Abstract
BACKGROUND
Prenatal programming of hypertension has been described in humans and in animal models that receive a prenatal insult, but the mechanism for the increase in blood pressure remains elusive.
METHODS
In male rats whose mothers received dexamethasone between days 15 and 18 of gestation systemic and urinary levels of angiotensin II were measured to determine whether angiotensin II was a potential factor for the generation (4 weeks of age) or maintenance (8 weeks of age) of hypertension.
RESULTS
A group 4- and 8-week-old male rats that were the product of a pregnancy where the mother received prenatal dexamethasone between days 15 and 18 of gestation had comparable plasma renin and angiotensin II levels to the offspring of vehicle-treated controls. Renal angiotensin II levels were not different at 4 and 8 weeks of age between the controls and the prenatal dexamethasone group. Urine angiotensin II/Creatinine levels, a reflection of filtered and renally generated and secreted angiotensin II, were higher at both 4 and 8 weeks of age in male rats that received prenatal dexamethasone compared to controls.
CONCLUSIONS
The high-urine angiotensin II levels in prehypertensive and hypertensive rats that were the product of mothers that received dexamethasone compared to vehicle suggest that luminal angiotensin II may play a role in the generation and maintenance of hypertension in this model of prenatal programming.
Keywords: blood pressure, hypertension, intrarenal renin–angiotensin system (RAS), prenatal programming
The fetal origin of adult diseases was originally hypothesized by Barker and colleagues 20 years ago.1,2 Since then numerous epidemiologic studies have shown a correlation between intrauterine insults and the risk of developing hypertension, diabetes, and ischemic heart disease in adults.3,4 This original observation by Barker and colleagues inspired investigators to develop animal models of prenatal programming of hypertension to understand the pathogenesis of programming of adult disease.
We have previously demonstrated that prenatal dexamethasone administered at specific times during pregnancy in rats results in offspring that have a reduction in nephron number and hypertension when they are studied as adults.5,6 The reduction in glomeruli by prenatal dexamethasone was likely due to altered branching morphogenesis.6,7 Prenatal dexamethasone resulted in hypertension when the rats were 8 weeks of age, similar to the age-dependent increase in blood pressure seen in rats whose mothers were fed a protein-deficient diet.6–8 The cause for the hypertension by prenatal dexamethasone remains unclear.
The renin–angiotensin system (RAS) is an important regulator of blood pressure. In addition to the systemic RAS, the kidney has all the synthetic machinery to generate angiotensin II.9 The intrarenal angiotensin II content was greater than can be explained on the basis of equilibration between plasma angiotensin II and intrarenal extracellular fluid.9–12 Either the systemic or the intrarenal RAS could be a factor in the generation or maintenance of hypertension in offspring of rats that received prenatal dexamethasone. The purpose of this study was to characterize both the systemic levels of angiotensin II and renal and urinary levels of angiotensin II in rats whose mothers were administered dexamethasone in young male rat offspring at 4 weeks of age, before they develop hypertension, and adult male rat offspring at 8 weeks of age, which were already hypertensive, and compare these results to offspring of mothers that received vehicle.
METHODS
Animals
Timed, pregnant Sprague–Dawley rats were purchased from Harlan Laboratories (Houston, TX). Pregnant rats arrived at our institution on day 12 of gestation. Intraperitoneal dexamethasone (American Regent, Shirley, NY) 0.2 mg/kg in dosage or saline vehicle injections were administered once daily between days 15 and 18 of gestation. Pregnant rats were used once, and rats from multiple litters were studied in each experiment.
We studied male offspring of the rats at the age of 4 weeks, as they are not hypertensive at this age, and at the age of 8 weeks as adults when hypertension was well established.5–13 Only males were studied, as females exposed to prenatal dexamethasone have an increase in blood pressure at 3–4 weeks of age.6 All experimental procedures conformed to the APS’s Guiding Principles in the Care and Use of Animals and the Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center (Dallas, TX).
Blood pressure
A group of 4- and 8-week-old male rats were trained by inflating a tail blood pressure cuff several times while being restrained in a Lucite tube daily for 4 days before measurement. On the day 5, the rats had their blood pressure measured five times with a IITC Model # 179 blood pressure analyzer (IITC, Woodland Hills, CA), with the mean of the results determined as the blood pressure for that animal.
Plasma renin activity
Rats were anesthetized with intraperitoneal Inactin (Sigma Chemical, St Louis, MO) 100 mg/kg in dosage. A midabdominal incision was performed, and blood was collected from the aorta with a syringe containing 5 mmol/l EDTA (GIBCO, Grand Island, NY). The samples were placed on ice and then were centrifuged at 1,500g at 4 °C for 30 min. The plasma was frozen at −80 °C until the radioimmunoassay was preformed. A Gamma Coat Plasma Renin Activity 125I RIA kit (DiaSorin, Stillwater, MN) was used to measure plasma renin activity by assaying angiotensin I generation.
Plasma and urine angiotensin II levels
Blood was collected in a similar manner as described previously, except that the syringe was flushed with heparin 1,000 units/ml (Baxter, Deerfield, IL). The blood (1 ml) was immediately transferred to a cooled tube (4 °C) containing EDTA (GIBCO) and enalaprilat at a final concentration of 5 mmol/l and 20 μmol/l, respectively (Bedford Laboratories, Bedford, OH). It has been shown that EDTA and enalaprilat prevent the in vitro degradation and generation of angiotensin II.11–14 The tube was centrifuged at 1,500g at 4 °C for 30 min, and the plasma was then frozen at −80 °C until angiotensin II was extracted and assayed.
For determination of urine angiotensin II, rats were placed in metabolic cages overnight with free access to food and water. The urine was collected in a tube containing 80 nmol enalaprilat and 50 μmol EDTA similar to that used described by Kobori et al.14,15 A quantity of 2 ml of urine was frozen at −80 °C until extraction and measurement of angiotensin II. Briefly, phenylbonded sodium phosphate–EDTA columns (Bond-Elut; Varian, Lake Forest, CA) were washed with 3 ml of 90% methanol in water and then twice with 6 ml of distilled deionized water. The sample was loaded on the column and washed twice with 3-ml distilled deionized water followed by 1.5 ml of hexane and 1.5 ml of chloroform. The angiotensin II retained on the column was eluted with 1 ml of 90% methanol in water and dried under vacuum in a Speed Vac overnight. The dried residue was reconstituted with 500 μl of enzyme immunoassay buffer. The angiotensin II measurement was performed using an angiotensin II enzyme immunoassay kit (Spi-Bio, Paris, France).
Renal angiotensin II content
Rats received an intraperitoneal injection of Inactin (Sigma Chemical) 100 mg/kg in dosage. A midabdominal incision was performed, and the left kidney was separated from perinephric fat. A clamp whose head was greater in size than the kidney was cooled in liquid nitrogen, and the left kidney was frozen in situ. The kidney was then removed and submerged in liquid nitrogen. Afterwards, the kidney was weighed and placed in a tube with 5 ml of methanol at −80 °C until study.
The kidney was thawed on ice and homogenized at 4 °C. The homogenate was centrifuged at 1,000g at 4 °C for 15 min. The supernatant was then dried overnight under vacuum in a Speed Vac. The dried residue was reconstituted in 1 ml of 50-mmol/l sodium phosphate (Sigma Chemical) with bovine serum albumin at 0.1 mg/ml and 1 mmol/l EDTA. The extraction and assay was performed as described previously, for plasma angiotensin II.
Statistics
Data are presented as mean ± s.e. of the mean. A Student’s t-test for unpaired analysis was used to determine the statistical significance for normally distributed results and the Mann–Whitney U-test was used to determine whether the data were not normally distributed. A P value at 0.05 or more was considered statistically significant.
RESULTS
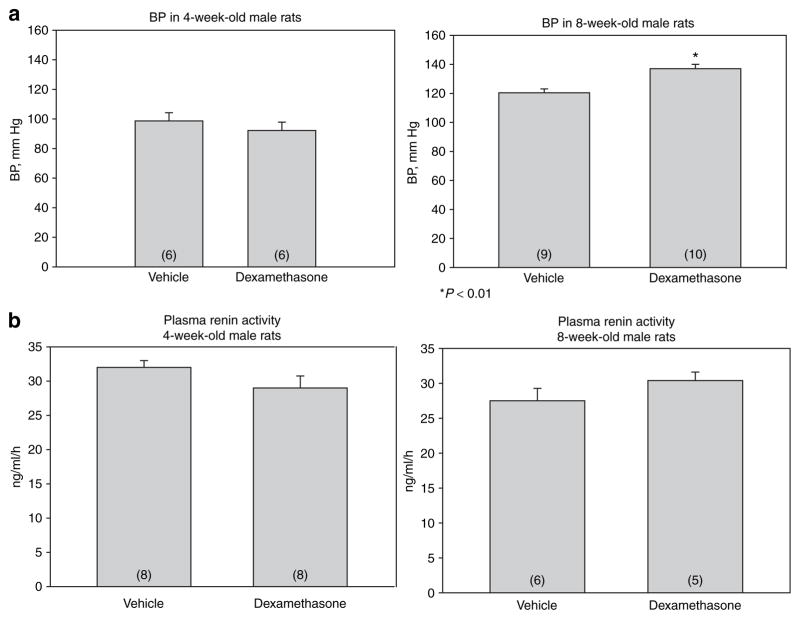
In the first group of experiments, we measured blood pressure in male rats at 4 and 8 weeks of age. As shown in Figure 1a, blood pressure in male rats that were the offspring of mothers that received prenatal dexamethasone were comparable to vehicle-treated controls at 4 weeks of age. At 8 weeks of age, the blood pressure of the male rats exposed to prenatal dexamethasone was significantly higher than the offspring of the vehicle-treated controls.
Figure 1.
Effect of prenatal dexamethasone on (a) blood pressure and (b) plasma renin activity in 4- and 8-week-old male rats. Pregnant rats were administered dexamethasone (0.2 mg/kg) body weight or saline vehicle daily between days 15 and 18 of gestation. (a) Blood pressure was measured in 4- and 8-week-old male rats in trained rats by tail cuff. (b) Plasma renin activity was measured in 4- and 8-week-old male rats that received either vehicle or prenatal dexamethasone. The difference in blood pressure was different at 8 weeks of age between vehicle and dexamethasone group using an unpaired Student’s t-test (P < 0.01). BP, blood pressure.
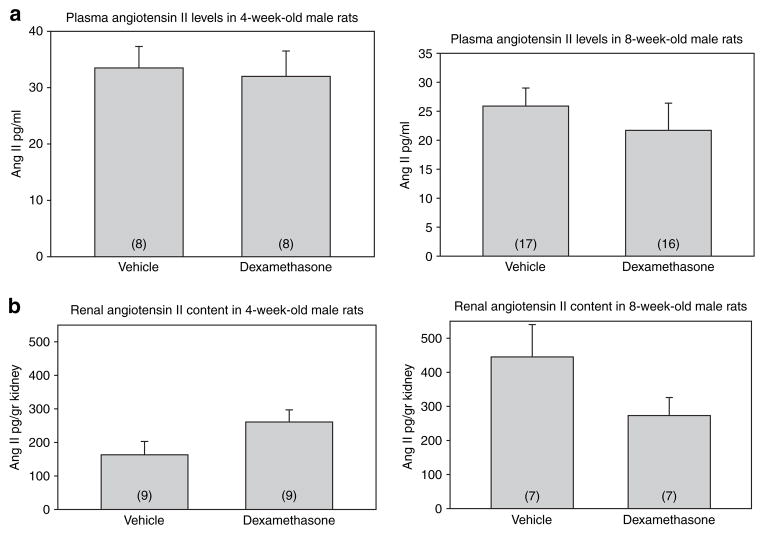
We next measured the effect of prenatal dexamethasone on plasma renin activity and angiotensin II levels. There was no difference in the plasma renin activity in the male offspring of rats that received dexamethasone and vehicle when they were studied at 4 weeks and 8 weeks of age (Figure 1b). As shown in Figure 2a, the plasma angiotensin II levels were comparable in the two groups at 4 and 8 weeks of age.
Figure 2.
Effect of prenatal dexamethasone on (a) plasma and (b) renal angiotensin II levels. (a) Plasma angiotensin II levels were measured in 4- and 8-week-old male rats that received either vehicle or prenatal dexamethasone. (b) Kidney angiotensin II content was measured in in situ snap frozen kidneys in male rats at 4 and 8 weeks of age that received prenatal dexamethasone or vehicle. Ang II, angiotensin II.
In the next experiments, we compared the renal angiotensin II content in rats that received prenatal dexamethasone to those that received vehicle. The results are shown in Figure 2b. The renal angiotensin II levels were comparable in prenatal dexamethasone-treated 4- and 8-week-old rats compared to the control rats. Thus, prenatal dexamethasone did not affect renal angiotensin II levels.
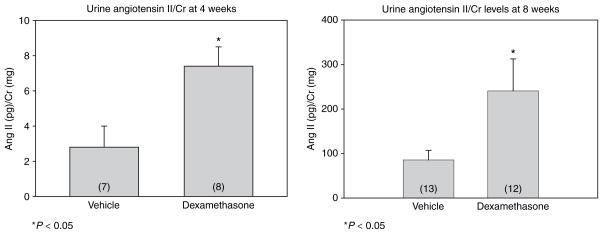
Finally, we assessed urinary angiotensin II levels that were normalized to urine creatinine levels (Figure 3). Urine angiotensin II has recently been shown to be a marker of the intrarenal RAS.16 Urine angiotensin II/Creatinine levels were significantly greater at 4 weeks of age in the male rats that were the offspring of rats that received prenatal dexamethasone compared to vehicle-treated control rats. At 8 weeks of age, the urinary angiotensin II/Creatinine levels were approximately tenfold higher in both groups. The difference between the control and prenatal dexamethasone-treated rats persisted in rats when studied at 8 weeks of age.
Figure 3.
Effect of prenatal dexamethasone on urinary angiotensin II levels. Urinary angiotensin II/Creatinine (Cr) levels were measured in 4- and 8-week-old rats that were exposed to prenatal dexamethasone. Urinary angiotensin II/Cr levels increased over tenfold in both groups between 4- and 8-week-old rats but was higher in the prenatal dexamethasone-treated group at both ages compared to vehicle controls (P < 0.05). The Mann–Whitney U-test was used to assess significance as the data were not normally distributed. Ang II, angiotensin II.
DISCUSSION
We have previously demonstrated that prenatal dexamethasone can cause hypertension in the male offspring at 8 weeks but not at 4 weeks of age.5,6 The cause for the increase in blood pressure is unknown at present, and this study examined the effect of prenatal dexamethasone on the systemic RAS and elements of the intrarenal and urinary intrarenal RAS. We find that there was no effect of prenatal dexamethasone on plasma renin, angiotensin II levels, or total renal angiotensin II in 4-week-old and 8-week-old rats compared to controls. However, urinary angiotensin II levels were higher for both 4- and 8-week-old rats whose mothers received prenatal dexamethasone compared to controls.
We have recently demonstrated that prenatal dexamethasone increases brush-border membrane NHE3 protein abundance (the proximal tubule Na+/H+ exchanger), the bumetanide-sensitive cotransporter, and the thiazide-sensitive cotransporter protein abundance in adult offspring compared to vehicle-treated controls.17 We also showed that in utero exposure to prenatal dexamethasone increases proximal tubule Na+/H+ exchanger activity and sodium absorption, as well as sodium absorption in the medullary, thick, ascending limb in adult male offspring compared to age-matched controls.13–18 As transport in several nephron segments has been shown to be affected by plasma or luminal angiotensin II, we examined whether either the systemic or urinary angiotensin II levels were different in animals receiving prenatal dexamethasone.
Both the systemic angiotensin and intrarenal RAS may be factors in generating or maintaining the hypertension in rats whose mothers were exposed to prenatal dexamethasone. Plasma angiotensin II binds to the basolateral membrane of proximal tubules and increases sodium reabsorption.19 Systemic angiotensin II has been shown to stimulate both proximal tubule and distal tubule sodium absorption that could lead to volume-mediated hypertension.19–23 In addition to the systemic RAS, the proximal tubule has all the synthetic machinery to synthesize angiotensin II, including angiotensinogen, renin, and angiotensin-converting enzyme.10–12,24–28 Angiotensin II was found to be secreted into the luminal fluid at levels ~100-fold greater than that measured in plasma.10–12 Luminal angiotensin II also regulates proximal29 and distal, convoluted, tubule sodium transport.21 In addition, the collecting duct has renin27,30 and can convert angiotensin I to angiotensin II, which has been shown to increase distal tubule sodium transport.22 The urinary angiotensin II not only reflects filtered and secreted proteins but also facilitates some insight into what is present in the tubular fluid in the distal tubule. Our results suggest that higher luminal angiotensin II levels in the distal nephron may be important in affecting sodium transport that could be a factor in hypertension observed after administering prenatal dexamethasone.
Previous studies looking at prenatal programming of hypertension have measured elements of the systemic RAS. Pulmonary angiotensin-converting enzyme activity was elevated in adult rats exposed to a 6% low-protein diet compared to 18% protein in the control, but maternal intake of a 9% and 12% protein diet leads to offspring that had angiotensin-converting enzyme activities that were not significantly different than control offspring.31,32 In rats that were the product of mothers who ingested a low-protein diet during pregnancy, plasma renin activity was lower than controls at 1 month and at 2 months but was higher than controls after 4 months.8,33,34 The increase in plasma renin activity in latter life may be the result of renal injury as these rats had proteinuria at this age.33 In the present study, we saw no effect of prenatal dexamethasone on plasma renin activity or angiotensin II levels at 4 or 8 weeks of age. Similar to our study, offspring of rats whose mothers were fed a low-protein diet did not have a difference in plasma angiotensin II levels, compared to control group mices at 4 weeks of age35 or at 13 weeks when hypertension was well established.32
The effect of prenatal dexamethasone on the RAS has been studied in sheep. The adult offspring of sheep administered dexamethasone on days 80 and 81 of gestation developed hypertension by 6 months of age.36,37 Sheep whose mothers received betamethasone had a higher percentage of active plasma renin at 6 months but not at 18 months of age.38 Plasma angiotensin II levels were comparable in the offspring of control and betamethasone- treated sheep studied at 6 months of age.36,38 However, at 18 months of age prenatal betamethasone group of sheep had a reduction in plasma angiotensin II levels. 38 Similar to what these studies revealed, the sheep do not have a consistent increase in plasma renin and angiotensin II during the period when they are hypertensive. Thus, the plasma RAS is likely not the cause for the hypertension in sheep.
The intrarenal RAS has been examined in pre- and early- hypertensive animals, as well as after hypertension has been well established. At 4 weeks of age, rats that were the product of mothers fed a low-protein diet had renal angiotensin I and II levels that were comparable to controls.35 In addition, renal angiotensin II levels were comparable to controls in 16-week-old rats that were the product of mothers with uteroplacental insufficiency.35–39 The results of these studies using different models of prenatal programming are comparable to our findings examining offspring of rats that received prenatal dexamethasone.
In the present study, we found that there was a higher urinary urine angiotensin II/Creatinine level in rats that were exposed to prenatal dexamethasone than that of vehicle controls at 4 weeks of age, which persisted at 8 weeks. This is consistent with increase in luminal angiotensin II and angiotensinogen levels, at least in the distal nephron. It will be of interest to determine whether luminal angiotensin II has an augmented effect on transport in the distal nephron in animals that receive a prenatal insult to possibly explain the increase in sodium transport and hypertension with prenatal programming.
Acknowledgments
This work was supported by NIH Grants DK41612 and DK78596 to M.B, T32 DK07257, and the O’Brien Center P30DK079328.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Barker DJ, Osmond C. Low birth weight and hypertension. BMJ. 1988;297:134–135. doi: 10.1136/bmj.297.6641.134-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJ, Bagby SP. Developmental antecedents of cardiovascular disease: a historical perspective. J Am Soc Nephrol. 2005;16:2537–2544. doi: 10.1681/ASN.2005020160. [DOI] [PubMed] [Google Scholar]
- 4.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 5.Ortiz LA, Quan A, Weinberg A, Baum M. Effect of prenatal dexamethasone on rat renal development. Kidney Int. 2001;59:1663–1669. doi: 10.1046/j.1523-1755.2001.0590051663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ortiz LA, Quan A, Zarzar F, Weinberg A, Baum M. Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension. 2003;41:328–334. doi: 10.1161/01.hyp.0000049763.51269.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh RR, Moritz KM, Bertram JF, Cullen-McEwen LA. Effects of dexamethasone exposure on rat metanephric development: in vitro and in vivo studies. Am J Physiol Renal Physiol. 2007;293:F548–F554. doi: 10.1152/ajprenal.00156.2007. [DOI] [PubMed] [Google Scholar]
- 8.Vehaskari VM, Aviles DH, Manning J. Prenatal programming of adult hypertension in the rat. Kidney Int. 2001;59:238–245. doi: 10.1046/j.1523-1755.2001.00484.x. [DOI] [PubMed] [Google Scholar]
- 9.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 10.Seikaly MG, Arant BS, Jr, Seney FD., Jr Endogenous angiotensin concentrations in specific intrarenal fluid compartments of the rat. J Clin Invest. 1990;86:1352–1357. doi: 10.1172/JCI114846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braam B, Mitchell KD, Fox J, Navar LG. Proximal tubular secretion of angiotensin II in rats. Am J Physiol. 1993;264:F891–F898. doi: 10.1152/ajprenal.1993.264.5.F891. [DOI] [PubMed] [Google Scholar]
- 12.Navar LG, Lewis L, Hymel A, Braam B, Mitchell KD. Tubular fluid concentrations and kidney contents of angiotensins I and II in anesthetized rats. J Am Soc Nephrol. 1994;5:1153–1158. doi: 10.1681/ASN.V541153. [DOI] [PubMed] [Google Scholar]
- 13.Dagan A, Gattineni J, Cook V, Baum M. Prenatal programming of rat proximal tubule Na+/H+ exchanger by dexamethasone. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1230–R1235. doi: 10.1152/ajpregu.00669.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–585. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary angiotensinogen as an indicator of intrarenal Angiotensin status in hypertension. Hypertension. 2003;41:42–49. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shao W, Seth DM, Navar LG. Augmentation of endogenous intrarenal angiotensin II levels in Val5-ANG II-infused rats. Am J Physiol Renal Physiol. 2009;296:F1067–F1071. doi: 10.1152/ajprenal.90596.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dagan A, Kwon HM, Dwarakanath V, Baum M. Effect of renal denervation on prenatal programming of hypertension and renal tubular transporter abundance. Am J Physiol Renal Physiol. 2008;295:F29–F34. doi: 10.1152/ajprenal.00123.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dagan A, Habib S, Gattineni J, Dwarakanath V, Baum M. Prenatal programming of rat thick ascending limb chloride transport by low-protein diet and dexamethasone. Am J Physiol Regul Integr Comp Physiol. 2009;297:R93–R99. doi: 10.1152/ajpregu.91006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris PJ, Young JA. Dose-dependent stimulation and inhibition of proximal tubular sodium reabsorption by angiotensin II in the rat kidney. Pflugers Arch. 1977;367:295–297. doi: 10.1007/BF00581370. [DOI] [PubMed] [Google Scholar]
- 20.Cogan MG. Angiotensin II: a powerful controller of sodium transport in the early proximal tubule. Hypertension. 1990;15:451–458. doi: 10.1161/01.hyp.15.5.451. [DOI] [PubMed] [Google Scholar]
- 21.Wang T, Giebisch G. Effects of angiotensin II on electrolyte transport in the early and late distal tubule in rat kidney. Am J Physiol. 1996;271:F143–F149. doi: 10.1152/ajprenal.1996.271.1.F143. [DOI] [PubMed] [Google Scholar]
- 22.Komlosi P, Fuson AL, Fintha A, Peti-Peterdi J, Rosivall L, Warnock DG, Bell PD. Angiotensin I conversion to angiotensin II stimulates cortical collecting duct sodium transport. Hypertension. 2003;42:195–199. doi: 10.1161/01.HYP.0000081221.36703.01. [DOI] [PubMed] [Google Scholar]
- 23.Peti-Peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002;13:1131–1135. doi: 10.1097/01.asn.0000013292.78621.fd. [DOI] [PubMed] [Google Scholar]
- 24.Ingelfinger JR, Zuo WM, Fon EA, Ellison KE, Dzau VJ. In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. J Clin Invest. 1990;85:417–423. doi: 10.1172/JCI114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moe OW, Ujiie K, Star RA, Miller RT, Widell J, Alpern RJ, Henrich WL. Renin expression in renal proximal tubule. J Clin Invest. 1993;91:774–779. doi: 10.1172/JCI116296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taugner R, Hackenthal E, Rix E, Nobiling R, Poulsen K. Immunocytochemistry of the renin-angiotensin system: renin, angiotensinogen, angiotensin I, angiotensin II, and converting enzyme in the kidneys of mice, rats, and tree shrews. Kidney Int Suppl. 1982;12:S33–S43. [PubMed] [Google Scholar]
- 27.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44:223–229. doi: 10.1161/01.HYP.0000135678.20725.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terada Y, Tomita K, Nonoguchi H, Marumo F. PCR localization of angiotensin II receptor and angiotensinogen mRNAs in rat kidney. Kidney Int. 1993;43:1251–1259. doi: 10.1038/ki.1993.177. [DOI] [PubMed] [Google Scholar]
- 29.Quan A, Baum M. Endogenous angiotensin II modulates rat proximal tubule transport with acute changes in extracellular volume. Am J Physiol. 1998;275:F74–F78. doi: 10.1152/ajprenal.1998.275.1.F74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prieto-Carrasquero MC, Kobori H, Ozawa Y, Gutiérrez A, Seth D, Navar LG. AT1 receptor-mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol Renal Physiol. 2005;289:F632–F637. doi: 10.1152/ajprenal.00462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langley SC, Jackson AA. Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diets. Clin Sci. 1994;86:217–222. doi: 10.1042/cs0860217. discussion 121. [DOI] [PubMed] [Google Scholar]
- 32.Langley-Evans SC, Jackson AA. Captopril normalises systolic blood pressure in rats with hypertension induced by fetal exposure to maternal low protein diets. Comp Biochem Physiol A Physiol. 1995;110:223–228. doi: 10.1016/0300-9629(94)00177-u. [DOI] [PubMed] [Google Scholar]
- 33.Manning J, Vehaskari VM. Low birth weight-associated adult hypertension in the rat. Pediatr Nephrol. 2001;16:417–422. doi: 10.1007/s004670000560. [DOI] [PubMed] [Google Scholar]
- 34.Manning J, Vehaskari VM. Postnatal modulation of prenatally programmed hypertension by dietary Na and ACE inhibition. Am J Physiol Regul Integr Comp Physiol. 2005;288:R80–R84. doi: 10.1152/ajpregu.00309.2004. [DOI] [PubMed] [Google Scholar]
- 35.Vehaskari VM, Stewart T, Lafont D, Soyez C, Seth D, Manning J. Kidney angiotensin and angiotensin receptor expression in prenatally programmed hypertension. Am J Physiol Renal Physiol. 2004;287:F262–F267. doi: 10.1152/ajprenal.00055.2004. [DOI] [PubMed] [Google Scholar]
- 36.Figueroa JP, Rose JC, Massmann GA, Zhang J, Acuña G. Alterations in fetal kidney development and elevations in arterial blood pressure in young adult sheep after clinical doses of antenatal glucocorticoids. Pediatr Res. 2005;58:510–515. doi: 10.1203/01.PDR.0000179410.57947.88. [DOI] [PubMed] [Google Scholar]
- 37.Shaltout HA, Figueroa JP, Rose JC, Diz DI, Chappell MC. Alterations in circulatory and renal angiotensin-converting enzyme and angiotensin-converting enzyme 2 in fetal programmed hypertension. Hypertension. 2009;53:404–408. doi: 10.1161/HYPERTENSIONAHA.108.124339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kantorowicz L, Valego NK, Tang L, Figueroa JP, Chappell MC, Carey LC, Rose JC. Plasma and renal renin concentrations in adult sheep after prenatal betamethasone exposure. Reprod Sci. 2008;15:831–838. doi: 10.1177/1933719108318599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grigore D, Ojeda NB, Robertson EB, Dawson AS, Huffman CA, Bourassa EA, Speth RC, Brosnihan KB, Alexander BT. Placental insufficiency results in temporal alterations in the renin angiotensin system in male hypertensive growth restricted offspring. Am J Physiol Regul Integr Comp Physiol. 2007;293:R804–R811. doi: 10.1152/ajpregu.00725.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]