Summary
T regulatory (Treg) cells enforce peripheral tolerance through regulation of diverse immune responses in a context-dependent manner. Okoye et al show one way in which Treg cells suppress Th1 cell responses is through non-autonomous gene silencing mediated by microRNA-containing exosomes.
T regulatory (Treg) cells employ a diverse set of mechanisms to enforce peripheral tolerance, reflecting both the complexity and plasticity of immune responses. Mechanisms of suppression include production of immunomodulatory cytokines [e.g. interleukin-10 (IL-10), transforming growth factor-β (TGF-β), IL-35)], the expression of inhibitory receptors [cytotoxic T lymphocyte antigen 4 (CTLA-4), cytokine sinks (e.g. IL-2 receptor α chain), and direct cytotoxic killing (granzymes, perforin) and several others (Shevach, 2009). These mechanisms have been validated by the identification of human and murine genetic defects that disable individual pathways, leading to immune dysregulation and autoimmunity. More recently, context dependent inhibition has emerged as strategy to finely tune the regulation of specific T helper cell responses. Treg cells appropriate partial or “aborted” forms of the transcriptional programs of respective target T helper (Th) cell types by expressing their master transcription factors and co-opting their function (Chaudhry et al., 2009; Zheng et al., 2009). For example, in the case of a Th1 response, Treg cells upregulate the expression of T-bet which in turn induces the expression of some Th1 related genes such as CX3CR1 but not others, enabling Treg cells to migrate to sites of Th1 cell-mediated inflammation while restraining their differentiation into Th1 cells (Koch et al., 2012).
In this issue of Immunity, Okoye et al. add to this list another mechanism of suppression, that of non-autonomous gene silencing mediated by miRNA-containing exosomes. Exosomes are 40–100 nM vesicles that are generated by the inward invagination of endosomal membranes to generate intraluminal vesicles in multivesicular bodies (Raposo and Stoorvogel, 2013). The latter are trafficked to the cell membrane by a Rab family GTPase-dependent mechanism where the exosomes are released. Exosome formation may proceed by a mechanism involving the endosomal sorting complex for transport, a set of conserved proteins involved in lysosomal and exosomal trafficking, or by an alternative mechanism involving lipid raft segregation in a ceramide-dependent manner. The capacity of exosomes to transfer miRNA and mRNA has been verified in many cell types (Robbins and Morelli, 2014). Okoye et al extended this concept to Treg cells by first identifying them as prolific producers of exosomes, whose release was hypoxia-sensitive and required Rab27a and Rab27b GTPases and ceramide. Importantly, Treg cell exosomes were laden with miRNA, the profile of which was distinct from those of Th1 and Th2 cells. The authors directly demonstrate that Treg cell exosomes transferred a specific set of miRNA to conventional T cells, including miR-155, Let7b and Let-7d, both in vitro and in vivo. Compromised transfer of Treg cell exosomal miRNAs to conventional T cells, either because of failed miRNA formation (Treg cell Dicer deficiency) or exosome release (Treg cell Rab27a- and Rab27b-deficient Treg cells) abrogated the capacity of Treg cells to prevent disease in a lymphopenia-induced model of colitis.
Okoye et al went on to demonstrate a specific role for exosomes in regulating Th1 responses. Purified exosomes from WT but not Dicer-deficient Treg cells added to in vitro Th1 cultures suppressed cell proliferation and IFN-γ production. Of the three mature exosomal Treg cell miRNAs that were identified in conventional T cells, Let-7d was specifically associated with the control of Th1 responses both in vitro and in vivo. Treg cells transfected with a Let 7-d inhibitor were compromised in their capacity to suppress Th1 cell proliferation and interferon-γ (IFN-γ) production in vitro and suppress colonic inflammation and IFN-γ expression by conventional T cell in the lymphopenia colitis model.
Employment by Treg cells of miRNA-mediated non-autonomous gene silencing as a suppressive mechanism offers several advantages. It redirects the transcriptional circuitry and cellular function of recipient conventional T cells in favor of a tolerogenic profile. As such, it is a particularly effective mediator of “infectious tolerance,” where the effects may range from the transient to the long-lasting. While the studies of Okoye et al were focused on the inhibition of Th1 cells by Treg exosome miRNA, this mechanism is well suited to context-dependent regulation of other Th cell responses. Specificity for a particular Th cell response may be tailored by the precise combination of miRNA delivered by Treg cell exosomes. Sensitivity of exosome release to hypoxia adds a further layer of control that may fine-tune exosome release in different regions of the gastrointestinal tract.
In addition to miRNA, exosomes also deliver other non-coding RNA, mRNA, proteins and lipids that have been implicated in immune regulation (Robbins and Morelli, 2014). Okoye et al demonstrate a wide range of transcripts enriched in Treg cell exosomes as compared to the parent cells, including those encoding chemokines, interleukins, collagen and matrix proteins, ephrins and others. The role of Treg cell exosomal mRNA and proteins in modulating target cells remains unknown, but seems likely to play a role in their immunomodulatory effects.
There are some caveats to the studies of Okoye et al, principle amongst which is the lack of clarity surrounding the differential contribution of non-autonomous gene silencing to Treg cell mediated regulation as compared to other well-established suppressive mechanisms. Most of the in vivo observations on the role of this pathway in peripheral tolerance were gleaned from studies using the lymphopenia-colitis model, which suffers from the limitations of an immunodeficient host and a lymphopenic environment. Additionally, Treg cell-specific ablation of RNaseIII enzymes involved in miRNA maturation, including Dicer and Drosha, results in rapidly fatal autoimmunity, while Rab27a- and Rab27b-double deficient mice lacking in Treg cell exosome release suffer a relatively mild inflammatory phenotype (Chong et al., 2008; Liston et al., 2008). These observations, while arguing for a dominant cell-intrinsic role for miRNA in controlling Treg cell functions, also hint at a more focused role for exosomal delivery of miRNA and other molecules in peripheral tolerance such as context-dependent Th cell regulation and maintenance of mucosal tolerance. Studies employing Treg cell lineage-specific genetic approaches that target the exosomal pathway in other disease models and rescue experiments of Foxp3 deficient mice with exosome sufficient or deficient Treg cells may further clarify the role of this pathway in peripheral tolerance. Its differential role in natural (thymic) versus induced Treg (iTreg) cell-mediated tolerance is also relevant, given the importance of the latter for mucosal tolerance.
While the work of Okoye et al centered on the regulation of T effector responses, it is easy to envision how Treg exosomes might impact the regulatory compartment. In an inflammatory environment, sentinel Treg cells might “educate” newly recruited Treg cells, For example, exosome-mediated transfer of miRNA-155 would decrease SOCS1 expression and increase STAT5 activation, based on data from Treg cell-specific ablation of miRNA-155 (Lu et al., 2009). The result would favor Treg homeostasis and stability. Similarly, enhancement of TGF-β signaling pathways in convential T cells might increase iTreg cell production. Overall, the results of Okoye et al foretell a number of immunoregulatory effects offered by Treg cell-mediated exosomal delivery of miRNA and other agents that will surely be the subject of future investigations.
Figure 1.
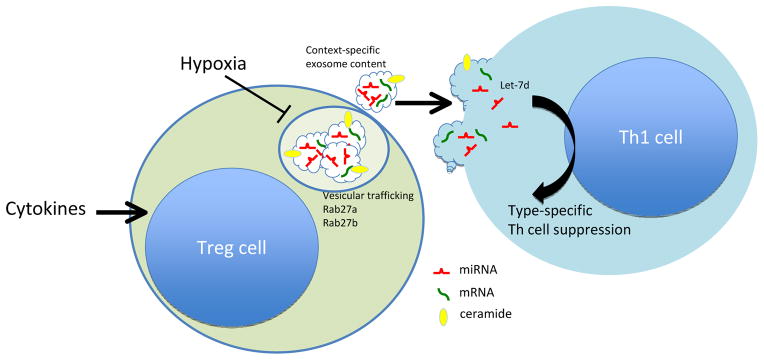
Treg cell exosomes suppress Th1 cell responses. Cytokines and metabolic factors condition Treg cell exosome content. Exosome generation and export depends upon ceramide and Rab GTPases (Rab27a, Rab27b), respectively. Delivery of exosomes containing the miRNA Let-7d to Th1 effector cells results in suppression of proliferation and cytokine secretion.
Acknowledgments
T.A.C. and C.B.W. are supported by National Institutes of Health grant R01 AI085090.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong MM, Rasmussen JP, Rudensky AY, Littman DR. The RNAseIII enzyme Drosha is critical in T cells for preventing lethal inflammatory disease. J Exp Med. 2008;205:2005–2017. doi: 10.1084/jem.20081219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch MA, Thomas KR, Perdue NR, Smigiel KS, Srivastava S, Campbell DJ. T-bet(+) Treg cells undergo abortive Th1 cell differentiation due to impaired expression of IL-12 receptor beta2. Immunity. 2012;37:501–510. doi: 10.1016/j.immuni.2012.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston A, Lu LF, O’Carroll D, Tarakhovsky A, Rudensky AY. Dicer-dependent microRNA pathway safeguards regulatory T cell function. J Exp Med. 2008;205:1993–2004. doi: 10.1084/jem.20081062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LF, Thai TH, Calado DP, Chaudhry A, Kubo M, Tanaka K, Loeb GB, Lee H, Yoshimura A, Rajewsky K, Rudensky AY. Foxp3-dependent microRNA 155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity. 2009;30:80–91. doi: 10.1016/j.immuni.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–383. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Chaudhry A, Kas A, deRoos P, Kim JM, Chu TT, Corcoran L, Treuting P, Klein U, Rudensky AY. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]


