Abstract
Background/Aims. To assess the effect of intravitreal bevacizumab injection (IVBI) for the treatment of macular edema due to infectious and noninfectious uveitides. Design. Retrospective interventional case series. Methods. A chart review was performed on all the patients who were diagnosed with uveitic macular edema (UME) and received 1.25 mg of IVBI at two referral centers in Riyadh, Saudi Arabia. All included patients had their visual acuity and macular thickness analyzed at baseline and at 1 and 3 months following IVBI and any sign of reactivation was noted. Results. The mean age of patients was 41 ± 16 years with a mean followup of 4 ± 1 months. Ten patients had idiopathic intermediate uveitis, 9 patients had Behcet's disease, 10 had idiopathic panuveitis, and twelve patients had presumed ocular tuberculosis uveitis. Following IVBI, the mean LogMAR visual acuity improved from 0.8 ± 0.8 at baseline to 0.4 ± 0.5 at 1 month and 0.3 ± 0.5 at 3 months (P < 0.002, at 3 months). The mean macular thickness was 430 ± 132 μm at baseline. Following IVBI macular thickness improved to 286 ± 93 μm at 1 month and to 265 ± 88 μm at 3 months of followup (P < 0.001, at 3 months). Conclusion. Bevacizumab was effective in the management of UME associated with both infectious and noninfectious uveitides. Intravitreal bevacizumab induced remission of UME with infectious uveitis and had no immunosuppressive effect against infectious agents.
1. Introduction
Uveitic macular edema (UME) occurs in up to 33% of uveitis cases and represents the most common cause of visual loss in patients with uveitis [1, 2]. The underlying pathophysiology of macular edema in uveitis is not well understood. However, several factors may play a role in the development of the edema including inflammatory cytokines, such as interferon gamma, interleukin 2, interleukin 6, interleukin 10, tumor necrosis factor alpha, and vascular endothelial growth factor (VEGF) [3–7].
In patients with uveitis and macular edema, greater concentrations of VEGF are upregulated compared to those without UME. Additionally, VEGF significantly stimulates and increases vascular permeability [7–10].
Early medical treatment is advocated to suppress intraocular inflammation and to prevent progressive and irreversible damage to the macular photoreceptors secondary to chronic and persistent UME [4]. Current management of UME includes the use of topical nonsteroidal anti-inflammatory, oral, periocular, and intraocular injections of corticosteroids as well as oral carbonic anhydrase inhibitors, systemic somatostatin analogs, interferon alpha, mycophenolate mofetil, and VEGF inhibitors [11–20]. However, uveitic macular edema may be nonresponsive to these treatments and continue to progress despite the control of ocular inflammation.
Bevacizumab is a recombinant humanized full-length monoclonal antibody against VEGF that has been used off-label for the treatment of age-related choroidal neovascularization (CNV) and other ocular pathologies that include UME [21–28]. Several clinical reports have described improved visual acuity and a reduction or resolution of macular edema in patients with noninfectious uveitis following intravitreal bevacizumab or ranibizumab injection as an adjunct therapy [10, 29–34]. However, the behavior and response of macular edema due to different etiologies have not been analyzed in detail. The present study aims to compare the effect of intravitreal bevacizumab in uveitic macular edema in patients with different etiologies: idiopathic intermediate uveitis, Behcet's disease, idiopathic panuveitis, and presumed ocular tuberculosis uveitis.
2. Patients and Methods
Patient charts were reviewed for cases of uveitic macular edema who had central 1.00 mm macular thickness by OCT of >250 μm and underwent intravitreal bevacizumab injection between June 2006 and June 2009 at King Khaled Eye Specialist Hospital (KKESH) and The Eye Center in Riyadh, Saudi Arabia. Four groups were included in the study: idiopathic intermediate uveitis (IIU), Behcet's disease (BD), idiopathic panuveitis (IPU), and presumed ocular tuberculosis uveitis (POTBU). The intravitreal dosage was 1.25 mg of bevacizumab (Avastin, Genentech/Roche) and repeated as required. Inclusion criteria were patients with refractory UME that was nonresponsive to topical, periocular, or intraocular injections of corticosteroids or different systemic therapy for uveitis within the previous 3 months. Patients with UME associated with epiretinal membrane or vitreomacular traction, pregnant patients, and patients who underwent cataract or intraocular surgeries during the study period were excluded. The study was approved by the IRB.
Demographic data on age and gender of the cohort were collected. The outcome measures included baseline logarithm of the minimal angle of resolution (LogMAR), visual acuity, and macular thickness. Data were collected at 1 and 3 months after intravitreal bevacizumab. The 1 mm central macular thickness was measured with optical coherence tomography (OCT) (Stratus III, Carl Zeiss Meditec, Dublin, CA, USA). The time of onset of macular edema or ocular complications and the follow-up period were recorded. The numbers of intravitreal injections of bevacizumab were recorded. Fluorescein angiography was performed on all patients to record the UME before and after treatment. All topical and systemic medications such as methotrexate, cyclosporine, azathioprine, steroids, infliximab, and antituberculosis therapy were continued during the follow-up period as required.
The diagnosis of presumed ocular tuberculosis was made based on clinical findings of chorioretinitis, granulomatous uveitis, positive PPD of 15 mm of induration or greater, positive response to antituberculosis therapy within 4 weeks, and exclusion of other causes of uveitis as previously reported [35]. Minimum followup was three months. The institutional review boards of both study centers approved this study.
2.1. Intravitreal Bevacizumab
After discussing the details of the intravitreal injection with each patient, all patients read and signed an informed consent prior to the procedure. The pupil was dilated, and topical anesthesia and topical moxifloxacin 0.5% were instilled. The lids and lashes were cleansed with povidone iodine 10% solution and a sterile drape was placed over the eye. A sterile lid speculum was inserted. Povidone iodine 5% ophthalmic solution was instilled and, after 90 seconds, rinsed with saline solution. A swab soaked in 5% povidone iodine was placed on the conjunctiva at the site of injection. A 0.05 mL solution containing 1.25 mg of bevacizumab was injected intravitreally. The bevacizumab was prepared in the compounding pharmacy. The injection site was 3.5 mm posterior to the limbus for phakic patients and 3 mm for pseudophakic and aphakic patients and injection was performed with a 30-gauge needle avoiding the horizontal meridians and aiming at the center of the globe. Broad spectrum antimicrobial eye drops were instilled at the end of the procedure and patients were instructed to continue topical antimicrobial drops four times daily for one week. Patients were requested to return at weekly intervals.
2.2. Control of Inflammation and Repeated Intravitreal Injections
Intraocular inflammation was graded during each follow-up visit based on the recommendations of the Standardization of Uveitis Nomenclature (SUN) working group [36]. The number of intravitreal injections of bevacizumab was correlated with the activity of the disease. Retreatments of intravitreal bevacizumab (up to one injection per month) were performed as required during the three-month follow-up period. The pre- and postinjection visual acuity was converted from Snellen to LogMAR scale.
2.3. Statistical Analyses
Descriptive statistics such as means, standard deviation, and percentages were calculated. Statistical analyses were performed to determine the mean change from baseline visual acuity to 1 month and 3 months of followup. The mean change from baseline retinal thickness using OCT was analyzed at 1 and 3 months. Statistical analyses were performed using repeated measure analyses of variance (ANOVA). All P values were two-sided and the significance level was set at 0.05. Data analyses were performed with SPSS for Windows version 11.0 (SPSS Inc., Chicago, IL, USA).
3. Results
The cohort comprised 41 patients of which 21 were female and 20 male. The mean age of patients was 41 ± 16 years with a mean followup of 4 ± 1 months. Patients were divided into four groups: idiopathic intermediate uveitis (10 patients) (Figure 1); Behcet's disease (9 patients); idiopathic panuveitis (10 patients); and presumed ocular tuberculosis uveitis group (12 patients) (Figure 2).
Figure 1.
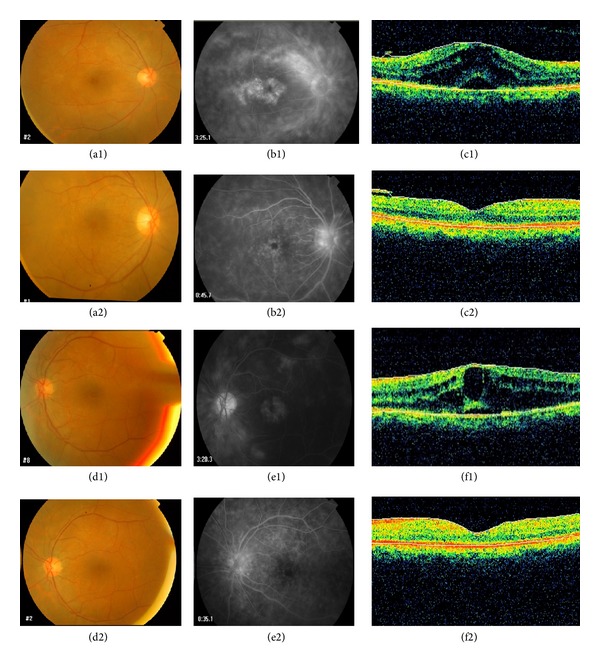
A 56-year-old female with bilateral idiopathic intermediate uveitis and chronic cystoid macular edema. (a1), (b1), and (c1) and (d1), (e1), and (f1) are the fundus photos, fluorescein angiograms, and optical coherence tomography prior to treatment with intravitreal bevacizumab in both eyes. (a2), (b2), and (c2) and (d2), (e2), and (f2) are the fundus photos, fluorescein angiograms and optical coherence tomography, after treatment with intravitreal bevacizumab, which show the response of CME after intravitreal bevacizumab.
Figure 2.
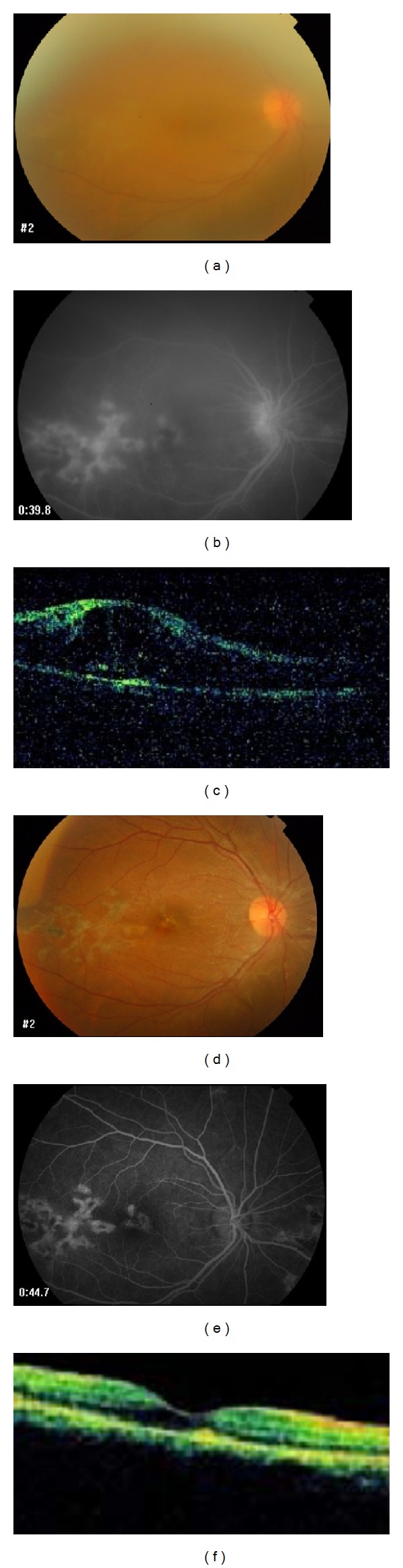
A 28-year-old female with presumed intraocular tuberculosis, choroiditis, and cystoid macular edema in the right eye. (a), (b), and (c) are the fundus photos, fluorescein angiograms, and optical coherence tomographies, prior to treatment with intravitreal bevacizumab. (d), (e), and (f) are the fundus photos, fluorescein angiograms, and optical coherence tomography, after treatment with intravitreal bevacizumab, which shows good response.
The mean LogMAR visual acuity for the study cohort improved from a baseline value of 0.8 ± 0.8 to 0.4 ± 0.5 at 1 month and 0.3 ± 0.5 at 3 months. The improvement in visual acuity at 3 months was statistically significant (P < 0.002) (Table 1). There was a continuous increase in mean visual acuity over the duration of followup in each group (Table 1). The baseline macular thickness for the study cohort was 430 ± 132 μm. Following intravitreal bevacizumab, the macular thickness improved to 286 ± 93 μm at 1 month and to 265 ± 88 μm at 3 months. The improvement in macular thickness at 3 months was statistically significant (P < 0.001) (Table 1).
Table 1.
Demographics, visual acuity, and macular thickness of patients with uveitic cystoid macular edema treated with intravitreal bevacizumab.
| IIU | BD | IPU | POTBU | P value | |
|---|---|---|---|---|---|
| Number of patients | 10 | 9 | 10 | 12 | |
| Mean age | 44 ± 16 | 34 ± 7 | 28 ± 13 | 43 ± 17 | |
| Mean followup | 4 ± 1 | 4 ± 1 | 4 ± 1 | 3.9 ± 2 | |
| Mean number of Avastin injections | 1.2 ± 0.4 | 1.7 ± 0.7 | 1.6 ± 0.7 | 1.6 ± 0.5 | |
| Mean initial VA | 0.5 ± 0.8 | 0.8 ± 0.8 | 0.8 ± 0.8 | 0.8 ± 0.5 | |
| Mean 1-month VA | 0.3 ± 0.4 | 0.4 ± 0.8 | 0.5 ± 0.8 | 0.5 ± 0.8 | |
| Mean 3-month VA | 0.2 ± 0.4 | 0.2 ± 0.5 | 0.3 ± 0.5 | 0.4 ± 0.5 | <0.002 |
| Mean initial OCT thickness (μm) | 437 ± 121 | 433 ± 179 | 342 ± 83 | 404 ± 134 | |
| Mean OCT thickness (1 month) (μm) | 314 ± 120 | 259 ± 102 | 270 ± 45 | 296 ± 94 | |
| Mean OCT thickness (3 months) (μm) | 246 ± 80 | 284 ± 106 | 239 ± 49 | 281 ± 110 | <0.001 |
P value (ANOVA) was assessed for the mean OCT retinal thickness and the mean LogMAR change in visual acuity form baseline.
IIU: idiopathic intermediate uveitis, BD: Behcet's disease, IPU: idiopathic panuveitis, POTBU: presumed ocular tuberculosis uveitis, VA: visual acuity, and OCT: optical coherence tomography.
The change in visual acuity and macular thickness for each group is presented in Table 1. All groups had an increase in mean visual acuity after intravitreal bevacizumab (Table 1). The greatest reduction in macular thickness occurred at 1 month in Behcet's disease group, but the edema reappeared by 3 months (Table 1). All other groups had a continuous reduction in macular thickness at 3 months (Table 1). The greatest reduction in macular thickness from baseline to 3 months occurred in the idiopathic intermediate uveitis group (Table 1).
Thirteen (32%) out of 41 patients received more than one intravitreal bevacizumab injection. Eight of these patients had uncontrolled intraocular inflammation and 5 (15%) of 33 patients (P < 0.001) had well-controlled intraocular inflammation.
No systemic or ocular complications were noted following intravitreal bevacizumab. A transient rise in intraocular pressure following intravitreal bevacizumab was observed in 14 (34%) patients.
4. Discussion
Uveitis is an important cause of ocular morbidity, as it can cause progressive, relentless destruction of visually important structures such as the macula. Immune-mediated inflammation of the uvea afflicts 1.15 per 1,000 individuals in the western hemisphere [37]. Chronic UME is frequently seen in patients with chronic uveitis. The therapeutic strategy for immune-mediated uveitis is evolving as new therapeutic modalities emerge. Immune-mediated insults initiate a chain of events at the cellular and molecular levels leading to an upregulation of several cytokines such as VEGF which is upregulated in patients with uveitis [5–8, 10].
Currently, there is no standard treatment for managing UME associated with chronic uveitis. Currently available treatment consists of topical nonsteroidal anti-inflammatory, oral, periocular, and intraocular injections of corticosteroids, as well as oral carbonic anhydrase inhibitors, systemic somatostatin analogs, and recently interferon alpha, mycophenolate mofetil, and VEGF inhibitors [11–20].
The outcomes of the current study indicate that intravitreal bevacizumab is effective, tolerable, and safe for the management of UME associated with uveitis. For example, there was a significant reduction in UME indicated by the decrease in macular thickness. Additionally, there was a concomitant improvement in visual acuity in patients suffering from idiopathic intermediate uveitis, panuveitis, Behcet's disease, and presumed ocular tuberculosis. These outcomes indicate that anti-VEGF treatment, which has no immunosuppressive effects may serve as a safe treatment for UME in patients with infectious uveitis. Our results concur with several reports that have described an improvement in macular edema and regression of ocular neovascularization following intravitreal bevacizumab for uveitis [7, 10, 29–31, 33]. The improvement of macular edema after intravitreal bevacizumab was transient and short-lived in several studies [30, 31, 38]. In this study, we found that adequate control of intraocular inflammation is associated with reduction in the number of intravitreal bevacizumab reinjection. Uncontrolled intraocular inflammation may lead to recurrence of UME which would warrant repeat injections of bevacizumab. We found that intravitreal bevacizumab with the control of inflammation affords long-term remission of UME. For example, only 5 out of 33 patients with controlled intraocular inflammation required more than one injection of intravitreal bevacizumab in comparison to 8 patients with uncontrolled active intraocular inflammation who received more than one injection (P < 0.001). Repeat injections were indicated in patients with active uveitis. We believe that bevacizumab is an important adjuvant treatment to appropriate therapies for the management of UME associated with infectious or noninfectious uveitis due to the lack of an immunosuppressive effect and the safety and efficacy.
Some limitations of this study include the retrospective review and short follow-up period. However, consecutive patients irrespective of outcome were selected over the time period of this study to mitigate some of the drawbacks.
In conclusion, cases with well-controlled intraocular inflammation that receive adjunct intravitreal bevacizumab result in long-term remission of UME. In cases of UME associated with infectious uveitis, the lack of immunosuppression from intravitreal bevacizumab treatment will not interfere with the immune response. Longer-term prospective studies are required to confirm the observation in this study.
Acknowledgments
This study was supported in part by a Fund from The Eye Foundation for Research in Ophthalmology, The Eye Center, Riyadh, Saudi Arabia, and the King Khaled Eye Specialist Hospital, Riyadh, Saudi Arabia. The authors have no proprietary or financial interest in any products or techniques described in this paper.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Rothova A, Suttorp-van Schulten MSA, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. The British Journal of Ophthalmology. 1996;80(4):332–336. doi: 10.1136/bjo.80.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lardenoye CWTA, van Kooij B, Rothova A. Impact of macular edema on visual acuity in uveitis. Ophthalmology. 2006;113(8):1446–1449. doi: 10.1016/j.ophtha.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 3.Guex-Crosier Y. The pathogenesis and clinical presentation of macular edema in inflammatory diseases. Documenta Ophthalmologica. 1999;97(3-4):297–309. doi: 10.1023/a:1002130005227. [DOI] [PubMed] [Google Scholar]
- 4.Okhravi N, Lightman S. Cystoid macular edema in uveitis. Ocular Immunology and Inflammation. 2003;11(1):29–38. doi: 10.1076/ocii.11.1.29.15582. [DOI] [PubMed] [Google Scholar]
- 5.Fine HF, Baffi J, Reed GF, Csaky KG, Nussenblatt RB. Aqueous humor and plasma vascular endothelial growth factor in uveitis-associated cystoid macular edema. American Journal of Ophthalmology. 2001;132(5):794–796. doi: 10.1016/s0002-9394(01)01103-5. [DOI] [PubMed] [Google Scholar]
- 6.van Kooij B, Rothova A, Rijkers GT, de Groot-Mijnes JDF. Distinct cytokine and chemokine profiles in the aqueous of patients with uveitis and cystoid macular edema. The American Journal of Ophthalmology. 2006;142(1):192–194. doi: 10.1016/j.ajo.2006.02.052. [DOI] [PubMed] [Google Scholar]
- 7.Gulati N, Forooghian F, Lieberman R, Jabs DA. Vascular endothelial growth factor inhibition in uveitis: a systematic review. British Journal of Ophthalmology. 2011;95(2):162–165. doi: 10.1136/bjo.2009.177279. [DOI] [PubMed] [Google Scholar]
- 8.Senger DR, Connolly DT, van de Water L, Feder J, Dvorak HF. Purification and NH2-terminal amino acid sequence of guinea pig tumor-secreted vascular permeability factor. Cancer Research. 1990;50(6):1774–1778. [PubMed] [Google Scholar]
- 9.Ziemssen F, Bartz-Schmidt KU, Grisanti S. (Side) effects of VEGF inhibition. Ophthalmologe. 2006;103(6):484–492. doi: 10.1007/s00347-006-1354-3. [DOI] [PubMed] [Google Scholar]
- 10.Weiss K, Steinbrugger I, Weger M, et al. Intravitreal VEGF levels in uveitis patients and treatment of uveitic macular oedema with intravitreal bevacizumab. Eye. 2009;23(9):1812–1818. doi: 10.1038/eye.2008.388. [DOI] [PubMed] [Google Scholar]
- 11.Rothova A. Medical treatment of cystoid macular edema. Ocular Immunology and Inflammation. 2002;10(4):239–246. doi: 10.1076/ocii.10.4.239.15589. [DOI] [PubMed] [Google Scholar]
- 12.Rojas B, Zafirakis P, Christen W, Markomichelakis NN, Foster CS. Medical treatment of macular edema in patients with uveitis. Documenta Ophthalmologica. 1999;97(3-4):399–407. doi: 10.1023/a:1002525619764. [DOI] [PubMed] [Google Scholar]
- 13.Hariprasad SM, Callanan D, Gainey S, He Y, Warren K. Cystoid and diabetic macular edema treated with nepafenac 0.1% Journal of Ocular Pharmacology and Therapeutics. 2007;23(6):585–589. doi: 10.1089/jop.2007.0062. [DOI] [PubMed] [Google Scholar]
- 14.Antcliff RJ, Spalton DJ, Stanford MR, Graham EM, Fytche TJ, Marshall J. Intravitreal triamcinolone for uveitic cystoid macular edema: an optical coherence tomography study. Ophthalmology. 2001;108(4):765–772. doi: 10.1016/s0161-6420(00)00658-8. [DOI] [PubMed] [Google Scholar]
- 15.van Kooij B, Rothova A, de Vries P. The pros and cons of intravitreal triamcinolone injections for uveitis and inflammatory cystoid macular edema. Ocular Immunology and Inflammation. 2006;14(2):73–85. doi: 10.1080/09273940500545684. [DOI] [PubMed] [Google Scholar]
- 16.Androudi S, Letko E, Meniconi M, Papadaki T, Ahmed M, Foster CS. Safety and efficacy of intravitreal triamcinolone acetonide for uveitic macular edema. Ocular Immunology and Inflammation. 2005;13(2-3):205–212. doi: 10.1080/09273940590933511. [DOI] [PubMed] [Google Scholar]
- 17.Whitcup SM, Csaky KG, Podgor MJ, et al. A randomized, masked, crossover trial of acetazolamide for cystoid macular edema in patients with uveitis. Ophthalmology. 1996;103(7):1054–1063. doi: 10.1016/s0161-6420(96)30567-8. [DOI] [PubMed] [Google Scholar]
- 18.Schilling H, Heiligenhaus A, Laube T, Bornfeld N, Jurklies B. Long-term effect of acetazolamide treatment of patients with uveitic chronic cystoid macular edema is limited by persisting inflammation. Retina. 2005;25(2):182–188. doi: 10.1097/00006982-200502000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Deuter CME, Kötter I, Günaydin I, Stübiger N, Doycheva DG, Zierhut M. Efficacy and tolerability of interferon alpha treatment in patients with chronic cystoid macular oedema due to non-infectious uveitis. British Journal of Ophthalmology. 2009;93(7):906–913. doi: 10.1136/bjo.2008.153874. [DOI] [PubMed] [Google Scholar]
- 20.Neri P, Mariotti C, Cimino L, Mercanti L, Giovannini A. Long-term control of cystoid macular oedema in noninfectious uveitis with Mycophenolate Mofetil. International Ophthalmology. 2009;29(3):127–133. doi: 10.1007/s10792-008-9200-z. [DOI] [PubMed] [Google Scholar]
- 21.Ip MS, Scott IU, Brown GC, et al. Anti-vascular endothelial growth factor pharmacotherapy for age-related macular degeneration: a report by the American Academy of Ophthalmology. Ophthalmology. 2008;115(10):1837–1846. doi: 10.1016/j.ophtha.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 22.Spaide RF, Laud K, Fine HF, et al. Intravitreal bevacizumab treatment of choroidal neovascularization secondary to age-related macular degeneration. Retina. 2006;26(4):383–390. doi: 10.1097/01.iae.0000238561.99283.0e. [DOI] [PubMed] [Google Scholar]
- 23.Avery RL, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ. Intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Ophthalmology. 2006;113(3):363–372. doi: 10.1016/j.ophtha.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 24.Krebs I, Lie S, Stolba U, Zeiler F, Felke S, Binder S. Efficacy of intravitreal bevacizumab (Avastin) therapy for early and advanced neovascular age-related macular degeneration. Acta Ophthalmologica. 2009;87(6):611–617. doi: 10.1111/j.1755-3768.2008.01312.x. [DOI] [PubMed] [Google Scholar]
- 25.Lynch SS, Cheng CM. Bevacizumab for neovascular ocular diseases. Annals of Pharmacotherapy. 2007;41(4):614–625. doi: 10.1345/aph.1H316. [DOI] [PubMed] [Google Scholar]
- 26.Ciulla TA, Rosenfeld PJ. Anti-vascular endothelial growth factor therapy for neovascular ocular diseases other than age-related macular degeneration. Current Opinion in Ophthalmology. 2009;20(3):166–174. doi: 10.1097/ICU.0b013e328329d173. [DOI] [PubMed] [Google Scholar]
- 27.Barkmeier AJ, Akduman L. Bevacizumab (Avastin) in ocular processes other than choroidal neovascularization. Ocular Immunology and Inflammation. 2009;17(2):109–117. doi: 10.1080/09273940802596534. [DOI] [PubMed] [Google Scholar]
- 28.Faghihi H, Roohipoor R, Mohammadi S-F, et al. Intravitreal bevacizumab versus combined bevacizumab-triamcinolone versus macular laser photocoagulation in diabetic macular edema. European Journal of Ophthalmology. 2008;18(6):941–948. doi: 10.1177/112067210801800614. [DOI] [PubMed] [Google Scholar]
- 29.Coma MC, Sobrin L, Onal S, Christen W, Foster CS. Intravitreal bevacizumab for the treatment of uveitic macular edema. Ophthalmology. 2007;114(8):1574.e1–1579.e1. doi: 10.1016/j.ophtha.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 30.Mackensen F, Heinz C, Becker MD, Heiligenhaus A. Intravitreal bevacizumab (avastin) as a treatment for refractory macular edema in patients with uveitis: a pilot study. Retina. 2008;28(1):41–45. doi: 10.1097/IAE.0b013e318156db75. [DOI] [PubMed] [Google Scholar]
- 31.Cervantes-Castañeda RA, Giuliari GP, Gallagher MJ, et al. Intravitreal bevacizumab in refractory uveitic macular edema: one-year follow-up. European Journal of Ophthalmology. 2009;19(4):622–629. doi: 10.1177/112067210901900417. [DOI] [PubMed] [Google Scholar]
- 32.Acharya NR, Hong KC, Lee SM. Ranibizumab for refractory uveitis-related macular edema. The American Journal of Ophthalmology. 2009;148(2):303–309. doi: 10.1016/j.ajo.2009.03.028. [DOI] [PubMed] [Google Scholar]
- 33.Al-Dhibi H, Khan AO. Bilateral response following unilateral intravitreal bevacizumab injection in a child with uveitic cystoid macular edema. Journal of AAPOS. 2009;13(4):400–402. doi: 10.1016/j.jaapos.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Lott MN, Schiffman JC, Davis JL. Bevacizumab in inflammatory eye disease. American Journal of Ophthalmology. 2009;148(5):711–717. doi: 10.1016/j.ajo.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamade IH, Tabbara KF. Complications of presumed ocular tuberculosis. Acta Ophthalmologica. 2010;88(8):905–909. doi: 10.1111/j.1755-3768.2009.01579.x. [DOI] [PubMed] [Google Scholar]
- 36.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. The American Journal of Ophthalmology. 2005;140(3):509–516. doi: 10.1016/j.ajo.2005.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California: the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111(3):491–500. doi: 10.1016/j.ophtha.2003.06.014. [DOI] [PubMed] [Google Scholar]
- 38.Ziemssen F, Deuter CM, Stuebiger N, Zierhut M. Weak transient response of chronic uveitic macular edema to intravitreal bevacizumab (Avastin) Graefe's Archive for Clinical and Experimental Ophthalmology. 2007;245(6):917–918. doi: 10.1007/s00417-006-0512-2. [DOI] [PubMed] [Google Scholar]


