Abstract
Nearly half of all HIV-1-positive individuals on combination antiretroviral therapy (cART) are afflicted with HIV-1-associated neurocognitive disorders (HAND). The most prevalent cognitive deficits observed in the cART era are those of attention and executive function. Presently, we sought to model deficits in attention and core components of executive function (inhibition, flexibility, and set-shifting) observed in HAND using the HIV-1 transgenic (Tg) rat, which expresses 7 of the 9 HIV-1 genes. Ovariectomized female Fischer HIV-1 Tg and non-transgenic control rats (ns=39–43) were tested in a series of operant tasks: signal detection, discrimination learning, reversal learning, and extradimensional set-shifting. The HIV-1 Tg animals attained the criterion of three sessions at 70% accuracy at a significantly slower rate than the control animals on all tasks with the exception of the extradimensional set-shifting task. Of the animals that met the criteria, there was no significant difference in percent accuracy in any task. However, the HIV-1 Tg rats showed a lower overall response rate in signal detection and discrimination learning. A discriminant function analysis classified the animals by genotype with 90.4% accuracy based on select measures of their performance. The functional consequences of chronic low-level expression of the HIV-1 proteins on attention, as well as inhibition and flexibility as core components of executive function, are apparent under conditions which resemble the brain proinflammatory immune responses and suppression of infection in HIV-1+ individuals under cART. Deficits in attention and core components of executive function may reflect an underlying impairment in temporal processing in HAND.
Keywords: HIV-1 transgenic rat, HIV-1-associated neurocognitive disorders, executive function, attention, AIDS, HIV
Introduction
The current estimate for the number of people living with HIV-1 worldwide is 35 million (UNAIDS 2013). HIV-1-associated neurocognitive disorders (HAND) continue to afflict up to 50% of patients on cART (Ances and Ellis 2007; Heaton et al. 2011), despite the significant reduction in the incidence of HIV-1 associated dementia (HAD) with the advent of combination antiretroviral therapy (cART) (Heaton et al. 2010; Sacktor et al. 2002). Given the increased life expectancy provided by cART, HAND is a significant health issue for millions, disrupting normal functioning of daily activities and posing an increased risk for early mortality (Vivithanaporn et al., 2010).
Before the introduction of cART, HIV-1+ individuals with HAND showed deficits primarily in information processing speed, sensory-perceptual skills, motor skills, and language. Current studies on HAND, which represent individuals on cART, demonstrate that attention, memory, and executive function are the cognitive domains most affected in HAND (Cysique et al., 2004; Garvey et al., 2009; Heaton et al., 2011). In particular, executive function shows the greatest decline as a function of HIV disease progression (Reger et al., 2002), which can have significant effects on the ability to perform important daily activities (Scott et al., 2011; Cattie et al., 2012).
Several complex behaviors that can be described as components of executive function, such as reversal learning and attentional set-shifting, have been demonstrated across species. Homologous subregions of the PFC in the rat, monkey, and human are associated with parallel components of executive function as well, suggesting that the rat is a useful and valid model of complex cognitive functions (Kesner and Churchwell 2011). Thus, executive function and attention, as the most affected domains in HAND, may be assessed with translatable results in the rat, with the use of measures selected to model specific cognitive functions. For the present study, we tested the HIV-1 Tg rat with signal detection, discrimination learning, reversal learning, and extradimensional set-shifting tasks. The signal detection task assesses the animal’s ability to sustain attention for a randomly presented stimulus, the presence or absence of which indicates which response to make (e.g., which lever to press) to receive a reinforcer. In the signal detection task, the number of hits (correct response during a signal trial) and correct rejections (correct response during a nonsignal trial) are measures of sustained attention to the stimulus. The number of misses (incorrect response during a signal trial) reflects a lapse of attention to the stimulus. The number of false alarms (incorrect response during nonsignal trial) reflects a failure of response inhibition. To assess core components of executive function, the animals were trained on series of tasks beginning with a basic stimulus discrimination task, in which the position of the stimulus indicates the correct response. In the reversal learning task, the stimulus-response contingencies are reversed, by which the correct response in the original discrimination task is now the incorrect response in the reversal learning task, and vice versa, thereby assessing flexibility and inhibition of responses learned in the previous discrimination task. Finally, the extradimensional set-shifting task assesses the animal’s ability to learn and respond correctly to a previously irrelevant dimension, without the removal of the original stimulus dimension. Thus, the new response rule requires shifting of attention to a different set of stimulus cues; i.e., assessing set-shifting.
The HIV-1 transgenic (Tg) rat, which expresses 7 of the 9 genes for HIV-1, resembles humans with HAND under cART, exhibiting low-level systemic and CNS inflammatory responses and the presence of HIV proteins in the brain (Royal et al., 2012). The HIV-1 Tg rat has not been used systematically to define and model cognitive impairments as per the most afflicted domains (Antinori et al. 2007). Thus, in the present study, we sought to model deficits in attention and executive function observed in HAND with the HIV-1 Tg rat, specifically the fundamental components of flexibility, inhibition, and set-shifting.
Methods
Animals
Ovariectomized female Fisher-344 HIV-1 transgenic (Tg; n=41) and non-transgenic control rats (n=43) were obtained from Harlan, Inc. (Indianapolis, IN) at 2-months of age and were group- or pair-housed throughout the experiment. Ovariectomized animals were used to remove the potential confounding effect of endogenous hormones and estrous cycle on cognitive performance. Animals were given approximately 4 g of rodent chow (Teklad Global Extruded Rodent Diet, Soy Protein-Free) daily, starting 1 week before testing, to maintain them at 85% of their normal body weight. Water was available ad libitum. The animals were maintained according to NIH guidelines in AAALAC-accredited facilities. The animal facility was maintained at 21° ± 2°C, 50% ± 10% relative humidity and had a 12-h light:12-h dark cycle with lights on at 0700 h (EST). The Institutional Animal Care and Use Committee of the University of South Carolina approved the project protocol; animal assurance number A3049-01.
Apparatus
Behavioral training and testing was conducted in 22 operant chambers located inside sound-attenuating chambers (Med Associates). One wall of each chamber consisted of two retractable levers, a pellet dispenser (45 mg) between the two levers, and a panel light above each lever and one above the pellet dispenser. A house light was located on the rear wall. Signal presentation, lever operation, reinforcement delivery, and data collection were controlled by a PC and Med-PC for Windows software (V 4.1.3; Med Associates).
Signal Detection Task
Starting at 3-months of age, animals were initially trained to press both levers on an FR-1 schedule of reinforcement for sucrose pellets (45 mg). To prevent side-bias, subjects were not rewarded for more than five consecutive presses on a single lever. After the animals achieved at least 40 reinforcers during the 42-min sessions for three consecutive days, with less than 20% variance across days, they were trained on the signal detection task.
Each signal detection session began with a 5-min habituation period. The houselight was off for the duration of the session. The presentation of signals (central panel light illumination) and non-signals (no illumination) was randomized over the 160 trials per session, with intertrial intervals of 9 ± 3 sec, during which time the levers remained retracted. Levers were extended 2 sec after each trial (signal or non-signal) began and remained extended for 6 sec for the animal to make a response. During signal trials, the light stimulus remained illuminated until the animal made a response, or until the levers retracted, whichever occurred first. For half of the animals, lever presses on the left lever during signal trials and on the right lever during non-signal trials were rewarded with a sucrose pellet (hits and correct rejections, respectively). The reverse set of rules was used for the other half of the subjects. Incorrect responses during signal trials (misses) and non-signal trials (false alarms) were not rewarded. An incorrect response was proceeded by up to three repetitions of the trial, or correction trials. Finally, failure to respond appropriately to the correction trials resulted in a forced-choice trial in which the same stimulus type was repeated (signal or non-signal) but only the correct lever was extended and remained extended until a response was made or 120 sec elapsed, whichever occurred first. Each animal was trained on the task each day until it achieved 70% or greater accuracy on three consecutive sessions. Accuracy was calculated as the total number of hits and correct rejections divided by the total number of correct and incorrect responses in a session.
Discrimination Task
All animals were tested on the discrimination task regardless of whether they met the criterion for the signal detection task. Each discrimination task session began with a 5-min habituation period. The houselight was off for the duration of the session. Left panel light and right panel light stimulus trials were presented randomly in a 160-trial session, with intertrial intervals of 9 ± 3 sec, during which time the levers remained retracted. The light stimulus during each trial was presented for 1 sec, followed by the presentation of both levers for 6 sec or until the animal made a response, whichever occurred first. Animals were rewarded for pressing the lever underneath the light stimulus. Following an incorrect response, animals were presented with three correction trials. If the animal again responded incorrectly, it was given a forced trial. Each animal was trained on the task until it achieved 70% or greater accuracy on three (nonconsecutive) sessions. Accuracy was calculated as the total number of correct responses divided by the total number of correct and incorrect responses in a session.
Reversal Learning Task
Only animals that met the criterion for the discrimination task were tested on the reversal learning task. Each reversal learning task session began with a 5-min habituation period. The houselight was off for the duration of the session. Left panel light and right panel light stimulus trials were presented randomly in a 160-trial session, with intertrial intervals of 9 ± 3 sec, during which time the levers remained retracted. The light stimulus during each trial was presented for 1 sec, followed by the presentation of both levers for 6 sec or until the animal made a response, whichever occurred first. The opposite set of response rules from the discrimination task were used in the reversal learning task; the animals were rewarded for pressing the lever on the opposite side of the light stimulus. Following an incorrect response, animals were presented with three correction trials. If the animal again responded incorrectly, it was given a forced trial. Each animal was trained on the task until it achieved 70% or greater accuracy on three (nonconsecutive) sessions. Accuracy was calculated as the total number of correct responses divided by the total number of correct and incorrect responses in a session.
Extradimensional Set-Shifting Task
All animals were tested on the extradimensional set-shifting task regardless of whether they met the criterion for the previous tasks. Each extradimensional set-shifting task session began with a 5-min habituation period. The houselight was off for the duration of the session. Left panel light and right panel light stimulus trials were presented randomly in a 160-trial session, with intertrial intervals of 9 ± 3 sec, during which time the levers remained retracted. The light stimulus during each trial was presented for 1 sec, followed by the presentation of both levers for 6 sec or until the animal made a response, whichever occurred first. Half of the animals were rewarded for pressing the left lever, and the other half were rewarded for pressing the right lever, regardless of the position of the light stimulus. Following an incorrect response, animals were presented with three correction trials. If the animal again responded incorrectly, it was given a forced trial. Each animal was trained on the task until it achieved 70% or greater accuracy on five consecutive sessions. Accuracy was calculated as the total number of correct responses divided by the total number of correct and incorrect responses in a session.
Statistical Analysis
All data were analyzed using SPSS Statistics 20 (IBM Corp., Somers, NY). For the signal detection task, percent accuracy, and hits, misses, correct rejections, and false alarms were analyzed with independent samples t-tests. For all tasks, a two-way mixed-factor analysis of variance (ANOVA) was used to analyze correct responses and errors, with genotype (HIV-1 Tg vs. control) as the between-subjects factor, and response type (correct responses vs. errors) as the within-subjects factor. Each animal’s performance on each measure was averaged across the first three days at which the animal performed with 70% accuracy, to provide the data for the analyses. Trials and errors to criterion were analyzed with independent sample t-tests, and sessions to criterion were analyzed with curve-fitting to assess the temporal process of acquisition. An alpha level of p≤0.05 was considered significant for all statistical tests. Sample sizes were chosen with the goal of sufficient statistical power (> 0.80) to maximize the likelihood of detecting subtle early alterations of expression of the HIV-1 transgene.
In addition, an exploratory discriminant function analysis was employed to determine which measures of executive function best differentiate group performance, and the extent to which the observed differences in measures of executive function correctly identified animals in regard to their group membership (HIV-1 Tg vs. control).
Results
Body Weight
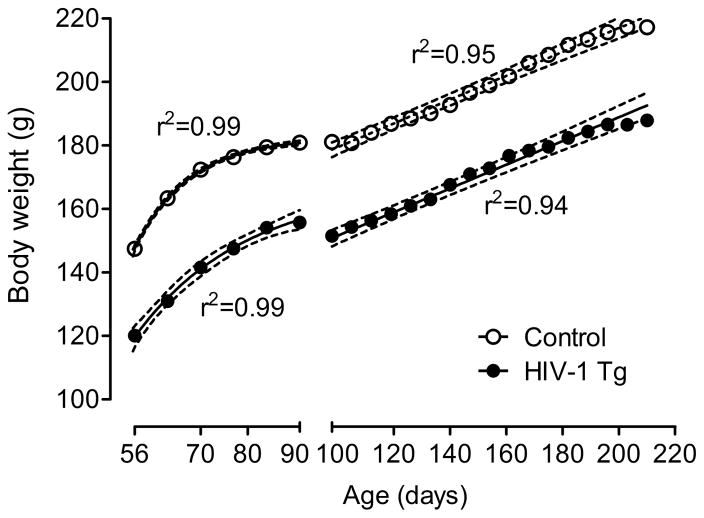
The HIV-1 Tg group weighed significantly less than the control group across the 6-month period during which they were tested, F(1, 80)=92.4, p≤0.001 (Fig. 1). Both groups increased significantly in body weight across this period [HIV-1 Tg: F(21, 798)= 128.0, p≤0.001; control: F(21, 882)=121.6, p≤0.001]. Through PD 91, both groups increased in weight according to a one-phase association curve fit. After this age, when food restriction was implemented, both groups increased in weight in a linear function. There was no significant difference in the slope of these lines, indicating that the groups did not differ in their rates of growth.
Fig. 1.
Mean body weight of the HIV-1 Tg animals and control animals across age with the best fit nonlinear regression for each group (± 95% CI). The HIV-1 Tg group weighed significantly less than the control group across the 6-month period during which they were tested. Both groups increased significantly in body weight across this period; most importantly, there was no evidence for any differential rate of growth. The x-axis break at 100 days indicates the point at which animals began food restriction, prior to testing.
Signal Detection
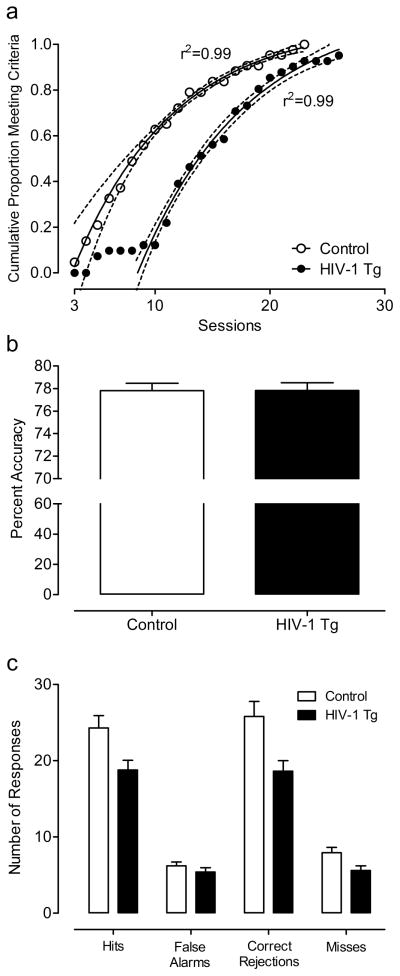
All controls and all but 2 HIV-1 Tg animals met the criterion of 70% accuracy on 3 consecutive sessions of the signal detection task within 1 month of daily testing. The HIV-1 Tg and control groups followed similar rates in achieving the criterion, but the HIV-1 Tg group showed an initial 7-day lag before any of the HIV-1 Tg animals met the criterion, reflected by their significantly greater number of trials [t(80)=−2.1, p≤0.05] and errors to criterion [t(80)=−2.4, p≤0.05] compared to the control group. In addition, a one-phase association regression analysis on the cumulative proportion of animals meeting the criterion over sessions revealed that a different curve was best fit to each group, F(3,27)=245.2, further illustrating the gap between groups in the number of sessions needed to reach criterion (Fig. 2) Once the animals met the criterion, percent accuracy was not significantly different between groups (3-day average: HIV-1 Tg: 77.8 ± 0.43; Control: 77.8 ± 0.42). However, the HIV-1 Tg animals responded significantly less than the control animals during the signal detection task, F(1,80)=8.2, p≤0.01. A significant genotype x response type interaction [F(1,80)=8.6, p≤0.01] suggested that the HIV-1 Tg animals displayed a different response profile than the control group. The HIV-1 Tg animals had fewer hits [t(80)=2.6, p≤0.05], misses [t(80)=2.5, p≤0.05], and correct rejections [t(80)=2.9, p≤0.005] than the control group, consistent with a lapse of attention. There was no significant difference in the number of false alarms, a putative index of response inhibition.
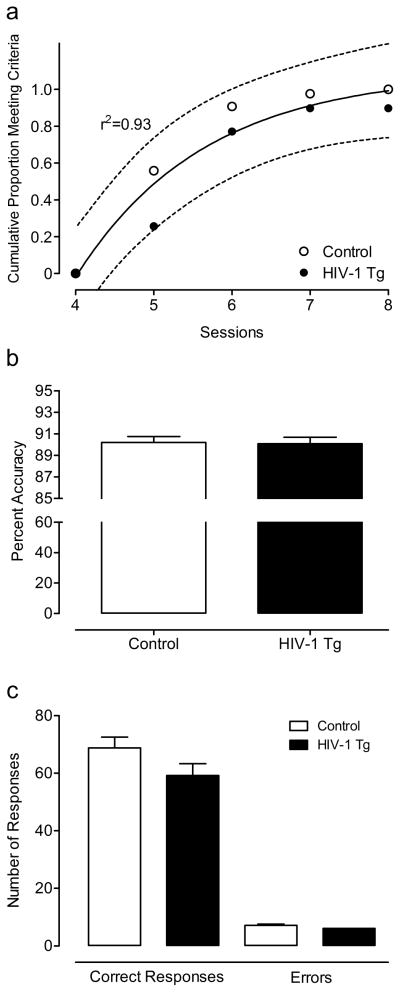
Fig. 2.
Performance on the signal detection task. One-phase association regression analysis on the cumulative proportion of animals meeting the criterion over sessions revealed that a different curve was best fit to each group, illustrating the gap between groups in the number of sessions needed to reach criterion (a). Percent accuracy was not significantly different between groups (3-day average: HIV-1 Tg: 77.8 ± 0.43; Control: 77.8 ± 0.42) (b). However, the HIV-1 Tg animals responded significantly less than the control animals during the signal detection task, with fewer hits, misses, and correct rejections than the control group, consistent with a lapse of attention (c). There was no significant difference in the number of false alarms, a putatvive index of response inhibition.
Discrimination Learning
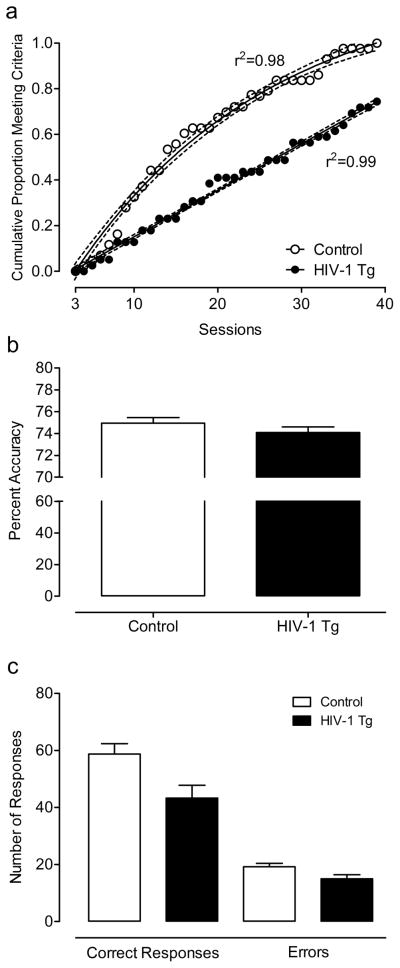
All controls and 29 HIV-1 Tg animals met the criterion of 70% accuracy on 3 sessions of the discrimination task within 40 days of daily testing. There was no significant difference between HIV-1 Tg and control animals in errors or trials to criterion (t≤1, p>0.8). However, regression analyses revealed that the rate at which each group achieved the criterion across sessions followed different functions; the control group showed a curvilinear one-phase association function (r2=0.98), whereas the HIV-1 Tg animals exhibited a linear function (r2=0.99), illustrating the slower acquisition of the task by the HIV-1 Tg animals over sessions (Fig. 3). Of the animals that met the criterion, there was no significant difference in percent accuracy (3-day average: HIV-1 Tg: 74.9±0.52; Control: 74.1±0.51). Despite attaining the same accuracy, the HIV-1 Tg animals that met the criterion made significantly fewer overall responses, F(1,70)=6.8, p≤0.05. A significant interaction between genotype and response type, F(1,70)=6.8, p≤0.05, indicates that there was a smaller difference between the number of correct responses and the number of errors in HIV-1 Tg group compared to control group.
Fig. 3.
Performance on the stimulus discrimination task. Regression analyses revealed that the rate at which each group achieved the criterion followed different functions; the HIV-1 Tg animals exhibited a linear function whereas the control group showed a curvilinear one-phase association function, illustrating the slower acquisition of the task by the HIV-1 Tg animals over sessions (a). There was no significant difference in percent accuracy (3-day average: HIV-1 Tg: 74.9 ± 0.52; Control: 74.1 ± 0.51) (b). Despite attaining the same accuracy, the HIV-1 Tg animals that met the criterion made significantly fewer overall responses, with a smaller difference between the number of correct responses and the number of errors in HIV-1 Tg group compared to control group (c).
Reversal Learning
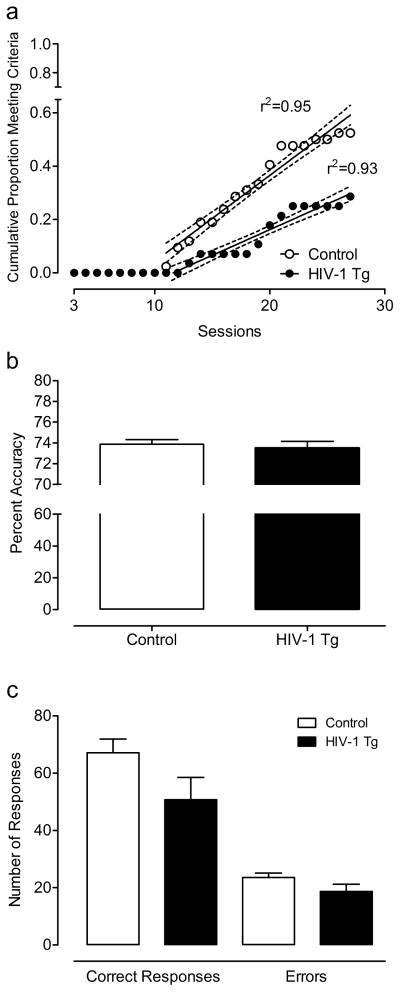
Of the subjects that met the criterion for the discrimination task and continued to the reversal learning task, 22 control animals and 8 HIV-1 Tg animals met the reversal learning task criterion of 70% accuracy over 3 sessions within 1 month of daily testing. There was no significant difference between HIV-1 Tg and control animals in errors or trials to criterion (t≤1, p>0.4). However, linear regression analysis on the cumulative proportion of animals that achieved the criterion over sessions revealed that each group met the criterion at different rates, demonstrated by significantly different slopes for each group’s linear function, F(1,30)=31.8, p≤0.001 (Fig. 4). Of the animals that met the criterion, there was no significant difference in percent accuracy (3-day average: HIV-1 Tg, 73.5 ± 0.66; Control, 73.9 ± 0.43). Similarly, there was no significant difference between groups in the total number of responses, and there was no interaction between genotype and response type.
Fig. 4.
Performance on the reversal learning task. Linear regression analysis on the cumulative proportion of animals that achieved the criterion over sessions revealed that each group met the criterion at different rates, demonstrated by significantly different slopes for each group’s linear function (a). Of the animals that met the criterion, there was no difference in percent accuracy (3-day average: HIV-1 Tg, 73.5 ± 0.66; Control, 73.9 ± 0.43) (b). There was also no significant difference between groups in the total number of responses, and there was no interaction between group and response type (correct responses or errors) (c).
Extradimensional Set-Shifting
All control animals and all but 4 HIV-1 Tg animals met the criterion for extradimensional set-shifting within 8 days of testing. The HIV-1 Tg and control animals did not differ in the number of trials or errors to criterion. A one-phase association regression analysis of the cumulative proportion of animals achieving the criterion over sessions revealed that the best-fit curves for each group were not significantly different (Fig. 5); thus, the HIV-1 Tg and control animals did not meet the criterion at different rates. The failure to find a significant effect of genotype on task acquisition was not readily dismissed by a lack of statistical power for detecting such an effect; sample size and effect size were sufficient to provide power of 0.8 to detect a 10% difference between groups as statistically significant in trials or errors to criterion. Of the animals that met the criterion within 8 days, there was no significant difference between groups in percent accuracy (5-day average: HIV-1 Tg, 90.1 ± 0.60; Control, 90.2 ± 0.56). The groups were not significantly different in the total number of responses and there was no interaction between genotype and response type.
Fig. 5.
Performance on the extradimensional set-shifting task. Nonlinear regression on the cumulative proportion of animals that achieved the criterion over sessions revealed that the HIV-1 Tg and control animals achieved the criterion at the same rate, represented by a single nonlinear regression curve (± 90% CI) (a). There was no significant difference between groups in percent accuracy (5-day average; HIV-1 Tg, 90.1 ± 0.60; Control, 90.2 ± 0.56) (b). The groups were not significantly different in the total number of responses and there was no interaction between group and response type (correct responses or errors) (c).
Discriminant Function Analysis
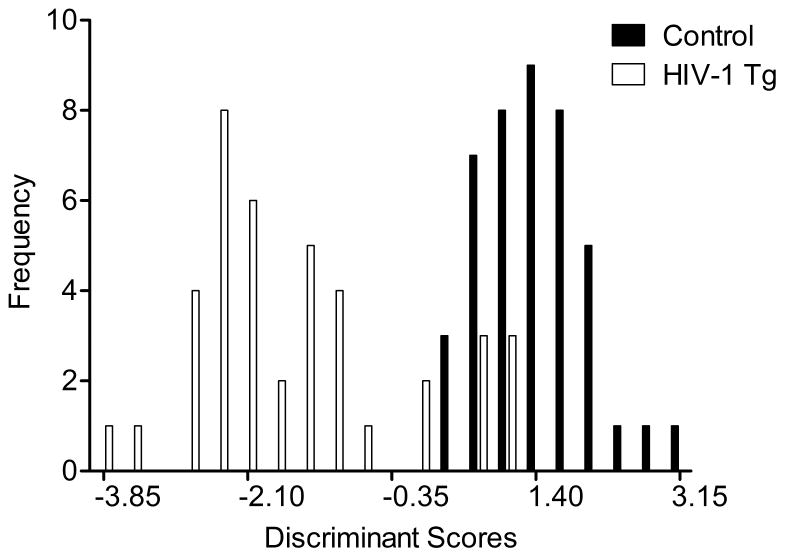
The effects of expression of the HIV-1 transgene on attention and core components of executive function were further explored by assessing the ability of various measures to correctly identify group membership of individual animals, using discriminant function analysis. A stepwise discriminant function analysis selected six variables (total number of correct responses, and trials and sessions to criterion on the signal detection task, as well as the number of trials and errors to criterion (log transformed) and total number of errors on the reversal learning task) which maximally separated the HIV-1 Tg and control animals (canonical correlation of 0.828) and correctly classified (jack-knifed) their group membership based on this function with 90.4% accuracy (F approximation of Wilks’ λ of 0.314, F(6,76)=27.7, p≤0.001) (Fig. 6).
Fig. 6.
Animal classification is illustrated as a function of the canonical variable representing the simplest linear function that best separated the HIV-1 Tg and control groups (canonical correlation 0.828) and correctly identified (jackknifed classification) group membership with 90.4% accuracy (100% of controls, and 80% of HIV-1 Tg animals).
Discussion
A series of operant tasks tapping attention and core components of executive function revealed prominent cognitive deficits in the HIV-1 Tg rat, prior to any documented neurological symptoms or clinical signs of wasting (Peng et al. 2010). In addition, a discriminant function analysis showed that certain measures of performance on the signal detection and reversal learning tasks can successfully discriminate between HIV-1 Tg and control animals. Thus, chronic low level exposure to HIV-1 proteins in the HIV-1 Tg rat, which resembles the suppression of infection with brain proinflammatory immune response in HIV-1 positive individuals under cART, primarily impairs sustained attention as well as inhibition and flexibility as core components of executive function. The cognitive deficits that define HAND in the cART era can be modeled and demonstrated in the HIV-1 Tg rat, providing opportunities to develop therapeutics for HAND.
In a signal detection task tapping sustained attention and inhibition as a core component of executive function, all of the HIV-1 Tg rats attained the criterion of three consecutive sessions at 70% or greater accuracy, but did so after a significantly greater number of trials than the control animals. Despite achieving the same level of accuracy as the control group, the HIV-1 Tg animals made significantly fewer responses. Specifically, the number of hits, misses, and correct rejections, but not false alarms, was lower in the HIV-1 Tg group. The ability of the HIV-1 Tg animals to maintain a high level of accuracy demonstrates that they can acquire the task, albeit more slowly and despite a reduced response rate. In addition, the HIV-1 Tg animals demonstrated response inhibition comparable to that of the control animals, as indicated by the low number of false alarms in both groups.
In a stimulus discrimination task which served to provide a baseline for the subsequent reversal learning and extradimensional set-shifting tasks, the HIV-1 Tg rats again exhibited a slower rate of acquisition of the criterion, which required three sessions at 70% or greater accuracy, as well as a significantly lower response rate compared to the control animals. As in the signal detection task, they were able to maintain a high level of accuracy despite a difference in the number of correct responses and errors compared to the control animals. Not all of the HIV-1 Tg animals acquired the task, and those that did acquire it took longer to achieve the criterion level of accuracy than the controls, suggesting an impairment in attentional processes. Although the discrimination task taps into sustained attention as the signal detection task does, it was a more challenging task, as only 75% of the HIV-1 Tg animals were able to achieve the criterion. The HIV-1 Tg animals may have had more difficulty in switching to a new response rule, in which they were required to respond to the position of the stimulus, rather than its presence or absence as in the signal detection task, thus providing evidence of their deficits in flexibility and inhibition. The discrimination task also presented an increase in attentional demands; the stimulus was only presented for 1 sec, whereas in the previously learned signal detection task, the animals were presented with the stimulus (for up to 6 sec) until they made a response on a lever, a contingency implemented after the animals failed to learn the task when a 1-sec stimulus was employed.
In the reversal learning task, which taps flexibility and inhibition, only a quarter of the HIV-1 Tg animals that were tested (i.e., those that did not meet the criterion for the discrimination task were not tested with reversal) were able to meet the criterion of three sessions at 70% or greater accuracy, and those animals reached the acquisition criterion more slowly than the control animals. The animals that met the criterion performed at the same accuracy level and did not show any difference in the total number of responses or in the number of correct responses or errors compared to the control animals. The HIV-1 Tg group’s impairment in flexibility and inhibition as core components of executive function was demonstrated by their difficulty in switching to a new stimulus-response contingency, thereby failing to inhibit responding to the previously rewarded stimulus. Most of the HIV-1 Tg animals were not able to acquire the reversal learning task, and those that did acquired it significantly more slowly than the control animals, consistent with a major impairment in the population of HIV-1 Tg rats.
In the extradimensional set-shifting task which tapped set-shifting, the HIV-1 Tg animals acquired the criterion of five consecutive sessions at 70% or greater accuracy at the same rate as the control group, performing with the same high level of accuracy. The HIV-1 Tg animals also responded at the same rate as the control animals throughout sessions, with no difference in the number of correct responses or errors compared to the control group. The extradimensional set-shifting task was undoubtedly the least difficult task for both groups, as all but 4 animals met the criterion within 8 days, as opposed to the other tasks which required up to 40 consecutive days to acquire. It is also the only task that did not demonstrate any difference in performance between the HIV-1 Tg and control animals. Set-shifting, a component of executive function which was uniquely targeted by the extradimensional set-shifting task, thus appears to be intact in the HIV-1 Tg animals.
A discriminant function analysis suggested that the behavioral profile of the HIV-1 Tg rats is quite distinct from that of the controls. The animals could be most accurately classified as HIV-1 Tg or control based on the number of trials or sessions to criterion and general responsiveness on the signal detection and reversal learning tasks. As described earlier, the HIV-1 Tg group had a significantly slower rate of acquisition of these tasks, and was also generally less responsive relative to controls. The poorer performance of the HIV-1 Tg group on the signal detection and reversal learning tasks demonstrates impairments in sustained attention, and flexibility and inhibition as core components of executive function, as a result of chronic low level expression of the HIV-1 proteins. Set-shifting, in contrast, as measured with the extradimensional set-shifting task, is spared in the HIV-1 Tg rat, illustrating that the expression of HIV-1 proteins does not cause gross cognitive deficits across all domains.
It is notable and worthy of acknowledging that despite the evidence for comparable levels of accuracy, lower rates of responding, compared to the control rats, were displayed by the HIV-1 Tg rats, most obviously in the signal detection and discrimination tasks. Typically, a lower response rate is indicative of attenuated motivation. In the face of greater cognitive demand, the subject may become less likely to be motivated to work for the reinforcer. A lower number of responses was observed in the HIV-1 Tg group during the signal detection and discrimination tasks, but in these and all other tasks, the HIV-1 Tg animals that met the criteria performed with the same high level of accuracy as the controls. The HIV-1 Tg animals also learned the fourth task (extradimensional shift task) at the same rate as the control animals. Therefore, it is unlikely that the animals were not sufficiently motivated. Furthermore, because both groups were maintained at 85% of the typical body weight for their respective genotype, one may reasonably infer that both the HIV-1 Tg and the control animals were highly motivated to work for the sucrose rewards. Although one cannot rule out lessened motivation as a contributory factor, the extant data suggest that food restriction did not induce differential motivation.
The selection of operant tasks in the present study was based on several factors. Each task tapped into basic, lower level components of executive function, which can be operationally defined in a fairly precise manner. In addition, there are well-established and relatively simple methods available to study each function. The preclinical assessment of sustained attention, and inhibition, flexibility, and set-shifting, as fundamental components of executive function, is essential in understanding deficits observed in more complex components of executive function, such as decision-making and planning. In the present study, assessments of specific aspects of executive function provided the opportunity to identify conditions under which the various components are deficient or intact in the HIV-1 Tg rat.
Sustained attention is the process by which one detects infrequent target stimuli over long periods of time. It has been characterized in both humans and rats, with analogous changes in performance related to varying task parameters, such as stimulus duration, intensity, and frequency (McGaughy and Sarter, 1995; Bushnell, 1998; Parasuraman and Davies, 1977). Most studies on sustained attention in HIV-1+ individuals have only reported measures of reaction time (for review, see Hardy and Hinkin, 2002), which is related more to psychomotor function than sustained attention per se. However, a few studies have reported reduced accuracy on sustained attention tasks by individuals with HIV-1 (Fein et al., 1995; Watkins et al., 2000), which may indicate specifically attentional deficits. In the present study, the signal detection, discrimination learning, and reversal learning tasks each tapped into sustained attention; the animals were required to make the appropriate response following the presentation (or absence) of a light stimulus in order to receive a reinforcer. Deficits in sustained attention in the HIV-1 Tg animals were apparent first in the signal detection task, demonstrated by the lag in acquisition of the task. A similar delay in task acquisition was observed with the subsequent discrimination and reversal learning tasks, again revealing deficits in sustained attention. The discrimination and reversal learning tasks also demonstrated impairments in core components of executive function.
The core components of executive function that were targeted for assessment in this study – flexibility, inhibition, and set-shifting – are both interrelated and dissociable, with the use of specific measures. Flexibility, as the ability to modify behavior to changing environmental contingencies, requires the formation of a new representation of a learned stimulus-response rule. In the stimulus discrimination task, the animals learned to respond to a different aspect of the light stimulus (position) than they had learned in the previous signal detection task (presence vs. absence); i.e., an intradimensional set-shift. In the reversal learning task, the animals learned to respond to a stimulus that was previously not reinforced, and vice versa, thus acquiring a new representation of the stimulus-response contingency that was learned in the discrimination task. The extradimensional set-shifting task required the animals to learn stimulus-response contingencies within a novel stimulus set; instead of responding to the position of the light stimulus, they learned to respond to the position of the lever. Inhibition is another component of executive function which is necessarily involved in learning new stimulus-response contingencies. In the discrimination and reversal learning and set-shifting tasks, the animal needed to deliberately inhibit responses that were previously reinforced in order to respond correctly to the present contingency.
Set-shifting has also been defined as a basic component of executive function, in which one disengages from a learned stimulus set and actively engages in novel one. It may be considered a composite of flexibility and inhibition, because learning a new stimulus-response contingency within a new stimulus set requires both a modification of a learned behavior as well as inhibition of previously learned responses. The extradimensional set-shifting task is distinct from the reversal learning task in that the latter does not involve shifting to a new stimulus set; it requires learning the opposite stimulus-response contingencies with the same stimulus set. Different brain regions have been associated with performance on each task; the lateral PFC of humans and nonhuman primates (Dias et al., 1996; Manes et al., 2002) and the mPFC of the rat (Birrell and Brown, 2000) support extradimensional set-shifting, whereas the orbitofrontal cortex (OFC) is associated with the reversal learning in nonhuman primates (Dias et al., 1996) and rats (McAlonan and Brown, 2003). The double dissociation of brain region and cognitive function may explain the difference in the HIV-1 Tg group’s performance on the reversal and extradimensional tasks. Although both tasks place demands on executive function, each requires the use of a different cognitive process and thus, presumably, a different brain region.
A common assessment of executive function in humans is the Wisconsin Card Sorting Test (WCST), in which the participant is instructed to match cards based on one of several possible categories (e.g., color, number, shape), which is not made explicit to the participant. The participant determines the sorting criterion, which changes after a certain number of correct responses, based on feedback from the test administrator or computer. With these task demands, the WCST targets flexibility, set-shifting or shifting between sorting criterion, and inhibition of prepotent responses based on the previously correct sorting criterion. It has been used extensively to characterize the deficits in executive function in individuals with HIV-1 (Tozzi et al. 1993; Basso and Bornstein, 2003; Heaton et al., 2011). Another typical test of executive function that has been employed in studies of HAND is the Stroop Color Word Test (Chang et al., 2002; Maki et al., 2009). The participant is instructed to state the color in which a word is presented, which, in the case of a word that is itself a color, requires inhibition of the prepotent response to read the word aloud as the answer. The use of the Stroop Color Word Test is thus limited to assessments of inhibition and does not tap flexibility per se or set-shifting. Flexibility as an executive function has been measured in terms of category fluency, revealing deficits in switching subcategories while producing a list of words in a given category (Iudicello et al., 2008).
The brain dopamine (DA) system has been implicated as a major target of HIV-1 infection. Reports of HIV-1 positive individuals with lower cerebrospinal fluid DA (Berger et al., 1994), Parkinsonian symptoms, sensitivity to DA receptor antagonists, or abnormalities in basal ganglia structure and function (Berger & Nath, 1997; Koutsilieri et al., 2002) provided some of the earliest lines of evidence that HIV-1 infection disrupts the DA system. Symptoms of HAND have also been associated with DA system dysfunction. In HIV-1+ individuals, significant reductions in DA levels in the substantia nigra are correlated with poor performance on learning and memory tasks (Kumar et al., 2011), and decreases in homovanillic acid have been associated with deficits in executive function (di Rocco et al., 2000). Reduced DA transporter (DAT) levels in the putamen and ventral striatum are found in HIV-1+ individuals with cognitive impairment (Wang et al. 2004; Chang et al., 2008). In vitro studies have shown that DAT is targeted by HIV-1 proteins Tat and gp120, resulting in transporter impairment (Aksenov et al. 2008; Ferris et al. 2009; Midde et al. 2013; Zhu et al. 2009; Zhu et al. 2011), due to direct protein-protein interactions (Zhu et al. 2009) involving an allosteric modulation of DAT by the Tat protein (Zhu et al. 2011). In addition, DA-dependent signaling has been identified as a mechanism of HIV-1 protein neurotoxicity (Aksenova et al. 2006; Silvers et al. 2007; Wallace et al. 2006).
Indeed, DA systems play a critical role in the processes of attention and executive function. Sustained attention depends on DA systems in the PFC, as evidenced by the differential effects of PFC-infused DA agonists and antagonists on sustained attention tasks (Granon et al. 2000; Chudasama and Robbins, 2004), and DA receptor activity regulates cortical ACh efflux (Moore et al., 1999; Zmarowski et al. 2005), which is essential in the mediation of attentional processes, as demonstrated with the signal detection task which taps sustained attention (McGaughy et al. 1996; Himmelheber et al. 2000). Executive function processes also rely on the integrity of DA systems; performance on the reversal learning task is disrupted by D2 receptor antagonists (Lee et al., 2007; Ridley et al., 1981) and DA depletion in the striatum (Clarke et al. 2011; O’Neill and Brown 2007), and is also correlated with D2 receptor activity in the striatum (Clatworthy et al, 2009; Kellendonk et al, 2006; Groman et al., 2011). Increases in DA in the rat PFC improve performance on the extradimensional set-shifting task (Tunbridge et al. 2004), whereas depletion of PFC DA impaired performance on the task in monkeys (Crofts et al., 2001). Rodent models of schizophrenia often incorporate the extradimensional set-shifting task to demonstrate deficits in executive function, which are attenuated by D2 antagonist antipsychotics (McLean et al. 2008; Rodefer et al., 2008; Tait et al., 2009).
Norepinephrine (NE) activity also plays a key role in attention and the core components of executive function. Performance on tasks that tap sustained attention is worsened when NE in the PFC is depleted, especially under conditions of increased attentional load (Aston-Jones and Cohen 2005; Carli et al. 1983; Milstein et al. 2007). Increased NE activity in the OFC has been associated with improved response inhibition (Bari et al., 2009; Robinson et al., 2008) and reversal learning (Seu and Jentsch, 2009). Lesions of the dorsal noradrenergic ascending bundle (Tait et al. 2007) and noradrenergic deafferentiation of rat mPFC (McGaughy et al. 2008) result in impaired performance on the extradimensional set-shifting task. NE activity has a significant impact on PFC function, via stimulation of α2A-receptors on dendritic spines of pyramidal neurons in the PFC, which strengthens synaptic connections by closing the ion channels on the spines, increasing the efficacy of synaptic inputs (Wang et al. 2007). Further study of neural network structures implicating NE as well as DA activity in the PFC of the HIV-1 Tg rat will be important in clarifying alterations underlying neurocognitive impairment of HAND.
Alterations in synaptodendritic networks caused by HIV-1 may underlie the observed deficits in executive function in HAND. Reductions in synaptodendritic complexity (Masliah et al. 1997; Everall et al., 1999) and alterations in BOLD signal changes (Chang et al. 2001) in frontal regions have been associated with HIV-1 associated neurocognitive impairments, even in less severe cases. In conjunction with changes in brain activity, HIV-1+ individuals also exhibit volume loss in the medial frontal and premotor cortices, as well as thinning of the prefrontal cortex, reflecting the deterioration of executive function (Thompson et al., 2005). In some cases, HIV-1+ individuals perform as well as HIV-1− individuals on attention and executive function tasks, but significant differences are still found in brain function. HIV-1+ individuals with normal cognitive function have shown decreased BOLD signal change in frontal regions, diminished functional frontostriatal connectivity, and a potentially compensatory activity increase in the parietal attentional networks while performing a semantic event-sequencing task (Melrose et al., 2008). Increases in BOLD signal changes in the prefrontal cortex were observed in HIV-1+ individuals during an attention and working memory task, although they performed as well as the control participants and also had similar scores on a battery of neuropsychological tests (Ernst et al. 2002). Axonal damage in frontostriatal fiber bundles as measured with diffusion tensor imaging has also been observed in HIV-1+ individuals, prior to any symptoms of dementia (Pfefferbaum et al., 2009). Neuronal abnormalities such as these may precede the onset of functional deficits, providing a screening measure to determine the need for preventive treatment for HAND.
Previous work in our lab has demonstrated temporal processing deficits in the HIV-1 Tg rat as measured with prepulse inhibition. The HIV-1 Tg animals, compared to controls, showed a relative insensitivity to interstimulus interval and an impaired development of perceptual sharpening in relation to the interstimulus interval with age (Moran et al., 2013). As with the present study, the deficits were observed prior to the onset of clinical symptoms or wasting. The temporal processing deficits that are displayed early in the expression of the HIV-1 transgene may underlie other cognitive impairments such as those observed in the present study. Fundamental to an assessment of temporal processing in attention and executive function is the manipulation of temporal aspects, such as stimulus duration, and deserves further exploration.
The need for a therapeutic for HAND is readily apparent, as cART cannot prevent or reverse the development of neurocognitive deficits, although it may help to lessen their severity. Characterizing early indicators of neurocognitive deficits is essential to identifying effective treatments, and the HIV-1 Tg rat provides a vehicle with which to do that. Effective gender-based treatments are of particular interest, as women represent a substantial percentage (approximately 52%) of all people currently living with HIV-1 (UNAIDS 2013), yet are underrepresented in clinical reports and descriptions of HAND (Maki and Martin-Thormeyer, 2009). Of the HIV-1 infections attributable to heterosexual contact in the U.S., approximately two-thirds have been in women, most of them young, minority, indigent women who use crack cocaine and who trade sex for crack, other drugs or money (CDC 2012). Even after adjusting for for age, education level, and race, HIV-1+ women display neurocognitive impairment relative to HIV-1− women (Manly et al., 2011). Modeling HAND in female HIV-1 Tg animals is thus a valid and important approach to studying the neurocognitive deficits of HIV-1.
The HIV-1 Tg rat is an excellent model of HIV-1+ humans on cART, with chronic low-level expression of viral proteins and suppressed infection (Royal et al., 2012), and it is also relevant to pediatric HIV-1, with constitutive expression of the virus beginning in the embryonic stage. In general, the HIV-1 Tg young adult female rats in the present study were found to be healthy (i.e., similar growth rate as controls), which is consistent with our prior studies (Moran et al., 2012; 2013a; 2013b; Webb et al., 2010). However, it is important to note that the excellent health of our HIV-1 Tg animals differs from the original description of the HIV-1 Tg rat. Reid et al. (2001) described several severe pathological phenotypes associated with the transgene expression (then located on chromosome 2 and 9), including three grades of cataracts, angiogenic corneas, kidney disease, ulcerative skin disease, respiratory disease, all accompanied by severe neurological disorders (hind limb paralysis) and wasting by a relatively young age (5–9 months). The HIV-1 Tg animals used in the current studies are a healthy derivation of these originally described phenotypes, on an inbred F344 background, with the transgene (now limited to chromosome 9), and a 100% penetrant phenotype of light to moderate cataracts. We have failed to find alterations in hearing, visual brightness detection, gustatory taste, growth rates, or locomotion. No gross peripheral organ pathology/general wasting was found in this contemporary HIV-1 Tg phenotype through 11 months of age (Moran et al., 2013b). Thus, the present moderate phenotype more closely resembles HAND, is suitable for long-term/aging studies, and should be considered distinct from the original descriptions of the most severe phenotypes of the HIV-1 Tg rat. The ability to use the HIV-1 Tg rat in long-term/aging studies is especially valuable, as an understanding of the progression of neurocognitive impairment in HAND is a critical next step in this research.
In summary, the HIV-1 Tg rats displayed impaired performance in a series of cognitive tasks, prior to any clinical signs of wasting, that implicate specific deficits in the processes of attention as well as flexibility and inhibition as core components of executive function. The functional consequences of chronic low level expression of the HIV-1 proteins on attention and executive function are apparent under conditions which resemble brain proinflammatory immune responses and suppression of infection in HIV-1+ individuals under cART. Deficits in attention and core components of executive function may reflect an underlying impairment in temporal processing in HAND.
Acknowledgments
This research was supported by the National Institute on Drug Abuse [RMB, Grant DA013137; CFM, Grant DA031604] and by the National Institute of Child Health and Human Development [CFM, Grant HD043680]. The authors thank Dr. Martin Sarter and Dr. Philip Bushnell for their expert consultation and generous sharing of their Med Associates computer programs for signal detection.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Aksenov MY, Aksenova MV, Silvers JM, Mactutus CF, Booze RM. Different effects of selective dopamine uptake inhibitors, GBR 12909 and WIN 35428, on HIV-1 Tat toxicity in rat fetal midbrain neurons. Neurotoxicology. 2008;29:971–977. doi: 10.1016/j.neuro.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenova MV, Silvers JM, Aksenov MY, Nath A, Ray PD, Mactutus CF, Booze RM. HIV-1 Tat neurotoxicity in primary cultures of rat midbrain fetal neurons: Changes in dopamine transporter binding and immunoreactivity. Neurosci Lett. 2006;395:235–239. doi: 10.1016/j.neulet.2005.10.095. [DOI] [PubMed] [Google Scholar]
- Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27:86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Bari A, Eagle DM, Mar AC, Robinson ES, Robbins TW. Dissociable effects of noradrenaline, dopamine, and serotonin uptake blockade on stop task performance in rats. Psychopharmacology. 2009;205:273–283. doi: 10.1007/s00213-009-1537-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MR, Bornstein RA. Effects of past noninjection drug abuse upon executive function and working memory in HIV infection. J Clin Exp Neuropsychol. 2003;25:893–903. doi: 10.1076/jcen.25.7.893.16489. [DOI] [PubMed] [Google Scholar]
- Berger JR, Kumar M, Kumar A, Fernandez JB, Levin B. Cerebrospinal fluid dopamine in HIV-1 infection. AIDS. 1994;8:67–71. doi: 10.1097/00002030-199401000-00010. [DOI] [PubMed] [Google Scholar]
- Berger JR, Nath A. HIV dementia and the basal ganglia. Intervirology. 1997;40:122–131. doi: 10.1159/000150539. [DOI] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell PJ. Behavioral approaches to the assessment of attention in animals. Psychopharmacology. 1998;138:231–259. doi: 10.1007/s002130050668. [DOI] [PubMed] [Google Scholar]
- Carli M, Robbins TW, Evenden JL, Everitt BJ. Effects of lesions to ascending noradenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradenergic bundle function based on selective attention and arousal. Behav Brain Res. 1983;9:361–380. doi: 10.1016/0166-4328(83)90138-9. [DOI] [PubMed] [Google Scholar]
- Cattie JE, Doyle K, Weber E, Grant I, Woods SP. Planning deficits in HIV-associated neurocognitive disorders: Component processes, cognitive correlates, and implications for everyday functioning. J Clin Exp Neuropsychol. 2012;34:906–918. doi: 10.1080/13803395.2012.692772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. New HIV Infections in the United States. 2012 http://www.cdc.gov/nchhstp/newsroom/docs/2012/HIV-Infections-2007-2010.pdf.
- Chang L, Ernst T, Witt MD, Ames N, Gaiefsky M, Miller E. Relationships among brain metabolites, cognitive function, and viral loads in antiretroviral-naïve HIV patients. Neuroimage. 2002;17:1638–1648. doi: 10.1006/nimg.2002.1254. [DOI] [PubMed] [Google Scholar]
- Chang L, Speck O, Miller EN, Braun J, Jovicich J, Koch C, Itti L, Ernst T. Neural correlates of attention and working memory deficits in HIV patients. Neurology. 2001;57:1001–1007. doi: 10.1212/wnl.57.6.1001. [DOI] [PubMed] [Google Scholar]
- Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, Fowler JS. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. Neuroimage. 2008;42:869–878. doi: 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chudasama Y, Robbins TW. Dopaminergic modulation of visual attention and working memory in the rodent prefrontal cortex. Neuropsychopharmacology. 2004;29:1628–36. doi: 10.1038/sj.npp.1300490. [DOI] [PubMed] [Google Scholar]
- Clarke HF, Hill GJ, Robbins TW, Roberts AC. Dopamine, but not serotonin, regulates reversal learning in the marmoset caudate nucleus. J Neurosci. 2011;16:4290–4297. doi: 10.1523/JNEUROSCI.5066-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clatworthy PL, Lewis SJ, Brichard L, Hong YT, Izquierdo D, Clark L, et al. Dopamine release in dissociable striatal subregions predicts the different effects of oral methylphenidate on reversal learning and spatial working memory. J Neurosci. 2009;29:4690–4696. doi: 10.1523/JNEUROSCI.3266-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts HS, Dalley JW, Collins P, Van Denderen JC, Everitt BJ, Robbins TW, et al. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cereb Cortex. 2001;11:1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: A combined study of two cohorts. J Neurovirol. 2004;10:350–357. doi: 10.1080/13550280490521078. [DOI] [PubMed] [Google Scholar]
- Dias R, Robbins TW, Roberts AC. Primate analogue of the Wisconsin Card Sorting Test: effects of excitotoxic lesions of the prefrontal cortex in the marmoset. Behav Neurosci. 1996;110:872–886. doi: 10.1037//0735-7044.110.5.872. [DOI] [PubMed] [Google Scholar]
- di Rocco A, Bottiglieri T, Dorfman D, Werner P, Morrison C, Simpson D. Decreased homovanillic acid in cerebrospinal fluid correlates with impaired neuropsychologic function in HIV-1-infected patients. Clin Neuropharmacol. 2000;23:190–194. doi: 10.1097/00002826-200007000-00004. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Jovicich J, Ames N, Arnold S. Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology. 2002;59:1343–1349. doi: 10.1212/01.wnl.0000031811.45569.b0. [DOI] [PubMed] [Google Scholar]
- Everall IP, Heaton RK, Marcotte TD, Ellis RJ, McCutchan JA, Atkinson JH, Grant I, Mallory M, Masliah E. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. Brain Pathol. 1999;9:209–217. doi: 10.1111/j.1750-3639.1999.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Biggins CA, Mackay S. Delayed latency of the event-related brain potential P3A component in HIV disease. Progressive effects with increasing cognitive impairment. Arch Neurol. 1995;52:1109–1118. doi: 10.1001/archneur.1995.00540350103022. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Frederick-Duus D, Fadel J, Mactutus CF, Booze RM. The human immunodeficiency virus-1-associated protein, Tat(1-86), impairs dopamine transporters and interacts with cocaine to reduce nerve terminal function: A no-net-flux microdialysis study. Neuroscience. 2009;159:1292–1299. doi: 10.1016/j.neuroscience.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey LJ, Yerrakalva D, Winston A. Correlations between computerized battery testing and a memory questionnaire for identification of neurocognitive impairment in HIV type 1-infected subjects on stable antiretroviral therapy. AIDS Res Hum Retroviruses. 2009;25:765–769. doi: 10.1089/aid.2008.0292. [DOI] [PubMed] [Google Scholar]
- Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J Neurosci. 2000;20:1208–15. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groman SM, Lee B, London ED, Mandelkern MA, James AS, Feiler K, et al. Dorsal striatal D2-like receptor availability covaries with sensitivity to positive reinforcement during discrimination learning. J Neurosci. 2011;31:7291–7299. doi: 10.1523/JNEUROSCI.0363-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy DJ, Hinkin CH. Reaction time performance in adults with HIV/AIDS. J Clin Exp Neuropsychol. 2002;24:912–929. doi: 10.1076/jcen.24.7.912.8391. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Ellis RJ, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, LeBlanc S, Corkran SH, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmelheber A, Sarter M, Bruno J. Increases in cortical acetylcholine release during sustained attention performance in rat. Cognit Brain Res. 2000;9:313–325. doi: 10.1016/s0926-6410(00)00012-4. [DOI] [PubMed] [Google Scholar]
- Iudicello JE, Woods SP, Weber E, Dawson MS, Scott JC, Carey CL, Grant I. Cognitive mechanisms of switching in HIV-associated category fluency deficits. J Clin Exp Neuropsychol. 2008;30:797–804. doi: 10.1080/13803390701779578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellendonk C, Simpson EH, Polan HJ, Malleret G, Vronskaya S, Winiger V, et al. Transient and selective overexpression of dopamine D2 receptors in the striatum causes persistent abnormalities in prefrontal cortex functioning. Neuron. 2006;49:603–615. doi: 10.1016/j.neuron.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Churchwell JC. An analysis of rat prefrontal cortex in mediating executive function. Neurobiol Learn Mem. 2011;96:417–431. doi: 10.1016/j.nlm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Koutsilieri E, Sopper S, Scheller C, ter Meulen V, Riederer P. Parkinsonism in HIV dementia. J Neural Transm. 2002;109:767–775. doi: 10.1007/s007020200063. [DOI] [PubMed] [Google Scholar]
- Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M. Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. J Neurovirol. 2011;17:26–40. doi: 10.1007/s13365-010-0003-4. [DOI] [PubMed] [Google Scholar]
- Lee B, Groman S, London ED, Jentsch JD. Dopamine D2/D3 receptors play a specific role in the reversal of a learned visual discrimination in monkeys. Neuropsychopharmacology. 2007;32:2125–2134. doi: 10.1038/sj.npp.1301337. [DOI] [PubMed] [Google Scholar]
- Maki PM, Cohen MH, Weber K, Little DM, Fornelli D, Rubin LH, Perschler P, Gould F, Martin E. Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women. Neurology. 2009;72:1661–1668. doi: 10.1212/WNL.0b013e3181a55f65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki PM, Martin-Thormeyer E. HIV, cognition and women. Neuropsychol Rev. 2009;19:204–14. doi: 10.1007/s11065-009-9093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manes F, Sahakian B, Clark L, Rogers R, Antoun N, Aitken M, et al. Decision-making processes following damage to the prefrontal cortex. Brain. 2002;125:624–639. doi: 10.1093/brain/awf049. [DOI] [PubMed] [Google Scholar]
- Manly JJ, Smith C, Crystal HA, Richardson J, Golub ET, Greenblatt R, Robison E, Martin EM, Young M. Relationship of ethnicity, age, education, and reading level to speed and executive function among HIV+ and HIV− women: the Women’s Interagency HIV Study (WIHS) Neurocognitive Substudy. J Clin Exp Neuropsychol. 2011;33:853–63. doi: 10.1080/13803395.2010.547662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. Ann Neurol. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behav Brain Res. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav Neurosci. 1996;110:247–265. doi: 10.1037//0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Ross RS, Eichenbaum H. Noradrenergic, but not cholinergic, deafferentation of prefrontal cortex impairs attentional set-shifting. Neuroscience. 2008;153:63–71. doi: 10.1016/j.neuroscience.2008.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughy J, Sarter M. Behavioral vigilance in rats: task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology. 1995;117:340–357. doi: 10.1007/BF02246109. [DOI] [PubMed] [Google Scholar]
- McLean SL, Beck JP, Woolley ML, Neill JC. A preliminary investigation into the effects of antipsychotics on sub-chronic phencyclidine-induced deficits in attentional set-shifting in female rats. Behav Brain Res. 2008;189:152–158. doi: 10.1016/j.bbr.2007.12.029. [DOI] [PubMed] [Google Scholar]
- Melrose RJ, Tinaz S, Castelo JMB, Courtney MG, Stern CE. Compromised fronto-striatal functioning in HIV: An fMRI investigation of semantic event sequencing. Behav Brain Res. 2008;188:337–347. doi: 10.1016/j.bbr.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Midde NM, Huang X, Gomez AM, Booze RM, Zhan CG, Zhu J. Mutation of tyrosine 470 of human dopamine transporter is critical for HIV-1 Tat-induced inhibition of dopamine transport and transporter conformational transitions. J Neuroimmune Pharmacol. 2013;8:975–987. doi: 10.1007/s11481-013-9464-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein JA, Lehmann O, Theobald DE, Dalley JW, Robbins TW. Selective depletion of cortical noradrenaline by anti-dopamine beta-hydroxylase-saporin impairs attentional function and enhances the effects of guanfacine in the rat. Psychopharmacology (Berl) 2007;190:51–63. doi: 10.1007/s00213-006-0594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore H, Fadel J, Sarter M, Bruno JP. Role of accumbens and cortical dopamine receptors in the regulation of cortical acetylcholine release. Neuroscience. 1999;88:811–822. doi: 10.1016/s0306-4522(98)00261-9. [DOI] [PubMed] [Google Scholar]
- Moran LM, Aksenov MY, Booze RM, Webb KM, Mactutus CF. Adolescent HIV-1 transgenic rats: evidence for dopaminergic alterations in behavior and neurochemistry revealed by methamphetamine challenge. Curr HIV Res. 2012;10:415–424. doi: 10.2174/157016212802138788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Booze RM, Mactutus CF. Time and time again: temporal processing demands implicate perceptual and gating deficits in the HIV-1 transgenic rat. J Neuroimmune Pharmacol. 2013a;8:988–997. doi: 10.1007/s11481-013-9472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Booze RM, Webb KM, Mactutus CF. Neurobehavioral alterations in HIV-1 transgenic rats: evidence for dopaminergic dysfunction. Exp Neurol. 2013b;239:139–147. doi: 10.1016/j.expneurol.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill M, Brown VJ. The effect of striatal dopamine depletion and the adenosine A2A antagonist KW-6002 on reversal learning in rats. Neurobiol Learn Mem. 2007;88:75–81. doi: 10.1016/j.nlm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Davies DR. A taxonomic analysis of vigilance performance. In: Mackie RR, editor. Vigilance: Theory, operational performance, and physiological correlates. Springer; Plenum, NY: 1977. pp. 559–574. [Google Scholar]
- Peng JS, Vigorito M, Liu XQ, Zhou DJ, Wu XW, Chang SL. The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART. J Neuroimmunol. 2010;218:94–101. doi: 10.1016/j.jneuroim.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Kemper CA, Deresinski S, Sullivan EV. Frontostriatal fiber bundle compromise in HIV infection without dementia. AIDS. 2009;23:1977–1985. doi: 10.1097/QAD.0b013e32832e77fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reger M, Welsh R, Razani J, Martin DJ, Boone KB. A meta-analysis of the neuropsychological sequelae of HIV infection. J Int Neuropsychol Soc. 2002;8:410–424. doi: 10.1017/s1355617702813212. [DOI] [PubMed] [Google Scholar]
- Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Jr, Hayes N, Jones O. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci. 2001;98:9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley RM, Haystead TA, Baker HF. An analysis of visual object reversal learning in the marmoset after amphetamine and haloperidol. Pharmacol Biochem Behav. 1981;14:345–351. doi: 10.1016/0091-3057(81)90401-9. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Eagle DM, Mar AC, Bari A, Banerjee G, Jiang X, et al. Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology. 2008;33:1028–1037. doi: 10.1038/sj.npp.1301487. [DOI] [PubMed] [Google Scholar]
- Rodefer JS, Nguyen TN, Karlsson JJ, Arnt J. Reversal of subchronic PCP-induced deficits in attentional set shifting in rats by sertindole and a 5-HT6 receptor antagonist: comparison among antipsychotics. Neuropsychopharmacology. 2008;33:2657–2666. doi: 10.1038/sj.npp.1301654. [DOI] [PubMed] [Google Scholar]
- Royal W, Zhang L, Guo M, Jones O, Davis H, Bryant JL. Immune activation, viral gene product expression and neurotoxicity in the HIV-1 transgenic rat. J Neuroimmunol. 2012;247:16–24. doi: 10.1016/j.jneuroim.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–142. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- Scott JC, Woods SP, Vigil O, Heaton RK, Grant I, Ellis RJ, Marcotte TD. Script Generation of Activities of Daily Living in HIV-Associated Neurocognitive Disorders. J Int Neuropsychol Soc. 2011;17:740–745. doi: 10.1017/S135561771100052X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seu E, Jentsch JD. Effect of acute and repeated treatment with desipramine or methylphenidate on serial reversal learning in rats. Neuropharmacology. 2009;57:665–672. doi: 10.1016/j.neuropharm.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JM, Aksenova MV, Aksenov MY, Mactutus CF, Booze RM. Neurotoxicity of HIV-1 tat protein: Involvement of D1 dopamine receptor. Neurotoxicology. 2007;28:1184–1190. doi: 10.1016/j.neuro.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait DS, Brown VJ, Farovik A, Theobald DE, Dalley JW, Robbins TW. Lesions of the dorsal noradrenergic bundle impair attentional set-shifting in the rat. Eur J Neurosci. 2007;25:3719–3724. doi: 10.1111/j.1460-9568.2007.05612.x. [DOI] [PubMed] [Google Scholar]
- Tait DS, Marston HM, Shahid M, Brown VJ. Asenapine restores cognitive flexibility in rats with medial prefrontal cortex lesions. Psychopharmacology. 2009;202:295–306. doi: 10.1007/s00213-008-1364-8. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4(+) T lymphocyte decline. Proc Natl Acad Sci USA. 2005;102:15647–15652. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi V, Narciso P, Galgani S, Sette P, Balestra P, Gerace C, Pau FM, et al. Effects of zidovudine in 30 patients with mild to end-stage AIDS dementia complex. AIDS. 1993;7:683–692. doi: 10.1097/00002030-199305000-00012. [DOI] [PubMed] [Google Scholar]
- Tunbridge EM, Bannerman DM, Sharp T, Harrison PJ. Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J Neurosci. 2004;24:5331–5335. doi: 10.1523/JNEUROSCI.1124-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS. Global Report: UNAIDS Report on the Global AIDS Epidemic 2013. 2013 http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2013/gr2013/UNAIDS_Global_Report_2013_en.pdf.
- Vivithanaporn P, Heo G, Gamble J, Krentz HB, Hoke A, Gill MJ, Power C. Neurologic disease burden in treated HIV/AIDS predicts survival A population-based study. Neurology. 2010;75:1150–1158. doi: 10.1212/WNL.0b013e3181f4d5bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DR, Dodson S, Nath A, Booze RM. Estrogen attenuates gp120-and tat(1-72)-induced oxidative stress and prevents loss of dopamine transporter function. Synapse. 2006;59:51–60. doi: 10.1002/syn.20214. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, Fowler JS. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004;127:2452–2458. doi: 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley A, Nou E, Mazer JA, McCormick DA, Arnsten AFT. α2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Watkins JM, Cool VA, Usner D, Stehbens JA, Nichols S, Loveland KA, Bordeaux JD, Donfield S, Asarnow RF, Nuechterlein KH. Attention in HIV-infected children: results from the Hemophilia Growth and Development Study. J Int Neuropsychol Soc. 2000;6:443–454. doi: 10.1017/s1355617700644028. [DOI] [PubMed] [Google Scholar]
- Webb KM, Aksenov MY, Mactutus CF, Booze RM. Evidence for developmental dopaminergic alterations in the human immunodeficiency virus-1 transgenic rat. J Neurovirol. 2010;16:168–173. doi: 10.3109/13550281003690177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Ananthan S, Mactutus CF, Booze RM. Recombinant human immunodeficiency virus-1 transactivator of transcription(1-86) allosterically modulates dopamine transporter activity. Synapse. 2011;65:1251–1254. doi: 10.1002/syn.20949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Mactutus CF, Wallace DR, Booze RM. HIV-1 Tat protein-induced rapid and reversible decrease in [H-3]dopamine uptake: Dissociation of [H-3]dopamine uptake and [H-3]2 beta-carbomethoxy-3-beta-(4-fluorophenyl)tropane (WIN 35,428) binding in rat striatal synaptosomes. J Pharmacol Exp Ther. 2009;329:1071–1083. doi: 10.1124/jpet.108.150144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zmarowski A, Sarter M, Bruno JP. NMDA and dopamine interactions in the nucleus accumbens modulate cortical acetylcholine release. Eur J Neurosci. 2005;22:1731–1740. doi: 10.1111/j.1460-9568.2005.04333.x. [DOI] [PubMed] [Google Scholar]