Abstract
To evaluate the safety, immunogenicity and batch consistency of Aleph inactivated split influenza vaccine, 3308 healthy Chinese people more than 3 years old were enrolled in a randomized, controlled, blinded study and divided into four age groups: 3–10 years, 11–17 years, 18–54 years, and more than 55 years. Each age group was then randomized (2:1) to receive either influenza vaccine or control vaccine (recombinant hepatitis B) for one dose. Also each influenza vaccine group was randomized (1:1:1) to receive three different batches of influenza vaccine. Systematic and local adverse reactions for 28 days after vaccination were recorded, and influenza antibody titer was determined by hemagglutination inhibition (HI) assay at 28 days after vaccination. There were significant differences in seroconversion and seroprotection rates achieved post-immunization of three strains of influenza antibody (H1N1, H3N2, B) between experimental group and control group in all age groups (P < 0.05). In addition, there were no statistically significant differences in local and systematic reaction rates after vaccination between the experimental and control group in all age groups (P > 0.05), except for the systematic reaction rates in the 18–54 years and ≥ 55 years age groups (P < 0.05). Thus, Aleph inactivated split influenza vaccine has good safety and immunogenicity.
Keywords: inactivated split influenza vaccine, safety, immunogenicity, batch consistency
Introduction
Influenza is an acute respiratory infectious disease caused by influenza virus. Primary influenza illness is characterized by the abrupt start of fever, sore throat, headache, myalgia, chills, anorexia, and extreme fatigue.1,2 Illness typically improves within a week. The risk of developing serious complications from influenza infection is elevated in persons at both age extremes as well as in those with certain underlying conditions.3,4 The most common serious complications of influenza include exacerbation of underlying chronic pulmonary and cardiopulmonary diseases, such as chronic obstructive pulmonary disease, asthma, and congestive heart failure, as well development of bacterial pneumonia usually associated with Streptococcus pneumonia, Staphylococcus aureus, or Hemophilus influenza.
Epidemics and pandemics of respiratory disease, consistent with influenza, have been recorded since the 16th century.5,6 During seasonal epidemics, large numbers of influenza infections can occur in all age groups. In most individuals, influenza is a self-limited illness, but serious secondary complications develop in some of those infected. The resulting illnesses, often requiring ambulatory medical care or hospitalization, substantially contribute to lost work and school time, overwhelmed hospitals and regional medical care systems, and increases in influenza-related hospitalizations and deaths.7-9 Few other infectious diseases have adversely affected the health and economies of global populations as consistently and extensively as influenza.
Influenza vaccination is considered as one of the most effective strategy for preventing influenza. WHO encourages influenza vaccine used in persons at increased risk for complications of influenza in all countries where epidemic surveillance is well established and where reduction of influenza and its complications are public health priorities.10 However, nearly all of the world’s vaccine production capacity is contained in nine countries, mostly in Western Europe and North America, and these countries utilize approximately 60% of the vaccine produced. Thus, comparatively little vaccine is utilized outside of these countries and in the developing world.11 Efforts to improve influenza vaccine capacity for pandemic influenza preparedness are likely to result in worldwide increases in influenza-manufacturing capacity for seasonal vaccine and encourage expanded use of annual vaccine.
Aleph influenza vaccine is a highly-purified inactivated, egg-based trivalent influenza vaccine made in China. It was initially licensed in China in 2005. The aim of this post-marketing clinical study was to assess the safety, immunogenicity and batch consistency of the split inactivated influenza vaccine produced by Aleph Biomedical Co., Ltd.
Results
Subjects and baseline analysis
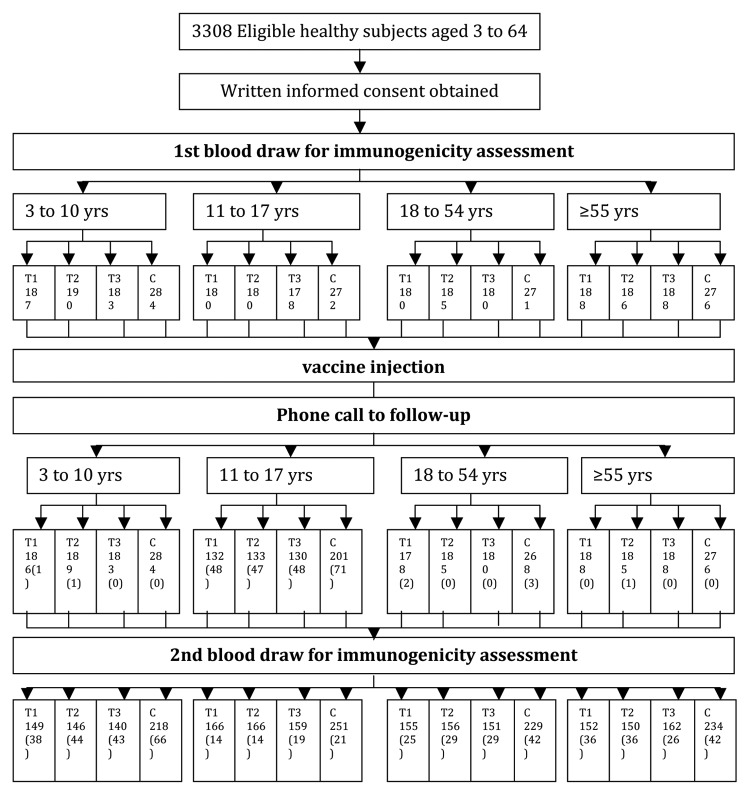
A total of 3308 healthy people more than 3 y old met the inclusion/exclusion criteria for the study and were randomized to the experimental (influenza vaccine) and control (hepatitis B) groups (2205 in experimental group and 1103 in control group). During the study, 222 subjects dropped out (56 cases did not agree to attend follow-ups while 166 subjects were lost to follow-up;,for the absence of participants who were students taking part of a special activity, so there was a much higher drop-out rate in the 11–17 y old group); 3086 (93%) people completed the safety observation (2057 in experimental group and 1029 in control group), and 2784 (84%) had serum antibody level data available from paired (pre- and post-immunization) blood samples (Table 1). There were no statistically significant differences in subjects’ age between the experimental and control groups in all age groups, nor among three experimental groups (three batches of influenza vaccine) (P > 0.05; Table 1). Also there were no statistically significant differences in the pre-immunization influenza antibody against three strains of influenza (H1N1, H3N2, B) geometric mean titer (GMT) between the experimental and control groups in all age groups, nor among three experimental groups (three batches of influenza vaccine) (P > 0.05; Table 2). From Table 2, the GMT of B strain before immunization was higher than other two strains in all four age groups. This indicated that there might be a inapparent infection of B strain in study area.
Table 1. Numbers and mean ages of subjects in the study groups.
| Age group | Subgroup | No. of subjects | Mean age ± SD (years) | No. completing observations | No. with paired serum samples |
|---|---|---|---|---|---|
| 3–10 y | T1 | 187 | 6.85 ± 2.36* | 186 | 149 |
| T2 | 190 | 6.93 ± 2.57 | 189 | 146 | |
| T3 | 183 | 6.74 ± 2.35 | 183 | 140 | |
| C | 284 | 6.89 ± 2.67 | 284 | 218 | |
| 11–17 y | T1 | 180 | 10.81 ± 4.81† | 132 | 166 |
| T2 | 180 | 11.13 ± 4.43 | 133 | 166 | |
| T3 | 178 | 11.04 ± 4.68 | 130 | 159 | |
| C | 272 | 10.74 ± 5.24 | 201 | 251 | |
| 18–54 y | T1 | 180 | 41.53 ± 11.06§ | 178 | 155 |
| T2 | 185 | 42.10 ± 11.60 | 185 | 156 | |
| T3 | 180 | 42.14 ± 10.14 | 180 | 151 | |
| C | 271 | 41.34 ± 10.89 | 268 | 229 | |
| ≥55 y | T1 | 188 | 59.86 ± 9.10¥ | 188 | 152 |
| T2 | 186 | 59.19 ± 14.42 | 185 | 150 | |
| T3 | 188 | 59.17 ± 11.54 | 188 | 162 | |
| C | 276 | 59.52 ± 10.22 | 276 | 234 |
P = 0.8981 vs control group; †P = 0.8557 vs control group; §P = 0.8326 vs control group; ¥P = 0.9145 vs control group.
Table 2. GMTs of H1N1, H3N2 and B antibodies before immunization in the study groups.
| Age group | Subgroup | No. of subjects | Antibody GMT (H1N1) | P value | Antibody GMT (H3N2) | P value | Antibody GMT (B) | P value |
|---|---|---|---|---|---|---|---|---|
| 3–10 y | T1 | 149 | 3.27 ± 4.47 | 1.294 | 4.33 ± 5.14 | 0.74 | 31.52 ± 4.13 | 0.676 |
| T2 | 146 | 2.97 ± 4.11 | 3.66 ± 4.80 | 36.94 ± 3.82 | ||||
| T3 | 140 | 3.41 ± 4.21 | 3.88 ± 4.65 | 30.74 ± 4.62 | ||||
| C | 218 | 3.41 ± 4.34 | 4.29 ± 5.08 | 34.02 ± 3.91 | ||||
| 11–17 y | T1 | 166 | 3.21 ± 4.13 | 0.276 | 3.09 ± 4.05 | 0.352 | 54.36 ± 4.04 | 0.837 |
| T2 | 166 | 3.89 ± 4.29 | 3.48 ± 4.18 | 51.63 ± 3.92 | ||||
| T3 | 159 | 2.86 ± 3.92 | 3.27 ± 4.06 | 47.52 ± 3.72 | ||||
| C | 251 | 3.37 ± 4.17 | 3.93 ± 4.21 | 51.21 ± 3.51 | ||||
| 18–54 y | T1 | 155 | 1.76 ± 3.22 | 0.334 | 1.64 ± 2.81 | 0.771 | 51.34 ± 4.98 | 0.892 |
| T2 | 156 | 1.75 ± 3.22 | 1.57 ± 2.75 | 58.40 ± 4.94 | ||||
| T3 | 151 | 1.83 ± 3.25 | 1.51 ± 2.62 | 55.53 ± 4.27 | ||||
| C | 229 | 1.52 ± 2.63 | 1.48 ± 2.51 | 56.60 ± 4.32 | ||||
| ≥55 y | T1 | 152 | 2.18 ± 3.71 | 0.455 | 3.09 ± 3.60 | 0.353 | 78.56 ± 2.13 | 0.709 |
GMT, geometric mean titer; H1N1, H3N2, B, three strains of influenza.
Immunogenicity results
The seroconversion rates (proportions of subjects with Influenza antibody GMT ≥ 1:40 or increase 4-folds in GMT) of experimental group after immunization in the 3–10 y age group were 86.5% (against H1N1, T1 85.9%, T2 83.7%, T3 90.0%), 94.7% (against H3N2, T1 94.0%, T2 95.2%, T3 95.0%), and 91.1% (against B, T1 91.3%, T2 89.9%, T3 92.1%), compared with 16.4% (against H1N1), 14.2% (against H3N2) and 36.5% (against B) in control group; in the 11–17 y age group were 93.5% (against H1N1, T1 97.0%, T2 91.6%, T3 91.9%), 98.0% (against H3N2, T1 98.8%, T2 97.0%, T3 98.1%), and 79.3% (against B, T1 75.3%, T2 81.3%, T3 81.3%), compared with 5.6% (against H1N1), 2.4% (against H3N2) and 4.4% (against B) in control group; in the 18–54 y age group were 88.3% (against H1N1, T1 89.1%, T2 87.2%, T3 88.7%), 76.3% (against H3N2, T1 73.7%, T2 77.6%, T3 77.5%), and 62.9% (against B, T1 60.9%, T2 60.9%, T3 66.9%), compared with 15.2% (against H1N1), 6.1% (against H3N2) and 20.0% (against B) in control group; in the ≥ 55 y age group were 85.6% (against H1N1, T1 83.0%, T2 86.7%, T3 87.0%), 95.9% (against H3N2, T1 94.8%, T2 96.7%, T3 96.3%), and 78.5% (against B, T1 75.8%, T2 81.3%, T3 78.4%), compared with 7.7% (against H1N1), 7.7% (against H3N2) and 6.0% (against B) in control group. The seroconversion rates of experimental groups were much higher than that of control groups. There were significant differences in seroconversion rates achieved post-immunization of three strains of influenza antibody (H1N1, H3N2, B) between experimental group and control group in all age groups (P < 0.05; Table 3). There were no significant differences in seroconversion rates achieved post-immunization of three strains of influenza antibody (H1N1, H3N2, B) among three experimental groups (three batches of influenza vaccine) in all age groups (P > 0.05; Table 3).
Table 3. Seroconversion rates of influenza antibody after immunization in the study groups.
| Age group | Subgroup | No. of subjects | Antibody seroconversion (H1N1) | Antibody seroconversion (H3N2) | Antibody seroconversion (B) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Percentage | P value | No. | Percentage | P value | No. | Percentage | P value | |||
| 3–10 y | T1 | 149 | 128 | 85.9 | 140 | 94 | 136 | 91.3 | |||
| T2 | 146 | 123 | 83.7 | <0.001* | 140 | 95.2 | <0.001* | 132 | 89.8 | <0.001* | |
| T3 | 140 | 126 | 90 | 0.285† | 133 | 95 | 0.870† | 129 | 92.1 | 0.779† | |
| C | 218 | 36 | 16.4 | 31 | 14.2 | 80 | 36.5 | ||||
| 11–17 y | T1 | 166 | 161 | 97 | 164 | 98.8 | 125 | 75.3 | |||
| T2 | 166 | 152 | 91.6 | <0.001* | 161 | 97 | <0.001* | 135 | 81.3 | <0.001* | |
| T3 | 159 | 147 | 91.9 | 0.081† | 157 | 98.1 | 0.499† | 130 | 81.3 | 0.301† | |
| C | 251 | 14 | 5.6 | 6 | 2.4 | 11 | 4.4 | ||||
| 18–54 y | T1 | 155 | 139 | 89.1 | 115 | 73.7 | 95 | 60.9 | |||
| T2 | 156 | 136 | 87.2 | <0.001* | 121 | 77.6 | <0.001* | 95 | 60.9 | <0.001* | |
| T3 | 151 | 134 | 88.7 | 0.854† | 117 | 77.5 | 0.661† | 101 | 66.9 | 0.458† | |
| C | 229 | 35 | 15.2 | 14 | 6.1 | 46 | 20 | ||||
| ≥55 y | T1 | 152 | 127 | 83 | 145 | 94.8 | 116 | 75.8 | |||
| T2 | 150 | 130 | 86.7 | <0.001* | 145 | 96.7 | <0.001* | 122 | 81.3 | <0.001* | |
| T3 | 162 | 141 | 87 | 0.537† | 156 | 96.3 | 0.675† | 127 | 78.4 | 0.505† | |
| C | 234 | 18 | 7.7 | 18 | 7.7 | 14 | 6 | ||||
Seroconversion rate, the proportion of subjects whose antibody GMT met the definition of seroconversion after vaccination; H1N1, H3N2, B, three strains of influenza; *P value between experimental and control groups; †P value between three experimental groups (three batches of influenza vaccine).
The seroprotection rates (proportions of subjects whose influenza antibody GMT ≥ 1:40) of experimental group after immunization in the 3–10 y age group were 87.6% (against H1N1, T1 87.9%, T2 85.0%, T3 90.0%), 99.3% (against H3N2, T1 100.0%, T2 100.0%, T3 97.9%), and 99.3% (against B, T1 99.3%, T2 98.6%, T3 100.0%), while they were 25.1% (against H1N1), 25.1% (against H3N2) and 72.6% (against B) in control group; in the 11–17 y age group were 95.3% (against H1N1, T1 97.6%, T2 94.0%, T3 94.4%), 100.0% (against H3N2, T1 100.0%, T2 100.0%, T3 100.0%), and 99.5% (against B, T1 99.4%, T2 99.4%, T3 98.8%), while they were 9.2% (against H1N1), 6.0% (against H3N2) and 30.7% (against B) in control group; in the 18–54 y age group were 90.3% (against H1N1, T1 90.4%, T2 89.7%, T3 90.7%), 78.2% (against H3N2, T1 75.6%, T2 80.1%, T3 78.8%), and 98.7% (against B, T1 98.7%, T2 98.7%, T3 98.7%), while they were 17.4% (against H1N1), 7.0% (against H3N2) and 52.2% (against B) in control group; in the ≥ 55 y age group were 88.8% (against H1N1, T1 86.3%, T2 89.3%, T3 90.7%), 96.1% (against H3N2, T1 94.8%, T2 96.7%, T3 96.9%), and 99.5% (against B, T1 99.3%, T2 99.3%, T3 100.0%), while they were 10.3% (against H1N1), 9.4% (against H3N2) and 49.1% (against B) in control group. The seroprotection rates of experimental groups were much higher than that of control groups. There were significant differences in seroprotection rates achieved post-immunization of three strains of influenza antibody (H1N1, H3N2, B) between experimental group and control group in all age groups (P < 0.05; Table 4). There were no significant differences in seroprotection rates achieved post-immunization of three strains of influenza antibody (H1N1, H3N2, B) among three experimental groups (three batches of influenza vaccine) in all age groups (P > 0.05; Table 4), except for influenza antibody against H3N2 in the 3–10 y age group (P < 0.05; Table 4).
Table 4. Seroprotection rates of influenza antibody after immunization in the study groups.
| Age group | Subgroup | No. of subjects | Antibody seroprotection (H1N1) | Antibody seroprotection (H3N2) | Antibody seroprotection (B) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Percentage | P value | No. | Percentage | P value | No. | Percentage | P value | |||
| 3–10 y | T1 | 149 | 131 | 87.9 | 149 | 100 | 148 | 99.3 | |||
| T2 | 146 | 125 | 85 | <0.001* | 147 | 100 | <0.001* | 145 | 98.6 | <0.001* | |
| T3 | 140 | 126 | 90 | 0.438† | 137 | 97.9 | 0.041† | 140 | 100 | 0.378† | |
| C | 218 | 55 | 25.1 | 55 | 25.1 | 159 | 72.6 | ||||
| 11–17 y | T1 | 166 | 162 | 97.6 | 166 | 100 | 165 | 99.4 | |||
| T2 | 166 | 156 | 94 | <0.001* | 166 | 100 | <0.001* | 165 | 99.4 | <0.001* | |
| T3 | 159 | 151 | 94.4 | 0.233† | 160 | 100 | † | 158 | 98.8 | 0.755† | |
| C | 251 | 23 | 9.2 | 15 | 6 | 77 | 30.7 | ||||
| 18–54 y | T1 | 155 | 141 | 90.4 | 118 | 75.6 | 154 | 98.7 | |||
| T2 | 156 | 140 | 89.7 | <0.001* | 125 | 80.1 | <0.001* | 154 | 98.7 | <0.001* | |
| T3 | 151 | 137 | 90.7 | 0.957† | 119 | 78.8 | 0.615† | 149 | 98.7 | 0.999† | |
| C | 229 | 40 | 17.4 | 16 | 7 | 120 | 52.2 | ||||
| ≥55 y | T1 | 152 | 132 | 86.3 | 145 | 94.8 | 152 | 99.3 | |||
| T2 | 150 | 134 | 89.3 | <0.001* | 145 | 96.7 | <0.001* | 149 | 99.3 | <0.001* | |
| T3 | 162 | 147 | 90.7 | 0.441† | 157 | 96.9 | 0.565† | 162 | 100 | 0.584† | |
| C | 234 | 24 | 10.3 | 22 | 9.4 | 115 | 49.1 | ||||
Seroprotection rate, the proportion of subjects whose antibody GMT met the definition of seroprotection after vaccination; H1N1, H3N2, B, three strains of influenza; *P value between experimental and control groups; †P value between three experimental groups (three batches of influenza vaccine).
The mean fold increase ratios of GMT in experimental group after immunization in the 3–10 y age group were 65.7 (against H1N1, T1 69.2, T2 70.9, T3 57.1), 89.2 (against H3N2, T1 90.2, T2 92.6, T3 84.9), and 28.5 (against B, T1 32.0, T2 24.0, T3 29.6); in the 11–17 y age group were 84.2 (against H1N1, T1 86.4, T2 70.6, T3 95.6), 60.2 (against H3N2, T1 65.1, T2 58.4, T3 57.2), and 8.9 (against B, T1 8.5, T2 8.4, T3 9.9); in the 18–54 y age group were 87.2 (against H1N1, T1 82.5, T2 87.3, T3 91.8), 38.9 (against H3N2, T1 32.8, T2 41.1, T3 42.7), and 6.3 (against B, T1 6.6, T2 6.0, T3 6.2); in the ≥ 55 y age group were 54.0 (against H1N1, T1 54.2, T2 58.4, T3 49.5), 47.5 (against H3N2, T1 45.8, T2 54.7, T3 41.9), and 6.8 (against B, T1 6.2, T2 6.7, T3 7.6) (Table 5).
Table 5. GMTs of H1N1, H3N2 and B antibodies and GMT mean ratio after immunization in the experimental groups.
| Age group | Subgroup | No. of subjects | Antibody GMT (H1N1) | Antibody GMT (H3N2) | Antibody GMT (B) | |||
|---|---|---|---|---|---|---|---|---|
| GMT (after) | mean ratio | GMT (after) | mean ratio | GMT (after) | mean ratio | |||
| 3–10 y | T1 | 149 | 226.12 ± 5.70 | 69.2 | 390.86 ± 2.72 | 90.2 | 1009.65 ± 3.77 | 32 |
| T2 | 146 | 210.33 ± 4.9 | 70.9 | 338.63 ± 2.75 | 92.6 | 886.09 ± 3.47 | 24 | |
| T3 | 140 | 194.73 ± 5.27 | 57.1 | 329.65 ± 2.98 | 84.9 | 909.59 ± 3.96 | 29.6 | |
| 11–17 y | T1 | 166 | 277.65 ± 3.09 | 86.4 | 201.31 ± 1.87 | 65.1 | 462.10 ± 2.40 | 8.5 |
| T2 | 166 | 274.19 ± 3.35 | 70.6 | 203.00 ± 2.05 | 58.4 | 432.23 ± 2.66 | 8.4 | |
| T3 | 159 | 273.79 ± 3.28 | 95.6 | 187.00 ± 1.99 | 57.2 | 472.58 ± 2.56 | 9.9 | |
| 18–54 y | T1 | 155 | 145.33 ± 3.60 | 82.5 | 53.64 ± 3.01 | 32.8 | 340.54 ± 2.74 | 6.6 |
| T2 | 156 | 152.83 ± 3.33 | 87.3 | 64.54 ± 2.66 | 41.1 | 352.86 ± 2.60 | 6 | |
| T3 | 151 | 168.29 ± 3.36 | 91.8 | 64.49 ± 3.16 | 42.7 | 342.81 ± 2.75 | 6.2 | |
| ≥55 y | T1 | 152 | 118.48 ± 3.43 | 54.2 | 141.58 ± 2.22 | 45.8 | 485.47 ± 3.18 | 6.2 |
| T2 | 150 | 154.19 ± 3.55 | 58.4 | 153.48 ± 2.32 | 54.7 | 496.37 ± 2.58 | 6.7 | |
| T3 | 162 | 135.41 ± 3.3 | 49.5 | 150.70 ± 2.23 | 41.9 | 534.73 ± 2.73 | 7.6 | |
GMT, geometric mean titer after immunization; H1N1, H3N2, B, three strains of influenza.
Furthermore, equivalence study was used to analysis the batch consistency of the study influenza vaccine. A range from -10% to 10% was set as the equivalence interval at the beginning of the study. From the study, the 95% CI when comparing the seroprotection rates of three batches of influenza vaccine were all included in the range from -10% to 10%, so it could be considered that three batches of influenza vaccine are equivalence (Table 6).
Table 6. 95% CI of seroprotection rates of influenza antibody after immunization between three batches of influenza vaccine.
| Age group | Subgroup | No. of subjects | Antibody seroprotection (H1N1) | Antibody seroprotection (H3N2) | Antibody seroprotection (B) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Percentage | 95%CI | No. | Percentage | 95%CI | No. | Percentage | 95%CI | |||
| ≥3 y | T1 | 622 | 566 | 91 | (–2.1%, 4.5%)1 | 578 | 92.9 | (–2.5%, 2.8%)1 | 619 | 99.5 | (–0.6%, 1.2%) 1 |
| T2 | 618 | 555 | 89.8 | (–2.4%, 3.8%)2 | 583 | 94.3 | (–2.1%, 3.5%)2 | 613 | 99.2 | - | |
| T3 | 612 | 561 | 91.7 | (–1.3%, 5.1%)3 | 573 | 93.6 | (–2.0%, 3.4%)3 | 609 | 99.5 | (–0.6%, 1.2%) 3 | |
1 95% CI when comparing the seroprotection rates of T1 and T2; 2, 95% CI when comparing the seroprotection rates of T1 and T3; 3, 95% CI when comparing the seroprotection rates of T2 and T3.
Safety findings
Within 30 min after vaccination, a total of 173 (5.6%) adverse events was reported. The most adverse reaction is mild fever. All the reactions had been resolved within 72 h after immunization without special treatments. There were no significant differences in 30 min reaction rates between experimental group and control group in all age groups (P > 0.05; Table 6), except for the 3–10 y age group which had slight difference between experimental and control groups (P < 0.05; Table6). There were no significant differences in 30 min reaction rates among three experimental groups (three batches of influenza vaccine) in all age groups (P > 0.05; Table 6).
Local adverse reactions occurring after vaccination mainly included erythema, induration, pain and itch. They were mild and had a short duration up to 3 d. A total of 180 subjects exhibited local reactions after immunization, which contained 115 subjects (5.21%) of experimental group and 65 subjects (5.89%) of control group. There were no statistically significant differences in local reaction rates after vaccination between the experimental and control groups in all age groups (P > 0.05; Table 6). There were no significant differences in local reaction rates between three experimental groups (three batches of influenza vaccine) in all age groups (P > 0.05; Table 6).
Systemic adverse reactions after vaccination mainly included headache, discomfort, fatigue, and myalgia. Very few subjects also exhibited allergy, nausea and vomiting. All systemic reactions were mild and had a short duration up to 4–5 d, causing no harm to any of the subjects. A total of 171 subjects exhibited systemic reactions after vaccination, which contained 110 subjects (4.98%) of experimental group and 61 subjects (5.53%) of control group. Although there were no significant differences in systematic reaction rates between experimental group and control group in the 3–10 y and 11–17 y age groups (P > 0.05; Table 6), the differences were found in the 18–54 y and ≥ 55 y age groups (P < 0.05; Table 6). There were no significant differences in systematic reaction rates among three experimental groups (three batches of influenza vaccine) in all age groups (P > 0.05; Table 6).
Discussion
Winter and spring were the most prevalent seasons for influenza virus in China, and influenza vaccination was very important for influenza prevention. Influenza immunization for free in the autumn season has been launching in Beijing by the government every year since 2007,which was promoted in Children under 18 y old and elders more than 60 y old. We have researched the influenza vaccine produced by GSK and Sanofi Pasteur before, they all have good safety and immunogenity. However, there was very few data and information published about influenza vaccine made in China. Thus, it was necessary to study influenza vaccine produced in China and used in Chinese people.
According to the quality standards of inactivated split influenza vaccine issued by European Medicines Agency, influenza vaccine antibody (HI antibody) seroconversion rate should be more than 40%, seroprotection rate should be more than 70% and the mean fold increase of GMT for HI antibody should be more than 2.5-fold after immunization.12
The present study was designed to evaluate the safety, immunogenicity and batch consistency of Aleph inactivated split influenza vaccine in people more than 3 y old, and thus provide a fully comprehension about influenza vaccine made in China. The study showed that the seroconversion rates of experimental group after immunization in all age groups were 62.9% to 98.0% in three strains of influenza antibody (H1N1, H3N2, B). They were all greater than 40% (the European Standards). They were in accordance with the results of other studies of influenza vaccine. In Tregnaghi’s study, the seroconversion rates of influenza vaccine made by Novartis after immunization were 68.0% to 92.0% in three strains of influenza antibody (H1N1, H3N2, B); the seroconversion rates of influenza vaccine made by GSK after immunization were 74.0% to 92.0% in three strains of influenza antibody (H1N1, H3N2, B).13 Consequently, there were significant differences in seroconversion rates achieved post-immunization of three strains of influenza antibody (H1N1, H3N2, B) between experimental group and control group in all age groups (P < 0.05). The seroprotection rates of experimental group after immunization in all age groups were 78.2% to 100.0% in three strains of influenza antibody (H1N1, H3N2, B). They were also greater than 70% (the European Standards). There were significant differences in seroprotection rates achieved post-immunization of three strains of influenza antibody (H1N1, H3N2, B) between experimental group and control group in all age groups (P < 0.05). The mean fold increase ratios of GMT in experimental groups after immunization were also greater than 2.5-folds (the European Standards), they were 49 to 96 in H1N1 strain, 32 to 93 in H3N2 strain, and 6 to 32 in B strain respectively. Safety findings showed that there were no statistically significant differences in local reaction rates after vaccination between the experimental and control groups (P > 0.05). Also there were no significant differences in systematic reaction rates between experimental groups and control groups in the 3–10 y and 11–17 y age groups (P > 0.05), but a slight differences were found in the 18–54 y and ≥ 55 y age groups (P < 0.05). The result of the study we just reported upside indicated that the split inactivated influenza vaccine produced by Aleph Biomedical Co., Ltd had reached the quality standards of inactivated split influenza vaccine issued by European Medicines Agency, it has good safety and immunogenicity.
In addition, in this study we also arranged subgroups which contained all the experimental group subjects to evaluate the batch consistency of the study vaccine. The subjects were all received the influenza vaccine with three different batch number. From the study, the 95% CI when comparing the seroprotection rates of three batches of influenza vaccine were all included in the range from −10% to 10% (Table 6). Just from the data, we could consider that three batches of influenza vaccine are equivalence. But as we described in “Materials and Methods,” the sample size evaluation in the study was determined according to the formula of two sample compare rate for excellent efficiency test (the experimental and control group), it was not determined by equivalence test (three batches of influenza vaccine), so we thought the conclusion of batch consistency still had some limits (Table 7).
Table 7. Reactions in 30 min, local reactions and systemic reactions during 28 d after vaccination in the study groups.
| Age group | Subgroup | No. of subjects | Reactions in 30minutes | Local reactions | Systemic reactions | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Percentage | P value | No. | Percentage | P value | No. | Percentage | P value | |||
| 3–10 y | T1 | 186 | 9 | 4.84 | 14 | 7.53 | 9 | 4.84 | |||
| T2 | 189 | 9 | 4.76 | 0.045* | 13 | 6.88 | 0.130* | 16 | 8.47 | 0.234* | |
| T3 | 183 | 18 | 9.78 | 0.079† | 10 | 5.43 | 0.710† | 19 | 10.33 | 0.137† | |
| C | 284 | 29 | 10.21 | 20 | 7.04 | 20 | 7.04 | ||||
| 11–17 y | T1 | 132 | 13 | 9.85 | 10 | 7.58 | 13 | 9.85 | |||
| T2 | 133 | 11 | 8.27 | 0.309* | 8 | 6.02 | 0.440* | 9 | 6.77 | 0.442* | |
| T3 | 130 | 14 | 10.77 | 0.785† | 7 | 5.38 | 0.754† | 8 | 6.15 | 0.488† | |
| C | 201 | 11 | 5.47 | 16 | 7.96 | 21 | 10.45 | ||||
| 18–54 y | T1 | 178 | 6 | 3.37 | 10 | 5.62 | 9 | 5.06 | |||
| T2 | 185 | 3 | 1.62 | 0.107* | 9 | 4.86 | 0.176* | 2 | 1.08 | 0.042* | |
| T3 | 180 | 11 | 6.11 | 0.072† | 16 | 8.89 | 0.253† | 8 | 4.44 | 0.846† | |
| C | 268 | 15 | 5.6 | 11 | 4.1 | 5 | 1.87 | ||||
| ≥55 y | T1 | 188 | 2 | 1.06 | 7 | 3.72 | 6 | 3.19 | |||
| T2 | 185 | 3 | 1.62 | 0.083* | 3 | 1.62 | 0.078* | 2 | 1.08 | 0.026* | |
| T3 | 188 | 6 | 3.19 | 0.597† | 8 | 4.26 | 0.313† | 9 | 4.79 | 0.107† | |
| C | 276 | 13 | 4.71 | 18 | 6.52 | 15 | 5.43 | ||||
P value between experimental and control groups; †P value between three experimental groups (three batches of influenza vaccine).
This paper only summarized a part of our research about the study TIV. The research also contained TIV immune persistence study. The negative control group was considered as the detection of the natural infection in the local area. Influenza which was a respiratory infectious disease, could induce recessive infection in local people from endemic area. This recessive infection could induce antibody to Influenza virus grow faster and higher than normal unvaccined people. Based on this situation, we could not estimate the exact vaccine immunogenicity if we choose control as another influenza vaccine. So we only chose negative vaccine as control.
In conclusion, this clinical study with large samples collection and comparison of different age groups indicated that this influenza vaccine had good safety and immunogenicity, and could be spreaded use in regular seasonal influenza vaccination and emergency vaccination when influenza outbreak. The study has lots of advantages, such as it has large samples and it is multicenter clinical trial. Except for the batch consistency of the vaccine as mentioned above, the study also has the limitation that it has only studied the immunogenicity of influenza vaccine, but hasn’t studied the protective effect of population because of the specificity of influenza disease. First, the mobile population in the study area had a huge proportion, so it was difficult to monitor the subjects for a long time. Second, because influenza surveillance was not incorporated into national legal infectious disease surveillance in China, there were no entrance for report through network. Third, we think there was not suitable for influenza surveillance only through clinical diagnose, experimental diagnose was also necessary for the surveillance. But lacking sufficient medical resources, we could not diagnose through experimental inspection. We have also been studying the immune persistence of influenza vaccine, which will be reported in a different paper.
Materials and Methods
Study design and subjects
We performed a randomized, controlled, blinded study in healthy people more than 3 y old without autoimmune diseases, acute illnesses and history of allergy who had no history of symptoms of upper respiratory infection within the last 6 mo, and had not previously received Influenza vaccination since 2008 (Fig. 1). In the study we employed negative control people in control group received HBV vaccination, the sample size evaluation was determined according to the formula of two sample compare rate for excellent efficiency test. We adopted 20% as the original positive percent in control group, we also estimate the seroconvention in experimental group was 90%, researchers considered seroconvention in experimental 60% more than seroconvention in control group as clinical reference. Significant level α = 0.025, study power 1-β = 0.80, sample size in control group of each age group was 161. Besides, SFDA did not specify the uniform requirements for sample size of vaccine post-marketing studying. Two thousand was the standard sample size for post-marketing drug evaluation from SFDA (The guiding principle of drug clinical trialpost-market). Finally we planned to recruit 2160 participants in experimental group and 1080 in control group totally. The study was performed in Zhuolu county and Xinglong county in Hebei Province in China. The subjects were divided into four age groups in a 1:1:1:1 ratio – they were 3–10 y old, 11–17 y old, 18–54 y old, and more than 55 y old. Each age group was then randomized (2:1) to an experimental (influenza) group and a control group (recombinant hepatitis B) via SAS 8.1 (45 per block; 30 to experimental group and 15 to control group). Meantime each experimental group was randomized (1:1:1) to receive three batches of influenza vaccine. We packed the experimental vaccine and control vaccine again in order to mix these vaccines. In the study, the blood collection doctors and the safety observation researchers could not realize which vaccine the participants received. The immunization schedule was as follows: Subjects in experimental group received one dose of influenza vaccine into the deltoid muscle by intramuscular injection, while subjects in control group received one dose of recombinant hepatitis B vaccine into the deltoid muscle by intramuscular injection. The immunization completed before the validity date of the study influenza vaccine, that is 30 April, 2012.
Figure 1. Study outline. Number in bracket is the number of drop-outs.
The study was approved by the Ethical Committee, and it was conducted in compliance with Good Clinical Practice guidance and the Declaration of Helsinki. Before enrollment, informed consent was obtained from subjects or guardians.
The study was registered in ClinicalTrials.gov, the identifier is NCT01758185.
Vaccine
The inactivated split influenza vaccine used in the study was manufactured by Aleph, and contained A/California/7/2009(H1N1) 15μg, A/Victoria/210/2009(H3N2) 15 μg and B/Brisbane/60/2008 15 μg per dose(0.5 ml). The vaccine batch numbers were 20110519, 20110521 and 20110522, and they were valid until 30 April, 2012. The recombinant hepatitis B vaccine used in the study was manufactured by Hissen, and contained HBsAg 10 μg per dose(0.5 ml). The vaccine batch number was 2010031004, and it was valid until May 5, 2013.
Safety evaluation
Safety observations were performed by the investigators in all subjects at 30 min and at 6, 24, 48, and 72 h after vaccinations. Symptoms such as redness, induration, skin rash, fever, vomiting, lethargy and loss of appetite were assessed and recorded. In addition, adverse events that occurred in the period from days 4 to 28 after vaccination were also recorded (subjects/guardians were told to call the study center if adverse events were experienced).
Immunogenicity evaluation
Two ml blood samples were obtained from subjects before vaccination and at 28 d after completion of the immunization schedule. Serum influenza antibody against the vaccine strains of H1N1, H3N2, and B were measured by hemagglutination-inhibition assay (HI), according to standard methods.12
The seroconversion of influenza antibody after immunization was defined as the influenza antibody GMT ≥ 1:40 if antibody GMT < l:l0 before vaccination. If antibody GMT ≥ l:10 before vaccination, the seroconversion would be also defined as the increase of GMT was 4-fold after immunization. The seroprotection of influenza antibody was defined as the antibody GMT ≥ 1:40. The proportion of subjects whose antibody GMT met the definition of seroconversion after vaccination was defined as seroconvention rate. The proportion of subjects whose antibody GMT met the definition of seroprotection after vaccination was defined as seroprotection rate.
Statistical analysis
The seroconversion rates and seroprotection rates of influenza antibody after immunization were determined. Vaccine safety was evaluated via the incidence rates of all post-vaccination local and systemic adverse reactions.
Epidata software was used for data input and SPSS 16.0 statistical software was applied for data processing. Comparisons between the study groups about seroconvertion rates and seroprotection rates were tested by χ2 and t test was used in comparing antibody geometric mean titer (GMT) in the study groups. The incidence rate of adverse reactions among the study groups was inspected by a χ2 test, with an inspection level of α = 0.05.
Disclosure of Potential Conflicts of Interest
The authors declare no potential conflicts of interest.
Funding
This study was supported by Aleph Biomedical Co., Ltd.
Acknowledgments
The study was approved by the Ethical Committee, and it was conducted in compliance with Good Clinical Practice guidance and the Declaration of Helsinki. Before enrollment, informed consent was obtained from subjects or guardians.
The study was registered in ClinicalTrials. gov, the identifier is NCT01758185.
Glossary
Abbreviations:
- HI
hemagglutination-inhibition assay
- GMT
geometric mean titer
- Seroconversion rate
the proportion of subjects whose antibody GMT met the definition of seroconversion after vaccination
- Seroprotection rate
the proportion of subjects whose antibody GMT met the definition of seroprotection after vaccination
References
- 1.Neuzil KM, O’Connor TZ, Gorse GJ, Nichol KL. Recognizing influenza in older patients with chronic obstructive pulmonary disease who have received influenza vaccine. Clin Infect Dis. 2003;36:169–74. doi: 10.1086/345668. [DOI] [PubMed] [Google Scholar]
- 2.Monto AS, Gravenstein S, Elliott M, Colopy M, Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch Intern Med. 2000;160:3243–7. doi: 10.1001/archinte.160.21.3243. [DOI] [PubMed] [Google Scholar]
- 3.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 4.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, Fukuda K. Influenza-associated hospitalizations in the United States. JAMA. 2004;292:1333–40. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 5.Creighton C. History of Epidemics in Britain, AD 664-1666. New York: Cambridge University Press; 1891. [Google Scholar]
- 6.Thompson T. Annals of influenza or epidemic catarrhal fever in Great Britain from 1510 to 1873. London: Sydenham Society; 1852. [Google Scholar]
- 7.Greene SK, Rett MD, Vellozzi C, Li L, Kulldorff M, Marcy SM, Daley MF, Belongia EA, Baxter R, Fireman BH, et al. Guillain-Barré Syndrome, Influenza Vaccination, and Antecedent Respiratory and Gastrointestinal Infections: A Case-Centered Analysis in the Vaccine Safety Datalink, 2009-2011. PLoS One. 2013;8:e67185. doi: 10.1371/journal.pone.0067185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gefenaite G, Tacken M, Bos J, Stirbu-Wagner I, Korevaar JC, Stolk RP, Wolters B, Bijl M, Postma MJ, Wilschut J, et al. Effectiveness of A(H1N1)pdm09 influenza vaccine in adults recommended for annual influenza vaccination. PLoS One. 2013;8:e66125. doi: 10.1371/journal.pone.0066125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frey S, Vesikari T, Szymczakiewicz-Multanowska A, Lattanzi M, Izu A, Groth N, Holmes S. Clinical efficacy of cell culture–derived and egg‐derived inactivated subunit influenza vaccines in healthy adults. Clin Infect Dis. 2010;51:997–1004. doi: 10.1086/656578. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Update: Influenza activity--United States, 2001-02 season. MMWR Morb Mortal Wkly Rep. 2002;51:276–9. [PubMed] [Google Scholar]
- 11.Stanley A, Orenstein PA. Vaccines, 6th Edition. Amsterdam: Elsevier; 2011. [Google Scholar]
- 12.Palmer DF, Dowle WR, Coleman MT, Schild GC. Advances in laboratory technicals for immunological diagnostics. Atlanta: U.S. Dept. of Health, Education, and Welfare 1975; 25–62. [Google Scholar]
- 13.Tregnaghi MW, Stamboulian D, Vanadía PC, Tregnaghi JP, Calvari M, Fragapane E, Casula D, Pellegrini M, Groth N. Immunogenicity, safety, and tolerability of two trivalent subunit inactivated influenza vaccines: a phase III, observer-blind, randomized, controlled multicenter study. Viral Immunol. 2012;25:216–25. doi: 10.1089/vim.2011.0063. [DOI] [PubMed] [Google Scholar]