Abstract
We evaluated the effect of a β-propiolactone (BPL)-inactivated coxsackievirus A16 (CA16) vaccine, using three immunogenicity evaluation and two animal challenge systems. A CA16 virus strain, named 419, was used as the production strain. Another CA16 strain, named 1131, was isolated and used as the challenge strain in intracerebral inoculation of neonatal mice for the calculation of median lethal dose (LD50). In the passive and maternal antibody-protection challenge systems, all results indicated that the vaccine could protect mouse pups from lethal challenge with the CA16 virus. In the immunogenicity systems, three types of animal (mouse, rat, and cynomolgus monkey), were immunized with the 419/CA16 vaccine. The dose–effect relationship and the antibody-generation routine were described. The CA16 vaccine induced a more potent serum antibody effect in rat than in mouse. The serum antibody titer was detectable more than 63 days after the initial vaccination. We also identified tools to evaluate the effect of the BPL-inactivated CA16 vaccine.
Keywords: coxsackievirus A16, enterovirus, vaccine, challenge systems, immunogenicity
Introduction
Hand, foot, and mouth disease (HFMD) has reached epidemic proportions in Asia and the Pacific area.1,2 The etiology of this viral disease, which mainly infects children, is unclear. The major causative agents of HFMD include coxsackieviruses A4, A5, A8, A10, A16, B3, and B7, and enterovirus 71.3 Of these different enterovirus strains, coxsackievirus A16 (CA16) is one of the major causative organisms of HFMD.4,5 Even in cold countries such as Norway, CA16 has been found extensively in patients with HFMD.6 Although infection with CA16 virus mainly induces mild symptoms, damage to the central nervous system can also be a pathological feature of CA16 infection.7 Therefore, it is important to investigate the protective effect of CA16 vaccines for the control and prevention of HFMD.
CA16 is a member of the human enterovirus A species (genus Enterovirus, family Picornaviridae) and was first isolated in 1951.8 CA16 is classified as genotypes A, B, and C. Genotype A and B viruses co-circulated before the 21st century, while genotype B is the major epidemic strain. In the 21st century, the genotype C viruses increased and gradually replaced genotype B as the predominant CA16 genetic lineage in Asia.9,10 Although CA16 is generally considered a relatively benign pathogen, mainly causing HFMD, there have been some cases of very serious infection caused by this virus. For example, a 15-mo-old boy died of myocarditis and intractable shock caused by CA16 infection.11 Of the 234 patients with HFMD hospitalized in Shengjing Hospital, 92 were infected with CA16 and, of these, 19 developed nervous system damage, including 2 with brainstem encephalitis and 1 with acute flaccid paralysis.12 Currently, the lethal mechanism of CA16 virus infection in humans is unclear.
A number of formaldehyde-inactivated EV71 vaccines have been reported,13,14 but as yet, there has been no successful development of a CA16 vaccine. We utilized β-propiolactone (BPL), a potent and safe inactivator, to produce an inactivated vaccine for prevention of CA16 spread.15,16 Because BPL can be hydrolysed at 37 °C, it can be considered a no-residue material compared with formalin.
We obtained different CA16 strains from the Chinese Center for Disease Control and Prevention. Based on the results of cross-neutralization experiments (data not shown), one CA16 strain was chosen as the vaccine production strain, and named 419/CA16, while another CA16 strain, named 1131/CA16, was used as the challenge virus. The vaccine production strain 419/CA16 belonged to the C1 subtype, and the challenge strain 1131/CA16 belonged to the C3 subtype, classified according to the analogy method.17 By using a different CA16 genotype to perform the challenge protection assay, we hoped to show a cross-protection effect of the vaccine against different CA16 strains.
To assess the protective effect of the CA16 vaccine, we analyzed its immunogenicity in different animal challenge systems: mouse, rat, and monkey. The antibody production protocol in these species is described in the Materials and Methods section.
In order to confirm the protective effect of this vaccine against the CA16 virus, we also established animal challenge systems. Although the rat was found to be a more sensitive species than mouse in the immunogenicity testing assays, we chose the latter as the challenge mode for evaluation of the vaccine effect because there was a high abortion rate observed in the rat challenge system when pregnant dams were injected with the CA16 vaccine.
In this paper, we describe the protective efficacy of a BPL-inactivated vaccine derived from the C type strain of CA16 (CA16-C) that is currently prevalent in China. Through these experiments, we produced a valuable vaccine for resisting CA16 virus infection, and using our vaccine evaluation system, we identified the infection mechanism of this virus in the mouse.
Results
Vaccine production
The antigen was purified (see Materials and Methods), and the purity was determined by SDS-PAGE (Fig. 1A). The identity of the capsid protein VP1 was confirmed by western blotting (Fig. 1B). Electrophoretic bands representing the viral capsid protein at the published molecular weight positions were observed: ~33 kDa for VP1, ~28 kDa for VP2, and ~26 kDa for VP3.18 The integrity of the viral particle was confirmed by electron microscopy (Fig. 2), which showed that the particle size of the virion was almost 30 nm. All these results indicated that this virus could be used as the vaccine production material.
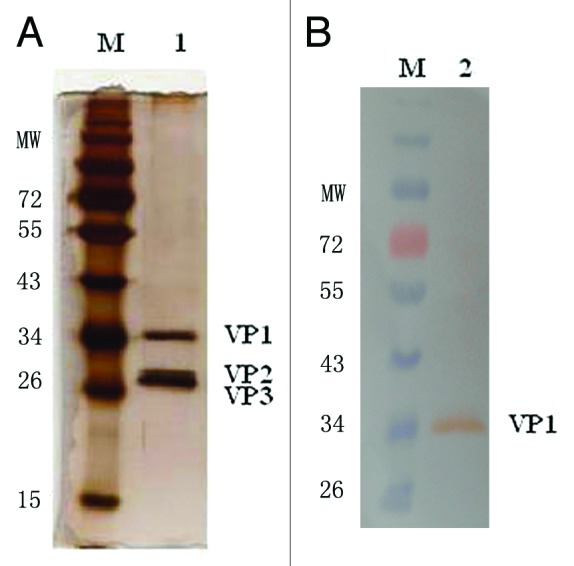
Figure 1. SDS-PAGE and western blot analyses of the virion. (A) SDS-PAGE of CA16 virion. Land 1 is the purified CA16 virion; (B) Western blot picture of CA16 Virion. Lane 2 is the purified virion which was incubated with the anti-VP1 polyclonal-antibody.
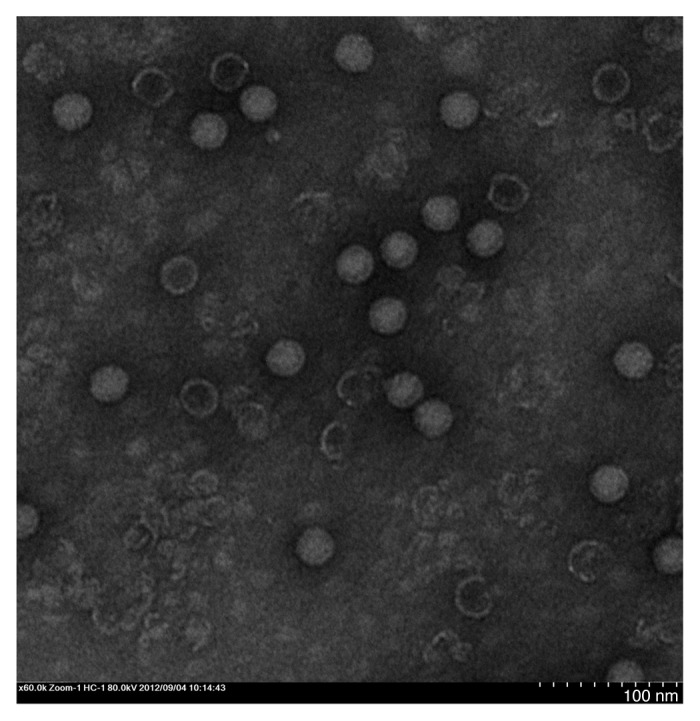
Figure 2. Electron microscopy of CA16 virions. Bar = 50 nm.
Immunogenicity of the CA16 vaccine in the mouse
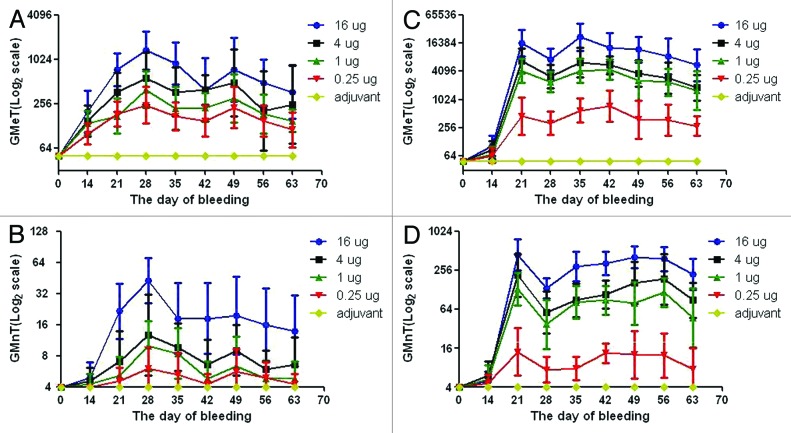
Mice (10 in each group) were given two intramuscular (IM) injections of 419/CA16 vaccine at 14-d intervals, at doses of 16, 4, 1, or 0.25 µg, and blood was collected until day 49 after the second vaccination, that is, day 63 after the first vaccination (immunization routine is shown in Fig. 3, data shown in Fig. 4A and B). Microneutralization assays and enzyme-linked immunosorbent assays (ELISAs) were performed to test the antibody titers. Neutralizing antibody titers of ≥8 and ELISA antibody titers of ≥100 were obtained for all mice (n = 10 per group) immunized with the 419/CA16 vaccines. However, mice had lower antibody levels than rats across all dosage groups. Such low neutralizing titers observed more than 2 mo post-vaccination indicated that the mouse is not the optimum system for evaluation of CA16 vaccine immunogenicity. The highest GMT of neutralizing antibodies ranged from 32 to 64, and the highest GMT of ELISA antibody ranged from 1024 to 2048. At day 63 after the first vaccination, the highest GMT of neutralization antibody and ELISA antibody were about 16 and 256, respectively, thus the titers had decreased by about 50–75%. No increase in antibody level was observed in control mice injected with aluminum hydroxide (Al(OH)3) adjuvant only. The results from two independent experiments were similar.
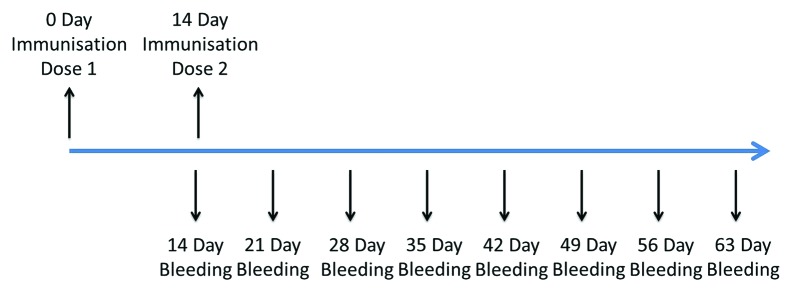
Figure 3. Timeline of mouse and rats immune procedures.
Figure 4. Serological responses of animals to immunization with the 419/CA16 vaccines. (A) the geometric mean ELISA titers of mouse; (B) the geometric mean neutralization titers of mouse; (C) the geometric mean ELISA titers of rats; (D) the geometric mean neutralization titers of rats; Error bars indicate the 95% confidence interval.
Immunogenicity of the CA16 vaccines in a rat system
We also used a rat immunogenicity system (data are shown in Fig. 4C and D), to investigate the antibody level and the duration of the immune response. The results (Fig. 4) indicated that all four dosage groups elicited production of a neutralizing antibody. At the sub-microgram (0.25 µg) dose, this vaccine also showed definite immunogenicity. Overall, the 1 µg dose group and >1 µg dose groups were significantly more effective in eliciting production of neutralizing antibodies. Specifically, the 1 µg dose of CA16 vaccine elicited a geometric mean titer (GMT) of nearly 130, whereas the 0.25 µg dose induced a lower GMT of about 14. The titer of the neutralizing antibody increased almost 10-fold with a 4-fold dose increase in vaccination dose. There was no similarly obvious variation in neutralizing antibody production between other doses; when the 16 µg group was compared with the 4 µg group, and the 4 µg group was compared with the 1 µg group, the titer of the neutralizing antibody was raised by almost 100% in both groups. Thus, the 1 µg/mouse dose was used as a suitable immunizing dose in the rat system.
We next examined the durability of the antibody responses in the different dosage groups. The rates showed a significant rise in antibody titer after the booster injection and also showed antibody durability. After a single booster immunization, all four dosage groups had neutralizing antibody responses that lasted for at least 2 mo. Interestingly, the antibody titer reached its peak at 21 d post-inoculation (DPI), and began to reduce at 28 DPI. Subsequently, the antibody titer increased again until 3–42 DPI, before slowly decreasing until 63 DPI. No increase in antibody was observed in the control rats (which were injected with Al(OH)3 adjuvant only). The results from two independent experiments were similar.
In the mouse immunogenicity system, there was a similar persistence of the antibody. The immunogenicity of CA16 in rats was confirmed to be higher than that in mice.
Immunogenicity of the CA16 vaccine in a monkey system
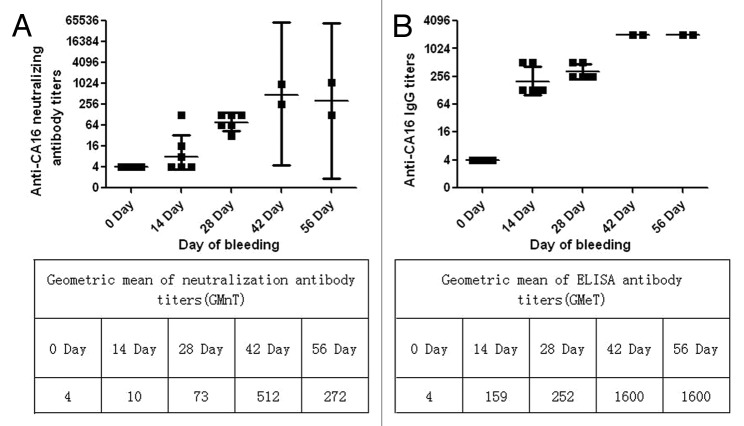
In the cynomolgus monkey system, each group was given three IM injections of the 419/CA16 vaccine at 14-d intervals, and blood was collected 28 d after the third vaccination (56 d after the first vaccination) (immunization routine is shown in Fig. 5, data are shown in Fig. 6). Microneutralization assays and ELISAs were performed to ascertain the neutralizing antibody and ELISA antibody titers. Neutralizing antibody titers of ≥8 and ELISA antibody titers of ≥128 were obtained for all vaccinated monkeys (Fig. 5). The neutralizing antibody titers were observed more than 2 mo post-inoculation, indicating long-term persistence of the anti-419/CA16 neutralizing antibodies. The immunological data (neutralizing antibody and ELISA antibody titers) suggested that the tested CA16 vaccines were immunogenic. No increase in antibody titers was observed in control monkeys injected with Al(OH)3 adjuvant only. The results from two independent experiments were similar.
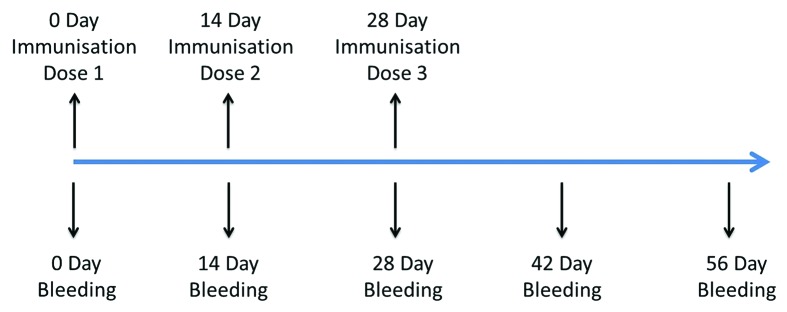
Figure 5. Timeline of cynomolgus monkeys immune procedures.
Figure 6. Antigen-specific neutralization and ELISA antibody levels in the serum of cynomolgus monkey. (A) Geometric mean of neutralization titers 56 d after prime and 28 d after boost vaccinations via the i.m route with CA16 vaccines containing 5 μg antigen. Each dot represents the individual titer of a monkey. (B) Geometric mean of ELISA titers 56 d after prime and 28 d after boost vaccinations via the i.m route with CA16 vaccines containing 5 μg antigen. In each Figure, horizontal bars indicate the geometric mean titers and error bars indicate the 95% confidence interval.
LD50 determination of 1131/CA16 virus
After continuous passages, the mouse-adapted CA16 strain 1131/CA16 was generated (data not shown) and the LD50 (50% lethal dose) was analyzed. The LD50 of the virus was estimated using the arithmetical method of Reed and Muench (Tables 1A and 1B; Fig. 7).19 The log doses, number of dead and surviving animals, cumulative number dead, cumulative number surviving, cumulative total, mortality rate and percentage mortality were assessed, and the LD50 was calculated as 0.081 of the 50% cell culture infective dose (CCID50). The low challenge dose required for LD50 in the sucking mouse system of 1131/CA16 indicates that this CA16 strain had very potent infective power in this animal system. There were no deaths in the control mice injected with culture medium instead of virus. The results from two independent experiments were similar.
Table 1A. The table for LD50 calculation.
| Group | Dilution fold | Dose (CCID50/Dose) | Log Dose | No.of Dead | No. of survival | Cumulative | Mortality rate | % mortality | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Dead | Survival | Total | ||||||||
| 1 | 1000000 | 0.0016 | −2.8010 | 0 | 8 | 0 | 18 | 18 | 0/18 | 0.00% |
| 2 | 100000 | 0.0158 | −1.8010 | 2 | 6 | 2 | 10 | 12 | 2/12 | 16.67% |
| 3 | 10000 | 0.1581 | −0.8010 | 5 | 4 | 7 | 4 | 11 | 7/11 | 63.64% |
| 4 | 1000 | 1.5811 | 0.1990 | 6 | 0 | 13 | 0 | 13 | 13/13 | 100.00% |
| 5 | 100 | 15.8114 | 1.1990 | 7 | 0 | 20 | 0 | 20 | 20/20 | 100.00% |
Estimation of median percent mortality, (MLD50); (50.0–16.67) ÷ (63.64–16.67) = 33.33 ÷ 46.97 = 0.7096; (−0.80103) – (−1.80103) = 1.0; ∴0.7096*1.0 = 0.7096; Antilog of −1.80103 + 0.7096 = Antilog of −1.09143; LD50 = 0.081 CCID50; Table 1B.
Table 1B.
| Dose (CCID50/Dose) | Log Dose |
|---|---|
| 0.0158 | −1.8010 |
| 0.1581 | −0.8010 |
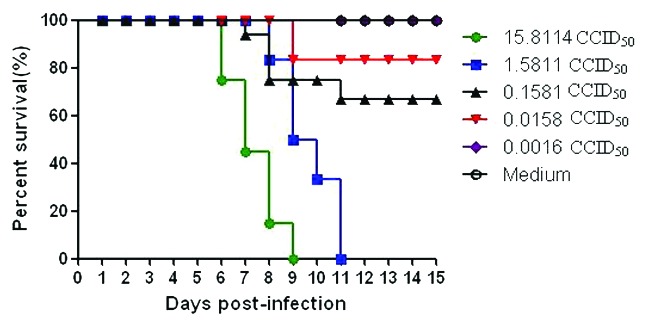
Figure 7. Intracerebral inoculation of 1131/CA16 results in dose-related mortality.
Virus loads in infant mouse tissues after virus challenge
Organs were extracted from challenged mice at several time points. The viruses were harvested (see Materials and Methods), and a viral plaque assay was used, with results expressed as plaque-forming units (PFU). For each mouse, virus levels in serum were analyzed. In addition, virus levels in eight different tissues (brain, heart, lung, liver, spleen, kidney, intestines, and hind-limb skeletal muscles) were analyzed. The tissues were dissected, homogenized, and centrifuged (see Materials and Methods), then the tissue supernatant was transferred to prepared 6-well plates, and used for the viral plaque assay.
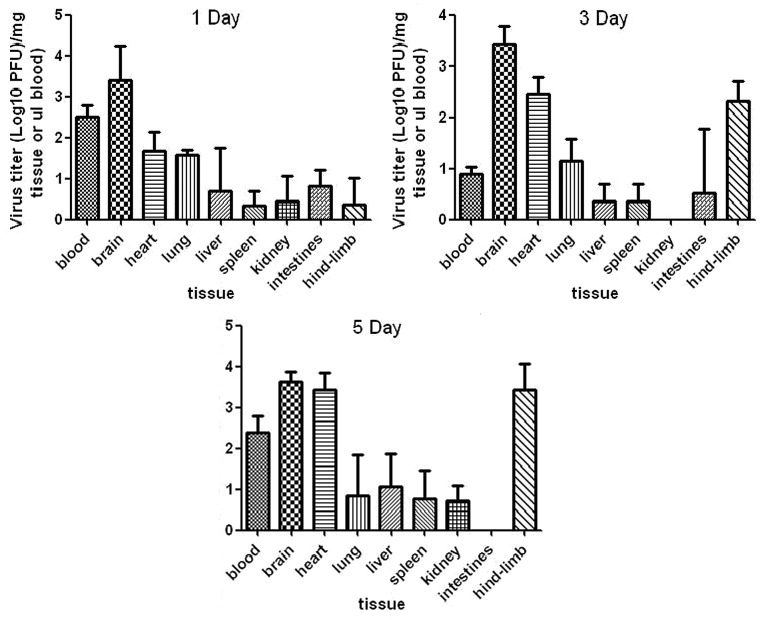
The trend in plaque change (PFU) is shown in Figure 8. Virus isolation revealed that 1131/CA16 replicated in different organs after inoculation. Specifically, no virus was isolated from kidney or intestine of injected mice on 3 or 5 DPI. Blood contained high virus titers (102.53 PFU/mg) at 1 DPI, which decreased at 3 DPI (100.9 PFU/mg, and increased again (102.39 PFU/mg) at 5 DPI. This might reflect serum immunoreaction in the suckling mice. In general, brain tissue contained the highest titers of virus (103.41 to 103.64 PFU/mg) at 1 to 5 DPI, which were approximately 0.5 to 1 log higher than those in muscle and blood. The viral loads in the hind-limb skeletal muscle and heart tissue steadily increased with time (from 100.38 to 103.44 PFU/mg for skeletal muscle, and from 101.66 to 103.43 PFU/mg for heart tissue). At 1 DPI, the virus was present in all tissues analyzed: intestine (100.83 PFU/mg), lung (101.58 PFU/mg), liver (100.69 PFU/mg), muscle (100.38 PFU /mg), brain (103.41 PFU /mg), spleen (100.35 PFU/mg), kidney (100.44 PFU/mg), and heart (103.43 PFU/mg). All this evidence indicated that the virus particles had spread throughout the whole body via the circulatory system from the injected position. In the control mice, no virus was detected in any of the analyzed tissues. The results from two independent experiments were similar.
Figure 8. Virus loads in infant mouse tissues. Virus titers in tissues are expressed as mean Log10 PFU ± standard error of mean per 1 mg tissue from groups of 3 mice at 1, 3, 5 d post-infection. Virus titers in sera are expressed as the Log10 PFU per mililiter.
Protective efficacy of the neutralizing antibody
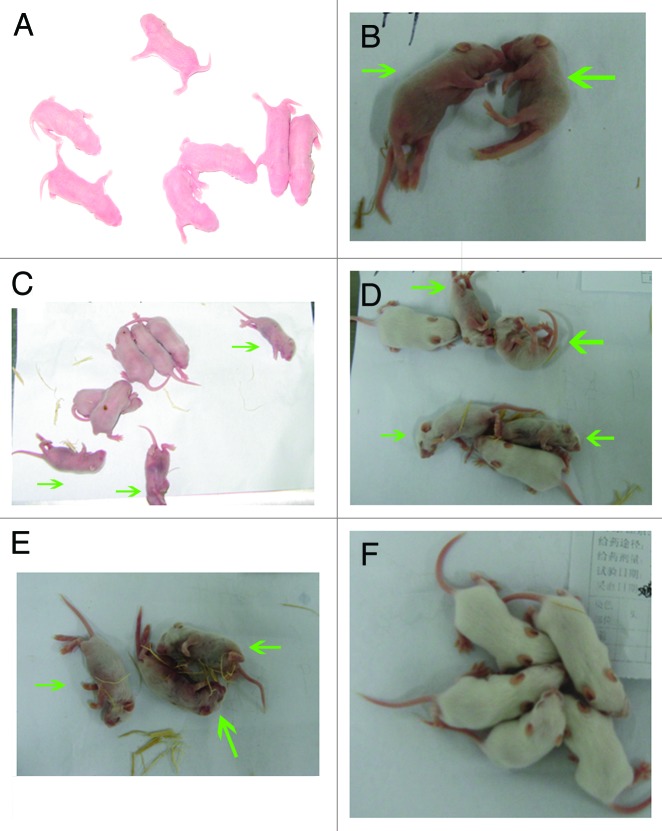
We analyzed the efficacy of 419/CA16 immunized serum in providing passive protection against the CA16 virus (Tables 2A and 2B; Fig. 9.) In the passive immunization protection assay, the anti-serum protected the mice from death, and the protection rate correlated with the different serum concentrations resulting from the different dilution ratios used in the different groups. Immunoprotection was determined by calculating the 50% effective dose (ED50). Five doses were chosen for determination of ED50, ranging from 0% to 100% mortality. Based on the survival rate correlated with the volume of injected serum, we calculated that the ED50 value was 0.477 U/mouse. The results illustrated that the protective effect could be achieved with a lower level of neutralizing antibodies. In the maternal antibody protection assay, single-injection and double-injection groups were used (Fig. 10). Blood samples were collected from the submandibular vein of each dam at intervals of 1 wk, until 5 wk after the first immunization. The antibody variation trends are shown in Figure 11. The pups of dams that were immunized with the 419/CA16 vaccine were fully protected from lethal challenge with 1131/CA16, whereas the offspring of the adjuvant-immunized pregnant mice were all dead 11 d after challenge with 1131/CA16. The variation in mouse lethality rate in the different vaccinated groups indicated that the 419/CA16 vaccine had a potent protective effect against 1131/CA16 infection. Results were shown as Kaplan–Meier survival curves in Figure 12. And the pictures of viral infection of natal mouse by CA16 virus were shown in Figure 13. The results of the passive protection assay, the neutralizing antibody test, and the ELISA antibody test clearly indicated that the levels of neutralizing antibody in pregnant mice were low, but still had protective efficacy. No increase in antibody level was observed in the control mice injected with Al(OH)3 adjuvant only, and all the control offspring died after 1131/CA16 challenge. The results from two independent experiments were similar.
Table 2A. The passive immunizing protection assay (in vivo).
| Group NO. | Serum dose(U/mouse) | serum dilution fold | No.of survival | Total | survival ratea |
|---|---|---|---|---|---|
| 1–1 | 12.00 | 0 | 8 | 8 | 100.00% |
| 1–2 | 3.00 | 4 | 7 | 8 | 87.50% |
| 1–3 | 0.75 | 16 | 5 | 8 | 62.50% |
| 1–4 | 0.19 | 64 | 2 | 8 | 25.00% |
| 1–5 | 0.09 | 128 | 0 | 8 | 0.00% |
a Survival rate of pups born to immunized dams after lethal viral challenge. Estimation of 50% effective dose (ED50). (50.0–25) ÷ (62.50–25.00) = 25 ÷ 37.5 = 0.67; (−0.125)–(−0.72) = 0.595;∴0.595*0.67 = 0.39865; Antilog of (−0.72 + 0.39865) = Antilog of −0.32135; ED50 = 0.477 U.
Table 2B.
| Serum dose(U/mouse) | Log Dose |
|---|---|
| 0.19 | −0.72 |
| 0.75 | −0.125 |
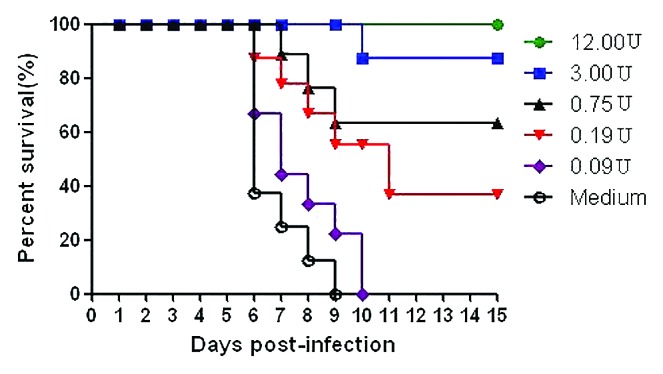
Figure 9. Passive immunizing protection studies in mouse. Results are shown as Kaplan–Meier survival curves. The mice (n = 8) were i.m. injected with the CA16 vaccines and then challenged with 1 × 102 LD50 of CA16 strain 1131. Control mouse were immunized with adjuvant only.
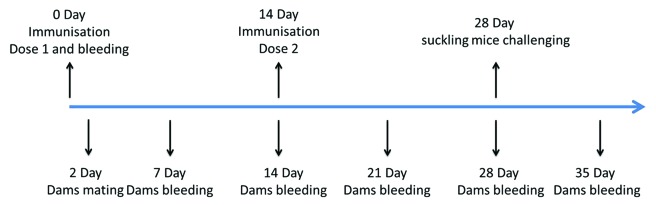
Figure 10. Timeline of mouse challenge procedures in the maternal antibody protection assay.
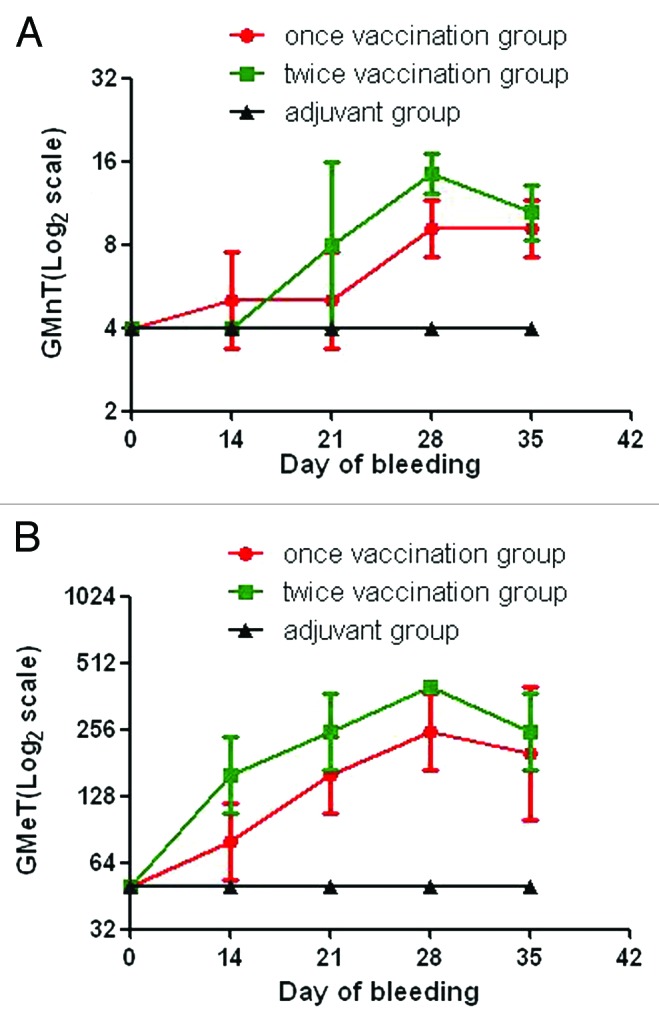
Figure 11. Serological responses of maternal mice to immunization with the 419/CA16 vaccines. (A) the geometric mean ELISA titers of maternal mice; Each point represents the geometric mean value (n = 10) ± 95% CI. (B) The geometric mean neutralization titers of maternal mice.
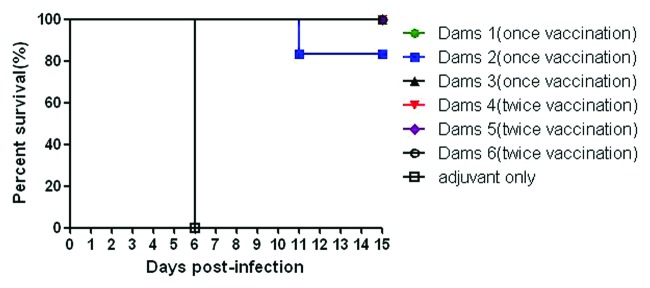
Figure 12. Maternal antibody protection studies in mice. Results are shown as Kaplan–Meier survival curves. The marternal mice (n = 8) were i.m. injected with the CA16 vaccine. After delivery (about 5 to 10 d after the boost), pups were i.c. challenged with 1 × 102 LD50 of 1131/CA16 (100 LD50/mouse) on postnatal day 3. Control mice were immunized with adjuvant only.
Figure 13. Viral infection of natal mouse by CA16 virus. (A) 0 d post challenge; (B), and (C) Mice at day 6 post infection are shown. The dead mice were indicated with green arrows; (D) and (E) 10 d post challenge. The dead mice were indicated with green arrows; (F) 14 d post challenge.
Discussion
CA16, which belongs to the family Picornaviridae and genus enterovirus (EV), was first isolated in South Africa almost 60 y ago. The first prototype strain of CA16, named CA16-G10, was first sequenced in 1994.8,10 In mainland China, CA16 has been found in the metabolic material of patients with HFMD. Sequencing and sequence alignment has shown that the majority of patients were infected with CA16, with most of the strains being genotype C. Thus in this study, we used CA16 genotype C strains as the production and challenge strains.
In contrast to previous studies,13,20 we chose not to use formalin for virus inactivation, but instead chose BPL, which has been used in a number of other studies.21,22 Although the BPL method can decrease the residual of inactivator, we did not perform biochemical analysis of the BPL-inactivated vaccine in this paper (unpublished in this paper), which is a limitation of this study. This virus was absorbed to the Al(OH)3 adjuvant at a concentration of 1 mg/mL. Because the antigen concentration was so low compared with that of the adjuvant, we considered that the antigen was completely adsorbed to the adjuvant.
To compare the antigenicity of the vaccine candidate, we first analyzed the immune response (neutralizing antibody titer, ELISA antibody titer, and vaccine durability) induced by the 419/CA16 vaccine in mice, rats, and cynomolgus monkeys, and subsequently performed vaccine protection studies in two different challenge systems.
If the neutralizing antibody elicited an effective protection response, the vaccinated animal would be immune against the relevant virus infection. Therefore, we first performed durability studies to determine whether the 419/CA16 vaccine could induce long-lasting neutralizing antibodies against the CA16 virus. We compared the immunogenicity of BPL-inactivated CA16 vaccine in three different animals, and then evaluated the protective effect of CA16 vaccine in two different neonatal mouse challenge systems. These animal systems confirmed the protective role of the vaccine in inducing neutralizing antibodies.
Using a maternal antibody protection study and an anti-serum protection study, we also demonstrated that the specific CA16 neutralizing antibody could block invasion of the virus and we were able to evaluate the protective efficacy of the CA16 vaccine. Because two virus strains of CA16 have been used in our article, so the cross-neutralization assay was important. However, the result of the cross-neutralization protection assay has not been published in the article (Because the data are shown in another unpublished article). In each animal experiment, the t test was used to analyze for significance; However, because the date was so complex, the t test results did not add to the figures of this article. It is possible that an observation period of 2 mo for antibody duration in immunogenicity assays is not sufficiently long. Persistence of neutralizing antibodies is important for the continuing protection ability of the CA16 vaccine, so this should be a focus of future study. Thus, there were several limitations in this study.
In the immunogenicity system, we first evaluated the capability of the BPL-inactivated vaccine to elicit the neutralizing and ELISA antibodies in rodents; specifically, in inbred BALB/c mice. In this animal system, there was poor immunogenicity. However, in another rodent system, the SD rat, a potent immunogenic reaction was observed after injection with the BPL-inactivated CA16 vaccine, and a similar immunogenic reaction was observed in the cynomolgus monkey. In summary, the BPL-inactivated vaccine had a potent ability to elicit neutralizing and ELISA antibodies in several species. Rats and cynomolgus monkeys had similarly significant results as animal systems for assessing vaccine immunogenicity, whereas mice had lower levels of neutralizing antibodies. Thus, we inferred from the monkey results that this vaccine is likely to produce a potent immunogenic reaction in humans. However, in the monkey immunogenicity assay, because only two monkeys were used on days 42 and 56, the limited sample number resulted in a large 95% CI value.
There were differences between this study and previously published studies on the immunogenicity of the CA16 vaccine in a mouse system.23 The levels of neutralizing antibody elicited in the mouse by the vaccine in the current study was not as high as those reported by others. However, ELISA showed an obvious increase in antibody level after immunization. One reason for the differences may be the difference in gene sequences between the production virus and the testing virus in the neutralization assay. We performed a large number of assays to inform our choice of challenge and neutralization assays for the viral strains, and found that the results of the challenge assays and neutralization assays were different from each other.
Compared with the VLP of CA16 produced by other researchers, although different injection and challenge methods were used, it seems that the whole virus-inactivated vaccine results in a lower neutralizing antibody titer and higher protection rate in the mouse system.24 All this evidence indicates that the vaccine protection ability should not be evaluated by a single method.
Animal challenge systems have been used for evaluating vaccine efficacy for many years, and they are considered the best type of vaccine evaluation system.25 In recent years, several EV71 vaccine challenge systems have been established, including neonatal mouse and cynomolgus animal systems.26,27 All these systems were able to elicit neutralizing antibodies and provide almost complete protection against EV71 virus infection.
In newborn mice, the CA virus has a potent lethal effect, but the reason for this lethality is unclear.8 In this study, we evaluated the potential of a BPL-inactivated CA16 virus as a vaccine candidate against CA16 infection. We separated the challenged natal mouse tissues to detect the CA16 virus titer on RD cells by viral plaque assay. We found tropism of the CA16 virus in different tissues. Based on the results of the viral plaque assay on different tissues, viral titer was consistently detected in brain and muscle tissues. It is possible that the CA16 virus has more potent affinity for muscle cells.
According to the LD50 determination assay, the 419/CA16 virus infected the mouse in a regular pattern. First, the neonatal mice were given intracerebral (IC) inoculation with virus that had been grown using brain cells as host. However, once the virus was injected, it dispersed into the serum, and was carried by the blood into every major organ. Thus, the viruses could be detected in most of the body tissues, but they were found to be most abundant in skeletal muscle and heart. We therefore inferred that the neonatal mice might have died as a result of the virus affecting the heart. However, we did not perform an assay to determine the viral location at the cellular level (for example, immunohistochemistry), thus this is a limitation of the study.
We compared the protective effect in both rat and mouse, but we found that the rat was not a good system for the challenge experiment, because the injected vaccine induced abortion in pregnant rats (data not shown). Owing to its complexity, the challenge experiment could not be performed in monkeys. Thus, this is another limitation of this study.
There were two neonatal mouse systems used to evaluate the protective effect in this study. In the passive immunization protection assay, the anti-serum protected mice from death after virus challenge. In the maternal antibody protection assay, rats were inoculated with either a single or double injection of the vaccine. All these systems illustrated that the vaccine had a potent protective effect against the CA16 virus.
In a previously published study,28 it was also observed that the CA16 virus has a strong pathological effect on newborn mice. To evaluate the protective effect of the CA16 vaccine, we also established two neonatal mouse challenge systems. Our results was consistent with the challenge assay results in the previous study.28 We also found that the protection rates of the vaccine in newborn mice were dose-dependent. Moreover, we analyzed the curve of antibody increase in different animals. All these data provide more detailed evidence to clarify the effect of the CA16 vaccine.
A number of different challenge routines have been performed in different studies.23,28 Thus we compared the intraperitoneal (IP) and IC routes of administration in mice (detailed data not shown). We found that the IC route led to a 100% death rate, but the IP route resulted in a lower death rate. We therefore chose the IC route as the most suitable challenge method for the CA16 virus.
In this paper, the LD50 value of the challenge virus was 0.081 CCID50/mouse, and in another published study, the LD50 was 1.2 PFU/mouse.28 Although the LD50 unit, CCID50, was not consistent with the PFU, there is a close relationship between the two types of virological assay. All these results indicated that the neonatal mouse was very sensitive to the CA16 virus. In the current study, the results of the viral loading assays were consistent with each other, and all the evidence indicated that focal myocarditis is the main cause of death from CA16 in the mouse. In the viral loading assay, the reason for virus deletion in the kidney and intestines of injected mice at the final two collection points was not clear. It is possible that the low detection rate in the viral plaque assay resulted in low sensitivity.
We examined the functional potential of CA16-neutralizing antibodies in vivo, and the results indicated that the anti-CA16 anti-serum was able to provide passive protection. There was almost 100% survival rate in suckling BALB/c mice that had been vaccinated before being challenged with a lethal dose of the homologous CA16 strain. Even though the titer of neutralizing antibodies elicited by the anti-serum was low, the protective effect still existed. Thus, the neonatal mouse could be protected from a lethal dose of CA16 by injecting a low titer of virus grown on RD cells.
Materials and Methods
Cells, vaccine, and virus strains
Vero cells (monkey kidney epithelial cell line, ATCC) and RD cells (rhabdomyosarcoma cell line, ATCC) were maintained in Eagle's minimum essential medium (MEM; Gibco), containing 2% or 10% fetal bovine serum (FBS; Gibco) plus 2 mmo/l L-glutamine.
The BPL-inactivated CA16 vaccine was manufactured by Hualan Biological Engineering Inc. In brief, the 419/CA16 strain was cultured in Vero cells in Eagle's MEM (Gibco) as above. Virus culture was performed as described previously.29,30 The virus culture bulk was harvested, and then purified as described in a previous study.31 The protein concentration was determined by the Lowry method. The antigen purity and identity was confirmed by SDS-PAGE and western blotting, as described previously.18 Anti-VP1 polyclonal antibody was produced in our laboratory (the antigen was CA16 VP1 expressed by Escherichia coli, the sequence from 419/CA16, which was immunized rabbit). Virus particles were subjected to negative staining with 2.0% phospho-tungstic acid aqueous solution, and transmission electron microscopy was performed (HT7700, Hitachi). The purified virions were absorbed onto Al(OH)3 adjuvant (1 mg/mL).
Two local isolates were used. The 1131/CA16 strain was a mouse-adapted strain, while the other, named 423/CA16, was used to perform the neutralization assay. Both were kindly provided by the Center for Disease Control and Prevention (CDC).
Viral RNA was extracted from the 1131/CA16 and 423/CA16 strains, and reverse transcription (RT)-PCR was performed to obtain the viral sequences; according to the results of the VP1 sequence alignment, the viruses belong to the C3 and C1 subtypes, respectively. The titers of 1131/CA16 (105.5 CCID50/mL) and 423/CA16 (106.53 CCID50/mL) were determined by CCID50 assay. All stock viruses were cultured on RD cells, and the titer rose to >105 CCID50/mL. The culture solution was subjected to three freeze–thaw cycles, centrifuged at 4000 × g for 10 min at 4 °C, and stored at −80 °C. The alignment results of the VP1 sequence between 1131/CA16 and 423/CA16 were shown as below, 93.5% DNA sequence identity and 98.3% protein sequence identity. These viruses were separated by our own laboratory, and the sequences have not been published previously.
Immunization strategy and approach in mice and rats
Inbred female BALB/c mice (aged 6–8 wk) and outbred Sprague–Dawley (SD) rats (aged 8–12 wk) (both from Experimental Animal Center, Hualan Biological Engineering Inc.) were used in the immunization experiments.
The animals were separated into four dosage groups (0.25, 1, 4, 16 µg/dose, 10/group), and each group was given IM injections of the relevant dose of BPL-inactivated 419/CA16 vaccine at weeks 0 and 2. Blood was collected at 1-wk intervals as shown in Figure 3. In addition, another 10 mice or rats were administered the Al(OH)3 adjuvant alone as a control.
All regulations (Animal Center, Hualan Biological Engineering Inc.) for animal welfare were strictly followed.
Immunization strategy and approach in cynomolgus monkeys
A total of 12 cynomolgus monkeys (6 male, 6 female, all aged 2–5 y; Experimental Animal Center, Hualan Biological Engineering Inc.) were divided into two dosage groups: 1 µg or 5 µg, with 6 animals per group. Animals were given IM injections of the relevant dose of BPL-inactivated 419/CA16 vaccine. The same doses of vaccines were administered three times at 2-wk intervals. In addition, another three monkeys were used as the control group, and were injected with the Al(OH)3 adjuvant (0.5 mg/monkey) only. Serum was collected to assay for neutralizing antibodies after the primary injection and the booster injection.
The injecting and bleeding protocols were performed as shown in Figure 3. All regulations (Animal Center, Hualan Biological Engineering Inc.) for animal welfare were strictly followed.
Microneutralization
Duplicate serum samples were diluted in Eagle's MEM (Gibco), from 1:8 to 1:1024 dilutions. The sera were then mixed with an equal volume containing 102 CCID50 of 423/CA16 virus in maintenance medium, consisting of MEM with 2% FBS (Invitrogen), 2 mmol/l l-glutamine (Sigma), and 1% penicillin/streptomycin (Invitrogen), then incubated at 37 °C for 1 h. The mixture was then added to RD cell monolayers in 96-well plates (Costar®; Corning Corporation). After 7 d of culture at 37 °C with 5% CO2, the neutralizing antibody titers were determined as the highest dilution for which no cytopathic effect was present in either of the duplicates (i.e., end-point of 100% neutralization). Neutralization data were analyzed using the GraphPad Prism 5.
Enzyme-linked immunosorbent assay
The purified 419/CA16 antigen was produced by the method described above, then 96-well ELISA plates (Costar®; Corning Corporation) were coated with 200 ng 419/CA16 virus antigen in 50 µL of 0.1 mol/L carbonate buffer pH 9.0 overnight at 4 °C. Plates were then blocked with a solution of 5% skim milk powder in phosphate-buffered saline (PBS) at 37 °C for 1 h. Duplicates of serum samples were diluted 1:100 in 50 µL blocking solution, and incubated in each well for 1 h at 37 °C. The plates were then drained of liquid, and washed five times with PBS containing 0.1% Tween-80. The appropriate HRP-conjugated secondary antibody (rabbit anti-mouse, anti-rat, or anti-monkey IgG; Sigma), diluted 1:1000 to 1:4000 in 50 µL wash buffer, was added, and the plates incubated at 37 °C for 1 h. Plates were then washed five times as before with the same PBS and Tween mixture described above, then 100 µL of substrate solution (3,3′,5,5′-tetramethylbenzidine (TMB) in H2O2) was added to each well. Plates were incubated at room temperature for 15 min, then 50 µL 1 mol/L H2SO4 stop solution was added to each well. The absorbance of the plates was read at 405 nm in an automated microtiter plate reader (SpectraMax® M2e; Molecular Devices Corporation). Each plate contained a negative serum well and a positive serum well as controls. Plates were considered positive if the numerical value of the reading was greater than 2.1 times the average absorbance of the negative serum controls from all plates.
50% lethal dose (LD50) determination
An inbred strain of mice, specific-pathogen-free BALB/c mice (Hualan Biological Engineering Inc.), was used for challenge with different dilutions of virus solution for LD50 calculation. In this assay, 3-d-old BALB/c mice (8 to 10 per group) were given IC inoculations with 10-fold serial dilutions of 1131/CA16. The control mice were injected with uninfected culture medium and kept in a separate cage from the infected mice. The mortality rate of the mice was observed daily until 15 DPI, and the percentage mortality was recorded. The LD50 was calculated by the method described by Reed and Muench. In addition, another 10 mice were used as a control group, and injected with the culture medium alone.
All regulations (Animal center, Hualan Biological Engineering Inc.) for animal welfare were strictly followed.
Virus loads and plaque assay
We used 12 experimental mice and 3 control mice to perform virus load assays. For these experiments, the mice were administered IC injections of 1131/CA16 (100 LD50/mouse) or uninfected culture medium. After the heart was punctured, blood samples were collected and stored at −80 °C. Tissue samples (brain, heart, lung, liver, spleen, kidney, intestines, and hind-limb skeletal muscle) were carefully and aseptically extracted from dead natal mice (3 mice for each time point) at days 1, 3, and 5 PDI. Tissues were weighed and cut into pieces, then homogenized with PBS (10% w/v). The tissue suspension was centrifuged at 3000 g for 10 min, and stored at −80 °C.
The concentration of RD cells was adjusted to 1 × 106 cell/mL, and plated into six-well tissue culture plates. Growth was observed under an inversion microscopy, and 100% confluency of the cell layer was usually observed after 15–24 h. The extracted cell suspension (100 µl) was added to each well (in duplicate), and the plates shaken gently to allow the solution to spread across the cell layer. A sterile solution of 4% agarose in hyperpure water (produced by Arium® 611UF, Sartorius Company) was prepared by autoclaving at 121 °C for 20 min, then 1 volume of medium and 0.11 volumes of liquid agarose were added with swirling to a container pre-warmed to 37 °C (1:10 dilution), which was then shaken vigorously to mix. Immediately afterwards, 2 mL of the agarose/growth medium mixture was added by pipette to each well, with the mixture being trickled down the side of the well to avoid disturbing the cell layer. The plates were moved to a humidified incubator at 37 °C with 5% CO2, and plaques were visible by day 7 after infection. After counting the plaques, the concentration of the initial vial suspension in PFU/mL was calculated.
Protective efficacy of neutralizing antibodies
To confirm the protective role of the humoral immune response, two experiments were performed. First, we used rabbit anti-419/CA16 serum (neutralizing antibody titer 600 units; Hualan Biological Engineering Inc.) to assess the efficacy of passive immunization in protecting mouse pups against CA16 challenge in vivo. BALB/c mice (3 d old; 8–10 per group) were given IP injection of 50 μL of diluted rabbit anti-CA16 serum (4-fold serially diluted: 0-, 4-, 16-, 64-, and 128-fold dilutions) or adjuvant only. Within 1 h after inoculation, each mouse was subjected to IC challenge with 100 LD50 of 1131/CA16. The mortality was then monitored and recorded daily after infection until 15 d after viral inoculation. The ED50 was calculated by the method described by Reed and Muench.
Maternal antibody protection was also studied. The 419/CA16 BPL-inactivated vaccine (adjusted with 1 mg/mL Al(OH)3) was prepared for the challenge assay. Two groups of mice were used, which were divided based on the number of injections. In the double-injection group, 8-wk-old female BALB/c mice (2/group) were given two IM injections, 2 wk apart, with either 0.5 mL BPL-inactivated 419/CA16 vaccine (1 µg/dose; experimental group), or adjuvant (control group). The mice were allowed to mate on the second day after the first injection. After delivery (about 5–10 d after the second [booster] injection), pups were subjected to IC challenge with 1131/CA16 (100 LD50/mouse) on postnatal day 3. Mortality among the challenged suckling mice was monitored until the 15th DPI.
In addition, two of the mouse dams were administered with the culture medium alone as a control, and their offspring were then challenged with the same dose of 1131/CA16 virus.
Statistical analysis
The results were expressed as mean, standard deviations (SD), Geometric mean and confidence interval 95%. LD50 were calculated as the method described by Reed and Muench. The anti-CA16 serum protection dosage was described as ED50 and calculated as the method described by Reed and Muench.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The work described in this manuscript was funded by Hualan Biological Engineering, Inc., Henan, People’s Republic of China. We are thankful to the center of Disease Control and Prevention of Henan province.
Glossary
Abbreviations:
- HFMD
Hand, foot and mouth disease
- CA16
Coxsackievirus A16
- EV71
Enterovirus 71
- BPL
β-propiolactone
- ELISA
Enzyme-linked immunosorbent assay
- GMT
geometric mean titer
- LD50
50% lethal dose
- ED50
50% protective dose
- CCID50
50% cell culture infectious dose
- CPE
cytopathic effect
- PFU
plaque forming units
- i.c
intracerebrally
- i.m
intramuscularly
References
- 1.Chan LG, Parashar UD, Lye MS, Ong FG, Zaki SR, Alexander JP, Ho KK, Han LL, Pallansch MA, Suleiman AB, et al. For the Outbreak Study Group Deaths of children during an outbreak of hand, foot, and mouth disease in sarawak, malaysia: clinical and pathological characteristics of the disease. Clin Infect Dis. 2000;31:678–83. doi: 10.1086/314032. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Deaths among children during an outbreak of hand, foot, and mouth disease--Taiwan, Republic of China, April-July 1998. MMWR Morb Mortal Wkly Rep. 1998;47:629–32. [PubMed] [Google Scholar]
- 3.Pallansch M, Roos R. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses, p 839–93 In Knipe DM, et al., editors. (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 4.Zhu J, Luo Z, Wang J, Xu Z, Chen H, Fan D, Gao N, Ping G, Zhou Z, Zhang Y, et al. Phylogenetic analysis of Enterovirus 71 circulating in Beijing, China from 2007 to 2009. PLoS One. 2013;8:e56318. doi: 10.1371/journal.pone.0056318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang XY, Kang K, Xu YL, Wei HY, Li XL, Ma H, You AG, Chen HM, Xu BL. [Etiology surveillance of hand-foot-mouth disease in Henan province between 2008 and 2011] Zhonghua Yu Fang Yi Xue Za Zhi. 2012;46:883–7. [PubMed] [Google Scholar]
- 6.Christensen A, Nordbø SA. [Enterovirus infections diagnosed in middle Norway during the period 1992-2001] Tidsskr Nor Laegeforen. 2003;123:3180–3. [PubMed] [Google Scholar]
- 7.Kusuda H, Kitamura S. Localization of coxsackie A16 viral antigen in various organs of newborn mice by the fluorescent antibody technique. Jpn J Microbiol. 1974;18:411–3. doi: 10.1111/j.1348-0421.1974.tb00828.x. [DOI] [PubMed] [Google Scholar]
- 8.Sickles GM, Mutterer M, Feorino P, Plager H. Recently classified types of Coxsackie virus, group A; behavior in tissue culture. Proc Soc Exp Biol Med. 1955;90:529–31. doi: 10.3181/00379727-90-22088. [DOI] [PubMed] [Google Scholar]
- 9.Oberste MS, Peñaranda S, Maher K, Pallansch MA. Complete genome sequences of all members of the species Human enterovirus A. J Gen Virol. 2004;85:1597–607. doi: 10.1099/vir.0.79789-0. [DOI] [PubMed] [Google Scholar]
- 10.Pöyry T, Hyypiä T, Horsnell C, Kinnunen L, Hovi T, Stanway G. Molecular analysis of coxsackievirus A16 reveals a new genetic group of enteroviruses. Virology. 1994;202:982–7. doi: 10.1006/viro.1994.1423. [DOI] [PubMed] [Google Scholar]
- 11.Wang CY, Li Lu F, Wu MH, Lee CY, Huang LM. Fatal coxsackievirus A16 infection. Pediatr Infect Dis J. 2004;23:275–6. doi: 10.1097/01.inf.0000115950.63906.78. [DOI] [PubMed] [Google Scholar]
- 12.Xu W, Liu CF, Yan L, Li JJ, Wang LJ, Qi Y, Cheng RB, Xiong XY. Distribution of enteroviruses in hospitalized children with hand, foot and mouth disease and relationship between pathogens and nervous system complications. Virol J. 2012;9:8. doi: 10.1186/1743-422X-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen CW, Lee YP, Wang YF, Yu CK. Formaldehyde-inactivated human enterovirus 71 vaccine is compatible for co-immunization with a commercial pentavalent vaccine. Vaccine. 2011;29:2772–6. doi: 10.1016/j.vaccine.2011.01.094. [DOI] [PubMed] [Google Scholar]
- 14.Ong KC, Devi S, Cardosa MJ, Wong KT. Formaldehyde-inactivated whole-virus vaccine protects a murine model of enterovirus 71 encephalomyelitis against disease. J Virol. 2010;84:661–5. doi: 10.1128/JVI.00999-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polley JR, Guerin MM. The use of beta-propiolactone for the preparation of virus vaccines. I. Selection of reaction conditions. Can J Microbiol. 1957;3:863–70. doi: 10.1139/m57-095. [DOI] [PubMed] [Google Scholar]
- 16.Polley JR, Guerin MM. The use of beta-propiolactone for the preparation of virus vaccines. II. Antigenicity. Can J Microbiol. 1957;3:871–7. doi: 10.1139/m57-096. [DOI] [PubMed] [Google Scholar]
- 17.Iwai M, Masaki A, Hasegawa S, Obara M, Horimoto E, Nakamura K, Tanaka Y, Endo K, Tanaka K, Ueda J, et al. Genetic changes of coxsackievirus A16 and enterovirus 71 isolated from hand, foot, and mouth disease patients in Toyama, Japan between 1981 and 2007. Jpn J Infect Dis. 2009;62:254–9. [PubMed] [Google Scholar]
- 18.Ranganathan S, Singh S, Poh CL, Chow VT. The hand, foot and mouth disease virus capsid: sequence analysis and prediction of antigenic sites from homology modelling. Appl Bioinformatics. 2002;1:43–52. [PubMed] [Google Scholar]
- 19.Reed LJ, Muench H. A simple method of estimating 50 percent end-points. Am J Hyg. 1938;27:493–7. [Google Scholar]
- 20.Bek EJ, Hussain KM, Phuektes P, Kok CC, Gao Q, Cai F, Gao Z, McMinn PC. Formalin-inactivated vaccine provokes cross-protective immunity in a mouse model of human enterovirus 71 infection. Vaccine. 2011;29:4829–38. doi: 10.1016/j.vaccine.2011.04.070. [DOI] [PubMed] [Google Scholar]
- 21.Kamaraj G, Lakshmi Narasu M, Srinivasan VA. Validation of betapropiolactone (BPL) as an inactivant for infectious bovine rhinotracheitis (IBR) virus. Res Vet Sci. 2008;85:589–94. doi: 10.1016/j.rvsc.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Refaie FM, Esmat AY, Mohamed AF, Mohamed WA. The effect of chemical inactivation of bovine viral diarrhea virus with beta-propiolactone and binary ethyleneimine on plasma proteins and coagulation factors. Egypt J Immunol. 2004;11:9–20. [PubMed] [Google Scholar]
- 23.Cai Y, Liu Q, Huang X, Li D, Ku Z, Zhang Y, Huang Z. Active immunization with a Coxsackievirus A16 experimental inactivated vaccine induces neutralizing antibodies and protects mice against lethal infection. Vaccine. 2013;31:2215–21. doi: 10.1016/j.vaccine.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Liu Q, Yan K, Feng Y, Huang X, Ku Z, Cai Y, Liu F, Shi J, Huang Z. A virus-like particle vaccine for coxsackievirus A16 potently elicits neutralizing antibodies that protect mice against lethal challenge. Vaccine. 2012;30:6642–8. doi: 10.1016/j.vaccine.2012.08.071. [DOI] [PubMed] [Google Scholar]
- 25.Herrlich A, Epp C, Mayr A. [Animal experimental studies on various smallpox vaccines in a cowpox challenge experiment on white mice] Z Immunitatsforsch Allerg Klin Immunol. 1968;135:237–45. [PubMed] [Google Scholar]
- 26.Dong C, Wang J, Liu L, Zhao H, Shi H, Zhang Y, Jiang L, Li Q. Optimized development of a candidate strain of inactivated EV71 vaccine and analysis of its immunogenicity in rhesus monkeys. Hum Vaccin. 2010;6:1028–37. doi: 10.4161/hv.6.12.12982. [DOI] [PubMed] [Google Scholar]
- 27.Mao Q, Li N, Yu X, Yao X, Li F, Lu F, Zhuang H, Liang Z, Wang J. Antigenicity, animal protective effect and genetic characteristics of candidate vaccine strains of enterovirus 71. Arch Virol. 2012;157:37–41. doi: 10.1007/s00705-011-1136-3. [DOI] [PubMed] [Google Scholar]
- 28.Mao Q, Wang Y, Gao R, Shao J, Yao X, Lang S, Wang C, Mao P, Liang Z, Wang J. A neonatal mouse model of coxsackievirus A16 for vaccine evaluation. J Virol. 2012;86:11967–76. doi: 10.1128/JVI.00902-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu CC, Lian WC, Butler M, Wu SC. High immunogenic enterovirus 71 strain and its production using serum-free microcarrier Vero cell culture. Vaccine. 2007;25:19–24. doi: 10.1016/j.vaccine.2006.06.083. [DOI] [PubMed] [Google Scholar]
- 30.Quesney S, Marvel J, Marc A, Gerdil C, Meignier B. Characterization of Vero cell growth and death in bioreactor with serum-containing and serum-free media. Cytotechnology. 2001;35:115–25. doi: 10.1023/A:1017589526145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong C, Liu L, Zhao H, Wang J, Liao Y, Zhang X, Na R, Liang Y, Wang L, Li Q. Immunoprotection elicited by an enterovirus type 71 experimental inactivated vaccine in mice and rhesus monkeys. Vaccine. 2011;29:6269–75. doi: 10.1016/j.vaccine.2011.06.044. [DOI] [PubMed] [Google Scholar]