAATGG CG |
| Ad7-AsiSI-ITR | AAGCGATCGC CTATCTATAT AATATACCTT ATAGATGG |
| Ad7-Recomb-F | GAGGCCACTC TTGAGTGCCA GCGAGGTTTA AACGCGGATA CAAAGTAAAA GGCACAGGAG |
| Ad7-Recomb-R | CTCCTGTGCC TTTTACTTTG TATCCGCGTT TAAACCTCGC TGGCACTCAA GAGTGGCCTC |
| Kan ori AsiSI-F | GCGATCGCTA ATTAACATGC ATGGATCCAT ATGCGGTGTG |
| Kan ori AsiSI-R | GCGATCGCTT AATTAAGAAT TAATTCGATC CTGAATGGCG AATGG |
Figure 7. Schematic of Ad7 gDNA cloning. The right and left arms of Ad7 were amplified with primers designed to incorporate a unique PmeI restriction site, an overlap of ~27 nucleotides and flanking AsiSI restriction sites (A). The 2 PCR products were fused together in a second round of amplification (B). A schematic representation of the cloning fragment is shown (C). The cloning PCR fragment is ligated to the Kanamycin resistance gene and the pBR322 origin of replication (D). The recomb cloner plasmid was digested and recombined with the Ad7 genomic DNA in vitro using BJ5183 electrocompetent cells (E). The complete Ad7 genomic DNA plasmid is shown (F).
The pAd4 and pAd7 complete genome plasmid (pAd4-complete and pAd7-complete gDNA Final, respectively) were confirmed by restriction enzyme analyses (Fig. 6F and Figure 7F). In order to confirm that the plasmids contained functional viral gDNA, the PacI, and AsiSI digested pAd4 and pAd7 plasmids were transfected into 293 cells using Polyfect (Qiagen) and wt Ad4 and Ad7 virus was confirmed by plaque formation and virus amplification.
Construction of shuttle plasmids
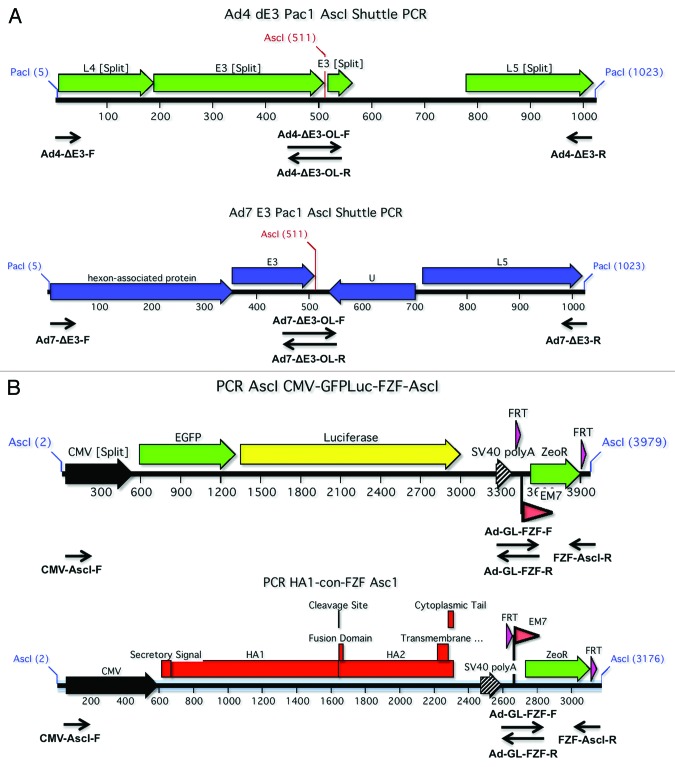
A series of shuttle plasmids were constructed in order to create the Ad4 and Ad7 E3 modified viruses. Table 2 shows the primers used to create the shuttle plasmids. Each shuttle was created using an overlapping PCR product that contained a unique AscI restriction enzyme site (Fig. 8A). The overlapping PCR products were amplified as previously described. The Ad4 and Ad7 E3 shuttle PCR products contained flanking PacI restriction sites. The final PCR products were cloned into the TOPO pCR8 cloning vector (Invitrogen, Carlsbad). Each shuttle was confirmed by sequencing.
Table 2. Primers for Ad4 and Ad7 Shuttle Plasmids.
| Ad4-ΔE3-F | TTAATTAACT GACTTTGGAG AGTTCTTCCT CGC |
| Ad4-ΔE3-OL-R | GTAATGGGGT AAGGGGTTAG TTGATGGCGC GCCTCAGCTT TTATTATACT CAGTAC |
| Ad4-ΔE3-OL-F | GTACTGAGTA TAATAAAAGC TGAGGCGCGC CATCAACTAA CCCCTTACCC CATTAC |
| Ad4-ΔE3-R | TTAATTAATG CAATGAGTTT TCCCGAGTCG TCAAG |
| Ad7-ΔE3-F | TTAATTAACC TATGTCGTCA CCGACCTCAA CAG |
| Ad7-ΔE3-OL-R | CAAGTAAGTG ATTTTTTATT AATTTTGGCG CGCCGATGGT AATCCGCACT CCGTGGGC |
| Ad7-ΔE3-OL-F | GCCCACGGAG TGCGGATTAC CATCGGCGCG CCAAAATTAA TAAAAAATCA CTTACTTG |
| Ad7-ΔE3-R | TTAATTAAGG GTCCTAGCGA CAAACCTATA GAG |
Figure 8. Construction of E3 shuttle plasmids and transgenes. In order to create replication-competent vectors, the nonessential E3 were genes replaced with either a HA1-con expression cassette or a GFPLuc fusion gene. The PCR products that were used to create the shuttle plasmids for Ad4 and Ad7 are shown (A). Both of the overlapping PCR products were designed to flank the E3 genes and contained unique AscI sites. The AscI flanked transgene expression cassettes are shown (B). The CMV expression cassettes were fused to a FRT flanked zeocin gene for selection of recombinants and ultimate removal by FLP recombinase.
The HA1-con and GFPLuc genes were cloned into the stratagene pShuttle-CMV plasmid (Startagene). The CMV-HA1-con-PolyA and CMV-GFPLuc-PolyA expression cassettes were fused to the FRT-Zeocin-FRT sequence by overlapping PCR. Table 3 shows the primers used to create the final PCR products. AscI restriction sites were incorporated into the PCR primers for ligation into the shuttle plasmids. Both PCR amplified transgenes were cloned into TOPO pCR8 (Invitrogen) and confirmed by sequencing. Schematic representations of the 2 transgene cassettes are shown in Figure 8B.
Table 3. Primers for Transgenes.
| CMV-AscI-F | GGCGCGCCCG CGTTACATAA CTTACGGTAA ATGGCC |
| Ad-GL-FZF-R | GCTAGCGATA TCGCGGCCGC GAGCTCATAA GATACATTGA TGAGTTTGGA CAAACCACAA CTAGAATGCA GTG |
| Ad-GL-FZF-F | CACTGCATTC TAGTTGTGGT TTGTCCAAAC TCATCAATGT ATCTTATGAG CTCGCGGCCG CGATATCGCT AGC |
| FZF-AscI-R | GGCGCGCCGA TCCGCGTTGC CTGCAGGTCG ACTCTA |
The final shuttle plasmids were created by digesting the shuttle plasmids with AscI and dephosphorylating with Antarctic alkaline phosphatase. The HA1-con-FZF and GFPLuc-FZF plasmids were also digested with AscI and the plasmid backbone was cut with MluI. The digested plasmids were ligated with T4 DNA ligase and transformed into XL-1 electrocompetent cells.
To make the recombinant Ad4 and Ad7 plasmids, the shuttle plasmids were digested with PacI, buffer exchanged, and cotransformed with 1.0 µg of pAd4 or pAd7 in BJ5183 electrocompetent cells. Recombinants were selected on LB containing Kanamycin and Zeocin. Recombinants were confirmed by restriction digestion analysis. Positive recombinant clones were transformed into XL-1 cells and maxiprepped using the Qiagen HI Speed Maxi kits (Qiagen).
Recombinant Ad4 and Ad7 rescue and purification
The recombinant Ad4 and Ad7 genomes were released from the plasmid backbone by digestion with PacI and AsiSI, respectively. The digested plasmids were buffer exchanged using a Strataprep PCR purification kit (Agilent Technologies). All transfections were performed in 6-well plates as described by Polyfect Transfection reagent (Qiagen). Viruses were released from infected cells by 3 freeze-thaw cycles and amplified in sequential passages in the complementing host cell lines. The viruses were amplified and purified as previously described for wt Ad4 and Ad7. All viral preps were quantitated by OD260. Infectious units of Ad4 and Ad7 expressing GFPLuc were determined by infecting 293 cells and quantitated by direct observation usning fluorescent microscopy.
In vivo luciferase activity
Luciferase activity induced by the Ad4 and Ad7 GFPLuc expressing viruses was determined using a Lumazone imaging system (Roper Scientific). Female BALB/c and CD46+ transgenic mice were anesthetized using ketamine/xylazine and 1010 vp of each virus was injected intramuscularly using a 27 gauge needle into both quadriceps in 2, 25 µl injections. For intranasal immunization, the mice were anesthetized and placed on their back. Virus was pipetted into the nares in 2 10 microliter instillations. Twenty-four hr. post-immunization, the mice were anasthesized with ketamine/xylazine and injected intraperitoneal with 200 μl of d-luciferin at a concentration of 20 mg/ml in PBS. The hair on the CD46+ transgenic mice was removed using a Wahl trimmer in order to improve luciferase detection. The mice were immediately placed into the Lumazone Imager and images were captured. All images were taken with a 10 min exposure and 4 × 4 binning using no filters and no photo-multiplication. Data analysis was performed on each image using background subtracted sum intensities as detected by the Lumazone Imaging Software and graphed using PrismGraphing Software.
Immunizations and influenza challenge
Protection against influenza challenge by the HA1-con expressing Ad4 and Ad7 vaccines was determined by serial dilution of the vaccine in BALB/c and CD46+ transgenic mice. All immunizations and challenges were performed using ketamine/xylazine anesthetized mice as previously described. Intranasal and intramuscular immunizations were performed as previously described for in vivo luciferase activity. Mice were immunized with 10-fold dilutions of the vaccine vectors from 1010 to 107 vp/mouse. Three weeks after immunization the mice were challenged with 100 MLD50 of mouse-adapted A/PR/8/34 influenza virus as previously described.11 The mice were observed for 2 wk for signs of disease, weight loss, and death. Mice that lost more than 25% of baseline body weight were humanely euthanized.
Ad neutralization assay
Ad7 neutralization was performed as previously described.45 Briefly, serial dilutions of plasma were made in cDMEM and incubated in triplicate for 1 h at 37 °C with 500 vp of Ad7 vector expressing the eGFP-luciferase fusion gene. The resulting solution was added to A549 cells in a black 96 well plate for 24 h and luciferase activity was measured. Data are expressed as the mean titer that reduced Ad7 luciferase activity by ≥90%.
In vitro luciferase activity
293 cells were infected with 104 vp/cell of either Ad4 or Ad7 virus expressing the GFPLuc gene. The luciferase activity was determined 24 h post-infection by lysing the cells with the reporter lysis 5X buffer. Luciferase activity was detected using the luciferase assay reagent (LAR) (Promega) as described by the manufacturer protocol. Briefly, 25 µl of 5X lysis buffer was added to each well of a 96-well plate and mixed. Then 50 µl of LAR was added and the luciferase activity was measured using a Beckman Coulter DTX 880 microplate reader.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the Biodefense and Emerging Infectious Diseases (BEI) Repository for reagents used in this study. We also thank Dr. Michael Barry for reagents and Ad genomic DNA. This work was supported by the National Institutes of Health, National Institute of Allergy and Infectious Diseases grant AI097241. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dudding BA, Top FH, Jr., Winter PE, Buescher EL, Lamson TH, Leibovitz A. Acute respiratory disease in military trainees: the adenovirus surveillance program, 1966-1971. Am J Epidemiol. 1973;97:187–98. doi: 10.1093/oxfordjournals.aje.a121499. [DOI] [PubMed] [Google Scholar]
- 2.Kitchen LW, Vaughn DW. Role of U.S. military research programs in the development of U.S.-licensed vaccines for naturally occurring infectious diseases. Vaccine. 2007;25:7017–30. doi: 10.1016/j.vaccine.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 3.Gaydos CA, Gaydos JC. Adenovirus vaccines in the U.S. military. Mil Med. 1995;160:300–4. [PubMed] [Google Scholar]
- 4.Buescher EL. Respiratory disease and the adenoviruses. Med Clin North Am. 1967;51:769–79. doi: 10.1016/s0025-7125(16)33045-0. [DOI] [PubMed] [Google Scholar]
- 5.Hyer RN, Howell MR, Ryan MAK, Gaydos JC. Cost-effectiveness analysis of reacquiring and using adenovirus types 4 and 7 vaccines in naval recruits. Am J Trop Med Hyg. 2000;62:613–8. doi: 10.4269/ajtmh.2000.62.613. [DOI] [PubMed] [Google Scholar]
- 6.Gurwith M, Lock M, Taylor EM, Ishioka G, Alexander J, Mayall T, Ervin JE, Greenberg RN, Strout C, Treanor JJ, et al. Safety and immunogenicity of an oral, replicating adenovirus serotype 4 vector vaccine for H5N1 influenza: a randomised, double-blind, placebo-controlled, phase 1 study. Lancet Infect Dis. 2013;13:238–50. doi: 10.1016/S1473-3099(12)70345-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoke CH, Jr., Snyder CE., Jr. History of the restoration of adenovirus type 4 and type 7 vaccine, live oral (Adenovirus Vaccine) in the context of the Department of Defense acquisition system. Vaccine. 2013;31:1623–32. doi: 10.1016/j.vaccine.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 8.Santra S, Sun Y, Korioth-Schmitz B, Fitzgerald J, Charbonneau C, Santos G, Seaman MS, Ratcliffe SJ, Montefiori DC, Nabel GJ, et al. Heterologous prime/boost immunizations of rhesus monkeys using chimpanzee adenovirus vectors. Vaccine. 2009;27:5837–45. doi: 10.1016/j.vaccine.2009.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weaver EA, Nehete PN, Nehete BP, Buchl SJ, Palmer D, Montefiori DC, Ng P, Sastry KJ, Barry MA. Protection against Mucosal SHIV Challenge by Peptide and Helper-Dependent Adenovirus Vaccines. Viruses. 2009;1:920–38. doi: 10.3390/v1030920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weaver EA, Nehete PN, Buchl SS, Senac JS, Palmer D, Ng P, Sastry KJ, Barry MA. Comparison of replication-competent, first generation, and helper-dependent adenoviral vaccines. PLoS One. 2009;4:e5059. doi: 10.1371/journal.pone.0005059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weaver EA, Rubrum AM, Webby RJ, Barry MA. Protection against divergent influenza H1N1 virus by a centralized influenza hemagglutinin. PLoS One. 2011;6:e18314. doi: 10.1371/journal.pone.0018314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lemckert AA, Sumida SM, Holterman L, Vogels R, Truitt DM, Lynch DM, Nanda A, Ewald BA, Gorgone DA, Lifton MA, et al. Immunogenicity of heterologous prime-boost regimens involving recombinant adenovirus serotype 11 (Ad11) and Ad35 vaccine vectors in the presence of anti-ad5 immunity. J Virol. 2005;79:9694–701. doi: 10.1128/JVI.79.15.9694-9701.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan WG, Jin HT, West EE, Penaloza-MacMaster P, Wieland A, Zilliox MJ, McElrath MJ, Barouch DH, Ahmed R. Comparative analysis of simian immunodeficiency virus gag-specific effector and memory CD8+ T cells induced by different adenovirus vectors. J Virol. 2013;87:1359–72. doi: 10.1128/JVI.02055-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbink P, Lemckert AA, Ewald BA, Lynch DM, Denholtz M, Smits S, Holterman L, Damen I, Vogels R, Thorner AR, et al. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J Virol. 2007;81:4654–63. doi: 10.1128/JVI.02696-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barouch DH, Kik SV, Weverling GJ, Dilan R, King SL, Maxfield LF, Clark S, Ng’ang’a D, Brandariz KL, Abbink P, et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine. 2011;29:5203–9. doi: 10.1016/j.vaccine.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mast TC, Kierstead L, Gupta SB, Nikas AA, Kallas EG, Novitsky V, Mbewe B, Pitisuttithum P, Schechter M, Vardas E, et al. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine. 2010;28:950–7. doi: 10.1016/j.vaccine.2009.10.145. [DOI] [PubMed] [Google Scholar]
- 17.Ludwig SL, Brundage JF, Kelley PW, Nang R, Towle C, Schnurr DP, Crawford-Miksza L, Gaydos JC. Prevalence of antibodies to adenovirus serotypes 4 and 7 among unimmunized US Army trainees: results of a retrospective nationwide seroprevalence survey. J Infect Dis. 1998;178:1776–8. doi: 10.1086/314498. [DOI] [PubMed] [Google Scholar]
- 18.Chengalvala M, Lubeck MD, Davis AR, Mizutani S, Molnar-Kimber K, Morin J, Hung PP. Evaluation of adenovirus type 4 and type 7 recombinant hepatitis B vaccines in dogs. Vaccine. 1991;9:485–90. doi: 10.1016/0264-410X(91)90033-3. [DOI] [PubMed] [Google Scholar]
- 19.Lubeck MD, Davis AR, Chengalvala M, Natuk RJ, Morin JE, Molnar-Kimber K, Mason BB, Bhat BM, Mizutani S, Hung PP, et al. Immunogenicity and efficacy testing in chimpanzees of an oral hepatitis B vaccine based on live recombinant adenovirus. Proc Natl Acad Sci U S A. 1989;86:6763–7. doi: 10.1073/pnas.86.17.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Natuk RJ, Lubeck MD, Chanda PK, Chengalvala M, Wade MS, Murthy SC, Wilhelm J, Vernon SK, Dheer SK, Mizutani S, et al. Immunogenicity of recombinant human adenovirus-human immunodeficiency virus vaccines in chimpanzees. AIDS Res Hum Retroviruses. 1993;9:395–404. doi: 10.1089/aid.1993.9.395. [DOI] [PubMed] [Google Scholar]
- 21.Alexander J, Ward S, Mendy J, Manayani DJ, Farness P, Avanzini JB, Guenther B, Garduno F, Jow L, Snarsky V, et al. Pre-clinical evaluation of a replication-competent recombinant adenovirus serotype 4 vaccine expressing influenza H5 hemagglutinin. PLoS One. 2012;7:e31177. doi: 10.1371/journal.pone.0031177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu KH, Lubeck MD, Davis AR, Bhat RA, Selling BH, Bhat BM, Mizutani S, Murphy BR, Collins PL, Chanock RM, et al. Immunogenicity of recombinant adenovirus-respiratory syncytial virus vaccines with adenovirus types 4, 5, and 7 vectors in dogs and a chimpanzee. J Infect Dis. 1992;166:769–75. doi: 10.1093/infdis/166.4.769. [DOI] [PubMed] [Google Scholar]
- 23.Artenstein AW. Vaccines for military use. Vaccine. 2009;27(Suppl 4):D16–22. doi: 10.1016/j.vaccine.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 24.Wold WS, Gooding LR. Region E3 of adenovirus: a cassette of genes involved in host immunosurveillance and virus-cell interactions. Virology. 1991;184:1–8. doi: 10.1016/0042-6822(91)90815-S. [DOI] [PubMed] [Google Scholar]
- 25.Lesokhin AM, Delgado-Lopez F, Horwitz MS. Inhibition of chemokine expression by adenovirus early region three (E3) genes. J Virol. 2002;76:8236–43. doi: 10.1128/JVI.76.16.8236-8243.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horwitz MS, Efrat S, Christen U, von Herrath MG, Oldstone MB. Adenovirus E3 MHC inhibitory genes but not TNF/Fas apoptotic inhibitory genes expressed in beta cells prevent autoimmune diabetes. Proc Natl Acad Sci U S A. 2009;106:19450–4. doi: 10.1073/pnas.0910648106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koehler DR, Martin B, Corey M, Palmer D, Ng P, Tanswell AK, Hu J. Readministration of helper-dependent adenovirus to mouse lung. Gene Ther. 2006;13:773–80. doi: 10.1038/sj.gt.3302712. [DOI] [PubMed] [Google Scholar]
- 28.Croyle MA, Chirmule N, Zhang Y, Wilson JM. PEGylation of E1-deleted adenovirus vectors allows significant gene expression on readministration to liver. Hum Gene Ther. 2002;13:1887–900. doi: 10.1089/104303402760372972. [DOI] [PubMed] [Google Scholar]
- 29.Kuzmin AI, Galenko O, Eisensmith RC. An immunomodulatory procedure that stabilizes transgene expression and permits readministration of E1-deleted adenovirus vectors. Mol Ther. 2001;3:293–301. doi: 10.1006/mthe.2000.0258. [DOI] [PubMed] [Google Scholar]
- 30.Nagao S, Kuriyama S, Okuda H, Tominaga K, Nakatani T, Tsujinoue H, Yoshiji H, Fukui H. Adenovirus-mediated gene transfer into tumors: evaluation of direct readministration of an adenoviral vector into subcutaneous tumors of immunocompetent mice. Int J Oncol. 2001;18:57–65. [PubMed] [Google Scholar]
- 31.Chirmule N, Raper SE, Burkly L, Thomas D, Tazelaar J, Hughes JV, Wilson JM. Readministration of adenovirus vector in nonhuman primate lungs by blockade of CD40-CD40 ligand interactions. J Virol. 2000;74:3345–52. doi: 10.1128/JVI.74.7.3345-3352.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McClane SJ, Chirmule N, Burke CV, Raper SE. Characterization of the immune response after local delivery of recombinant adenovirus in murine pancreas and successful strategies for readministration. Hum Gene Ther. 1997;8:2207–16. doi: 10.1089/hum.1997.8.18-2207. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Li ZY, Liu Y, Persson J, Beyer I, Möller T, Koyuncu D, Drescher MR, Strauss R, Zhang XB, et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat Med. 2011;17:96–104. doi: 10.1038/nm.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartman ZC, Appledorn DM, Serra D, Glass O, Mendelson TB, Clay TM, Amalfitano A. Replication-attenuated Human Adenoviral Type 4 vectors elicit capsid dependent enhanced innate immune responses that are partially dependent upon interactions with the complement system. Virology. 2008;374:453–67. doi: 10.1016/j.virol.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oglesby TJ, Allen CJ, Liszewski MK, White DJ, Atkinson JP. Membrane cofactor protein (CD46) protects cells from complement-mediated attack by an intrinsic mechanism. J Exp Med. 1992;175:1547–51. doi: 10.1084/jem.175.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H, Beyer I, Persson J, Song H, Li Z, Richter M, Cao H, van Rensburg R, Yao X, Hudkins K, et al. A new human DSG2-transgenic mouse model for studying the tropism and pathology of human adenoviruses. J Virol. 2012;86:6286–302. doi: 10.1128/JVI.00205-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuschner RA, Russell KL, Abuja M, Bauer KM, Faix DJ, Hait H, Henrick J, Jacobs M, Liss A, Lynch JA, et al. Adenovirus Vaccine Efficacy Trial Consortium A phase 3, randomized, double-blind, placebo-controlled study of the safety and efficacy of the live, oral adenovirus type 4 and type 7 vaccine, in U.S. military recruits. Vaccine. 2013;31:2963–71. doi: 10.1016/j.vaccine.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 38.Nam JH, Na HN, Atkinson RL, Dhurandhar NV. (2013) Genomic stability of adipogenic human adenovirus 36. Int J Obes (Lond). [DOI] [PubMed] [Google Scholar]
- 39.Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, Fullman N, Naghavi M, Lozano R, Lopez AD. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–31. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 40.Fukuda MM, Klein TA, Kochel T, Quandelacy TM, Smith BL, Villinski J, Bethell D, Tyner S, Se Y, Lon C, et al. AFHSC-GEIS Malaria and Vector Borne Infections Writing Group Malaria and other vector-borne infection surveillance in the U.S. Department of Defense Armed Forces Health Surveillance Center-Global Emerging Infections Surveillance program: review of 2009 accomplishments. BMC Public Health. 2011;11(Suppl 2):S9. doi: 10.1186/1471-2458-11-S2-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitman TJ, Coyne PE, Magill AJ, Blazes DL, Green MD, Milhous WK, Burgess TH, Freilich D, Tasker SA, Azar RG, et al. An outbreak of Plasmodium falciparum malaria in U.S. Marines deployed to Liberia. Am J Trop Med Hyg. 2010;83:258–65. doi: 10.4269/ajtmh.2010.09-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chuang I, Sedegah M, Cicatelli S, Spring M, Polhemus M, Tamminga C, Patterson N, Guerrero M, Bennett JW, McGrath S, et al. DNA prime/Adenovirus boost malaria vaccine encoding P. falciparum CSP and AMA1 induces sterile protection associated with cell-mediated immunity. PLoS One. 2013;8:e55571. doi: 10.1371/journal.pone.0055571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vogels R, Zuijdgeest D, van Rijnsoever R, Hartkoorn E, Damen I, de Béthune MP, Kostense S, Penders G, Helmus N, Koudstaal W, et al. Replication-deficient human adenovirus type 35 vectors for gene transfer and vaccination: efficient human cell infection and bypass of preexisting adenovirus immunity. J Virol. 2003;77:8263–71. doi: 10.1128/JVI.77.15.8263-8271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berge TO, England B, Mauris C, Shuey HE, Lennette EH. Etiology of acute respiratory disease among service personnel at Fort Ord, California. Am J Hyg. 1955;62:283–94. doi: 10.1093/oxfordjournals.aje.a119779. [DOI] [PubMed] [Google Scholar]
- 45.Sprangers MC, Lakhai W, Koudstaal W, Verhoeven M, Koel BF, Vogels R, Goudsmit J, Havenga MJ, Kostense S. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. J Clin Microbiol. 2003;41:5046–52. doi: 10.1128/JCM.41.11.5046-5052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.