1. Introduction
In a constantly changing environment, observers are continually flooded with a barrage of sensory information. Because of the limited capacity of the visual system, only a subset of this information can be processed in depth, at the cost of competing information. Thus, a mechanism is needed to select only those cues for further processing that are relevant to guide motivated behavior. Theories of selective attention (Hillyard et al., 1973) converge to suggest that allocation of attention to competing representations is influenced and ultimately determined by a combination of stimulus properties (such as intensity and saliency) and attentional top-down signals, which can bias competition among stimuli for neural representation (Desimone and Duncan, 1995; Reynolds and Heeger, 2009).
Emotionally arousing stimuli attract attentional resources based on their inherent stimulus significance, optimizing their processing in sensory systems (Bradley et al., 2003), which may be at the cost of processing concurrent information (Ihssen et al. 2007). Empirically, task-irrelevant appetitive and aversive stimuli (as distractors) interfere with a variety of perceptual and cognitive tasks (Schimmack and Derryberry, 2005) suggesting that they are powerful competitors for limited capacity. Evidence for distraction by the presence of emotional stimuli in the visual domain comes from a broad base of behavioral, physiological, electrocortical, and neuroimaging studies (Öhman et al., 2001; Sabatinelli et al., 2005; Müller et al., 2008; Wangelin et al., 2011; Shafer et al., 2012; Wieser et al., 2012).
For several decades, clinical and translational research has examined the role of heightened attention to fear-related stimuli (hypervigilance) in patients diagnosed with disorders of fear and anxiety (Öhman and Mineka, 2001). Hypervigilance, or attentional bias, has been implicated as an important factor in the etiology and maintenance of disorders in the fear and anxiety spectrum (Becker et al., 2001; see Bar-Haim et al., 2007 for review). Recently, training procedures have been developed building on these findings, and have demonstrated that reducing hypervigilance has been effective in reducing symptom report in anxiety patients (McNally, 2007; Amir et al., 2009;). Studies investigating the mechanisms mediating hypervigilance have consistently found heightened sensitivity to visual features associated with specific phobic objects in paradigms as diverse as categorical perception and visual search (Kolassa et al., 2007a; Straube et al., 2007; Lipka et al., 2011; see Heeren et al., for review).
Alternatively, a hypervigilance-avoidance hypothesis of fear processing (e.g., Mogg et al., 1989; Mathews, 1990;) posits a sequence in which initial hypervigilance is subsequently followed by perceptual avoidance of the fear-relevant stimulus in observers high in trait anxiety. Evidence of fear-related avoidance (Bögels and Mansell, 2004) has been less consistently found but has been most robust in studies of eye-tracking (Mogg et al., 2000, Garner et al., 2006). For instance, Wieser at al. (2009) presented angry, happy, and neutral faces to students characterized by high or low fear of negative evaluation and found that high-fear participants initially looked more at emotionally arousing faces than to neutral faces (hypervigilance), but that this pattern reversed in the second half of the period (avoidance). Similarly, Pflugshaupt and colleagues (Pflugshaupt et al., 2005) demonstrated that compared to controls, observers with spider phobia initially fixated at locations closer to spiders, but subsequently fixated at locations further away from spiders.
To examine processing of specific fear cues at the level of lower-tier visual cortical processing, we employed steady-state visual evoked potentials (ssVEPs; Regan, 1989), which measure the amplitude of neural activity elicited by a task-relevant stimulus that is flickering at a known rate; competition by a distractor can therefore be measured as an attenuation of the task-evoked processing. Because ssVEPs possess high signal-to-noise ratios and excellent psychometric reliability, they may be particularly suitable for studying individual differences in terms of perception or attention, for instance differences between high and low fearful individuals. An additional advantage of ssVEPs is that they are readily converted to a time-varying measure of neural population activity in visual cortex, providing an opportunity to quantify the time course of competition for processing resources in a neurophysiologically meaningful way (Müller et al., 2008). By studying the time-varying ssVEP amplitude for distractors and task arrays separately, researchers can assess fluctuations in visual cortical engagement both within and between control and high-fear subjects, and across conditions. Capitalizing on the strengths of this approach, the present study addresses inter-individual differences regarding both the time course and neurophysiological locus of distraction effects seen in observers high in snake fear.
Using ssVEPs in non-anxious observers, Müller and colleagues (2008) and Hindi Attar et al. (2010) both found a reduction in the overall ssVEP amplitude evoked by flickering dots when the dots were superimposed on emotionally arousing, compared to neutral, distractor pictures. Quantifying interference using face-evoked ssVEPs in socially anxious observers, (Wieser et al., 2012) observed competition between a social threat cue and a task stimulus only when task demands were high and the distractors and the task stimuli overlapped.
Here, we examined the extent to which individual differences in self-reported fear of snakes modulates ssVEP amplitude across an interval in which fear-relevant images serve as distractors. Task-relevant stimuli were moving dots, flickering at 8.57 Hz, which moved randomly or with brief intervals of coherent motion; the participants’ task was to detect coherent motion of the flickering dots. Distractor pictures were presented in the background of the moving dot display (i.e. overlapping) and included snake pictures, as well as emotionally arousing content depicting erotica and violence, as well as control pictures. We expected to replicate previous findings of attenuated ssVEP amplitude for the moving dot task when emotional, compared to neutral, pictures served as distractors. Thus, for instance, we expected both high-fear participants and controls to show reduction of ssVEP amplitude when pictures of violence were distractors.
Of central importance was ssVEP task-evoked amplitude when snake pictures served as distractors: we expected ssVEP amplitude to show more attenuation than pictures of violence for high-fear participants, whereas the opposite was expected for controls. To test hypotheses regarding whether hypervigilance and/or avoidance are supported by a direct neural index of time-varying visual cortical engagement, the following predictions were made: If sustained hypervigilance in the presence of the fear cue presentation characterizes phobic processing (Kolassa et al., 2007b; Wieser et al., 2011; Wieser et al., 2012), the data would indicate reduced ssVEP amplitude for dots superimposed over snake pictures that persists across the presentation interval for high-fear participants. Alternatively, a hypervigilance-avoidance hypothesis predicts that an initial reduction in ssVEP amplitude for dots appearing over fear-relevant stimuli for high-fear participants will be followed by enhanced ssVEPs, indicating perceptual avoidance.
2. Methods
2.1 Participants
Forty-one female right-handed undergraduate students at the University of Florida provided written consent following the guidelines proposed by the University of Florida’s Institutional Review Board and received either course credit or compensation (20 USD) for their participation. All participants were screened for photic epilepsy. Half of the sample was selected based on a prescreening inventory given to 561 undergraduate students participating in the Introduction to Psychology course: Students scoring within the top 15% on the 30-item snake fear questionnaire (SNAQ; Klorman et al., 1974) were contacted and recruited into the high-fear group. Participants in this group scored well above the 85th percentile in the SNAQ (mean 20.58, SD 3.53, 85th percentile for female students = 17). The remaining participants were unselected and reported common levels of snake fear (SNAQ mean = 6.31, SD = 3.78). All participants included in the final analysis performed the coherent motion detection task at a minimal level of 60% or better (average: 72.9% correct, range: 61.68–98.3% correct), which is consistent with accuracy thresholds used in similar paradigms employing a challenging motion detection task (Müller et al., 2008; Hindi Attar et al., 2010). Three of the forty-one students participating in the experiment performed the task at less than 60% correct, and were excluded from the final analysis. The data from thirty-eight participants (age range: 18–25, mean age: 18.8) with normal to corrected-to-normal vision were included in the final analysis. Due to incomplete responses from three participants, stimulus-rating data was included only for thirty-five participants.
2.2 Stimuli and procedure
Stimuli were presented centrally on a 23-inch Samsung SyncMaster SA950 LED monitor, set at a resolution of 1680 × 1050 with a refresh rate of 120 frames per second (i.e., 8.33 ms refresh interval). Erotic couples, neutral people at work, and mutilated human bodies composed the pleasant, neutral, and unpleasant stimulus categories, respectively. To evaluate individual differences between participant groups with respect to snake fear, snake pictures were added to the unpleasant stimulus set. Categories of kitten (pleasant) and cow pictures (neutral) were included to provide animal content as an added control for the snake pictures. Accordingly, each valence category was divided into two subgroups (pleasant: erotica, kittens; neutral: people at work, cows; unpleasant: mutilation, snakes), with 20 pictures in each subset totaling to 120 pictures.
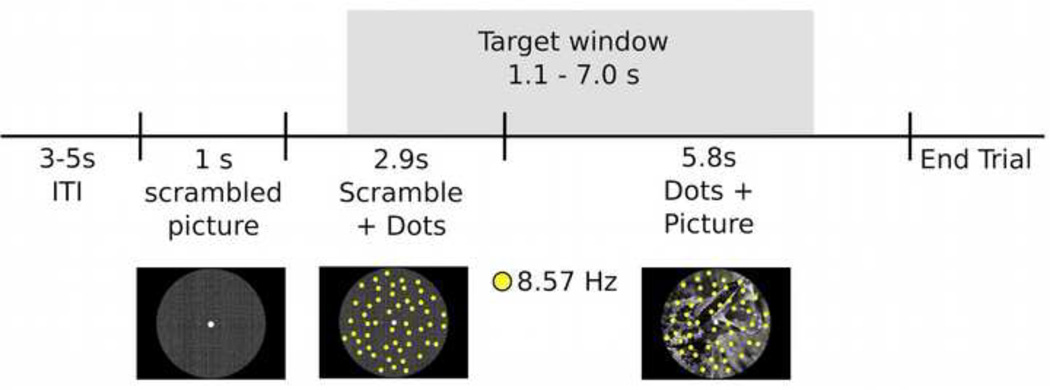
Pictures were selected from the International Affective Picture System (IAPS; Lang et al., 1997) based on normative valence and arousal ratings using the Self-Assessment Manikin (SAM; Bradley and Lang, 1994) 9-point scale. Additional images were selected from the public domain to complete balanced human and animal picture categories. For all IAPS images, valence and arousal ratings for neutral pictures were 6.28 and 4.15, for pleasant 7.01 and 5.84, and for unpleasant images 2.84 and 5.9, respectively. All stimuli were controlled for visual complexity, measured as jpeg size, and were matched for luminance using scripts from the Matlab image processing toolbox. Luminance was then measured for the entire screen using a Gossen (Nürnberg, Germany) luminance meter and was 80.02 cd/m2 on average. Picture stimuli were circular in nature, and were cropped and adjusted such that the defining element of each picture was positioned at the center of a circle (see Figure 1).
Figure 1.
Time sequence for a single trial: intervals of coherent motion could occur between 1.17 – 7 seconds post-stimulus onset (target window). Each trial lasted 9.751 seconds with a variable 3–5 second inter-trial interval.
Each trial began with a 1 second presentation of an IAPS image with individual pixels scrambled, to avoid contamination of the ssVEP with transient responses to the luminance gradient created by stimulus onset. Next, a total of 150 yellow dots (each 0.3 × 0.3 degrees of visual angle) were superimposed upon the scrambled image for 2917 ms. The scrambled background picture was then replaced by either a pleasant, neutral, or unpleasant picture which remained on the screen for the duration of the trial (5834 ms; Figure 1). All picture stimuli were grayscale pictures subtending a viewing angle of 6.9° at a viewing distance of 170 cm. Dots were distributed randomly across pictures and flickered at a rate of 8.57 Hz. Background images and the overlapping flickering dots remained inside the circle (6.9° visual angle) at all times.
The yellow dots were “on” for 6 frames and “off” for 8 frames. All dots remained in continuous motion throughout the trial and each dot changed its position by 0.04 degrees in a random direction with every ssVEP cycle (i.e. 8.57 times/sec). In a random subset of 50% of the trials, 100% of the dots moved coherently in the same direction (target), and participants were instructed to respond to coherent motion events with a mouse click, as quickly and as accurately as possible. Coherent motion of the targets occurred in one of four diagonal directions (45°, 135°, 225°, 315°) at random. In an effort to produce a difficult and demanding detection task, coherent motion lasted for only 4 successive cycles of 8.57 Hz (i.e., 466.64 ms). Targets occurred unpredictably once (in 58 of the 120 trials) or twice (in 4 of the 120 trials) in a given trial, with the remaining 58 trials consisting of random movement of the dots. The first possible coherent motion event was at 1170 ms (i.e., 10 cycles) after stimulus onset and the last coherent motion event was at 7000 ms (i.e., 60 cycles). Targets occurring during the scrambled image were not included in the behavioral analysis. Trials with dual targets were inserted to ensure attention was directed to the task for the entire duration of the trial, and such trials were not included in the final analysis. As a result, only single-target or no-target trials occurring during picture presentation were included in the final analysis. Participants were instructed to click the mouse as soon as coherent motion was detected. Each trial lasted for 9751 ms, with interstimulus intervals randomly varying between 3000 and 5000 ms. Fixation was facilitated by presenting a white fixation dot at the center of the screen (i.e. circle).
Prior to the experiment, all participants completed the SNAQ and performed 15 practice trials to become familiar with the stimulation and task. In the training session, 8 of the trials contained a target (coherent motion of the flickering dots), with one of those targets being a double target. Following the experiment, participants rated each of the 120 affective picture stimuli used in the experiment in pseudo-randomized order on the dimensions of affective valence and arousal, using a paper and pencil version of the SAM.
2.3 EEG recording
Electrophysiological data were collected from the scalp using a 257-sensor net (EGI, Eugene, OR). Scalp impedance for each sensor was kept below 60 kΩ, which is recommended for this high input impedance amplifier (200 mΩ input impedance, see Keil et al. 2014). The EEG was collected continuously with a sampling rate of 250 Hz (16-bit resolution) and were band-pass filtered online in the 0.1–90 Hz frequency range using a hardware elliptical filter. The vertex electrode (Cz) was used as the recording reference. Further processing and filtering was performed offline.
2.4 EEG reduction and analyses
Continuous data were low-pass filtered offline at a frequency (3dB point) of 40 Hz (12th order Butterworth filter with 24 dB / octave roll-off implemented in Matlab) prior to segmenting. Single epochs of 9200 ms in length (400 ms pre- and 8800 ms post-dot onset) were then extracted from the continuous EEG signal. Using the artifact rejection procedure proposed by Junghöfer et al. (2000) trials with artifacts were identified based on the distribution of statistical parameters of EEG epochs (absolute value, standard deviation, maximum of the differences) and were extracted across time points and channels. Sensors contaminated with artifacts were replaced by statistically weighted, spherical spline interpolated values, and a maximum of 25 channels was set for interpolation. Trials with spatially concentrated bad sensors were excluded as well, as these would invalidate interpolation for approximated sensors (see Junghöfer et al., 2000, for a more detailed description). As a result, each of the six picture conditions retained an average of 14 trials (SD = 0.28) which did not differ by condition (p > 0.59). To the extent that the signal of interest (the ssVEP) is concentrated in one specific frequency band, previous work has established that stable estimates of the time varying ssVEP amplitude are possible with trial counts between 10 and 20 [e.g., Keil et al., 2003; Wieser et al., 2011,]. To ensure satisfactory signal quality, we submitted each participants’ data to the circular T-square statistic (Victor and Mast, 1991), which formally tests the temporal stability of the entrained brain signal at a given driving frequency. To this end, the entire ssVEP viewing epoch for each experimental condition was segmented in non-overlapping epochs containing 4 cycles each, and then submitted to the circular T-square algorithm. This algorithm can be used to test for the presence of an evoked signal at the frequency of interest, taking both phase and amplitude information into account. All participants included in this study showed reliable (defined as p < 0.05 for the Chi-square distributed circular T-square at site Oz and its nearest neighbors) evoked oscillations at the driving frequency. This suggests satisfactory signal-to-noise ratios with the trial counts available in this experiment.
2.5 Steady-state visual evoked potential analyses
Artifact-free epochs of the voltage data were averaged for the six picture categories by group. Time-varying amplitude at the stimulation frequency of 8.57 Hz was extracted by means of a Hilbert transformation of the time-domain averaged data using in-house MATLAB scripts: Data were filtered with a 10th-order Butterworth band-pass filter having a width of .5 Hz around the center frequency of 8.57 Hz. Then the time-varying amplitude was extracted as the complex conjugate of the band-pass filtered signal and the Hilbert-transformed analytic signal, for each time point. Data were then temporally smoothed applying a linear moving average, with a window length of 420 ms, corresponding to the time resolution of the time-varying amplitude, which was 421 ms full width at half maximum of the filter’s impulse response.
2.6 Statistical analyses: Performance & SAM ratings
The percentage of correctly identified targets (hits) and false alarms were calculated for each distractor condition and participant. Button presses occurring after 150 ms post-target onset (coherent motion) were accepted as correct detections. False alarms were calculated as the percentage of trials in which a response was made (e.g., clicking the mouse) in the absence of a coherent motion target. Behavioral sensitivity, a measure of detection sensitivity (Macmillan and Creelman, 2004), was calculated by subtracting the number of false alarms from the number of hits for each condition comparison. Differences among picture conditions were evaluated by means of omnibus repeated-measures analysis of variance (ANOVA) with factors of hedonic content (pleasant, neutral, unpleasant), category (humans, animals) and group (high snake fear, controls).
2.7 Statistical analysis: ssVEP time course
Permutation-controlled t-tests for each sampling point and scalp location were conducted to assess the time course of ssVEP amplitude using the sample-by-sample data which has the best temporal resolution for assessing time-varying ssVEP amplitude In these analyses, t-tests were calculated at each EEG sensor and sampling point for each distractor content which compared ssVEP amplitude between high snake fear and control participants. Significance thresholds for each comparison were calculated by computing 500 electrode by time point matrices of t-tests based on random permutations of the existing data, i.e., with group membership shuffled. The statistic for each topography and time point entered a reference distribution, whose 2.5% tails served as the criterion for statistical significance (see McGinnis and Keil, 2011 for a similar procedure). Comparisons reaching significance (as determined by the permutation corrected t-tests) indicated scalp locations and sampling points at which the high-fear group was significantly different from the control group, for each condition separately.
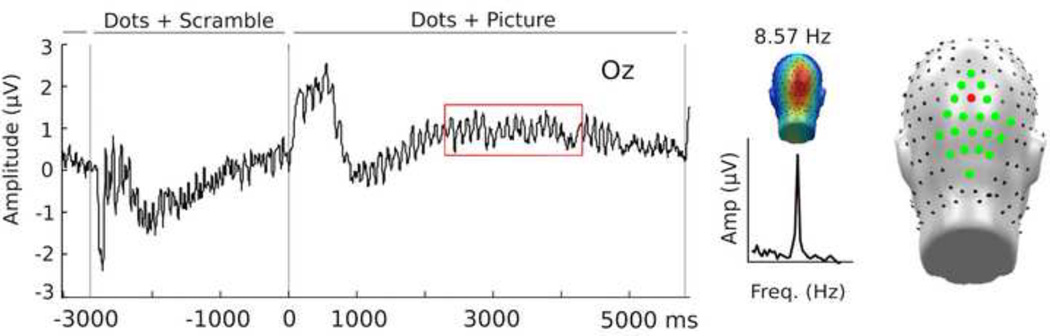
To assess the full factorial statistical model of the mixed design of the present study, and to complement time course analyses, ssVEP amplitudes were averaged in an occipito-parietal cluster of electrodes including Oz and its 24 nearest neighbors (EGI sensors: 116–119 123–127 135–139 147–150 157–159; see Figure 2), resulting in posterior regional mean amplitudes. These amplitudes were then subjected to a z-transformation to address inter-individual variability in amplitude. To evaluate modulations in ssVEP amplitude for different distractor content, ssVEP amplitude was deviated from a baseline period (−1500-300 ms pre-stimulus onset) in four time windows following picture onset (T1: 380–1100 ms, T2: 1100–2100 ms, T3: 2100–3100 ms, T4: 3100–4100 ms). These time windows were selected based on visual inspection of the datar and to capture the temporal dynamics related to ssVEP amplitude modulation of distractor contents, across the viewing epoch. The initial 700 ms time window was selected to immediate capture effects related to picture onset (expected based on earlier research; Müller et al., 2008), and was followed by three 1000 ms time windows selected to sample any effects related to sustained picture viewing. A linear mixed model analysis (implemented in SPSS ©) was conducted with fixed effects of hedonic content (pleasant, neutral, unpleasant), category (human, animal) and time, and subject as a random variable, nested within group.
Figure 2.
Left panel: Grand average time domain of the ssVEP signal averaged across all stimulus conditions and participants, plotted at sensor Oz. Middle panel, top: Grand average topographical distribution of ssVEP amplitude across all subjects and conditions in the time window between 2200 and 4200 ms (red box) after stimulus onset. Middle panel, bottom: Grand average of the frequency spectra across subjects and conditions, demonstrating a reliable peak at the driving frequency of the flickering dots, 8.57 Hz. Right: Representation of electrode placement; sensor Oz is highlighted in red and sensors used in the cluster analyses are highlighted in green.
3. Results
3.1 Behavioral data & SAM ratings
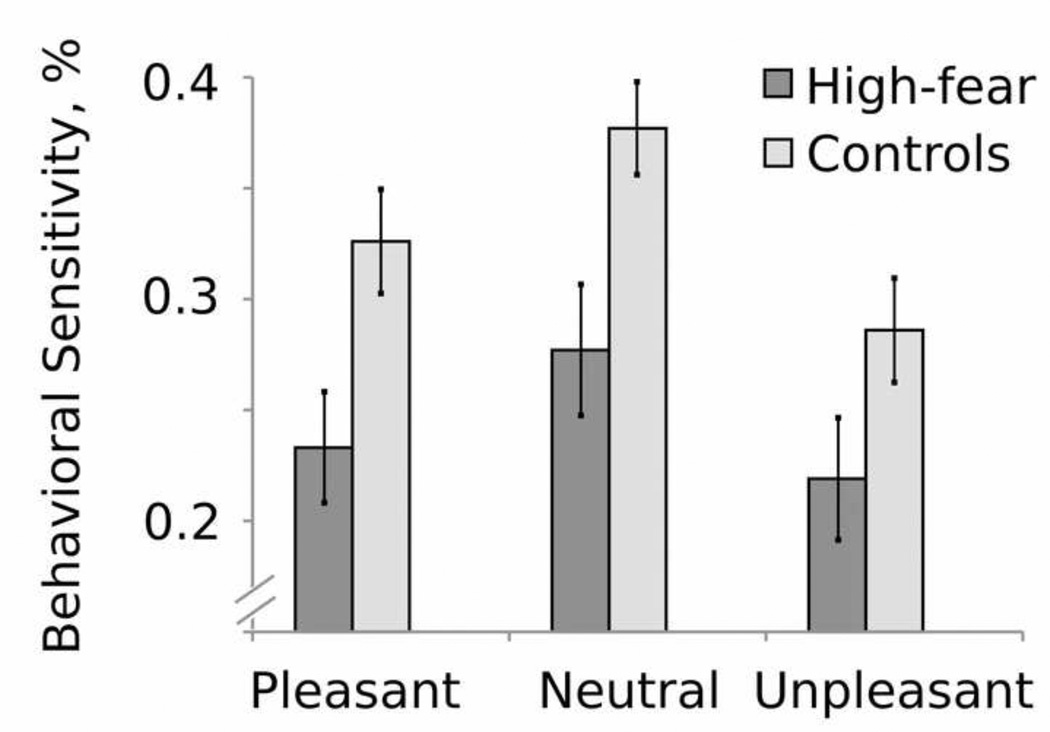
Performance on the coherent motion detection task was affected by hedonic picture content (F1,33 = 4.33, p = 0.017), with a significant quadratic trend in behavioral sensitivity indicating that, compared to neutral distractors, emotionally engaging pictures (both pleasant and unpleasant) prompted poorer performance (F(l, 36) = 5.411, p = 0.024; see Figure 3).
Figure 3.
Group averages of behavioral performance (hits - false alarms) for high-fear (dark gray) and controls (light gray) for pleasant, neutral, and unpleasant picture contents.
As expected, high-fear participants rated pictures of snakes as more unpleasant (t33 = 3.89, p < 0.0001) and more arousing (t33 = 2.99, p = 0.005) than did control participants. High-fear participants also rated pictures of erotica (t33 = 2.28, p = 0.03) and mutilation (t33 = 3.23, p = 0.004) as slightly more unpleasant than did the control participants. High-fear participants also rated pictures of mutilation (t33 = 2.29, p = 0.03) as being more arousing than did controls.
3.2 ssVEPs
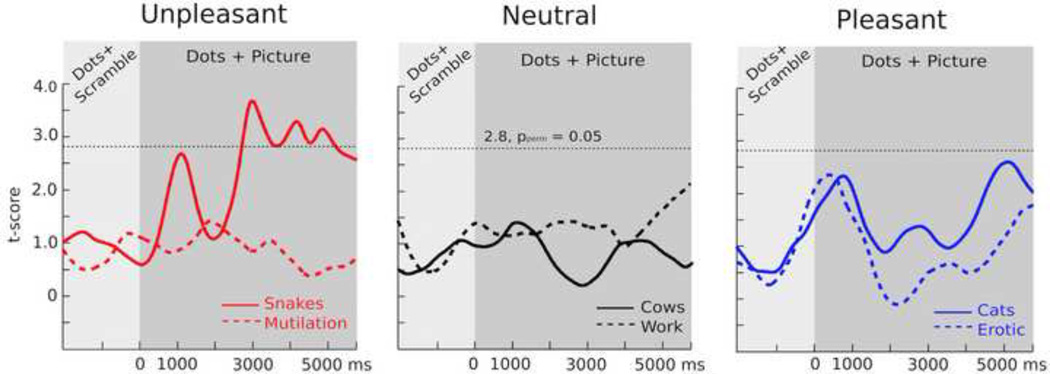
The flickering dots in the task-relevant stream reliably evoked steady-state responses at the expected frequency of 8.57 Hz, as illustrated in Figure 2, with the greatest overall ssVEP amplitudes across all experimental conditions occurring for sensor Oz and its nearest neighbors. The grand mean time-varying energy of the signal over occipital sensors as quantified by the Hilbert transform is shown in Figure 4. As illustrated in Figure 5, permutation t-tests conducted on each individual sampling point (critical value of t = 2.8 at the pperm < 0.05; dashed line) identified when ssVEP amplitudes were statistically different between high-fear and control participants for each distractor type. As illustrated in Figure 5, high-fear participants differed significantly in ssVEP amplitude from controls only when snake pictures served as distractors, and this difference persisted across the distractor interval. Differences in ssVEP amplitude between high-fear participants and controls were more pronounced later in the distractor interval, which is inconsistent with a hypothesis of initial vigilance followed by avoidance.
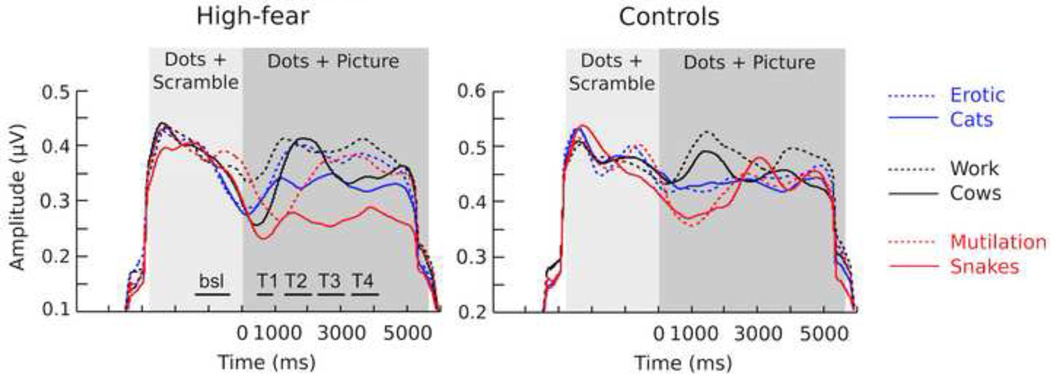
Figure 4.
Hilbert transformed data for high-fear and control subjects for all conditions, averaged over a cluster of occipito-parietal electrodes; averaged amplitudes were calculated for sequential 600-ms time bins. The light gray panel indicates presentation of the dots and scrambled image (2900 ms before stimulus onset) and the dark gray panel indicates simultaneous presentation of the dots and the background image) total duration of 5800 ms). Note different scales.
Figure 5.
Permutation corrected waveforms for unpleasant (left), neutral (center), and pleasant (right) conditions. The critical value for conditions reaching significance at the pperm = 0.05 level is indicated by the dotted black line (t = 2.8). Conditions above the significance level indicate group differences. The time scale refers to the simultaneous onset of the dots and the background picture.
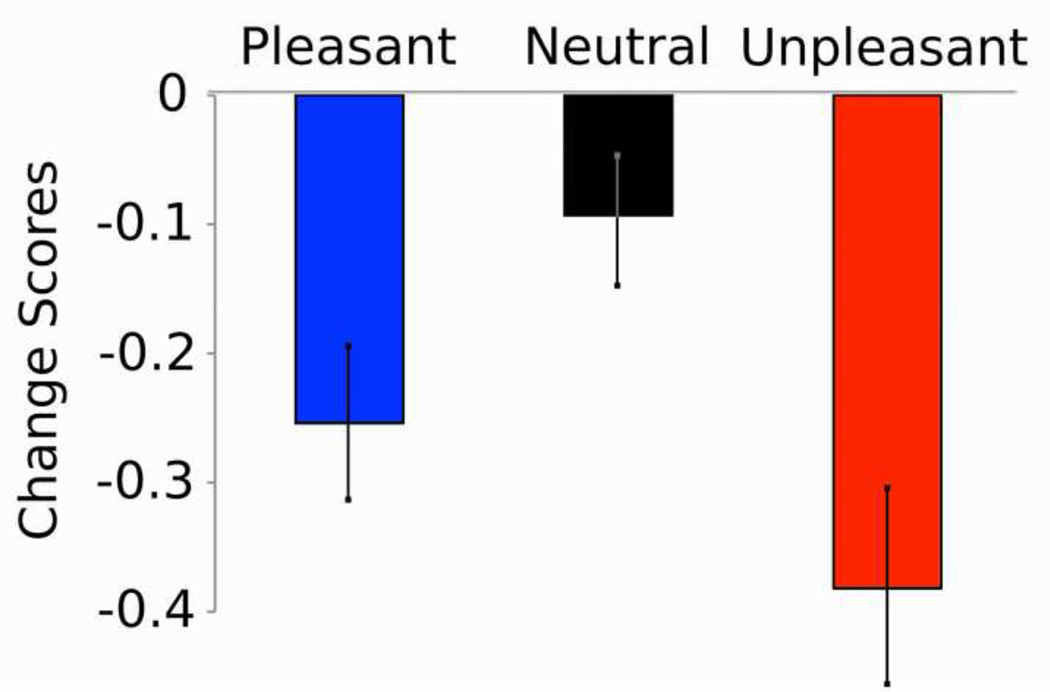
A mixed model linear analysis assessed differences in ssVEP amplitude as a function of distractor contents for each group. Significant modulation of ssVEP amplitude varied as a function of hedonic content, F(2,828) = 18.4, p < 0.0001, category, F(l, 828) = 3.9, p = 0.048 and time, F(3,828) = 4.9, p = 0.002. Replicating previous research, over all picture contents, dot-evoked ssVEP amplitudes were attenuated when emotionally evocative (either pleasant or unpleasant), compared to neutral pictures served as distractors (Pleasant vs. Neutral, p = 0.002; Unpleasant vs. Neutral, p < 0.0001; Figure 6).
Figure 6.
Mean ssVEP amplitude (calculated as the change from baseline) averaged across high-fear and control groups, for pleasant, neutral, and unpleasant picture content.
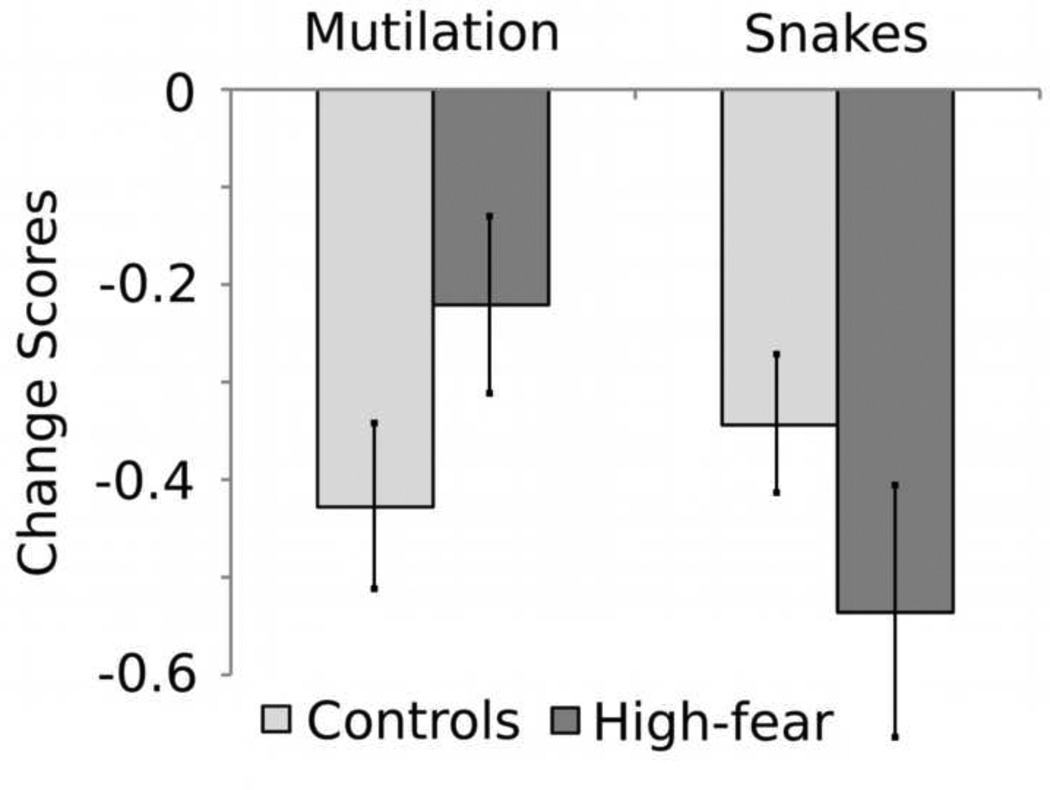
These main effects were qualified by a three-way interaction of hedonic content, category, and group, F(2,828) = 6.5 , p = 0.002, indicating that ssVEP amplitude was differentially modulated as a function of picture content and category for high-fear participants and controls. Follow-up tests of this interaction assessed effects of group and category separately for each hedonic content. For pleasant pictures, as expected, high-fear participants and controls did not differ in their pattern of ssVEP modulation as a function of category, p > .3. For neutral pictures, a significant interaction of group and category F(l, 252) = 4.7, p = 0.03 indicated that pictures of people at work showed slightly less ssVEP reduction than cows (p = 0.056) for high-fear participants. Means for pleasant and neutral category comparisons can be found in Table 1. Most importantly, a significant interaction of group and category, F(l, 252) = 12.3, p = 0.001 indicated that snakes prompted a larger ssVEP reduction than mutilations for high-fear participants, as illustrated in Figure 7. Here, the high-fear group demonstrated a greater reduction in ssVEP amplitude for snake pictures than for mutilation pictures t18 = 2.15, p = 0.045. The control group did not show this effect, t18 = 0.6, p = 0.56.
Table 1.
Mean ssVEP amplitude calculated as the change from baseline for erotica, cat, people at work, and cow conditions for high-fear and control groups.
| Group | ||
|---|---|---|
| Category | Controls | High-fear |
| Erotic | −0.149 | −0.323 |
| Cats | −0.242 | −0.302 |
| Work | 0.089 | −0.195 |
| Cows | −0.12 | −0.148 |
Figure 7.
Mean ssVEP amplitude (calculated as the change from baseline) for mutilation and snake categories for high-fear (dark gray) and control (light gray) subjects.
4. Discussion
The current study investigated the extent to which ssVEP amplitude and the associated time course of visual competition was modulated by task-irrelevant fearful stimuli and a foreground task. For all participants, a reduction in ssVEP amplitude for task-related stimuli was found when task-irrelevant, but emotionally arousing distractors were present, consistent with previous studies (e.g., Müller et al., 2008; Hindi Attar et al., 2010). This supports the notion that emotionally arousing cues compete for limited resources at the level of the visual cortex (Keil et al. 2005; Hajcak et al., 2013). Importantly, for participants reporting high levels of snake fear, the reduction in task-related ssVEP amplitude was greatest when snakes served as distractors, even when compared to other aversive content. In addition, there was no evidence that initial hypervigilance shifts to perceptual avoidance later in the interval for high-fear participants. Rather, the reduction in task-evoked ssVEP amplitude was most pronounced later in the distractor interval, consistent with sustained hypervigilance.
Preferential and sustained cortical processing of fear-relevant cues in the absence of avoidance has been demonstrated in several studies assessing attentional capture by phobia-relevant stimuli (McTeague et al., 2011, Wieser et al., 2011, Wieser et al., 2012), whereas patterns of hypervigilance-avoidance have been less consistent, and most robust in eye-tracking studies (Pflugshaupt et al., 2005; Wieser et al., 2009). To the extent that ocular control is a complex mechanism which involves widespread cortical communication along with coordinated activity in several cortical and sub-cortical structures (Sommer and Wurtz, 2008), it is conceivable that a dynamic sequence of hypervigilance and avoidance is specific to eye movements. Importantly, oculomotor data do not inform on covert shifts of attention, whereas the ssVEP technique is sensitive to covert attention processes, providing a potential explanation for sustained hypervigilance in the absence of avoidance in studies using the ssVEP.
Previous studies examining competition effects in healthy controls have shown modulation of perceptual arousal to begin around 400 ms after stimulus onset and persist for a few hundred milliseconds (Müller et al., 2008). This parallels the pattern of ssVEP amplitude modulation demonstrated by the control group in the present study (see Figure 4, right). Group differences between anxious and non-anxious observers, however, emerged later in the viewing epoch. These differences in ssVEP amplitude began 1100 ms after picture onset, and reliably indicated that the greatest amplitude reduction occurred specifically in the snake condition for fearful observers, an effect that persisted as long as the fear-relevant image remained on screen. Thus, while all participants showed early sensitivity to distraction, only snake fearful observers displayed sustained interference, induced specifically by the snake pictures. This finding is consistent with work by Wieser et al. (2012), who observed a prominent visual competition effect for threatening faces, observed only in individuals with high social anxiety. Specifically, these authors found that participants high in social anxiety showed diminished visual engagement for task stimuli that were superimposed on an angry, but not neutral or happy faces, which was maintained throughout the viewing period (∼3000 ms). Thus, automatic attentional bias toward a threatening stimulus seems to reliably persist as long as the threat stimulus remains visible.
In the clinical and translational research literature, hypervigilance in the context of fear-relevant cues is seen as dysfunctional attentional and perceptual processes that may cause or contribute to the maintenance of phobias (McNally, 2007). Thus, objective and quantitative measures of selective attention to threat have important applications in diagnosis and treatment of anxiety disorders (Bögels and Mansell, 2004; Amir et al., 2009) as well as other disorders that involve the dysregulation of affective processing (Kemp et al., 2004; Donaldson et al., 2007). To the extent that the ssVEP technique employed here is sensitive to electrocortical modulation related to inter-individual differences (e.g., differential amplitude modulation of a fear-relevant stimulus in a fearful participant versus a control), applications in the clinical research arena are conceivable: In assessment, these methods could be used to objectively identify patients with dysfunctional attentional resource allocation to fear-related stimuli. In addition, inter-individual differences in the time course of hypervigilance versus perceptual avoidance could be a novel way to assign patients to individualized treatment and in predicting treatment outcome. Specifically, modulatory responses of distraction by or interference of fear-related stimuli over the course of treatment may be examined and quantified using the ssVEP, which may have implications for therapeutic treatments aiming to direct attention away from a threat cue. It should be noted that replication of these findings in a clinically anxious population is an essential next step before this measure may be used as a reliable diagnostic tool. However, previous studies assessing emotional reactivity to fear-relevant stimuli have found significant differences using both fMRI (Sabatinelli et al., 2005) and EEG (Weiser et al., 2012) in sub-clinical populations. In future research, anxiety patients diagnosed with small animal phobia may aid in confirming and generalizing these findings.
An interesting question prompted by the current data is the extent to which the reduction of the task-evoked ssVEP signal when snakes serve as distractors is accompanied by enhancement of the visual processing of the snake stimulus, confirming a trade-off between task and distractor stimuli. Hindi Attar et al. (2012) have shown cost effects related to processing unpleasant distractors in healthy subjects, thus it is plausible to predict similar reciprocal amplitude effects in snake-fearful populations. Future ssVEP studies could address this issue by utilizing another advantage of the ssVEP technique: when two stimuli flickering at different rates are simultaneously presented in an overlapping spatial array, the electrocortical signature of the processing of each separate stimulus can be calculated and separated in the frequency domain, a technique referred to as “frequency tagging.” Thus, frequency-tagging of both the task-relevant and distractor stimuli represents a promising avenue for examining the hypothesis that fear-related background pictures “withdraw” resources from the foreground detection task. Such a trade-off would be in line with the biased competition model of attention, which suggests that competition is greatest when conflicting stimuli activate overlapping visual areas (Desimone, 1998).
In conclusion, the results from the present study provide support for the hypothesis that task-irrelevant but emotionally engaging stimuli act as strong competitors, interfering with the visual processing of a concurrent task stimulus. For high-fear participants, task-evoked ssVEP reductions were larger when fear cues served as distractors, compared to other aversive content, and compared to control participants. Moreover, the attenuation of task-evoked ssVEPs when fear cues were present in the array was sustained across the temporal interval for participants reporting high snake fear, consistent with a hypothesis of sustained hypervigilance.
Acknowledgement
The authors would like to thank Cintya Larios and Tyler Jarrett for their help in data acquisition. This research was supported by National Institute of Mental Health Grants R01 MH084932-02 and R01 MH097320, and by a grant from the Spanish Government (I+D+i PSI2009-07066), awarded to Andreas Keil.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amir N, Beard C, Taylor CT, Klumpp H, Elias J, Burns M, Chen X. Attention training in individuals with generalized social phobia: A randomized controlled trial. J Consulting and Clinical Psychology. 2009;77:961–973. doi: 10.1037/a0016685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Becker ES, Rinck M, Margraf J, Roth WT. The emotional Stroop effect in anxiety disorders: General emotional or disorder specificity? Journal of Anxiety Disorders. 2001;15(3):147–159. doi: 10.1016/s0887-6185(01)00055-x. [DOI] [PubMed] [Google Scholar]
- Bögels SM, Mansell W. Attention processes in the maintenance and treatment of social phobia: hypervigilance, avoidance and self-focused attention. Clinical Psychology Review. 2004;24(7):827–856. doi: 10.1016/j.cpr.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring Emotion - the Self-Assessment Mannequin and the Semantic Differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Sabatinelli D, Lang PJ, Fitzsimmons JR, King W, Desai P. Activation of the visual cortex in motivated attention. Behavioral Neuroscience. 2003;117:369–380. doi: 10.1037/0735-7044.117.2.369. [DOI] [PubMed] [Google Scholar]
- Desimone R. Visual attention mediated by biased competition in extrastriate visual cortex. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 1998;353:1245–1255. doi: 10.1098/rstb.1998.0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Donaldson C, Lam D, Mathews A. Rumination and attention in major depression. Behavior Research and Therapy. 2007;45(11):2664–2678. doi: 10.1016/j.brat.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Garner M, Mogg K, Bradley BP. Orienting and mantenance of gaze to facial expressions in social anxiety. Journal of Abnormal Psychology. 2006;115(4):760–770. doi: 10.1037/0021-843X.115.4.760. [DOI] [PubMed] [Google Scholar]
- Hajcak G, MacNamara A, Foti D, Ferri J, Keil A. The dynamic allocation of attention to emotion: simultaneous and independent evidence from the late positive potential and steady state visual evoked potentials. Biological Psychology. 2013;92(3):447–455. doi: 10.1016/j.biopsycho.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Heeren A, De Raedt R, Koster EH, Philippot P. The (neuro)cognitive mechanisms behind attention bias modification in anxiety: proposals based on theoretical accounts of attentional bias. Frontiers in Human Neuroscience. 2013;7:119. doi: 10.3389/fnhum.2013.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyard SA, Hink RF, Schwent VL, Picton TW. Electrical signs of selective attention in the human brain. Science. 1973;182:177–180. doi: 10.1126/science.182.4108.177. [DOI] [PubMed] [Google Scholar]
- Hindi Attar C, Andersen SK, Müller MM. Time course of affective bias in visual attention: convergent evidence from steady-state visual evoked potentials and behavioral data. Neuroimage. 2010;53:1326–1333. doi: 10.1016/j.neuroimage.2010.06.074. [DOI] [PubMed] [Google Scholar]
- Hindi Attar C, Müller MM. Selective attention to task-irrelevant emotional distractors is unaffected by the perceptual load associated with a foreground task. PLoS One. 2012 doi: 10.1371/journal.pone.0037186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihssen N, Heim S, Keil A. The Costs of emotional attention: Affective processng inhibits subsequent lexico-semantic analysis. Journal of Cognitive Neuroscience. 2007;19(12):1932–1949. doi: 10.1162/jocn.2007.19.12.1932. [DOI] [PubMed] [Google Scholar]
- Junghöfer M, Elbert T, Tucker DM, Rockstroh B. Statistical control of artifacts in dense array EEG/MEG studies. Psychophysiology. 2000;37:523–532. [PubMed] [Google Scholar]
- Keil A, Debener S, Gratton G, Junghöfer M, Kappenman ES, Luck SJ, Luu P, Miller GA, Yee CM. Committee report: publication guidelines and recommendations for studies using electroencephalography and magnetoencephalography. Psychophysiology. 2014;51(1):1–21. doi: 10.1111/psyp.12147. [DOI] [PubMed] [Google Scholar]
- Keil A, Gruber T, Müller MM, Moratti S, Stolarova M, Bradley MM, Lang PJ. Early modulation of visual perception by emotional arousal: evidence from steady-state visual evoked brain potentials. Cognitive, Affective, and Behavioral Neuroscience. 2003;3(3):195–206. doi: 10.3758/cabn.3.3.195. [DOI] [PubMed] [Google Scholar]
- Keil A, Moratti S, Sabatinelli D, Bradley MM, Lang PJ. Additive effects of emotional content and spatial selective attention on electrocortical facilitation. Cerebral Cortex. 2005;15(8):1187–1197. doi: 10.1093/cercor/bhi001. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Silberstein RB, Armstrong SM, Nathan PJ. Gender differences in the cortical electrophysiological processing of visual emotional stimuli. NeuroImage. 2004;21(2):632–646. doi: 10.1016/j.neuroimage.2003.09.055. [DOI] [PubMed] [Google Scholar]
- Klorman R, Weerts TC, Hastings JE, Melamed BG, Lang PJ. Psychometric Description of Some Specific-Fear Questionnaires. Behavior Therapy. 1974;5:401–409. [Google Scholar]
- Kolassa IT, Buchmann A, Lauche R, Kolassa S, Partchev I, Miltner WH, Musial F. Spider phobics more easily see a spider in morphed schematic pictures. Behavior and Brain Functions. 2007b;3:59. doi: 10.1186/1744-9081-3-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolassa I, Kolassa S, Musial F, Miltner WH. Event-related potentials to schematic faces in social phobia. Cognition and Emotion. 2007a;21:1721–1744. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Attention and Orienting: Sensory and motivational processes. Hillsdale, N.J: Lawrence Erlbaum Associates; 1997. Motivated attention: Affect, activation, and action; pp. 97–135. [Google Scholar]
- Lipka J, Miltner WH, Straube T. Vigilance for threat interactions with amygdala responses to subliminal threat cues in specific phobia. Biological Psychiatry. 2011;70(5):472–478. doi: 10.1016/j.biopsych.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection Theory: A User’s Guide. 2, Revised ed. Taylor & Francis; 2004. [Google Scholar]
- Mathews A. Why worry? the cognitive function of anxiety. Behaviour Research and Therapy. 1990;28(6):455–458. doi: 10.1016/0005-7967(90)90132-3. [DOI] [PubMed] [Google Scholar]
- McGinnis EM, Keil A. Selective processing of multiple features in the human brain: effects of feature type and salience. PloS One. 2011;6:e16824. doi: 10.1371/journal.pone.0016824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally RJ. Mechanisms of exposure therapy: how neuroscience can improve psychological treatments for anxiety disorders. Clinical Psychology Review. 2007;27:750–759. doi: 10.1016/j.cpr.2007.01.003. [DOI] [PubMed] [Google Scholar]
- McTeague LM, Shumen JR, Wieser MJ, Lang PJ, Keil A. Social Vision: Sustained perceptual enhancement of affective facial cues in social anxiety. NeuroImage. 2011;54:1615–1654. doi: 10.1016/j.neuroimage.2010.08.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogg K, Mathews A, Weinman J. Selective processing of threat cues in anxiety states: a replication. Behavior Research and Therapy. 1989;27:317–323. doi: 10.1016/0005-7967(89)90001-6. [DOI] [PubMed] [Google Scholar]
- Mogg K, Millar N, Bradley BP. Biases in eye movements to threatening facial expressions in generalized anxiety disorder and depressive disorder. Journal of Abnormal Psychology. 2000;109(4):695–704. doi: 10.1037//0021-843x.109.4.695. [DOI] [PubMed] [Google Scholar]
- Müller MM, Andersen SK, Keil A. Time course of competition for visual processing resources between emotional pictures and foreground task. Cerebral Cortex. 2008;18:1892–1899. doi: 10.1093/cercor/bhm215. [DOI] [PubMed] [Google Scholar]
- Öhman A, Flykt A, Esteves F. Emotion drives attention: detecting the snake in the grass. Journal of Experimental Psychology: general. 2001;130:466–478. doi: 10.1037/0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Öhman A, Mineka S. Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychological Review. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Pflugshaupt T, Mosimann UP, von Wartburg R, Schmitt W, Nyffeler T, Müri RM. Hypervigilance-avoidance pattern in spider phobia. Journal of Anxiety Disorders. 2005;19(1):105–116. doi: 10.1016/j.janxdis.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Regan D. Human Brain Electrophysiology: Evoked Potentials and Evoked Magnetic Fields in Science and Medicine. New York: Elsevier; 1989. [Google Scholar]
- Reynolds JH, Heeger DJ. The normalization model of attention. Neuron. 2009;61(2):168–185. doi: 10.1016/j.neuron.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Fitzsimmons JR, Lang PJ. Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage. 2005;24:1265–1270. doi: 10.1016/j.neuroimage.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Schimmack U, Derryberry D. Attentional interference effects of emotional pictures: threat, negativity, or arousal? Emotion. 2005;5:55–66. doi: 10.1037/1528-3542.5.1.55. [DOI] [PubMed] [Google Scholar]
- Shafer AT, Matveychuk D, Penney T, O’Hare AJ, Stokes J, Dolcos F. Processing of Emotional Distraction is both automatic and modulation by attention: Evidence from an event-related fMRI investigation. Journal of Cognitive Neuroscience pp. 2012:1233–1252. doi: 10.1162/jocn_a_00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Visual perception and corollary discharge. Perception. 2008;37:408–418. doi: 10.1068/p5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T, Mentzel HJ, Miltner WH. Waiting for spiders: Brain avtivation during anticipatory anxiety in spider phobics. Neuroimage. 2007;37(4):1427–1436. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Wangelin BC, Low A, McTeague LM, Bradley MM, Lang PJ. Aversive picture processing: effects of a concurrent task on sustained defensive system engagement. Psychophysiology. 2011;48:112–116. doi: 10.1111/j.1469-8986.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- Wieser MJ, McTeague LM, Keil A. Sustained preferential processing of social threat cues: Bias without competition? Journal of Cognitive Neuroscience. 2011:1973–1986. doi: 10.1162/jocn.2010.21566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser MJ, McTeague LM, Keil A. Competition effects of threatening faces in social anxiety. Emotion. 2012;12:1050–1060. doi: 10.1037/a0027069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser MJ, Puli P, Wyers P, Alpers GW, Muhlberger A. Fear of negative evaluation and the hypervigilance-avoidance hypothesis: An eye-tracking study. Journal of Neural Transmission. 2009;116(6):717–723. doi: 10.1007/s00702-008-0101-0. [DOI] [PubMed] [Google Scholar]
- Victor JD, Mast J. A new statistic for steady-state evoked potentials. Electroencephalography and Clinical Neurophysiology. 1991;78(5):378–388. doi: 10.1016/0013-4694(91)90099-p. [DOI] [PubMed] [Google Scholar]