Abstract
A live attenuated influenza A(H1N1)pdm 2009 vaccine was developed and distributed in India in 2010. We estimated the vaccine effectiveness (VE) against laboratory-confirmed pandemic H1N1 (pH1N1) infections in patients with influenza-like illness who visited five tertiary care hospitals in Pune, India during June–December 2010. Swab specimens were analyzed for influenza pH1N1 by reverse transcriptase polymerase chain reaction (RT-PCR). VE was estimated using the test-negative case-control study design and logistic regression. A total of 784 patients (253 cases, 531 controls) were analyzed. The unadjusted overall VE was 75.5% (95% confidence interval [CI] 42.1–89.7), while the adjusted VE was 76% (95% CI 42.1–89.7). We conclude that the live attenuated influenza A(H1N1)pdm 2009 vaccine was effective in our study population, which has opened prospects for using this platform for trivalent formulations.
Keywords: pandemic, influenza A(H1N1)pdm 2009, live attenuated vaccine, effectiveness, case control
Introduction
In the spring of 2009, a new type of H1N1 influenza A virus infection was detected in Mexico. Due to its quick spread, it caused a major concern worldwide.1,2 This led the World health Organization (WHO) to declare a pandemic of its highest level 6 on 11 June 2009. This action indicated widespread transmission in the community.1,3
The first case in India was reported in Hyderabad on 16 May 2009,4 and the first death was of a 14-y-old girl in Pune, on 3 August 2009. Hyderabad is situated 557 km southeast to Pune. These events and the quick spread in the community caused panic in public.5 From May 2009 until the week ending on 2 January 2011, India reported 46 142 laboratory confirmed influenza A(H1N1)pdm 2009 cases and 2728 deaths.6,7 Maharashtra state alone reported 9972 proven cases of influenza A(H1N1)pdm 2009 and 937 deaths. These were the largest numbers in any state of India.6 The city of Pune in Maharashtra reported the highest numbers of cases and deaths among all cities of India.5
To meet the challenge, Serum Institute of India Ltd (SIIL), Pune-following a technology transfer, including transferring reassortant strain from Russia which was facilitated by WHO- developed a live attenuated influenza vaccine (LAIV) against the A(H1N1)pdm 2009 strain. This monovalent vaccine (Nasovac®) was administered by intranasal spray. SIIL also developed an inactivated A(H1N1)pdm 2009 vaccine which was licensed after clinical trials;8 however only LAIV was marketed since its production was more economical, faster, and less resource intensive as compared with the inactivated vaccine.
The vaccine was found highly effective in preventing influenza A(H1N1)pdm 2009 in ferrets.9 After immunogenicity and safety was demonstrated in clinical studies (Prasad S Kulkarni, unpublished data),10 the vaccine was licensed by the Drug Controller General of India (DCGI) on 3 July 2010. One intra-nasal dose of 0.5 ml was recommended for adults and children ≥3 y of age. More than 2.5 million doses were distributed India of which over one million doses were used in Maharashtra alone.
Since Pune had the maximum number of influenza A(H1N1)pdm 2009 cases in the country and most of the vaccine was used in this city, a case control study was conducted in Pune to estimate the effectiveness of Nasovac®. During the pandemic, other inactivated H1N1 vaccines as well as trivalent inactivated vaccines containing pH1N1 were available in India.
Although inactivated vaccines were also used in the city during this period, the numbers are estimated to be much lower than with live vaccine.
Although India falls in the northern hemisphere, the seasonal pattern of influenza is more like southern hemisphere, with major peaks in the month of June to September which is related with the monsoon across major parts of the country and minor peaks in winter i.e., November to January.
Results
Demographics
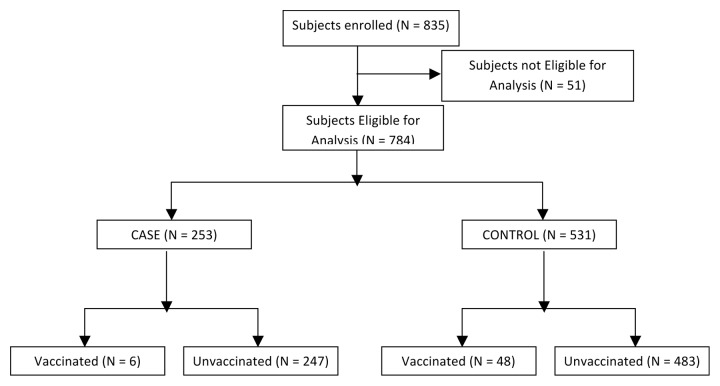
Data was collected from 835 subjects. From these, 51 subjects (6.1%) were excluded due to non-eligibility. Thus, data from 784 subjects (Fig. 1 and Table 1) were analyzed which included 253 (32.27%) cases and 531(67.73%) controls. The age ranged from 3 y in both groups to 79 and 87 y, respectively. There was no difference in age, gender distribution, body mass index (BMI), or in the numbers of subjects with high risk conditions or those hospitalized for influenza like illness (ILI). However, there were more deaths among cases than among controls. 168 (66.40%) cases and 369 (69.49%) controls were interviewed personally or by telephone in addition to checking their hospital records, whereas from 85 (33.60%) cases and 162 (30.51%) controls, the information was collected from the hospital records alone. (P = 0.411).
Figure 1. Subject disposition.
Table 1. Demographic characteristics.
| Characteristics | Case (n = 253) |
Control (n = 531) |
P value | |
|---|---|---|---|---|
| Male | N (%) | 146 (57.7) | 313 (58.9) | 0.757 |
| Age | Mean(SD) | 28.98 (12.9) | 30.43 (18.8) | 0.770 |
| Median | 27 | 26 | ||
| Range | 3.00–79.00 | 3.00–87.00 | ||
| Height | Mean(SD) | 160.08 (16.3) | 154.77 (21.1) | 0.017 |
| Median | 164 | 161.50 | ||
| Range | 74.00–185.00 | 86.00–188.90 | ||
| Weight | Mean(SD) | 61.32 (16.1) | 56.44 (20.8) | 0.020 |
| Median | 63 | 58 | ||
| Range | 15.00–102.00 | 7.26–102.00 | ||
| BMI | Mean(SD) | 23.58 (4.4) | 22.77 (5.5) | 0.100 |
| Median | 23.40 | 22.95 | ||
| Range | 11.50–36.90 | 9.90–47.10 | ||
| High risk conditions | N (%) | 54 (21.3) | 144 (27.1) | 0.095 |
| Swabs were obtained within 4 d after onset of ILI | N (%) | 206 (81.4) | 399 (75.1) | 0.106 |
| Hospitalizations for ILI | N (%) | 240 (94.9) | 505 (95.1) | 0.862 |
| Deaths | N (%) | 8 (3.2) | 3 (0.6) | 0.007 |
| Vaccinated | N (%) | 6 (2.4) | 48 (9.1) | 0.001 |
| Unvaccinated | N (%) | 247 (97.6) | 483 (91.0) | |
| Treatment with anti-viral medications | N (%) | 252 (99.6) | 500 (94.2) | <0.0001 |
Out of 6 vaccinated cases, 5 subjects received Oseltamivir / Zanamivir as a treatment of ILI, out of which one received within 21 d of vaccination. While out of 48 vaccinated controls, 43 received Oseltamivir / Zanamivir out of which 9 received within 21 d of vaccination.
Vaccine effectiveness (VE)
The unadjusted overall VE was 75.5% [95% CI 42.1- 89.7]. The adjusted overall VE was similar, 76.0% (95% CI 42.1–89.7). The effect of confounders on overall VE was found insignificant as follows; age (P value = 0.2436), gender (P value = 0.6711), and presence of high risk medical conditions as per the Advisory Committee on Immunization Practices (ACIP) criteria (P value = 0.0890). VE when different intervals between vaccination and onset of ILI were considered, are presented in Table 2. When estimated among people from whom the swabs had been collected within 4 d from the onset of illness, the VE was 77.3% (95% CI 34.8–92.1)
Table 2. VE stratification according to interval between date of vaccination and onset of ILI.
| Period | Unadjusted VE %, (CI) | Adjusted VE %, (CI) |
|---|---|---|
| Overall | 75.5% (95% CI 42.1–89.7) | 76.0% (95% CI 42.1–89.7) |
| Period 1 | ||
| (Difference between date of Vaccination and Illness Onset Date is greater than 7 d) | 75 (40.8–89.5) | 75.1 (40.9–89.5) |
| Period 2 | ||
| (Difference between date of Vaccination and Illness Onset Date is greater than 14 d) | 76.7 (40.4–90.9) | 76.5 (39.8–90.8) |
| Period 3 | ||
| (Difference between date of Vaccination and Illness Onset Date is greater than 21 days) |
78.9 (40–92.5) | 78.6 (39.2–92.5) |
Discussion
The study demonstrated good effectiveness of the newly developed, locally manufactured vaccine. The overall vaccine effectiveness against laboratory confirmed disease was 75.5%. Similar design, in which the test-negative individuals have served as controls to the test-positive group have been previously described to assess the effectiveness of seasonal and pandemic vaccines in North America and Europe.11-16
Our results are comparable to the earlier inactivated A(H1N1)pdm 2009 influenza vaccines in three case control studies which assessed effectiveness against laboratory-confirmed H1N1 infection: 73.4% effectiveness in Korea,17 72% effectiveness in England and Scotland18 and 86% effectiveness in Manitoba (Canada)19 reported. A trivalent influenza vaccine for 2010 influenza season showed effectiveness of 79% in another case control study in Australia,20 and recently, a meta-analysis of five observational studies showed the median VE to be 69% (range 60- 93%) for monovalent A(H1N1)pdm 2009 vaccine.21 Another case control study in Spain found adjusted effectiveness of 54% against laboratory-confirmed influenza-related hospitalizations during the 2010–2011 influenza season.22 Although in our study, majority of patients (around 95%) were hospitalized, the overall effectiveness was better than this. To our knowledge, this is the only study which assessed effectiveness of any H1N1 vaccine in India. In fact, case control studies are rare in India and in many developing countries.
The interval from the ILI onset to specimen collection might affect the VE estimates. Earlier studies,17,20,23 have used as their cut-off an interval of 4 d. In India patients usually visit first a general practitioner before then perhaps being referred to a tertiary care hospital where the facilities for swabbing nasal/throat exist. Therefore, we selected an interval of 7 d, but found still an overall VE of 75.5%. However, if only those people were examined from whom a swab was obtained within 4 d, VE was almost similar, 76.7%.
Most studies12,17-19,24 have examined the results also for the vaccination-to-disease interval of ≥ 14 d. We looked at our results for >7, >14, or >21 d postvaccination, and found the VE to be 75.0%, 76.7%, and 78.9%. This demonstrated that the vaccine gave protection from day 7 d onwards.
In India, the uptake of seasonal influenza vaccines is generally low. It is not part of the government’s immunization program, although it is recommended by the Indian Academy of Pediatrics for children at risk. However, in case of this pandemic, the public awareness was very high which resulted in widespread use of the vaccine in Pune which had the maximum activity of the infection. The price of the vaccine was affordable (around 4 US dollars) which meant that people from all classes could take the vaccine. On some occasions, free vaccination camps were conducted by non-governmental organizations. Thus the study population was quite representative of the general population in the city.
Our study has many strengths. It was done in a real life situation at a place where the infection and the vaccine usage was most prevalent in India. The test negative design afforded a right control group. Using the laboratory confirmed cases only gave a validated result. The study had some limitations. An age-stratified VE would have been useful, however this was not possible because of small number of cases. Second, the date of vaccination was not available in some subjects. For this reason, VE was calculated after imputing this date as that when the vaccine become available. Although date of vaccination is important during pandemic situations to avoid misclassification of exposure, this approximation has been used also in other case-control studies.18 Protection due to sub-clinical infections cannot be ruled out in some cases. Also some persons may have received vaccine after the infection. However any such effect would have happened in the control arm as well, thus nullifying any impact on the effectiveness figure.
To conclude, a single dose of the intranasal Indian-made vaccine (Nasovac®) was found well protective against laboratory confirmed A(H1N1)pdm 2009 infection in subjects ≥3 y of age. Significant, close to 80% effectiveness was observed from day 7 postvaccination. The results demonstrate high effectiveness of the LAIV platform. Based on the same platform, a trivalent formulation has been developed and assessed clinically in India. It is under regulatory process of marketing authorization.
Subjects and Methods
Setting and population
The present retrospective case control study was conducted at five tertiary care hospitals of Pune in 2011–2012. Since the vaccine was licensed for use in age group ≥3 y, the potential subjects of age ≥3 y who presented to the hospitals with the symptoms and signs of ILI during 19 July 2010–31 December 2010 were identified from hospital records and were contacted telephonically. ILI was defined as a fever ≥100 °F and a cough and/or sore throat. Since A(H1N1)pdm 2009 had already become a major public health problem, most of such patients were swabbed for confirming the diagnosis.
The vaccine was launched in India on 05 July 2010, hence individuals reporting ILI from 19 July 2010 (i.e., two weeks after the launch of the vaccine) were selected. Confirmed cases in Pune started tapering in September and came down to zero in the second half of December and hence individuals reporting ILI until 31 December 2010 were selected. The vaccination campaigns were conducted by private physicians in hospitals and clinics on payment of charges. On some occasions, free vaccination camps were conducted by non-governmental organizations. Thus the study population was quite representative of the general population in the city.
A case was defined as a patient whose throat or nasal swab was collected within 7 d of the disease onset, and the influenza A(H1N1)pdm 2009 strain was identified by reverse transcriptase polymerase chain reaction (RT-PCR). All samples were tested by rRT-PCR following the Centers for Disease Control and Prevention (CDC) protocol.25 The controls were ILI patients whose throat or nasal swab were found negative for the A(H1N1)pdm 2009 virus.
An individual was excluded from the study if he or she was younger than 3 y, had received inactivated H1N1 vaccine, the Nasovac® vaccination status remained unknown, evidence of previous laboratory confirmed influenza in the 2009–10 season, ILI developed within 7 d of vaccination, or the swab test result was not available. The majority of swabs were tested at the National Institute of Virology (NIV), Pune, which is a WHO and CDC –qualified reference laboratory for the PCR methodology. A few swabs were tested at two private laboratories namely Religare laboratories and Dr Lal’s Pathology Lab, Pune although they also followed the same methodology. During the study period, most of the hospitalized ILI patients were swabbed in large hospitals.
Study procedures
All potential subjects identified from hospital records were contacted by telephonic calls and/or home visits and interviews were conducted. The information obtained from the personal/telephonic interviews was verified using the hospital records. In case of non-traceable individuals who were eligible as per the criteria, interviews were not possible and relevant information was collected from the hospital records, if it was available. If the records did not have required information, such subjects were not included.
Demographic and clinical information including date of birth/ age, gender, date of onset of ILI, date of specimen collection, influenza vaccination status, vaccination date in case of vaccinated subjects, and history of medical conditions was collected for eligible individuals.
Individuals were defined as high risk if they had any of the following medical conditions that increased the risk of influenza complications like chronic pulmonary (including asthma), cardiovascular (except hypertension), renal, hepatic, neurologic, hematologic, or metabolic disorders (including diabetes mellitus); residents of nursing homes and other chronic-care facilities; morbidly obese (body-mass index is 40 or greater); health-care personnel; household contacts and caregivers of children aged younger than 5 y and adults aged 50 y and older; and household contacts and caregivers of persons with medical conditions that put them at higher risk for severe complications from influenza.
Study vaccine
Nasovac® is manufactured by Serum Institute of India Ltd. It is stored at 2–8 °C until use, and is used in a one-dose regimen. The virus strain used in Nasovac® is antigenically similar to the A/California/7/2009 strain and is attenuated, cold-adapted, and temperature-sensitive virus designed for vaccination.
The vaccine strain is derived from highly stable, attenuated influenza A master donor viruses (MDV). This MDV is attenuated in nature and replicates efficiently at 25 °C-33 –°C but does not replicate efficiently at temperatures above 39 °C; these phenotypic traits have respectively been designated as attenuated (att), cold-adapted (ca), and temperature-sensitive (ts) phenotypes. Genetic reassortment method is used to generate vaccine strain such that the genome composition consists of the six internal genes namely PB1, PB2, PA, NP, M, and NS of the MDV and the two HA and NA gene segments of the wild type virus A/California/07/2009. This 6:2 reassortant virus possess stable phenotypic traits of the MDV and the antigenic properties conferred by the HA and NA of the WHO recommended pandemic H1N1 virus. Embryonated hens eggs are used as a substrate for seed virus preparation and bulk vaccine consisting of partially purified monovalent virus pool.
After reconstitution with 2.5 ml of sterile water, each vial consists of 5 doses (each of 0.5 ml). 0.25 ml of reconstituted vaccine was administered in each nostril. Each dose of 0.5 ml contains not less than 107 EID50 of the live attenuated influenza virus re-assortant of the A(H1N1)pdm 2009 virus, partially hydrolysed gelatin 2.5% and sorbitol 5.0%. The batches of Nasovac® were marketed after the in-house quality control testing and the release by the Central Drug Laboratories (CDL), Kasauli.
Statistical analysis
Using an α error of 0.05, a power of 0.80, and an odds ratio of 0.4 in a population with 20% vaccine coverage, 181 cases and 181 controls were required. SAS 9.2 was used for statistical testing. For age, the mean, standard deviation (SD), median, and range are given, whereas the gender was expressed as the number and percentage. Demographic and baseline characteristics between the cases and controls were compared by Fisher’s exact test (categorical data) / Kruskal Wallis test (continuous data). For vaccine effectiveness, potential confounders considered were age, gender, presence of high-risk medical conditions, and receipt of oseltamivir / zanamivir within 21 d of vaccination. With logistic regression the overall vaccine effectiveness (%) was calculated as (1-odds ratio) × 100. VE was further estimated according to the stratification of interval between the date of vaccination and the onset of ILI i.e., 7-d, 14-d, and 21-d periods from vaccination to ILI. VE was also estimated for the population in which swabs were collected within 4 d of onset of ILI. Unknown date of vaccination was imputed as 05 July 2010 i.e., the day when Nasovac® became available in market. In case of date of onset of ILI missing, the date of sample collection minus seven days was considered as date of onset of ILI. For unknown date of swab collection, the date of receipt of swab in laboratory or date of report, was considered. The results are expressed as numbers and percentages, along with 95% confidence intervals (95% CI).
Disclosure of Potential Conflicts of Interest
P.S.K. and S.M. are employed by Serum Institute of India Ltd. V.P. is employed by a CRO which was contracted by the sponsor for statistical analysis.
Funding
The study was funded by Serum Institute of India Ltd, Pune.
Ethics
The Institutional Ethics Committees of each study site approved the study. For all potential subjects, interviews were conducted after obtaining a written consent. For the subjects who could not be available for a meeting, but agreeing for a telephonic interview, a verbal consent was obtained. For subjects ≥3 to 17 y, parents/legal guardians were requested for consent, as was an assent obtained from subjects aged 13 to 17 y.
Glossary
Abbreviations:
- ACIP
Advisory Committee on Immunization Practices
- CI
confidence interval
- ILI
influenza like illness
- WHO
World Health Organization
- RT-PCR
reverse transcriptase polymerase chain reaction
- pH1N1
pandemic H1N1
- CDC
Centre for Disease Control and Prevention
- LAIV
live attenuated influenza vaccine
- VE
vaccine effectiveness
- EID
egg infective dose
- NIV
National Institute of Virology
- SIIL
Serum Institute of India Ltd
- CDL
Central Drug Laboratories
- SD
standard deviation
References
- 1.Centers for Disease Control and Prevention (CDC) Outbreak of swine-origin influenza A (H1N1) virus infection - Mexico, March-April 2009. MMWR Morb Mortal Wkly Rep. 2009;58:467–70. [PubMed] [Google Scholar]
- 2.World Health Organization. Pandemic (H1N1) 2009 - update 69. [Accessed 21 August 2012]. Available from: http://www.who.int/csr/don/2009_10_09/en/index.html
- 3.World Health Organization. World now at the start of 2009 influenza pandemic. http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en /index.html (Accessed June 11, 2009).
- 4.Ministry of Health and Family Welfare. Government of India: Update of influenza A (H1N1) as on 17th May, 2009. Press release dated 17th May, 2009. [Accessed on 21 August 2012]. Available from: http://pib.nic.in/newsite/erelease.aspx?relid=48762].
- 5.Mishra AC, Chadha MS, Choudhary ML, Potdar VA. Pandemic influenza (H1N1) 2009 is associated with severe disease in India. PLoS One. 2010;5:e10540. doi: 10.1371/journal.pone.0010540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ministry of Health and Family Welfare. Government of India: Situational updates. [Accessed on 15 March 2011]. Available from: http://mohfw-h1n1.nic.in/documents/PDF/SituationalUpdatesArchives/december2010/ Situational%20Updates%20on%2027.12.2010.pdf
- 7.Ministry of Health and Family Welfare. Government of India: Epidemiological trends. [Accessed on 21 August 2012]. Available from: http://mohfw-h1n1.nic.in/Epidemiological.html
- 8.Kulkarni PS, Manjunath K, Agarkhedkar S, Group of SII IIV Studies Safety and immunogenicity of an adjuvanted whole virion, inactivated A (H1N1) 2009 influenza vaccine in young and elderly adults, and children. Vaccine. 2012;31:20–2. doi: 10.1016/j.vaccine.2012.10.081. [DOI] [PubMed] [Google Scholar]
- 9.Stittelaar KJ, Veldhuis Kroeze EJ, Rudenko L, Dhere R, Thirapakpoomanunt S, Kieny MP, Osterhaus AD. Efficacy of live attenuated vaccines against 2009 pandemic H1N1 influenza in ferrets. Vaccine. 2011;29:9265–70. doi: 10.1016/j.vaccine.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 10.Dhere R, Yeolekar L, Kulkarni P, Menon R, Vaidya V, Ganguly M, Tyagi P, Barde P, Jadhav S. A pandemic influenza vaccine in India: from strain to sale within 12 months. Vaccine. 2011;29(Suppl 1):A16–21. doi: 10.1016/j.vaccine.2011.04.119. [DOI] [PubMed] [Google Scholar]
- 11.Puig-Barberà J, Arnedo-Pena A, Pardo-Serrano F, Tirado-Balaguer MD, Pérez-Vilar S, Silvestre-Silvestre E, Calvo-Mas C, Safont-Adsuara L, Ruiz-García M, Surveillance and Vaccine Evaluation Group during the autumn 2009 H1N1 pandemic wave in Castellón, Spain Effectiveness of seasonal 2008-2009, 2009-2010 and pandemic vaccines, to prevent influenza hospitalizations during the autumn 2009 influenza pandemic wave in Castellón, Spain. A test-negative, hospital-based, case-control study. Vaccine. 2010;28:7460–7. doi: 10.1016/j.vaccine.2010.09.042. [DOI] [PubMed] [Google Scholar]
- 12.Van Buynder PG, Dhaliwal JK, Van Buynder JL, Couturier C, Minville-Leblanc M, Garceau R, Tremblay FW. Protective effect of single-dose adjuvanted pandemic influenza vaccine in children. Influenza Other Respir Viruses. 2010;4:171–8. doi: 10.1111/j.1750-2659.2010.00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skowronski DM, De Serres G, Crowcroft NS, Janjua NZ, Boulianne N, Hottes TS, Rosella LC, Dickinson JA, Gilca R, Sethi P, et al. Canadian SAVOIR Team Association between the 2008-09 seasonal influenza vaccine and pandemic H1N1 illness during Spring-Summer 2009: four observational studies from Canada. PLoS Med. 2010;7:e1000258. doi: 10.1371/journal.pmed.1000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savulescu C, Valenciano M, de Mateo S, Larrauri A, cycEVA Study Team Estimating the influenza vaccine effectiveness in elderly on a yearly basis using the Spanish influenza surveillance network--pilot case-control studies using different control groups, 2008-2009 season, Spain. Vaccine. 2010;28:2903–7. doi: 10.1016/j.vaccine.2010.01.054. [DOI] [PubMed] [Google Scholar]
- 15.Fleming DM, Andrews NJ, Ellis JS, Bermingham A, Sebastianpillai P, Elliot AJ, Miller E, Zambon M. Estimating influenza vaccine effectiveness using routinely collected laboratory data. J Epidemiol Community Health. 2010;64:1062–7. doi: 10.1136/jech.2009.093450. [DOI] [PubMed] [Google Scholar]
- 16.Belongia EA, Kieke BA, Donahue JG, Greenlee RT, Balish A, Foust A, Lindstrom S, Shay DK, Marshfield Influenza Study Group Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004-2005 season to the 2006-2007 season. J Infect Dis. 2009;199:159–67. doi: 10.1086/595861. [DOI] [PubMed] [Google Scholar]
- 17.Song JY, Cheong HJ, Heo JY, Noh JY, Choi WS, Park DW, Lee J, Jeong HW, Kee SY, Kim WJ. Effectiveness of the pandemic influenza A/H1N1 2009 monovalent vaccine in Korea. Vaccine. 2011;29:1395–8. doi: 10.1016/j.vaccine.2010.12.050. [DOI] [PubMed] [Google Scholar]
- 18.Hardelid P, Fleming DM, McMenamin J, Andrews N, Robertson C, SebastianPillai P, Ellis J, Carman W, Wreghitt T, Watson JM, et al. Effectiveness of pandemic and seasonal influenza vaccine in preventing pandemic influenza A(H1N1)2009 infection in England and Scotland 2009-2010. Euro Surveill. 2011;16:19763. [PubMed] [Google Scholar]
- 19.Mahmud S, Hammond G, Elliott L, Hilderman T, Kurbis C, Caetano P, Van Caeseele P, Kettner J, Dawood M. Effectiveness of the pandemic H1N1 influenza vaccines against laboratory-confirmed H1N1 infections: population-based case-control study. Vaccine. 2011;29:7975–81. doi: 10.1016/j.vaccine.2011.08.068. [DOI] [PubMed] [Google Scholar]
- 20.Fielding JE, Grant KA, Garcia K, Kelly HA. Effectiveness of seasonal influenza vaccine against pandemic (H1N1) 2009 virus, Australia, 2010. Emerg Infect Dis. 2011;17:1181–7. doi: 10.3201/eid1707.101959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:36–44. doi: 10.1016/S1473-3099(11)70295-X. [DOI] [PubMed] [Google Scholar]
- 22.Puig-Barberà J, Díez-Domingo J, Arnedo-Pena A, Ruiz-García M, Pérez-Vilar S, Micó-Esparza JL, Belenguer-Varea A, Carratalá-Munuera C, Gil-Guillén V, Schwarz-Chavarri H. Effectiveness of the 2010-2011 seasonal influenza vaccine in preventing confirmed influenza hospitalizations in adults: a case-case comparison, case-control study. Vaccine. 2012;30:5714–20. doi: 10.1016/j.vaccine.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fielding JE, Grant KA, Papadakis G, Kelly HA. Estimation of type- and subtype-specific influenza vaccine effectiveness in Victoria, Australia using a test negative case control method, 2007-2008. BMC Infect Dis. 2011;11:170. doi: 10.1186/1471-2334-11-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J, Xu F, Lu L, Lu M, Miao L, Gao T, Ji W, Suo L, Liu D, Ma R, et al. Safety and effectiveness of a 2009 H1N1 vaccine in Beijing. N Engl J Med. 2010;363:2416–23. doi: 10.1056/NEJMoa1006736. [DOI] [PubMed] [Google Scholar]
- 25.CDC protocol of realtime RTPCR for influenza A (H1N1). Geneva: World Health Organization, (2009). [Accessed on 20 July 2009]. Available from: http://www.who.int/csr/resources/publications/swineflu/CDCRealtimeRTPCR_SwineH1Assay-2009_20090428.pdf