Abstract
Palivizumab monthly injections throughout the RSV season prevent severe respiratory syncytial virus (RSV) disease in preterm infants ≤ 35 wGA. However, some RSV guidelines currently recommend stopping palivizumab after 3 months of age in the midst of the RSV season. This article evaluates the need for full-season dosing by reviewing the pharmacokinetic properties of palivizumab and RSV hospitalization (RSVH) risk as a function of chronologic age. Precise human palivizumab protective levels are not established. Clinical trials show significant interpatient variability in palivizumab serum trough concentrations. Partial season dosing is associated with increased risk of RSVH. For late-preterm infants, data suggest that the risk of RSVH remains elevated through at least 6 months of age. Monthly, full-season palivizumab dosing provides the only empirically proven protection from RSVH. In conclusion, late-preterm infants are at significant risk for RSVH through at least 6 months of age and would benefit from dosing throughout the RSV season.
Keywords: palivizumab, preterm infants, monthly dosing, guidelines, respiratory syncytial virus
Introduction
Palivizumab, an IgG1 monoclonal antibody, is approved for the prevention of serious lower respiratory tract disease caused by respiratory syncytial virus (RSV) in pediatric patients at high risk of RSV disease. Palivizumab provides passive immunity through a dosing regimen of 15 mg/kg monthly throughout the RSV season. To control drug costs, some guideline bodies have attempted to amend this approved dosing regimen. Current American Academy of Pediatrics (AAP) guidelines1 for RSV prophylaxis with palivizumab in the US and the British Columbia Immunoprophylaxis Committee guidelines2 in British Columbia, Canada, recommend less than full-season dosing for some or all high-risk infants. These variations generally assume that the RSV protection threshold for palivizumab is well defined, is similar for all patients, and that palivizumab behaves pharmacokinetically like a small molecule drug or medication. The variations in the dosing of palivizumab are based on the belief that the risk of RSV hospitalization (RSVH) decreases significantly after 3 mo of age1 or that, because of higher mean serum palivizumab levels after multiple doses, high-risk infants will maintain their protection for longer than a month following the last dose.3
This review will discuss the evidence for monthly dosing of palivizumab throughout the RSV season from a pharmacokinetic perspective and with regard to the risk of RSVH in high-risk infants by chronologic age during the season.
Historical Overview of Guidelines
In 2003, AAP guidelines for RSV prophylaxis in high-risk infants recommended that infants requiring medical therapy due to chronic lung disease (CLD) or congenital heart disease (CHD) and preterm infants born ≤35 wk gestational age (wGA) receive ≤5 successive monthly doses of palivizumab to ensure continual coverage throughout the RSV season.4 In 2009, the AAP guidelines were revised for preterm infants 32–34 wGA to recommend dosing only through 90 d of age, with a maximum of 3 doses, regardless of when during the season the third dose was given.5 These new recommendations were based on the rationale that “once an infant has passed 90 days of age, the risk of hospitalization attributable to RSV lower respiratory tract disease is reduced.”5
In Canada, the British Columbia Immunoprophylaxis Committee revised its guidelines for RSV prophylaxis in 2012 to state that all high-risk infants are eligible to receive a maximum of 4 doses of palivizumab; in 2013, the exception of qualifying children with certain cardiac conditions, who may receive a maximum of 5 doses, was added.”2 Preterm infants 29–34 wGA are further restricted to a maximum of 3 doses because they are viewed as lower risk than preterm infants ≤28 wGA and infants with CLD or CHD. These variations in dosing appear to be driven by cost-reduction goals and supported by a retrospective literature review comprising preterm infants over a range of gestational ages.6 This analysis reported that therapeutic levels of palivizumab result in protective trough levels of 30 µg/mL for 152–171 d after the fourth dose and 120–138 d after the third dose and that the “calculated average half life for the first PVZ IM (palivizumab delivered intramuscularly - Ed.) dose ranged from 23 to 32 days but, by the fourth dose, the average half life increased to a range of 26 to 40 days.”6
Passive Vs. Active Immunity
Unlike a vaccine that induces long-lasting immunity, palivizumab is an exogenous antibody that degrades over time and needs to be replenished monthly to allow for continued protection against RSV.7 Vaccines are recommended to be given to preterm infants at the same dose and same chronologic age as term infants.8 Even though immunogenicity may be decreased, antibody concentrations usually remain protective. Vaccine guidelines that are consistent for all infants (term or preterm) limit confusion with healthcare providers and parents. The same reasoning for guideline consistency can be applied to RSV prophylaxis recommendations for preterm infants.
Palivizumab Pharmacokinetics
Nearly 30 y ago, it was reported that IgG1 antibodies generally have a half-life of approximately 20 d.9 RespiGam® (MedImmune), an intravenous immunoglobulin polyclonal antibody preparation, prevented severe RSV disease when given monthly to high-risk infants.10 Palivizumab was developed as a monoclonal IgG1 antibody to be given intramuscularly with the same monthly dosing. A preclinical cotton rat model demonstrated that animals with existing palivizumab mean serum concentrations of 25–30 µg/mL at the time of RSV challenge achieved a mean 2-log (99%) lower RSV pulmonary viral load compared with groups of sham-treated animals. Subsequently, a serum palivizumab concentration of 40 µg/mL was required to achieve this 2-log (99%) reduction in viral load in all animals.11 These data have been interpreted by some to define a protective serum concentration level of palivizumab of 40 µg/mL that could be extrapolated from the animal model into humans. However, other aspects of this model have not been shown to mirror human pathology of RSV, and a protective threshold in humans has not been established.12-14
A randomized, double-blind, placebo-controlled dose escalation trial in 62 high-risk infants demonstrated that infants who received 15 mg/kg of palivizumab were more likely to have trough concentrations (30 d following the first and second dose) >40 µg/mL compared with infants who received 10 mg/kg: 71% and 86% vs. 25% and 38%, for the first and second doses, respectively. The mean palivizumab serum half-life for intravenous administration was 20 d.15
A subsequent open-label dose-escalation trial was conducted in 65 high-risk infants using 5, 10, or 15 mg/kg of palivizumab given in ≤5 doses intramuscularly.16 In this small study, at 15 mg/kg of palivizumab, a majority of infants had trough concentrations of >40 µg/mL 30 d after each of 5 monthly injections. The mean serum half-life for palivizumab given intramuscularly was 24 d.16 Based on a combination of these data from the cotton rat model, dose-escalation studies, and the observed half-life, palivizumab 15 mg/kg administered monthly was chosen as the appropriate regimen for study in all subsequent clinical trials evaluating efficacy.
A randomized, double-blind, placebo-controlled trial of palivizumab was conducted in 1502 infants with prematurity (≤35 wGA) or children aged ≤24 mo with bronchopulmonary dysplasia (BPD)17 with the primary endpoint of RSVH prevention. All subjects were administered palivizumab 15 mg/kg monthly for 5 successive doses, with the first dose starting at the beginning of the RSV season. Palivizumab prophylaxis resulted in an overall 55% relative reduction in RSVH (placebo, 10.6% vs palivizumab, 4.8%; P < 0.001). Preterm infants without BPD who received palivizumab had a 78% relative reduction in RSVH (placebo, 8.1% vs palivizumab, 1.8%; P < 0.001).17 The same dosing regimen proved effective in a second randomized, placebo-controlled trial in 1287 children aged ≤24 mo with hemodynamically significant CHD.18 In this study, a 45% relative reduction in RSVH was observed (placebo, 9.7% vs palivizumab, 5.3%).
Although the monthly palivizumab dosing regimen was based on the pharmacokinetic studies described above, the selection of 5 monthly doses for the clinical trial simply reflected the average length of the RSV season across all sites in the US, Canada, and the United Kingdom. This dosing schedule, used despite the known variation in timing at regional/local levels, provided a simplified study design across an international dosing protocol. The US RSV season at the national level is typically defined as November through March.19 Consistent with prior studies, serum trough concentrations (± SD) in the aforementioned palivizumab study were found to be 37 ± 21, 57 ± 41, 68 ± 51, and 72 ± 50 µg/mL before doses 2, 3, 4, and 5, respectively.17 Although mean serum trough concentrations were higher with each dose, the increase in mean concentrations is smaller with each successive dose as steady-state is reached. This broad range of palivizumab serum trough concentrations highlights the wide interpatient variability observed in subjects: 25% of infants had serum trough concentrations <40 µg/mL before dose 5.20 An important limitation of these data was that each subject had a level drawn at only 2 time points: the first before dose 5 and the second at a single time point before dose 2, 3, or 4.17
Recently, a population model was developed to better describe the pharmacokinetics of palivizumab to address these misconceptions. The model was derived from analyzing all palivizumab levels in clinical studies in children and adults (n = 1800).21 In total, 1684 of the 1800 subjects (93.5%) were children. These 1684 pediatric subjects had a gestational age ranging from 22–40 wGA and a range of body weights from 2–15 kg. In all groups modeled, there was close agreement between serum concentrations observed in the study and those predicted by the model.17 The model confirmed the significant interpatient variability of palivizumab serum trough concentrations, which prevents prediction of the serum concentration in an individual infant. The model also demonstrated that the half-life of palivizumab remains constant over the dosing period.21
The aforementioned pharmacokinetic model investigated different covariates of gestational age, chronologic age, and weight and found that only the weight of the infant was a significant variable in determining the pharmacokinetics of palivizumab.21 These 2 important concepts—interpatient variability and a constant half-life—support the need for monthly weight-based dosing of palivizumab throughout the RSV season in all patient groups. A related analysis comparing palivizumab concentrations of subjects who receive 5 doses with those only receiving 3 doses, demonstrated that if palivizumab dosing is stopped after 3 doses, serum concentrations during the fourth and fifth month will fall below the fifth percentile concentration seen in infants receiving 5 monthly doses in 52% and 85% of infants, respectively.20
Clinical Impact of Full-Season Dosing Vs. Missed or Delayed Dosing
Given substantial interpatient variability and the lack of a well-defined protective antibody concentration threshold, it is important to focus on the clinical impact of missed, delayed, or inconsistent dosing. The Palivizumab Outcomes Registry prospectively followed 19 548 high-risk infants who received palivizumab during 4 RSV seasons (2000–2001 to 2003–2004). The analysis of this registry used 2 definitions of compliance: one based on number of expected doses of palivizumab (eg, a patient receiving the first dose in November would be compliant if receiving 5 doses total) and the other including those infants receiving ≥2 doses of palivizumab who received all doses within 35 d of the previous dose. Analysis of compliance defined by the number of expected doses did not reveal statistically significant differences between compliant and noncompliant populations. There was, however, a significantly higher RSV breakthrough hospitalization rate in infants who did not receive all doses within 35 d compared with those who did (1.65% vs 1.16%, P = 0.007).22
A pharmacy-dispensing analysis in which 10 390 infants who received palivizumab were evaluated23 showed that when palivizumab compliance was defined as dosing gaps of ≤35 d, RSVH breakthrough rates were higher in noncompliant vs. compliant infants (1.4% vs 3.1%; odds ratio, 2.2 [95% CI: 1.4, 3.5]; P < 0.001). These results were also observed in 2 recent retrospective cohort studies evaluating outcomes in insurance claims databases during the 6 RSV seasons between 2003–2004 and 2008–2009.24,25
Risk of RSVH During Infancy as a Function of Chronologic Age
Pharmacokinetic data, dosing compliance, and length of dosing regimens are important factors affecting RSVH risk; however, the most confusing aspects of the recent shift in recommendations by national bodies to change dosing regimen comes in the interpretation of chronologic age.
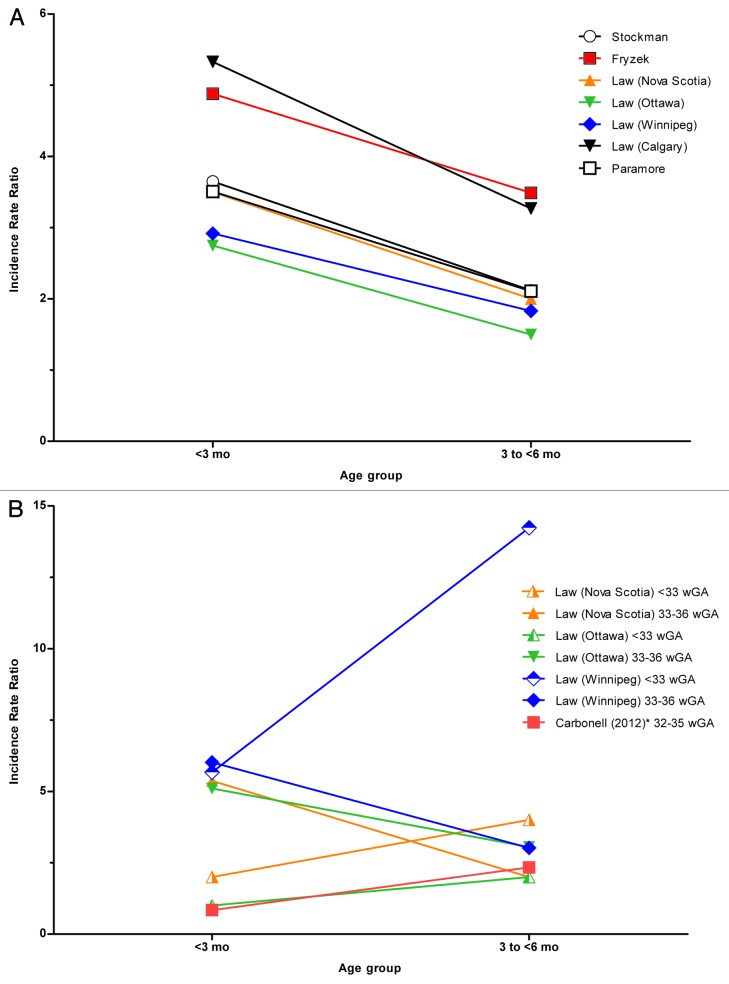
To further assess the confusion surrounding age, we asked whether there was a difference in risk of RSVH by chronologic age for preterm infants compared with term infants. This question directly affects the US recommendation regarding the number of palivizumab doses for preterm infants 32–34 wGA. Population studies involving predominantly term infants (Table 1) found that the highest RSVH rates occurred in infants aged <3 mo (Fig. 1).26,29-32 Several studies have examined RSVH risk by chronologic age in preterm infants (Table 2). These studies are generally of a different design than those evaluating all infants and use a prospective cohort design in which age at the start of the RSV season was the primary focus. When infants were followed up through the entire RSV season, studies found that the highest risk lies in preterm infants aged <3 mo at the start of the RSV season.36-38 In studies in Spain and Canada, chronologic age ≤10 wk at the onset of the RSV season and birth in season served as independent risk factors for RSVH in infants 33–35 wGA.37-40 It is clear from these studies that age at the commencement of the season, and not simply age during the season, was the basis of investigation, and extrapolating to establish cut-off ages during the season would be suspect.
Table 1. All infants: RSV-related hospitalization rates as a function of chronologic age.
| Reference | Study design | Study population | RSV ascertainment | RSVH rates |
|---|---|---|---|---|
| Hall et al., 2013*27 | Prospective, population surveillance study from NVSN | US children <24 mo of age with ARI from 2000–2005 | Active testing by RT-PCR | Range, 13.5–25.9/1000 infants <3 mo† Range, 4.8–10.3/1000 infants 3 to <6 mo‡ Range, 3.4–5.6/1000 infants 6 to <9 mo§ Range, 2.9–3.8/1000 infants 9 to <12 mo|| |
| Stockman et al., 2012¶32 | Retrospective analysis of NHDS | US children <5 y of age with RSV-like coded hospitalization from 1997−2006 | ICD-9-CM codes for both RSV-specific and nonspecific (% calculated from NVSN data from Nov–Apr, 30% bronchiolitis and 20% pneumonia codes) | 48.9/1000 infants ≤2 mo (95% CI: 36.6, 61.2) 28.4/1000 infants 3–5 mo (95% CI: 21.3, 35.5) 13.4/1000 infants 6–11 mo (95% CI: 10.7, 16.1) |
| Rossi et al., 2007#31 | Retrospective case-control study | Italian children <4 y of age with lower respiratory tract infection from 2000–2004 | Active testing during study years with EIA | Age <3 mo associated with increased RSVH (OR, 8.46 [95% CI: 3.09, 23.19]) 3–5 mo (OR, 4.15 [95% CI: 1.51, 11.45]) 6–11 mo (OR, 2.47 [95% CI: 0.88, 6.93]; P < 0.0001) ≥12 mo (referent) |
| Law et al., 1998**29 | Prospective cohort study | Canadian infants ≤12 mo of age from 1993–1994 in 4 provinces | Active testing by EIA, DFA, and culture RSVH rates vary by Canadian province, which accounts for wide range of rates |
Range, 1.6–3.5/1000 infants <3 mo Range, 0.98–2.2/1000 infants 3 to <6 mo Range, 0.3–1.2/1000 6 to <12 mo |
| Holman et al., 2004††28 | Retrospective analysis of NHDS | US children <12 mo of age with RSV-coded hospitalization from 2000−2001 | ICD-9-CM codes specific for RSV, RSV bronchiolitis, or RSV pneumonia | 41.9/1000 infants <6 mo (95% CI: 31.7, 52.1) 12.8/1000 infants 6–11 mo (95% CI: 9.6, 16.0) |
| Fryzek et al., 2011††‡‡26 | Retrospective analysis of NHDS | US children <24 mo of age with RSV-coded hospitalization from 1998−2006 | ICD-9-CM codes specific for RSV, RSV bronchiolitis, or RSV pneumonia | RR, 4.88 for infants <3 mo RR, 3.49 for infants 3–6 mo >6 to <12 mo (referent) |
| Paramore et al., 2004§§30 | Retrospective analysis of 3 US federally funded databases | US children <5 y of age with RSV-coded hospitalization in 2000 | ICD-9-CM codes specific for RSV, RSV bronchiolitis, or RSV pneumonia | 32.03/1000 infants <3 mo 19.26/1000 infants 3–6 mo 9.12/1000 infants 6–12 mo (referent) |
| Vicente et al., 2003††33 | Retrospective analysis of discharge records from a public hospital | Spanish children <5 y of age with hospitalization from 1996–2000 | ICD-9-CM codes specific for bronchiolitis and acute bronchiolitis | 46.7/1000 infants <3 mo 36.8/1000 infants <6 mo 25.5/1000 infants <12 mo (referent) |
ARI, acute respiratory infection; DFA, direct fluorescent antibody; EIA, enzyme immunoassay; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; NHDS, National Hospital Discharge Survey; NVSN, New Vaccine Surveillance Network; OR, odds ratio; RR, relative rates; RSV, respiratory syncytial virus; RSVH, respiratory syncytial virus hospitalization; RT-PCR, reverse transcriptase polymerase chain reaction. *10% of infants were preterm. †Confidence intervals for the 3-mo age groupings were not published. Hall et al.27 defined a child as being <1 mo of age if the child had not yet reached age 1 mo, 1 mo of age if the child was 1 to <2 mo of age, etc. The following hospitalization rates per 1000 children and 95% CI (rate [95% CI]) for the individual month age groups were reported from this study as: <1 mo, 13.5 (10.3, 17.1); 1 mo, 25.9 (21.3, 30.8); 2 mo, 14.3 (11.1, 17.8). ‡Hospitalization rates and 95% CI (rate [95% CI]): 3 mo, 10.3 (7.7, 13.5); 4 mo, 8.9 (6.3, 11.8); 5 mo, 4.8 (2.9, 7.0). §Hospitalization rates and 95% CI (rate [95% CI]): 6 mo, 4.1 (2.5, 6.2); 7 mo, 5.6 (3.6, 8.0); 8 mo, 3.4 (1.8, 5.2). ||Hospitalization rates and 95% CI (rate [95% CI]): 9 mo, 3.8 (2.1, 6.0); 10 mo, 3.7 (2.0, 5.7); 11 mo, 2.9 (1.5, 4.8). 4% of infants had congenital heart disease, chronic respiratory distress, or premature birth. #11.7% of infants were preterm. **18% of infants were preterm. ††Study did not mention whether data included information from preterm infants. ‡‡Additional data were provided by JP Fryzek by personal communication on June 3, 2013. §§0.3% of infants were preterm.
Figure 1. (A) Incidence Rate Ratio (Term Infants). (B) Incidence Rate Ratio (Preterm Infants). Legend reflects source of data. Ratio calculated by dividing reported incidence rate by referent incidence rate. No rates were reported for Calgary for preterm infants >3 mo of age. Data included in this figure were calculated from all studies available in the English-language medical literature (1998–2012, National Library of Medicine), which contained the following: data available to enable a calculation of the incidence rate ratio, data for infants <3 mo of age, data for infants 3 to <6 mo of age, and a referent group defined as infants 6–12 mo of age. *Incidence rate ratio only available for those born before the start of the RSV season; the exposure time varied for those born during the RSV season.
Table 2. Preterm infants: RSV-related hospitalization rates as a function of chronologic age.
| Study design | Study population | RSV ascertainment | RSVH rates | |
|---|---|---|---|---|
| Boyce et al., 200034 | Retrospective cohort study of TN Medicaid data files | Children <3 y of age enrolled in TN Medicaid: 1989–1993 | ICD-9-CM codes for both RSV-specific and nonspecific bronchiolitis, pneumonia from Nov–Apr | 79.8/1000 for infants 33–35 wGA <6 mo of age (IRR, 1.8 [95% CI: 1.5, 2.1]) 34.5/1000 for infants 33–35 wGA 6 to <12 mo of age (IRR, 2.3 [95% CI: 1.7, 3.0]) 44.1/1000 term infants <6 mo of age (referent) 15/1000 term infants 6 to <12 mo of age (referent) |
| Carbonell-Estrany et al., 2001*36 | Prospective, observational study | ≤32 wGA, ≤12 mo of age, Spain: 1999–2000 | Passive testing by EIA, DFA, and culture | ≤32 wGA, >3 mo of age at RSV season start associated with decrease in RSVH (OR, 0.44 [95% CI: 0.25, 0.77]; P = 0.004) |
| Figueras-Aloy et al., 2004*37 | Prospective case-control study | 33–35 wGA, ≤12 mo of age, Spain: 2002–2003 | Passive testing by EIA, DFA, and culture | 33–35 wGA, ≤10 wk of age at RSV season start associated with increase in RSVH (OR, 3.95 [95% CI: 2.65, 5.90]) |
| Figueras-Aloy et al., 2008*38 | Prospective case-control study | 32–35 wGA, ≤12 mo of age, Spain: 2005–2007 | Passive testing by EIA, DFA, and culture | 32–35 wGA, ≤10 wk of age at RSV season start associated with increase in RSVH (OR, 2.99 [95% CI: 2.23, 4.01]) |
| Law et al., 199829 | Prospective cohort study | Canadian infants ≤12 mo of age from 1993–1994 with data only regarding preterm infants in study | Active testing by EIA, DFA, and culture RSVH rates vary by Canadian province, which accounts for wide range of rates |
Range, 5.0–16.9/1000 infants <33 wGA <3 mo of age Range, 10.0–42.4/1000 for infants <33 wGA 3 to <6 mo of age Range, 2.98–5.0/1000 for infants <33 wGA 6 to <12 mo of age Range, 6.7–25.3/1000 for infants 33–36 wGA <3 mo of age Range, 5.4–12.7/1000 for infants 33–36 wGA 3 to <6 mo of age Range, 1.9–4.2/1000 for infants 33–36 wGA 6 to <12 mo of age |
| Law et al., 2004*39 | Prospective, observational study | 33–35 wGA, ≤12 mo of age, Canada: 2001–2003 | Rapid antigen or viral culture | Birth Nov–Jan associated with increased risk of RSVH (OR, 4.89 [95% CI: 2.57, 9.29]) |
| Carbonell et al., 2012†35 | Prospective, 2-cohort study | 32–35 wGA; discharged during RSV season or ≤6 mo of age at start of RSV season, Spain: 2005–2006 and 2006–2007 RSV seasons | DFA, EIA, or culture | Born inside RSV season Infants 32–35 wGA <3 mo of age: 32.2/1000 Infants 32–35 wGA <6 mo of age: 38.3/1000 Born outside RSV season Infants 32–35 wGA <3 mo of age: 8.5/1000 Infants 32–35 wGA <6 mo of age: 32.2/1000 Infants 32–35 wGA >6 mo of age (referent): 10.0/1000 |
DFA, direct fluorescent antibody; EIA, enzyme immunoassay; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; IRR, incidence rate ratio; OR, odds ratio; RSV, respiratory syncytial virus; RSVH, respiratory syncytial virus hospitalization; wGA, weeks gestational age. *Study group or publication referenced by American Academy of Pediatrics Committee on Infectious Diseases 2009 Policy Statement for Palivizumab.5 †Additional data were provided by BS Rodgers-Gray by personal communication on July 3, 2013.
An important bias to consider in evaluating these studies is exposure time. Infants born during the RSV season will have less exposure time than those born at the onset of the RSV season. Many of the infants <3 mo at season start would have been aged >3 mo when hospitalized during the RSV season. Few studies have examined the risk of RSVH in preterm infants based on age at time of event. A large Canadian study in 4 different provinces compared RSVH of term infants with preterm infants as a function of age during the season, adjusting for exposure time. This study found that although term infants seem to be at greatest risk for RSVH in the first 3 mo (Table 1 and Fig. 1), late-preterm infants had elevated risk through 6 mo and a similar risk to that of term infants in the 6- to 11-mo time frame.29 This study of preterm infants was not cited by the 2009 AAP guidelines in establishing their 90-d age cutoff. Preterm infants ≤32 wGA also showed increased risk in the first 3 mo but showed the highest rate in the 3- to 6-mo time period (Table 2 and Fig. 1).29 In another study of RSVH in the first year of life among preterm infants 32–35 wGA, RSV risk was examined as a function of chronologic age during the RSV season.35 Risk of RSVH was determined by chronologic age from birth through 12 mo of age for those born within and outside of the RSV season. The risk of RSVH in preterm infants 32–35 wGA extended to approximately 200 d (6.5 mo) of age. For those born outside the RSV season, the study found a median time to hospitalization of approximately 5 mo (148 d) of age compared with approximately 2 mo (58 d) of age for those born within the season.35 When current AAP eligibility criteria for 32–35 wGA were applied to the study population, only 52% of infants hospitalized with RSV were identified as candidates for RSV prophylaxis, with an overall predictive accuracy of 57%.
Physiological Reasons for Continued Risk of RSVH in Preterm Infants Through 6 Months of Age
There are clear physiological reasons why preterm infants maintain this recognized high risk of RSVH for extended periods significantly longer than term infants. Current knowledge in the field is that deficits in the immune system and lung development issues are responsible for sustained increased RSVH risk. Preterm infants have lower levels of circulating immune globulin, as transplacental maternal antibody transfer predominantly occurs in the third trimester.41 Additionally, a recent study showed that infants have lower absolute counts of all B- and T-cell subsets other than regulatory T cells.42 Preterm infants also have interrupted lung development impacting airway and alveolar development.43-45 Alveoli, which are important determinants of gas exchange, develop during the first 2 y of life in term infants.45 Preterm infants have very few fully developed alveoli at birth which are not consistently present until 36 wGA. Lack of alveoli with resulting poor airway exchange and ineffective tethering leading to airway collapse has been cited as a mechanism for abnormal lung development in preterm infants 32–36 wGA.45 Examination of lung function in 31 preterm infants 33–36 wGA without respiratory disease found that, when evaluated at corrected term age, these healthy late-preterm infants had decreased respiratory compliance and increased vascular resistance compared with term infants.44 In the first few months of life, and at a 1-y follow-up, preterm infants 30–34 wGA (n = 26) were found to have significantly lower forced expiratory flows which persisted into the second year of life compared with term infants (n = 24).43
Conclusion
The efficacy of monthly dosing with palivizumab to reduce hospitalization among preterm infants during the 5-mo RSV season has been firmly established in randomized trials and in clinical practice. Although there is substantial evidence supporting the need for full-season, monthly dosing with palivizumab, current treatment guidelines in the US and in British Columbia, Canada do not fully support this treatment regimen for all preterm infants. Because of a lack of a well-defined antibody correlate of RSV protection along with marked interpatient variability in serum palivizumab concentrations, it seems imprudent to over-extrapolate from successful clinical trials to recommend alternative dosing schemes. It is challenging to identify a protective serum level as palivizumab is used prophylactically and the RSV inoculum varies considerably among subjects. Like all monoclonal antibodies, palivizumab levels change with time adding to the difficulty of identifying a precise level that is associated with a reduction in RSV hospitalization.
While the endpoint for animal models is reduction of lung viral concentrations, the endpoint for infants is reduction of RSV hospitalization. These endpoints have some correlation, but within a group of children, we do not have the ability to precisely identify a human protective level for RSV hospitalization. For the above reasons, a protective level of palivizumab in humans has not been established. However, data are available from the initial randomized, placebo-controlled trial of palivizumab in preterm infants and infants with BPD regarding the relationship between disease severity and palivizumab levels at the time of RSV hospitalization; these data suggest that levels up to 92 µg/mL provide additional clinical benefit as evidenced by a lower proportion of RSV-hospitalized subjects requiring ICU admission.46
Palivizumab pharmacokinetic data highlight the significant decline in mean population serum concentrations that will occur if dosing is stopped midseason: a decline that would likely place preterm infants at increased risk for serious RSV disease if partial-season dosing was implemented. Epidemiologic data also provide evidence for the appropriateness of full-season dosing. Increased rates of breakthrough RSVH have been noted with infants who have received less than full-season dosing. Despite new recommendations for discontinuing dosing at 3 mo chronologic age, information supporting such actions is sparse. Limited data exist regarding the risk of severe RSV in preterm infants as a function of chronologic age during the RSV season, because most studies have evaluated risk as a function of age at season start. More data are needed regarding the risk of serious RSV disease among preterm infants by chronologic age, adjusting for infant-time exposure and age at the start of the season. Thus, evidence supporting the use of chronologic age as a criterion for discontinuing RSV prophylaxis midseason is lacking, given that RSVH risk in late-preterm infants appears to remain increased through at least 6 mo of life. The impact of leaving vulnerable infants at risk with this unsupported change in protective strategies has yet to be determined.
Discloure of Potential Conflicts of Interest
DM is an employee of MedImmune and receives stock options for AstraZeneca, the parent company of MedImmune. PC has received payment for lectures, including service on speakers bureaus, from AbbVie. JD has consulted (including advisory boards) for Alios, Alnylam, AstraZeneca, Crucell, the Genomics Institute of the Novartis Research Foundation, Gilead, Janssen, MedImmune, MicroDose, and Novartis. All consultancies, with the exception of that for MedImmune, were to develop/discover new therapies or preventions for RSV. JD has provided expert testimony on behalf of the Henry M Jackson Foundation for the Advancement of Military Medicine and Wilson, Kehoe, Winningham, LLC. JD received grant funding to his institution on his behalf from Alios, Alnylam, AstraZeneca, Gilead, and MicroDose. JD has received honoraria for lectures, including service on speakers bureaus, from MedImmune.
Acknowledgments
This study was sponsored by MedImmune. The authors would like to thank Christopher Ambrose, MD, for helpful suggestions and a critical review of the manuscript. We would also like to thank Dr Jon P Fryzek and Dr Barry S Rodgers-Gray for providing additional data for analysis. Editorial assistance was provided by Complete Healthcare Communications, Inc. and was funded by MedImmune.
Glossary
Abbreviations:
- AAP
American Academy of Pediatrics
- BPD
bronchopulmonary dysplasia
- CHD
congenital heart disease
- CLD
chronic lung disease
- RSV
respiratory syncytial virus
- RSVH
respiratory syncytial virus hospitalization
- wGA
weeks gestational age
References
- 1.American Academy of Pediatrics. Respiratory syncytial virus. In: Pickering LK, Baker CJ, Kimberlin DW, Long SS, eds. Red Book: 2012 Report of the Committee on Infectious Diseases. 29th ed. Elk Grove Village, IL: American Academy of Pediatrics, 2012:609-17. [Google Scholar]
- 2.Child Health BC. BC guidelines for RSV infection immunoprophylaxis: 2013-2014 RSV season. [cited 2014 Feb 20]. Available from: http://www.childhealthbc.ca/guidelines/category/17-rsv-immuno-prophylaxis-guidelines
- 3.Haller AA, Mitiku M, MacPhail M. Bovine parainfluenza virus type 3 (PIV3) expressing the respiratory syncytial virus (RSV) attachment and fusion proteins protects hamsters from challenge with human PIV3 and RSV. J Gen Virol. 2003;84:2153–62. doi: 10.1099/vir.0.19079-0. [DOI] [PubMed] [Google Scholar]
- 4.American Academy of Pediatrics Committee on Infectious Diseases and Committee on Fetus and Newborn Revised indications for the use of palivizumab and respiratory syncytial virus immune globulin intravenous for the prevention of respiratory syncytial virus infections. Pediatrics. 2003;112:1442–6. doi: 10.1542/peds.112.6.1442. [DOI] [PubMed] [Google Scholar]
- 5.Committee on Infectious Diseases From the American Academy of Pediatrics: Policy statements--Modified recommendations for use of palivizumab for prevention of respiratory syncytial virus infections. Pediatrics. 2009;124:1694–701. doi: 10.1542/peds.2009-2345. [DOI] [PubMed] [Google Scholar]
- 6.Solimano A, Kwan E. Revisiting palivizumab (PVZ) dosing recommendations based on published pharmacokinetics data. Presented at: Pediatric Academic Societies; April 28-May 1, 2012; Boston, MA. [Google Scholar]
- 7.Synagis® (palivizumab). Full Prescribing Information, MedImmune, LLC., Gaithersburg, MD, 2013. [Google Scholar]
- 8.Saari TN, American Academy of Pediatrics Committee on Infectious Diseases Immunization of preterm and low birth weight infants. Pediatrics. 2003;112:193–8. doi: 10.1542/peds.112.1.193. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton RG. Human IgG subclass measurements in the clinical laboratory. Clin Chem. 1987;33:1707–25. [PubMed] [Google Scholar]
- 10.The PREVENT Study Group Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics. 1997;99:93–9. doi: 10.1542/peds.99.1.93. [DOI] [PubMed] [Google Scholar]
- 11.Johnson S, Oliver C, Prince GA, Hemming VG, Pfarr DS, Wang SC, Dormitzer M, O’Grady J, Koenig S, Tamura JK, et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis. 1997;176:1215–24. doi: 10.1086/514115. [DOI] [PubMed] [Google Scholar]
- 12.Bem RA, Domachowske JB, Rosenberg HF. Animal models of human respiratory syncytial virus disease. Am J Physiol Lung Cell Mol Physiol. 2011;301:L148–56. doi: 10.1152/ajplung.00065.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeVincenzo JP. A new direction in understanding the pathogenesis of respiratory syncytial virus bronchiolitis: how real infants suffer. J Infect Dis. 2007;195:1084–6. doi: 10.1086/512622. [DOI] [PubMed] [Google Scholar]
- 14.Welliver TP, Garofalo RP, Hosakote Y, Hintz KH, Avendano L, Sanchez K, Velozo L, Jafri H, Chavez-Bueno S, Ogra PL, et al. Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis. 2007;195:1126–36. doi: 10.1086/512615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Subramanian KN, Weisman LE, Rhodes T, Ariagno R, Sánchez PJ, Steichen J, Givner LB, Jennings TL, Top FH, Jr., Carlin D, et al. Safety, tolerance and pharmacokinetics of a humanized monoclonal antibody to respiratory syncytial virus in premature infants and infants with bronchopulmonary dysplasia. MEDI-493 Study Group. Pediatr Infect Dis J. 1998;17:110–5. doi: 10.1097/00006454-199802000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Sáez-Llorens X, Castaño E, Null D, Steichen J, Sánchez PJ, Ramilo O, Top FH, Jr., Connor E. Safety and pharmacokinetics of an intramuscular humanized monoclonal antibody to respiratory syncytial virus in premature infants and infants with bronchopulmonary dysplasia. The MEDI-493 Study Group. Pediatr Infect Dis J. 1998;17:787–91. doi: 10.1097/00006454-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 17.The IMpact-RSV Study Group Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–7. doi: 10.1542/peds.102.3.531. [DOI] [PubMed] [Google Scholar]
- 18.Feltes TF, Cabalka AK, Meissner HC, Piazza FM, Carlin DA, Top FH, Jr., Connor EM, Sondheimer HM, Cardiac Synagis Study Group Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. 2003;143:532–40. doi: 10.1067/S0022-3476(03)00454-2. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC) Respiratory syncytial virus--United States, July 2007-June 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1203–6. [PubMed] [Google Scholar]
- 20.La Via WV, Notario GF, Yu XQ, Sharma S, Noertersheuser PA, Robbie GJ. Three monthly doses of palivizumab are not adequate for 5-month protection: A population pharmacokinetic analysis. Pulm Pharmacol Ther. 2013;26:666–71. doi: 10.1016/j.pupt.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 21.Robbie GJ, Zhao L, Mondick J, Losonsky G, Roskos LK. Population pharmacokinetics of palivizumab, a humanized anti-respiratory syncytial virus monoclonal antibody, in adults and children. Antimicrob Agents Chemother. 2012;56:4927–36. doi: 10.1128/AAC.06446-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frogel M, Nerwen C, Cohen A, VanVeldhuisen P, Harrington M, Boron M, Palivizumab Outcomes Registry Group Prevention of hospitalization due to respiratory syncytial virus: results from the Palivizumab Outcomes Registry. J Perinatol. 2008;28:511–7. doi: 10.1038/jp.2008.28. [DOI] [PubMed] [Google Scholar]
- 23.Berger J, Fensterheim L, O’Rourke J. The importance of Synagis compliance in preventing hospitalizations. Pediatr Res. 2003;53:468A–9A. [abstract #2646] [Google Scholar]
- 24.Krilov LR, Weiner LB, Wade SW, Smith DM, Masaquel AS. Non-compliance with palivizumab and increased risk of respiratory syncytial virus hospitalization among a Medicaid population. Presented at: Pediatric Academic Societies Annual Meeting; April 28-May 1, 2012; Boston, MA. [Google Scholar]
- 25.Stewart DL, Ryan KJ, Seare JG, Pinsky B, Becker L, Frogel M. Association of RSV-related hospitalization and non-compliance with Palivizumab among commercially insured infants: a retrospective claims analysis. BMC Infect Dis. 2013;13:334. doi: 10.1186/1471-2334-13-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fryzek JP, Martone WJ, Groothuis JR. Trends in chronologic age and infant respiratory syncytial virus hospitalization: an 8-year cohort study. Adv Ther. 2011;28:195–201. doi: 10.1007/s12325-010-0106-6. [DOI] [PubMed] [Google Scholar]
- 27.Hall CB, Weinberg GA, Blumkin AK, Edwards KM, Staat MA, Schultz AF, Poehling KA, Szilagyi PG, Griffin MR, Williams JV, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics. 2013;132:e341–8. doi: 10.1542/peds.2013-0303. [DOI] [PubMed] [Google Scholar]
- 28.Holman RC, Curns AT, Cheek JE, Bresee JS, Singleton RJ, Carver K, Anderson LJ. Respiratory syncytial virus hospitalizations among American Indian and Alaska Native infants and the general United States infant population. Pediatrics. 2004;114:e437–44. doi: 10.1542/peds.2004-0049. [DOI] [PubMed] [Google Scholar]
- 29.Law B, Macdonald N, Langley J, Mitchell I, Stephens D, Wang E, Robinson J, Boucher F, McDonald J, Dobson S. Severe respiratory syncytial virus infection among otherwise healthy prematurely born infants: What are we trying to prevent? Paediatr Child Health. 1998;3:402–4. doi: 10.1093/pch/3.6.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paramore LC, Ciuryla V, Ciesla G, Liu L. Economic impact of respiratory syncytial virus-related illness in the US: an analysis of national databases. Pharmacoeconomics. 2004;22:275–84. doi: 10.2165/00019053-200422050-00001. [DOI] [PubMed] [Google Scholar]
- 31.Rossi GA, Medici MC, Arcangeletti MC, Lanari M, Merolla R, Paparatti UD, Silvestri M, Pistorio A, Chezzi C, Osservatorio RSV Study Group Risk factors for severe RSV-induced lower respiratory tract infection over four consecutive epidemics. Eur J Pediatr. 2007;166:1267–72. doi: 10.1007/s00431-007-0418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stockman LJ, Curns AT, Anderson LJ, Fischer-Langley G. Respiratory syncytial virus-associated hospitalizations among infants and young children in the United States, 1997-2006. Pediatr Infect Dis J. 2012;31:5–9. doi: 10.1097/INF.0b013e31822e68e6. [DOI] [PubMed] [Google Scholar]
- 33.Vicente D, Montes M, Cilla G, Perez-Yarza EG, Perez-Trallero E. Hospitalization for respiratory syncytial virus in the paediatric population in Spain. Epidemiol Infect. 2003;131:867–72. doi: 10.1017/S0950268803008926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boyce TG, Mellen BG, Mitchel EF, Jr., Wright PF, Griffin MR. Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. J Pediatr. 2000;137:865–70. doi: 10.1067/mpd.2000.110531. [DOI] [PubMed] [Google Scholar]
- 35.Carbonell X, Fullarton JR, Gooch KL, Figueras-Aloy J. The evolution of risk factors for respiratory syncytial virus-related hospitalisation in infants born at 32-35 weeks’ gestational age: time-based analysis using data from the FLIP-2 study. J Perinat Med. 2012;40:685–91. doi: 10.1515/jpm-2011-0248. [DOI] [PubMed] [Google Scholar]
- 36.Carbonell-Estrany X, Quero J, IRIS Study Group Hospitalization rates for respiratory syncytial virus infection in premature infants born during two consecutive seasons. Pediatr Infect Dis J. 2001;20:874–9. doi: 10.1097/00006454-200109000-00010. [DOI] [PubMed] [Google Scholar]
- 37.Figueras-Aloy J, Carbonell-Estrany X, Quero J, IRIS Study Group Case-control study of the risk factors linked to respiratory syncytial virus infection requiring hospitalization in premature infants born at a gestational age of 33-35 weeks in Spain. Pediatr Infect Dis J. 2004;23:815–20. doi: 10.1097/01.inf.0000136869.21397.6b. [DOI] [PubMed] [Google Scholar]
- 38.Figueras-Aloy J, Carbonell-Estrany X, Quero-Jiménez J, Fernández-Colomer B, Guzmán-Cabañas J, Echaniz-Urcelay I, Doménech-Martínez E, IRIS Study Group FLIP-2 Study: risk factors linked to respiratory syncytial virus infection requiring hospitalization in premature infants born in Spain at a gestational age of 32 to 35 weeks. Pediatr Infect Dis J. 2008;27:788–93. doi: 10.1097/INF.0b013e3181710990. [DOI] [PubMed] [Google Scholar]
- 39.Law BJ, Langley JM, Allen U, Paes B, Lee DS, Mitchell I, Sampalis J, Walti H, Robinson J, O’Brien K, et al. The Pediatric Investigators Collaborative Network on Infections in Canada study of predictors of hospitalization for respiratory syncytial virus infection for infants born at 33 through 35 completed weeks of gestation. Pediatr Infect Dis J. 2004;23:806–14. doi: 10.1097/01.inf.0000137568.71589.bd. [DOI] [PubMed] [Google Scholar]
- 40.Carbonell-Estrany X, Figueras-Aloy J, Law BJ, Infección Respiratoria Infantil por Virus Respiratorio Sincitial Study Group. Pediatric Investigators Collaborative Network on Infections in Canada Study Group Identifying risk factors for severe respiratory syncytial virus among infants born after 33 through 35 completed weeks of gestation: different methodologies yield consistent findings. Pediatr Infect Dis J. 2004;23(Suppl):S193–201. doi: 10.1097/01.inf.0000144664.31888.53. [DOI] [PubMed] [Google Scholar]
- 41.Yeung CY, Hobbs JR. Serum-gamma-G-globulin levels in normal premature, post-mature, and “small-for-dates” newborn babies. Lancet. 1968;1:1167–70. doi: 10.1016/S0140-6736(68)91865-5. [DOI] [PubMed] [Google Scholar]
- 42.Correa-Rocha R, Pérez A, Lorente R, Ferrando-Martínez S, Leal M, Gurbindo D, Muñoz-Fernández MÁ. Preterm neonates show marked leukopenia and lymphopenia that are associated with increased regulatory T-cell values and diminished IL-7. Pediatr Res. 2012;71:590–7. doi: 10.1038/pr.2012.6. [DOI] [PubMed] [Google Scholar]
- 43.Friedrich L, Pitrez PM, Stein RT, Goldani M, Tepper R, Jones MH. Growth rate of lung function in healthy preterm infants. Am J Respir Crit Care Med. 2007;176:1269–73. doi: 10.1164/rccm.200703-476OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McEvoy C, Venigalla S, Schilling D, Clay N, Spitale P, Nguyen T. Respiratory function in healthy late preterm infants delivered at 33-36 weeks of gestation. J Pediatr. 2013;162:464–9. doi: 10.1016/j.jpeds.2012.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colin AA, McEvoy C, Castile RG. Respiratory morbidity and lung function in preterm infants of 32 to 36 weeks’ gestational age. Pediatrics. 2010;126:115–28. doi: 10.1542/peds.2009-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Forbes ML, Kumar VR, Yogev R, et al. Association between palivizumab level and probability of respiratory syncytial virus admission to the pediatric intensive care unit among high-risk infants. Presented at: IDWeek; October 2-6, 2013; San Francisco, CA. [Google Scholar]