Abstract
Background
Substantial complexity has been introduced into treatment regimens for patients with human immunodeficiency virus (HIV) infection. Many drug-related problems (DRPs) are detected in these patients, such as low adherence, therapeutic inefficacy, and safety issues. We evaluated the impact of pharmacist interventions on CD4+ T-lymphocyte count, HIV viral load, and DRPs in patients with HIV infection.
Methods
In this 18-month prospective controlled study, 90 outpatients were selected by convenience sampling from the Hospital Dia–University of Campinas Teaching Hospital (Brazil). Forty-five patients comprised the pharmacist intervention group and 45 the control group; all patients had HIV infection with or without acquired immunodeficiency syndrome. Pharmaceutical appointments were conducted based on the Pharmacotherapy Workup method, although DRPs and pharmacist intervention classifications were modified for applicability to institutional service limitations and research requirements. Pharmacist interventions were performed immediately after detection of DRPs. The main outcome measures were DRPs, CD4+ T-lymphocyte count, and HIV viral load.
Results
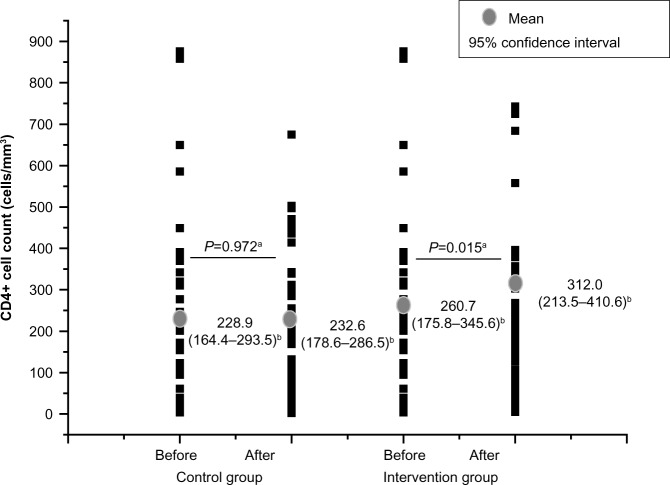
After pharmacist intervention, DRPs decreased from 5.2 (95% confidence interval [CI] =4.1–6.2) to 4.2 (95% CI =3.3–5.1) per patient (P=0.043). A total of 122 pharmacist interventions were proposed, with an average of 2.7 interventions per patient. All the pharmacist interventions were accepted by physicians, and among patients, the interventions were well accepted during the appointments, but compliance with the interventions was not measured. A statistically significant increase in CD4+ T-lymphocyte count in the intervention group was found (260.7 cells/mm3 [95% CI =175.8–345.6] to 312.0 cells/mm3 [95% CI =23.5–40.6], P=0.015), which was not observed in the control group. There was no statistical difference between the groups regarding HIV viral load.
Conclusion
This study suggests that pharmacist interventions in patients with HIV infection can cause an increase in CD4+ T-lymphocyte counts and a decrease in DRPs, demonstrating the importance of an optimal pharmaceutical care plan.
Keywords: pharmaceutical care, HIV, clinical pharmacy, CD4+ T lymphocyte count, AIDS, pharmacy service
Introduction
With the increasing number, availability, and rapid emergence of new information on antiretroviral (ARV) agents, a substantial complexity has been introduced into treatment regimens for patients with human immunodeficiency virus (HIV) infection.1 This may lead to low adherence to ARV regimens, which directly affects therapeutic efficacy. The risk of treatment failure, commonly associated with resistance to ARV agents, increases as adherence to therapy decreases.2 Clinical parameters used as predictors of therapeutic efficacy are CD4+ T-lymphocyte count and HIV viral load. CD4+ T-lymphocyte count is the most significant predictor of disease progression and survival. Lower CD4+ counts are associated with a greater risk of disease progression.3,4 CD4+ counts from 350 to 500 cells/mm3 are associated with risks of ≤5% across all age and HIV ribonucleic acid strata, whereas the risk of acquired immunodeficiency syndrome (AIDS) progression substantially increases at CD4+ counts <350 cells/mm3. The greatest risk increase occurs as CD4+ counts fall to <200 cells/mm3.3,5
Due to ARV regimen complexity, many drug-related problems (DRPs) are detected in both inpatients and outpatients.6–8 A DRP is an undesirable patient experience involving drug therapy that actually or potentially interferes with the desired patient outcome. The pharmacist is a key professional in detecting and preventing DRPs by providing information, answering questions, and helping the patient to improve his or her self-care. In this context, the function assigned to the pharmacist-educator is one of the most important to promote the rational use of medicines.6,7,9,10
The public health system in Brazil is internationally recognized for its delivery of optimal treatment to patients with HIV at no cost since 1996. It is estimated that 217,000 patients have already been treated.11,12 Many similarities are observed when comparing Brazilian and American HIV treatment guidelines, suggesting that both can provide optimal care to patients with HIV (Table 1).4,13
Table 1.
Comparison of the Brazilian and United Stated guidelines for human immunodeficiency virus treatment in relation to drug-resistance testing at the time of antiretroviral therapy (ART) initiation, initiating ART in treatment-naïve patients, and initial combination regimens for antiretroviral-naïve patients
| Drug-resistance testing at the time of ART initiation | Initiating ART in treatment-naïve patients | Initial combination regimens for antiretroviral-naïve patients | |
|---|---|---|---|
| Brazil4 | People infected by partner in use of ART (recent or in the past); pregnant women with HIV infection | All patients with HIV infection with symptomatic HIV; active tuberculosis; CD4+ count ≤500 cells/mm3; coinfection with hepatitis B virus; pregnant women; established cardiovascular disease; AIDS-defining malignancies with no indication of chemotherapy or radiotherapy. | AZT/3TC + EFV or TDF/3TC + EFV, regarding each patient’s characteristics and presence of renal dysfunction or anemia not related to HIV infection. |
| United States13 | People with HIV infection at entry into care regardless of whether ART was initiated immediately or deferred/pregnant women with HIV infection | All patients with HIV infection, to reduce the risk of disease progression. | 1) Based on the NNRTI: EFV/TDF/FTC 2) Based on PI/r: ATV/r + TDF/FTC or DRV/r + TDF/FTC 3) Based on integrase inhibitor: RAL + TDF/FTC. |
Abbreviations: AIDS, acquired immunodeficiency syndrome; ATV/r + TDF/FTC, atazanavir/ritonavir + tenofovir/emtricitabine; AZT/3TC, zidovudine/efavirenz; DRV/r + TDF/FTC, darunavir/ritonavir + tenofovir/emtricitabine; EFV, efavirenz; EFV/TDF/FTC, efavirenz/tenofovir/emtricitabine; HIV, human immunodeficiency virus; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI/r, protease inhibitors/ritonavir; RAL + TDF/FTC, raltegravir + tenofovir/emtricitabine; TDF/3TC, tenofovir/lamivudine.
However, suboptimal adherence to drug therapy greatly contributes to viral resistance and treatment failure.14 In an effort to slow viral resistance; improve treatment effectiveness; and identify, prevent, and solve DRPs, clinical pharmacist intervention can be of benefit to patients with HIV.10,15 Several studies with various population profiles demonstrated a significant decrease in medication errors at institutions where pharmacists performed interventions alongside the clinical team.10,16–19 A systematic review and meta-analysis showed that pharmacists providing direct patient care had favorable effects across various patient outcomes, health care settings, and disease states.20
Clinical pharmacy began to be officially discussed in Brazil in the mid-1990s. Since then, clinical pharmacy and pharmaceutical care services have been introduced in group discussions and research throughout the country in both private and public institutions, resulting in important developments in the field.21,22 Academically, general pharmacists are trained to work in various profession areas, and clinical pharmacists can now specialize. As is the case in many countries, more studies assessing the impact of clinical pharmacy services in Brazil are needed on patient outcomes and safety.23,24
This article contributes to the literature in many ways. Its purpose is to verify the relevance of performing pharmacy care in patients with HIV infection through the analysis of its effect on DRPs, CD4+ T-lymphocyte counts, and HIV viral load.
Materials and methods
In this prospective controlled study, patients were selected by convenience sampling between February 2009 and August 2010 at the Hospital Dia–University of Campinas Teaching Hospital (Campinas, Brazil). The research was approved by the Research Ethics Committee of the Faculty of Medical Sciences, University of Campinas.
Eligibility criteria
Outpatients with HIV or AIDS aged 18–60 years and with a body mass index of <30 kg/m2 were eligible if they were receiving ARV treatment with no changes at the inclusion visit or within the previous 4 weeks. Patients aged >60 years were not included in the study because higher HIV infection progression and mortality rates have been identified among older patients compared with younger patients.25 Additionally, advanced age is associated with a greater number of medications for comorbidities, drug interactions, adverse reactions, and toxicity.26 Obese patients were not included because they present higher incidences of hyperlipidemia, hypertension, and insulin resistance, and because some HIV medications, such as protease inhibitors, can cause weight gain and fat accumulation, it would not be possible to determine whether weight gain was related to the medication or to the background disease in such patients.27–30
Excluded from participation were patients who were prisoners, who were unable to return for appointments or examinations, who died during the study period, who had severe psychiatric disease that limited comprehension, who did not follow previously scheduled appointments, and who were pregnant.
Groups, outcome measures, and classifications
Patients were assigned to an intervention group or a control group. On days when the clinical pharmacy team was in Hospital Dia for appointments, medical charts of patients being attended were analyzed for fit with the study criteria, and those eligible were assigned to the intervention group. On days when the clinical pharmacy staff was not in Hospital Dia, patients were evaluated for eligibility and assigned to the control which was subsequently revised by the clinical pharmacy team.
Patients in the intervention group were asked to attend appointments with a clinical pharmacist after their routine physician appointments during the study period. Those in the control group received the usual care without appointments with the clinical pharmacists.
For the intervention group, at least two pharmacist appointments of 30–60 minutes each were performed. The first appointment was named visit one. Visit two was defined as the second pharmacist appointment with new CD4+ count and HIV viral load results. Some patients attended more than one pharmacist appointment after visit one, but for study purposes, only visits defined as one and two were considered. For the control group, visits one and two were defined as medical appointments that included examination results. Patients in the intervention group who had fewer than two pharmacist appointments and patients in both groups who were referred to another health service during the study were excluded.
Pharmacist appointments began with data collection through patient interview regarding medical history. Besides patient reports about health and ongoing treatments, other information was obtained from family members or caregivers and other health professionals. The theoretical framework for the pharmaceutical appointment relied on Pharmacotherapy Workup, a process where pharmacists use rational decision-making to assess a patient’s drug-related needs, identify drug therapy problems, develop a care plan, conduct follow-up evaluations, and ensure that all drug therapies are safe and effective.31 The Pharmacotherapy Workup was modified according to our institution’s service limitations and research requirements. Forms used to record data and guide the pharmacist appointments were prepared by the study pharmacists.
The pharmacist team comprised two pharmacists responsible for performing the clinical pharmacist appointments and two pharmacists responsible for DRP analysis. The primary end point was the comparison between DRPs identified at baseline and at the end of the study for the intervention group. Secondary end points were the comparison between change in CD4+ and HIV viral load from baseline to study end in both the intervention and the control groups.
Before the pharmacist appointments, the patient’s medical history was reviewed using his or her medical chart. The following data were collected for each intervention group patient: age, ethnicity, HIV diagnosis in years, HIV treatment in years, CD4+ count, HIV viral load, comorbidities, and ARV regimen. Patient symptoms, signs, complaints, medications in use, and self-reported nonadherence data were obtained through an interview during the pharmacist appointment. These data were analyzed by separate pharmacists on the team to identify and classify the DRPs related to all medications, including ARV therapy. For the control group, the same data were collected from the medical chart at the time when the patient was included in the study, but the pharmacist appointment was not performed, and additional data regarding medication were not collected; thus, the DRPs could not be accurately identified.
DRPs were individually identified and classified by two pharmacists, in seven categories: 1) unnecessary drug therapy: drug therapy is unnecessary because there is no clinical indication; 2) needs additional drug therapy: additional drug therapy is required to treat or prevent a medical condition; 3) ineffective drug: drug is not effective in producing the desired response; 4) dosage too low: dosage is too low to produce the desired response; 5) adverse drug reaction: drug causes an adverse reaction; 6) dosage too high: dosage results in undesirable effects; 7) noncompliance: the patient is not able or willing to take the drug regimen appropriately.31 After identifying DRPs, the clinical pharmacists developed a pharmacist intervention plan comprising measures to be taken by the patient and medical team in upcoming appointments. The three main pharmacist goals were to resolve DRPs, prevent DRPs, and encourage the adoption of healthy habits (quality-of-life intervention).
Pharmacist interventions consisted of guiding patients, mainly with regard to adherence to the prescribed therapeutic regimen, and suggesting medication changes to physicians when needed. Pharmacist interventions were performed in case of problems with dosage, drug–drug and drug–food interactions, side effects, and adverse reactions. Verbal and written information about pharmaceutical interventions was provided to the patients and physicians.
A community pharmacy specializing in ARV treatment, also located at the University of Campinas, was responsible for providing the ARV treatment.
Statistical analysis
A 95% confidence interval (CI) was applied to determine the extent of variation in data. Collected data were analyzed using a paired t-test to compare primary and secondary end points before and after interventions. For nonparametric data and because of the small sample size, DRP reduction, total pharmacist interventions, increase of CD4+ T-lymphocyte count, and reduction in HIV viral load for the intervention group were analyzed by Spearman correlation test. A two-sided P<0.05 was considered statistically significant for all tests.
Results
A total of 115 patients with HIV infection from Hospital Dia were screened between February 2009 and August 2010. Among them, 111 patients met the study inclusion criteria and were assigned to either the intervention group (n=57) or control group (n=54). Four patients could not be included in the study because of obesity (n=3) and being older than 60 (n=1). Consent was obtained from all patients participating in the study. During the study, 12 patients in the intervention group were excluded: six were transferred to another health care center, and six did not attend at least two pharmacist appointments. From the control group, nine patients were excluded: seven were transferred to another health care center, and two died. Thus, the final sample included 90 patients (n=45 for both groups). The average interval between visit one and visit two for both groups was 5.47±3.94 months, and the average duration of each visit was 1 hour. The average number of pharmacist appointments per patient in the intervention group was 3.0±1.1 (range =2–5).
Table 2 shows the baseline characteristics of the control and intervention groups. The groups were similar in age, sex, ethnicity, time of HIV diagnosis, treatment, and laboratory baseline values. The intervention group presented with a mean basal CD4+ count of 260.7 cells/mm3, and the control group presented with 228.9 cells/mm3. Eighty-nine percent of intervention group patients versus 93% of control group patients presented with comorbidities.
Table 2.
Comparison of the initial characteristics (baseline) of the control group (n=45) and the intervention group (n=45)
| Characteristics | CG | IG | P-valuea |
|---|---|---|---|
| Age (average ± CI, years) | 41.5 (39.2–43.8) | 42.0 (39.1–44.9) | 0.778 |
| Men, % (n) | 55.5 (25) | 55.5 (25) | 1.000 |
| Ethnicity, % (n) | |||
| Caucasian | 64.4 (29) | 57.8 (26) | 0.522 |
| Biracial | 24.4 (11) | 28.9 (13) | 0.816 |
| Asian | 0.0 (0) | 2.2 (1) | 0.320 |
| Black | 11.1 (5) | 11.1 (5) | 1.000 |
| HIV diagnosis (average ± CI, years) | 8.3 (6.9–9.7) | 8.7 (6.2–11.2) | 0.758 |
| Treatment time (average ± CI, years) | 7.5 (6.3–8.7) | 6.7 (5.2–8.2) | 0.409 |
| Tablets per dayb (average ± CI, number) | 8.6 (7.1–10.1) | 8.6 (7.5–9.7) | 0.961 |
| CD4+ basal lymphocytes (average ± CI, cells/mm³) | 228.9 (164.4–293.5) | 260.7 (175.8–345.6) | 0.589 |
| Basal viral load <50 copies/mm³ (%) | 34.0 | 44.0 | 0.285 |
| Basal hemoglobin (average ± CI, g/dL) | 12.4 (11.6–13.2) | 12.7 (12.2–13.2) | 0.411 |
| Presented comorbidities, % (n) | 93.3 (42) | 88.8 (40) | 0.464 |
| Hepatitis C | 33.3 (15) | 13.3 (6) | 0.025 |
| Smoking | 28.8 (13) | 11.1 (5) | 0.035 |
| Neurotoxoplasmosis | 13.3 (6) | 15.5 (7) | 0.767 |
| Antiretroviral regimen, % | |||
| Zidovudine/lamivudine + efavirenz | 15.5 | 15.4 | 0.910 |
| Lamivudine + tenofovir + lopinavir/ritonavir | 22.2 | 7.7 | 0.212 |
| Zidovudine/lamivudine + tenofovir + lopinavir/ritonavir | 11.1 | 15.4 | 0.753 |
| Zidovudine/lamivudine + lopinavir/ritonavir | 11.1 | 0.0 | 0.198 |
| Lamivudine + tenofovir + efavirenz | 11.1 | 15.4 | 0.753 |
| Others | 28.9 | 46.1 | 0.150 |
Notes:
Student’s t-test considering two-tailed test
prescription medicines, not only antiretroviral agents.
Abbreviations: CG, control group: not assisted by the pharmacist; CI, confidence interval; HIV, human immunodeficiency virus; IG, intervention group: assisted by the pharmacist.
Table 3 shows DRPs, the primary end point. At visit one, 5.2 (95% CI =4.1–6.2) potential DRPs per patient were found for a total of 227. Most of these DRPs (3.0 per patient) were due to adverse reactions. After pharmacist intervention, DRPs per patient decreased to 4.2 (95% CI =3.3–5.1, P=0.043), illustrating the relationship between potential DRPs observed before and after pharmacist intervention and their frequency in the study (in absolute numbers).
Table 3.
Comparison of drug-related problems before and after the pharmaceutical intervention
| DRPs Types | Before PI
|
After PI
|
P-valuea | ||
|---|---|---|---|---|---|
| Number | Average (CI) | Number | Average (CI) | ||
| Unnecessary | 1 | 0.0 (0.0–0.1) | 1 | 0.0 (0.0–0.1) | 1.000 |
| Additional | 70 | 1.6 (1.2–2.0) | 50 | 1.3 (0.9–1.7) | 0.016 |
| Ineffective | 7 | 0.2 (0.1–0.3) | 4 | 0.1 (0.0–0.2) | 0.183 |
| Dosage too low | 3 | 0.1 (0.0–0.2) | 0 | 0.0 (0.0) | 0.323 |
| Adverse reaction | 111 | 2.9 (2.4–3.4) | 96 | 2.6 (2.1–3.1) | 0.141 |
| Dosage too high | 17 | 0.5 (0.3–0.7) | 17 | 0.5 (0.2–0.8) | 1.000 |
| Noncompliance | 18 | 0.5 (0.2–0.7) | 17 | 0.8 (0.3–0.8) | 0.859 |
| Total | 227 | 5.2 (4.1–6.2) | 171 | 4.2 (3.3–5.1) | 0.043 |
Note:
Student’s t-test considering two-tailed test
Abbreviations: CI, confidence interval; DRPs, drug-related problems; PI, pharmaceutical intervention.
A total of 122 interventions were proposed, with an average of 2.7 interventions per patient. Among them, 33 (27%) were to solve DRPs, including 28 pharmacist–patient interventions. Seventy-nine (65%) interventions were to prevent DRPs, including 63 pharmacist–patient interventions. Ten (8%) interventions were related to improving quality of life. Figure 1 shows the most-performed pharmacist interventions and prevalence of pharmacist–patient/physician interventions. All pharmacist interventions were accepted by physicians, and among patients, the interventions were well accepted during the appointments, but compliance with the interventions was not measured.
Figure 1.
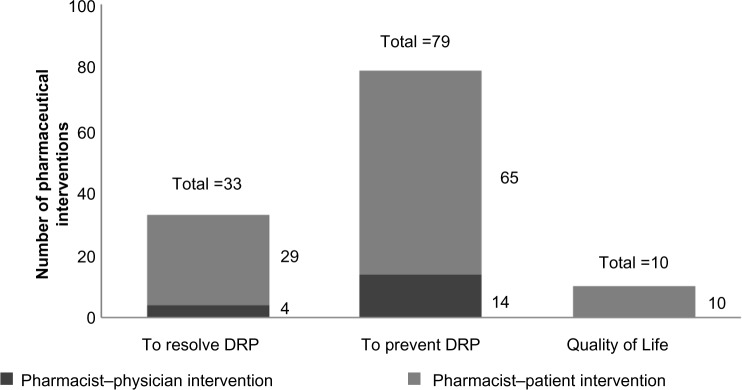
Pharmaceutical interventions performed by the clinical pharmacists.
Abbreviation: DRP, drug-related problem.
Figure 2 shows a significant increase in the CD4+ T-lymphocyte count for the intervention group after the study period (P=0.015). However, no significant difference in CD4+ counts after the study was observed for the control group.
Figure 2.
Comparison of the mean CD4+ count before and after the study period for the control group (n=45) and intervention group (n=45).
Notes: aStudent’s t-test considering two-tailed test; b95% confidence interval considered.
Variation of HIV viral load results before and after pharmacist interventions for both groups were not significant (intervention group, P=0.154; control group, P=0.226). At baseline, 46% of the intervention group and 19% of the control group had an HIV viral load of <50 copies/mL; after the study period, these rates increased to 61% and 40%, respectively.
DRP reduction, total pharmacist interventions, increase of CD4+ T-lymphocyte count, and reduction in HIV viral load did not correlate significantly (data not shown).
Discussion
Pharmacist contributions to the optimization of drug therapy may be evaluated through indirect measures (eg, DRPs) and direct measures, which provide the most conclusive evidence (eg, clinical outcomes).32 A study with 70 patients who received pharmacist interventions during a 4-year period showed that direct patient care led to an increase in treatment compliance and clinical benefits and demonstrated that an average of 1.7 interventions per patient were needed.33
Data from the current study show that patients with HIV infection managed simultaneously by a clinical pharmacy team and their physicians benefited from a decrease in the number of DRPs. At visit one, a high number of potential DRPs per patient were found, which is consistent with a study where medical records were prospectively reviewed in 83 patients with HIV infection who received ARV therapy for 20 months; 176 DRPs were identified in 71 of these patients, including inappropriate prophylaxis for opportunistic infections, too-low and too-high dosages, adverse drug reactions due to ARV drugs, and drug–drug interactions between the ARV drug and another medication.7 In the current study, the most prevalent DRP identified was adverse reaction. Other researchers have also found high frequencies of inappropriate dosage and safety problems.34,35 One showed that the most common issues in hospitalized patients with HIV infection were contraindicated drug–drug combinations and dosing errors.35
The current study shows that DRPs per patient decrease significantly with clinical pharmacist intervention, which is consistent with another study demonstrating that clinical pharmacist involvement leads to significantly more DRPs being identified compared with usual care.36 Other studies have demonstrated that clinical pharmacists not only can effectively identify, solve, and prevent clinically significant DRPs and their outcomes but also have assisted the multidisciplinary team in improving ARV treatment adherence and rational drug use and diminishing drug-related health problems.33,36,37
The detection of DRP reduction, however, is not sufficient. It is also necessary to demonstrate positive clinical outcomes. The current study demonstrates that patients undergoing pharmaceutical care had a significant increase in CD4+ counts when compared before and after the study period, whereas the control group did not show this increase. This difference in CD4+ count was the most relevant between the study groups. In the intervention group, the difference between CD4+ counts at both measured moments (ie, visits one and two) was ~51.3 cells/mm3. Immunologic responses of 50 cells/mm3 had previously been correlated with a reduced risk of developing opportunistic infections,38 suggesting a trend toward improvement of ARV treatment adherence in the intervention group as previously demonstrated by other studies.4,6,15,33,39 Researchers demonstrated that patients with HIV under pharmaceutical care have significantly improved CD4+ counts, decreased HIV viral load, and fewer toxic effects related to ARV drugs.39 Toxicity reduction improves patient quality of life and treatment adherence.40–42 A cohort study in 2,234 patients showed that those patients who had pharmacy team involvement in their care adhered more to ARV therapy than those who did not have pharmacy team involvement.43 On the contrary, the current study demonstrates that both the intervention and control groups had similar results relative to HIV viral load. Further studies are needed over a longer monitoring period to allow for a better analysis.
The clinical pharmacists in the current study performed >70% of the pharmacist–patient intervention. This finding demonstrates that patients with HIV need orientation regarding ARV treatment, which clinical pharmacists are well qualified to provide.
This study is one of the first efforts in Brazil to analyze the contribution of a clinical pharmacy team to clinical and safety outcomes in patients with HIV receiving ARV therapy. We intentionally measured only clinical outcomes, such as CD4+ counts and DRPs, rather than subjective outcomes, such as quality of life, to show quantitatively the benefits of pharmaceutical care in this patient population.
This study suggests that pharmaceutical intervention can increase CD4+ T-lymphocyte counts and decrease DRPs. Aside from its limitations, the study contributes to the discussion of clinical care in both national and international health care scenarios. The study also shows the importance of an optimal pharmaceutical care plan and that the distribution of ARV treatments should be associated with educational initiatives. Further studies are encouraged to confirm the initiative of this program.
Limitations
This study has several limitations. First, the patients were allocated for control or intervention groups based on convenience sampling, which can lead to selection bias. Second, the pharmacists were not blinded, which may have influenced the DRP identification and quantification process. However, the pharmacists were not the same as those who interviewed the patients during their appointments, minimizing this influence. Third, it was not possible to quantify DRPs in the control group. Although the control group did not participate in pharmaceutical appointments, DRP quantification could have been obtained through medical chart revision. However, the medical charts were not complete and are not as useful for gathering accurate information as is a pharmaceutical appointment. Fourth, some relevant information that could contribute to the study was not obtained, such as pill count. Due to Hospital Dia characteristics and study requirements, all pharmacist appointments were performed by two pharmacists, which is unusual in pharmaceutical care services owing to financial issues. These research singularities were a result of study adaptations to the requirements of Hospital Dia. Finally, CD4+ counts and comorbidities were not identical in both study groups at inclusion. However, the difference between the groups regarding CD4+ count was not statistically significant. These limitations are already being addressed by the research team in current studies.
Acknowledgments
This work was supported by State of São Paulo Research Foundation (Fapesp), Coordination for the Improvement of Higher Level Personnel (Capes), National Council for Scientific and Technological Development (CNPQ), and Student Support Service (SAE) of University of Campinas (Unicamp).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Martin S, Wolters PL, Calabrese SK, et al. The Antiretroviral Regimen Complexity Index. A novel method of quantifying regimen complexity. J Acquir Immune Defic Syndr. 2007;45(5):535–544. doi: 10.1097/QAI.0b013e31811ed1f1. [DOI] [PubMed] [Google Scholar]
- 2.Anderson AM, Bartlett JA. Changing antiretroviral therapy in the setting of virologic relapse: review of the current literature. Curr HIV/AIDS Rep. 2006;3(2):79–85. doi: 10.1007/s11904-006-0022-1. [DOI] [PubMed] [Google Scholar]
- 3.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 4.Ministry of Health and Health Surveillance Secretariat (Brazil) National STD/AIDS Programme. Recommendations on Antiretroviral Therapy in HIV-infected Adults and Adolescents. Final rules. Fed Regist. 2012. [Accessed July 24, 2014]. pp. 37–57. Available from: http://www.aids.gov.br/sites/default/files/anexos/publicacao/2012/52140/consenso_adulto2012_principais_mudancas_pdf_11946.pdf.
- 5.Langford SE, Ananworanich J, Cooper DA. Predictors of disease progression in HIV infection: a review. AIDS Res Ther. 2007;4:11. doi: 10.1186/1742-6405-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saberi P, Dong BJ, Johnson MO, Greenblatt RM, Cocohoba JM. The impact of HIV clinical pharmacists on HIV treatment outcomes: a systematic review. Patient Prefer Adherence. 2012;6:297–322. doi: 10.2147/PPA.S30244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mok S, Minson Q. Drug-related problems in hospitalized patients with HIV infection. Am J Health Syst Pharm. 2008;65(1):55–59. doi: 10.2146/ajhp070011. [DOI] [PubMed] [Google Scholar]
- 8.de Maat MM, de Boer A, Koks CH, et al. Evaluation of clinical pharmacist interventions on drug interactions in outpatient pharmaceutical HIV-care. J Clin Pharm Ther. 2004;29(2):121–130. doi: 10.1111/j.1365-2710.2003.00541.x. [DOI] [PubMed] [Google Scholar]
- 9.Strand LM, Morley PC, Cipolle RJ, Ramsey R, Lamsam GD. Drug-related problems: their structure and function. DICP. 1990;24(11):1093–1097. doi: 10.1177/106002809002401114. [DOI] [PubMed] [Google Scholar]
- 10.Nunes P, Pereira B, Nominato J, et al. Pharmaceutical intervention and prevention of drug related problems. Braz J Pharm Sci. 2008;44(4):691–699. Portuguese. [Google Scholar]
- 11.Okie S. Fighting HIV – lessons from Brazil. N Engl J Med. 2006;354(19):1977–1981. doi: 10.1056/NEJMp068069. [DOI] [PubMed] [Google Scholar]
- 12.Brazil, Ministry of Health . Aids No Brasil. Departamento de DST, AIDS e Hepatites Virais/SVS/MS; 2012. [Accessed April 30, 2013]. Avaliable from: http://www.aids.gov.br/sites/default/files/anexos/page/2010/36364/aids_no_brasil_2012_17137.pdf. [Google Scholar]
- 13.Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines For the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. Department of Health and Human Services; 2013. [Accessed April 27, 2013]. Avaliable from: http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. [Google Scholar]
- 14.Neogi U, Heylen E, Shet A, et al. Long-term efficacy of first line antiretroviral therapy in Indian HIV-1 infected patients: a longitudinal cohort study. PLoS One. 2013;8(1):e55421. doi: 10.1371/journal.pone.0055421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma A, Chen DM, Chau FM, Saberi P. Improving adherence and clinical outcomes through an HIV pharmacist’s interventions. AIDS Care. 2010;22(10):1189–1194. doi: 10.1080/09540121003668102. [DOI] [PubMed] [Google Scholar]
- 16.Heelon M, Skiest D, Tereso G, et al. Effect of a clinical pharmacist’s interventions on duration of antiretroviral-related errors in hospitalized patients. Am J Health Syst Pharm. 2007;64(19):2064–2068. doi: 10.2146/ajhp070072. [DOI] [PubMed] [Google Scholar]
- 17.de Lyra DP, Kheir N, Abriata JP, da Rocha CE, Dos Santos CB, Pelá IR. Impact of pharmaceutical care interventions in the identification and resolution of drug-related problems and on quality of life in a group of elderly outpatients in Ribeirão Preto (SP), Brazil. Ther Clin Risk Manag. 2007;3(6):989–998. [PMC free article] [PubMed] [Google Scholar]
- 18.Blix HS, Viktil KK, Moger TA, Reikvam A. Characteristics of drug-related problems discussed by hospital pharmacists in multidisciplinary teams. Pharm World Sci. 2006;28(3):152–158. doi: 10.1007/s11096-006-9020-z. [DOI] [PubMed] [Google Scholar]
- 19.Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;166(9):955–964. doi: 10.1001/archinte.166.9.955. [DOI] [PubMed] [Google Scholar]
- 20.Chisholm-Burns MA, Kim Lee J, Spivey CA, et al. US pharmacists’ effect as team members on patient care: systematic review and meta-analyses. Med Care. 2010;48(10):923–933. doi: 10.1097/MLR.0b013e3181e57962. [DOI] [PubMed] [Google Scholar]
- 21.Ivama AM, Noblat L, de Castro MS, et al. Consenso Brasileiro de Atenção Farmacêutica: Proposta. Organização Pan Americana da Saúde [Brazilian Consensus of Pharmaceutical Care: Proposal. Pan American Health Organization (OPAS)]; 2002. [Accessed April 30, 2013]. Available from: http://bvsms.saude.gov.br/bvs/publicacoes/PropostaConsensoAtenfar.pdf. [Google Scholar]
- 22.Pereira L, de Freitas O. The evolution of pharmaceutical care and the prospect for Brazil. Braz J Pharm Sci. 2008;44(4):601–612. Portuguese. [Google Scholar]
- 23.Zhu M, Guo DH, Liu GY, et al. Exploration of clinical pharmacist management system and working model in China. Pharm World Sci. 2010;32(4):411–415. doi: 10.1007/s11096-010-9407-8. [DOI] [PubMed] [Google Scholar]
- 24.King RC, Fomundam HN. Remodeling pharmaceutical care in Sub-Saharan Africa (SSA) amidst human resources challenges and the HIV/AIDS pandemic. Int J Health Plann Manage. 2010;25(1):30–48. doi: 10.1002/hpm.982. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen N, Holodniy M. HIV infection in the elderly. Clin Interv Aging. 2008;3(3):453–472. doi: 10.2147/cia.s2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasse B, Ledergerber B, Furrer H, et al. Swiss HIV Cohort Study Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis. 2011;53(11):1130–1139. doi: 10.1093/cid/cir626. [DOI] [PubMed] [Google Scholar]
- 27.Bavinger C, Bendavid E, Niehaus K, et al. Risk of cardiovascular disease from antiretroviral therapy for HIV: a systematic review. PLoS One. 2013;8(3):e59551. doi: 10.1371/journal.pone.0059551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friis-Møller N, Sabin CA, Weber R, et al. Data Collection on Averse Events of Anti-HIV Drugs (DAD) Study Group Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349(21):1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 29.Dubé MP. Disorders of glucose metabolism in patients infected with human immunodeficiency virus. Clin Infect Dis. 2000;31(6):1467–1475. doi: 10.1086/317491. [DOI] [PubMed] [Google Scholar]
- 30.Crum-Cianflone N, Roediger MP, Eberly L, et al. Infectious Disease Clinical Research Program HIV Working Group Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS One. 2010;5(4):e10106. doi: 10.1371/journal.pone.0010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cipolle RJ, Strand L, Morley PC. Pharmaceutical Care Practice: The Clinician’s Guide. 2nd ed. New York, NY: McGraw-Hill; 2004. [Google Scholar]
- 32.Viktil KK, Blix HS. The impact of clinical pharmacists on drug-related problems and clinical outcomes. Basic Clin Pharmacol Toxicol. 2008;102(3):275–280. doi: 10.1111/j.1742-7843.2007.00206.x. [DOI] [PubMed] [Google Scholar]
- 33.Geletko SM, Poulakos MN. Pharmaceutical services in an HIV clinic. Am J Health Syst Pharm. 2002;59(8):709–713. doi: 10.1093/ajhp/59.8.709. [DOI] [PubMed] [Google Scholar]
- 34.Rastegar DA, Knight AM, Monolakis JS. Antiretroviral medication errors among hospitalized patients with HIV infection. Clin Infect Dis. 2006;43(7):933–938. doi: 10.1086/507538. [DOI] [PubMed] [Google Scholar]
- 35.Carcelero E, Tuset M, Martin M, et al. Evaluation of antiretroviral-related errors and interventions by the clinical pharmacist in hospitalized HIV-infected patients. HIV Med. 2011;12(8):494–499. doi: 10.1111/j.1468-1293.2011.00915.x. [DOI] [PubMed] [Google Scholar]
- 36.Viktil KK, Blix HS, Moger TA, Reikvam A. Interview of patients by pharmacists contributes significantly to the identification of drug-related problems (DRPs) Pharmacoepidemiol Drug Saf. 2006;15(9):667–674. doi: 10.1002/pds.1238. [DOI] [PubMed] [Google Scholar]
- 37.Pastakia SD, Corbett AH, Raasch RH, Napravnik S, Correll TA. Frequency of HIV-related medication errors and associated risk factors in hospitalized patients. Ann Pharmacother. 2008;42(4):491–497. doi: 10.1345/aph.1K547. [DOI] [PubMed] [Google Scholar]
- 38.Binquet C, Chêne G, Jacqmin-Gadda H, et al. Groupe d’Epidémiologie Clinique du SIDA en Aquitaine Modeling changes in CD4-positive T-lymphocyte counts after the start of highly active antiretroviral therapy and the relation with risk of opportunistic infections: the Aquitaine Cohort, 1996–1997. Am J Epidemiol. 2001;153(4):386–393. doi: 10.1093/aje/153.4.386. [DOI] [PubMed] [Google Scholar]
- 39.March K, Mak M, Louie SG. Effects of pharmacists’ interventions on patient outcomes in an HIV primary care clinic. Am J Health Syst Pharm. 2007;64(24):2574–2578. doi: 10.2146/ajhp070048. [DOI] [PubMed] [Google Scholar]
- 40.Mocroft A, Youle M, Moore A, et al. Reasons for modification and discontinuation of antiretrovirals: results from a single treatment centre. AIDS. 2001;15(2):185–194. doi: 10.1097/00002030-200101260-00007. [DOI] [PubMed] [Google Scholar]
- 41.Pádua CA, César CC, Bonolo PF, Acurcio FA, Guimarães MD. High incidence of adverse reactions to initial antiretroviral therapy in Brazil. Braz J Med Biol Res. 2006;39(4):495–505. doi: 10.1590/s0100-879x2006000400010. [DOI] [PubMed] [Google Scholar]
- 42.Morillo Verdugo R, Gil Navarro MV, Abdel-Kader Martín L, Castillo Muñoz A, Baños Roldán U, Artacho Criado S. Analysis of the causes and predictive factors for discontinuing treatment with tenofovir in pretreated HIV patients. Farm Hosp. 2007;31(4):200–205. doi: 10.1016/s1130-6343(07)75374-6. Spanish. [DOI] [PubMed] [Google Scholar]
- 43.Hirsch JD, Gonzales M, Rosenquist A, Miller TA, Gilmer TP, Best BM. Antiretroviral therapy adherence, medication use, and health care costs during 3 years of a community pharmacy medication therapy management program for Medi-Cal beneficiaries with HIV/AIDS. J Manag Care Pharm. 2011;17(3):213–223. doi: 10.18553/jmcp.2011.17.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]