Abstract
Objective
Withholding insulin for weight control is a dangerous practice among individuals with type 1 diabetes; yet little is known about the factors associated with this behavior. Studies of nondiabetic individuals with weight concerns suggest that eating in a disinhibited manner (e.g., binge eating) predicts the use of maladaptive compensatory strategies (e.g., self-induced vomiting). The purpose of this study was to test whether individuals with type 1 diabetes are less restrained in their eating when they think their blood glucose (BG) is low and whether this contributes to insulin omission for weight control purposes and subsequently higher hemoglobin A1c (HbA1c).
Methods
Two-hundred and seventy-six individuals with type 1 diabetes completed an online survey of eating behaviors, insulin dosing and most recent HbA1c. We used structural equation modeling to test the hypothesis that disinhibited eating when blood sugar is thought to be low predicts weight-related insulin mismanagement, and this, in turn, predicts higher HbA1c.
Results
The majority of participants endorsed some degree of disinhibition when they think their blood glucose is low (e.g., eating foods they do not typically allow) and corresponding negative affect (e.g., guilt/shame). The frequency of disinhibited eating was positively associated with weight-related insulin mismanagement. Controlling for age, sex, education, and insulin pump use, the model explained 31.3% of the variance in weight-related insulin mismanagement and 16.8% of the variance in HbA1c.
Conclusion
Addressing antecedents to disinhibited eating that are unique to type 1 diabetes (e.g., perceived BG level), and/or guilt or shame for unrestrained eating when hypoglycemic may reduce weight-related insulin omission.
Keywords: Insulin Mismanagement, Diabetes, Eating Disorder
Insufficient insulin dosing among individuals with diabetes leads to higher hemoglobin A1c (HbA1c) [1-2], increased hospitalizations [1], early and severe diabetes-related medical complications [3], and a three-fold increase in premature death [4]. Intentional insulin omission is common among 20% of diabetic patients [5], and particularly among young women with type 1 diabetes with prevalence estimates ranging from 30% to 57% [5-10]. Recent studies have indicated that diet non-adherence [5] and dissatisfaction with body weight are associated with the most extreme and purposeful insulin omission [3] among individuals with type 1 diabetes. This is in contrast to individuals with type 2 diabetes, for whom age, income, pain, and the embarrassment of injections have been found to be better predictors [5].
Insulin omission, while exceedingly dangerous, is an effective weight control strategy. Administering insufficient insulin results in excretion of glucose into the urine (i.e., glycosuria) [11-12]. Thus, rather than this sugar being absorbed by muscle and fat cells, it is essentially “purged,” allowing the individual to eat without the impact of additional unwanted calories [13]. While this may seem like a convenient method of weight control, withholding insulin is potentially life-threatening and may lead to diabetic ketoacidosis or cause additional irreparable nerve damage [7, 14]. The prevalence of intentional insulin omission among individuals with type 1 diabetes suggests that there might be unique features of this chronic illness that increase the risk for engaging in maladaptive weight control strategies. One factor may be increased body dissatisfaction among individuals with type 1 diabetes due to the weight gain associated with insulin therapy, combined with a highly effective method for weight loss [15]. However, interventions that effectively decrease body dissatisfaction among individuals with type 1 diabetes fail to improve diabetes management and glycemic control (e.g., HbA1c) [16-18], suggesting the presence of other factors that influence the decision to withhold insulin on a particular day, or for a particular meal or snack. Identifying these factors might allow for the development of sensitive and specific interventions for this patient population.
Among individuals without type 1 diabetes, attempting to restrain eating in a rigid or extreme manner paradoxically increases the risk of disinhibited eating (eating more than one intends to in an uncontrolled way) [19]. This is particularly likely to happen when there is an elimination of foods that are enjoyed. For example, in typical populations, an individual who attempts to adhere to the dietary rule “I must not eat cake” is more likely to overeat when cake is consumed (referred to as the abstinence-violation effect) [20-21]. Of importance, even imagining one will have to restrain eating (e.g., start a diet) in the future results in otherwise healthy individuals eating in a disinhibited manner in anticipation of the deprivation that is to come [22]. Among typical populations, disinhibited eating is associated with guilt and shame, and for some individuals without type 1 diabetes, attempts to reduce the risk of weight gain via self-induced vomiting, excessive exercise or other problematic strategies [23]. Although strategies such as vomiting and intense exercise would be expected to be less common among diabetic patients due to an increased risk for hypoglycemia, breaking dietary restraint in this manner may lead to insulin omission as a way to minimize the impact of excess calories on weight and re-establish a sense of control (by controlling weight).
Individuals with type 1 diabetes must be more cognizant of food choices than their non-diabetic counterparts, and carefully plan food intake and insulin administration to ensure that sufficient insulin is available to meet their body's needs [24-25]. Although flexible eating and insulin dosing is currently recommended (e.g., allowing occasional desserts, but dosing insulin adequately for these desserts) [26-27], the restraints imposed by type 1 diabetes may lead some individuals to approach their condition as a strict diet, adopting rigid dietary rules that leave them feeling deprived (e.g., “no eating between meals,” “no sweets”). At the same time, diabetes might also set up conditions to violate self-imposed dietary rules (e.g., consuming “junk food”) when BG is low or rapidly declining. Although it is necessary to respond to BG decline by consuming fast-acting carbohydrate (and in some situations this may include highly processed foods or desserts), in some cases, this may lead to eating more of these foods than one intends and generate psychological distress (feeling a loss of control or guilt and shame), increasing risk of weight-related insulin omission as a maladaptive compensatory strategy. If this occurred regularly (for example, when BG is only relatively low compared to an individual's average BG) it could have a significant negative impact on HbA1c.
In the current study, we administered an online survey to individuals diagnosed with type 1 diabetes. The survey assessed the experience of eating and insulin dosing and diabetes management. We hypothesized that disinhibited eating when BG is thought to be low and associated psychological sequelae would predict weight-related insulin omission, which in turn would predict higher HbA1c.
Research Design and Methods
Participants and procedure
The sample was drawn from two major medical centers in the Southeastern United States. Participants learned about the study through email announcements and flyers displayed in patient areas. The study was described to participants as an online survey related to eating attitudes and diabetes management for individuals with type 1 diabetes. No other information was provided about the content of the questions in the advertising materials. Participants had to voluntarily access the survey online. Participants provided demographic information and completed questions about their eating behavior, diabetes regimen, history of complications, and most recent HbA1c. They also completed the Diabetes Eating Problems Survey-Revised (DEPS-R)[28]. The survey was conducted from 2010-2012 and delivered using Qualtrics.com®, an online survey platform. All procedures were approved by the respective institutional review boards.
Measures
Disinhibited eating when BG is perceived to be low
There are no established self-report measures that query the experience of eating when BG is thought to be dropping or low. Thus, we developed and administered four individual items; two behavioral and two emotional indicators of disinhibited eating. To assess behavioral indicators of Disinhibited Eating, participants were asked whether they relinquished control over food type and food amount when they think their BG is low: “When you think your blood sugar is low, do you eat foods that you do not normally “allow” yourself to have (e.g., chips, candy, etc.)?” (Allow Foods: Q2); and “When you think your blood glucose is low, do you continue to eat until you feel better, rather than waiting 15 minutes or so between servings to see if your symptoms remit?” (Continue to Eat: Q3). For emotional indicators of disinhibited eating, participants were asked the following: “Do you feel like you lose control over your eating when your blood sugar is low?” (Loss of Control: Q1); and “Does eating in a way that is out of your normal routine, for example, having a snack in between meals, when your blood sugar is low, make you feel guilty, shameful or regretful?” (Guilt/Shame: Q4). Of note, participants were not asked whether they checked their blood sugar at these times, or whether they were below 70 mg/dL. Thus, this construct is best described as disinhibition in response to perceived (rather than actual) low BG.
Item responses were on 6-point Likert scales, ranging either from 0 (Never) to 5 (Always), or from 0 (Not at All) to 5 (Very Much). For each of the four items, if individuals endorsed the presence of the behavior or experience (i.e., responded with a 1 or greater), they were asked the subsequent item assessing the frequency at which this behavior or experience occurred (1=Less than once a month; 6=Daily). If the participant responded with 0 (Never) the subsequent frequency item was not asked. This method shortened the survey administration time for individuals for whom items were not relevant. If this subsequent frequency item was not asked, individuals received a 0 (Never) for the frequency item, rather than it being coded as “missing.” The recoded frequency items of each variable (i.e., Loss of Control: Q1; Allow Foods: Q2; Continue to Eat: Q3; and Guilt/Shame: Q4) were used in subsequent analyses.
Weight-related insulin mismanagement
Although there is not an established measure that specifically assesses weight-related insulin mismanagement, there is a scale that assesses diabetes-specific eating disorder symptomatology (i.e., DEPS-R [28]), which includes relevant items that may function as indicators of this form of insulin omission. The DEPS-R is a self-report measure assessing eating disorder behavior and attitudes over the past four weeks. The DEPS-R consists of 16-items using a 6-point scale ranging from 0 (Never) to 5 (Always) with higher scores indicating greater eating disorder symptomatology. Scores above 20 on the DEPS-R have been associated with greater insulin omission and higher HbA1c among adolescents with type 1 diabetes [28]; and thus may suggest clinically significant eating disorder symptomatology. Five items on the DEPS-R assess manipulation of the diabetic treatment regimen for weight control purposes or related attitudes. We used three of the five items, including: “I try to keep my blood sugar high so that I will lose weight” (Keep BG High: DEPS-R9), “I try to eat to the point of spilling ketones in my urine” (Spill Ketones: DEPS-R10), and “I feel fat when I take all of my insulin” (Feel Fat: DEPS-R11). The remaining two DEPS-R items that assess manipulation of the diabetic treatment regimen for weight control purposes include: “After I overeat, I don't take enough insulin to cover the food” and “After I overeat, I skip my next insulin dose.” These items were not used because they reference overeating which overlaps with the predictor variable of Disinhibited Eating. Including these two items as indicators of insulin omission would thus inappropriately increase the association between the predictor (Disinhibited Eating) and the outcome (Weight-Related Insulin Mismanagement).
Analytic strategy
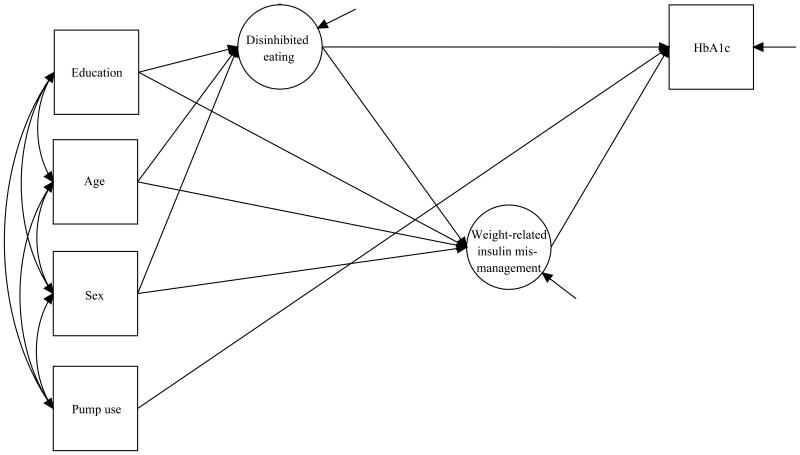
We used structural equations modeling [29] to examine the relationships between Disinhibited Eating, Weight-Related Insulin Mismanagement, and HbA1c (Figure 1). The SEM framework provided several advantages [30-31]. Specification of the measurement part of the model (i.e., the links from each latent variable to its observed indicators), allowed us to use multiple indicators of each construct of interest and to directly model measurement error. Estimation of the structural part of the model allowed us to examine directional associations among latent variables, observed variables, as well as to account for the effects of putative covariates (i.e., age, sex, education, and insulin pump use). Unlike the traditional regression-based approaches, the SEM approach allows for a simultaneous estimation of all hypothesized effects within a given model, thereby preserving the α-level in small to moderate samples. The SEM method also provided us with alternative estimation options that accommodated our items' ordinal response categories and positive skew (see Measurement Model section below). We report model fit indices as appropriate for all analyses, and use the following previously suggested cutoff values for acceptable fit: ≥.95 for Comparative Fit Index (CFI); <.06 for Root Mean Square Error Of Approximation (RMSEA); ≤.08 for Standardized Root Mean Square Residual (SRMR); and <.90 for Weighted Root Mean Square Residual (WRMR) (for review, see [32]). All latent variable modeling was carried out in Mplus (version 7) [33].
Figure 1. Hypothesized relationships among disinhibited eating, weight-related insulin mismanagement and HbA1c.
Measurement model
First, we established and confirmed the reliable assessment of our constructs of interest (i.e., Disinhibited Eating and Weight-Related Insulin Mismanagement) [31]. Four indicators (i.e., Loss of Control: Q1; Allow Foods: Q2; Continue to Eat: Q3; and Guilt/Shame: Q4) comprised the Disinhibited Eating latent variable (see Measures section for descriptions of the four relevant items). We initially used Exploratory Factor Analysis (EFA) – a data-driven approach that identifies unobservable latent variables that account for associations among indicator variables – on a randomly selected half of participants (n=138). To address the positive skew, we employed maximum likelihood with robust standard errors (MLR) – an Mplus parameter estimator that is robust to non-normality in observations. EFA results examining 1 through 4 factors showed that the one-factor solution had an eigenvalue of 2.57, and was the only solution with an eigenvalue above 1. Model fit indices showed that the model provided excellent fit to these data: χ2 (2, N=138)=2.122, p=.967; CFI=.999; RMSEA=.021 (90% CI [0.000, 0.171]); SRMR=.017. All four factor loadings exceeded the threshold of .32, exhibiting the following factor loadings: 0.988 (SE=.033) for “Loss of Control: Q1;” 0.566 (SE=0.078) for “Allow Foods: Q2;” 0.778 (SE=0.045) for “Continue to Eat: Q3;” and 0.564 (SE=0.066) for “Guilt/Shame: Q4.” Results using the modindices command showed no residual correlations among indicator variables above minimum value of 3.8. Next, we ran a Confirmatory Factor Analysis (CFA) on the second half of our sample (n=138), in order to formally test whether the one-factor model fit these data well. Factor variance was set to 1 to allow estimation of factor loadings, item intercepts, and residual variances. Three participants had missing values on all indicator variables, and were excluded from analysis. Results supported the hypothesis that the unidimensional model fit these data well, showing excellent fit indices: χ2 (2, N=135)=0.940, p=.625; CFI=1.000; RMSEA<.001 (90% CI [0.00, 0.137]); SRMR=.014. All standardized loadings for the Disinhibited Eating factor were statistically significant, ranged from 0.652 to .810, and ranged from .425 to .657 in the amount of item variance accounted for by the Disinhibited Eating factor. As a final step, we computed a reliability coefficient of the 4-item Disinhibited Eating measure on the full sample, using a latent variable approach to assess measurement reliability [34-35]. The measure exhibited good reliability, with the coefficient estimated at 0.810 (SE=0.020; 95% CI [0.770, 0.849]).
The availability of only three indicators of Weight-Related Insulin Mismanagement (see Measures section for descriptions of the four relevant items) precluded us from carrying out the split sample strategy utilized to derive the Disinhibited Eating latent variable, as well as alternative strategies, such as multiple group analysis. With three indicators of Weight-Related Insulin Mismanagement, the model was just-identified, and did not allow for standard evaluation of model fit statistics. Thus, we settled on an EFA approach in establishing the Weight-Related Insulin Mismanagement latent variable. Due to the high positive skew of these variables, we recoded the ordinal responses into binary responses, and chose an estimator that is appropriate for categorical data analysis – the weighted least squares mean and variance-adjusted estimator (WLSMV). Additionally, WLSMV estimator accommodated missing data while applying more stringent assumptions than the default full information maximum likelihood [36], provided good parameter and chi-square estimates [37-39], and performed well in small to moderate sample sizes [40-41]. EFA results on the full sample showed that the eigenvalue for the 1-factor solution was 2.625, exceeding the suggested cut-off value of 1.0. The factor loadings were 0.965 (SE=.060) for DEPS-R9; 0.946 (SE=0.101) for DEPS-R10; and 0.796 (SE=0.060) for DEPS-R11. The modindices command showed that there were no residual correlations among indicator variables above the minimum value of 3.8.
Structural equations model
We used the SEM framework to assess the fit of the hypothesized model (Figure 1), where disinhibited eating (latent variable) predicts weight-related insulin mismanagement (latent variable) and HbA1c; and weight-related insulin mismanagement (latent variable) predicts HbA1c. WLSMV estimator was used to accommodate binary- and ordinal-scale observed variables. Error variances were freely estimated and set to be uncorrelated. Given previous work that has shown that insulin pump therapy is related to HbA1c level [42], that age, sex, and education are associated with eating behaviors [43-44], and insulin therapy adherence [45-46], we included insulin pump use, age, sex, and education as covariates. As a final step, we examined the significance test of the total, direct and indirect effects, to report whether Weight-Related Insulin Mismanagement is a significant mediator of the relationship between Disinhibited Eating and HbA1c.
Supplementary analyses
We conducted secondary analyses to more fully elucidate the Disinhibited Eating construct. We first investigated whether individual items of the Disinhibited Eating construct may point to distinct underlying mechanisms whereby particular aspects of disinhibited eating (e.g., losing control over eating when one's BG is low) may be differentially linked with insulin management and HbA1c. Thus, we ran four supplementary SEM models where the Disinhibited Eating construct was substituted with each of the four individual items, to examine if the results are substantially different from those with the Disinhibited Eating latent variable. Second, we examined whether individual differences in Disinhibited Eating items were associated with clinically significant eating disorder symptomatology.
Results
Sample characteristics
Two-hundred seventy-six individuals participated in the study (Table 1). Participants' ages ranged from 18 to 76 with a mean of 43.5 years (SD=13.71). Most of the sample was female (68.5%, n=189), Caucasian (89.5%; n=247), and had completed at least some college or technical school (96.4%). The majority of participants reported childhood onset of type 1 diabetes, with 63 individuals diagnosed at or before the age of 10, and 74 diagnosed during pre-teen to teenage years. Over half of the participants reported receiving insulin pump therapy (69.6%), the rest were either on multiple injections daily, or a single standard daily dosing with additional injections as needed. Self-reported HbA1c ranged from 4.9 to 15.0% (30 to 140 mmol/mol), with 139 individuals above the recommended 7% (53 mmol/mol) and 46 above 8% (64 mmol/mol). Eighty-two participants (29.7%) reported that they had been told that their diabetes was poorly controlled. Complications were reported by 49.3% (n=136) of the sample, with the most common being diabetic retinopathy, diabetic neuropathy, and diabetic ketoacidosis. Twenty-two percent of the current sample of adults with type 1 diabetes scored above 20 on the DEPS-R (M=15.20, SD=9.50) and scores were significantly correlated with patients' self-reported HbA1c (M=7.34, SD=1.27), Pearson r correlation coefficient of .44 (p<.001).
Table 1. Demographic characteristics of participants (N=276).
| Characteristic | M (SD) or Percent | Missing (%) |
|---|---|---|
| Age (yrs) | 43.5 (13.7) | 0.0 |
| Gender (% female) | 68.5 | 0.0 |
| Race/Ethnicity | 0.4 | |
| Caucasian (%) | 89.5 | |
| Non-Hispanic Black (%) | 8.0 | |
| Hispanic (%) | 1.1 | |
| Other (%) | 1.1 | |
| Marital Status | 0.4 | |
| Never Married (%) | 23.9 | |
| Married (%) | 64.9 | |
| Separated/divorced (%) | 9.4 | |
| Widowed (%) | 1.4 | |
| Education | 0.4 | |
| High school/GED (%) | 3.3 | |
| Some college (or technical school) (%) | 24.6 | |
| College graduate (%) | 42.8 | |
| Graduate degree (%) | 29.0 | |
| Diabetes Complications (%) | 49.3 | 0.0 |
| Age of diagnosis | 20.9 (13.5) | 2.5 |
| Poorly controlled or uncontrolled diabetes (%) | 29.8 | 0.4 |
| Insulin pump therapy (%) | 69.6 | 0.0 |
| HbA1c | 7.3 (1.3) | 9.4 |
Eating experience when BG is low
Most participants (88%) reported that when they thought their blood sugar was low, they continued to eat until they felt better rather than waiting 15 minutes or so between servings to see if symptoms would remit at least once in a while, with 45.3% of these individuals doing so at least 2-3 times a month. A significant portion of participants (20%) indicated that they engaged in this behavior at least weekly indicating this is a fairly regular behavior for many individuals. Similarly, the majority of participants (87.7%) reported that they eat foods that they would not normally permit themselves to have (e.g., candy) when they think their blood sugar is low at least once in a while, with 52.9% of these individuals doing so at least 2-3 times a month. Many participants (27.5%) reported that this occurs at least weekly.
Over 40% of the sample indicated that they feel as though they lose control over their eating when their BG is low [reporting a 3 or greater on a scale from 0 (Not at All) to 5 (Very Much)]. Thirty-five percent of individuals indicated that they felt like they lost control over their eating in the context of low blood sugar at least 2-3 times a month. A smaller, yet substantial, portion of the sample (16%) reported feeling guilty, shameful, or regretful when BG required that they break their normal routine of meals and snacks [reporting at least a 3 on a scale from 0 (Not at All) to 5 (Very Much)]. For some individuals (21%), this was a fairly frequent experience, occurring at least several times a month.
Structural equations modeling
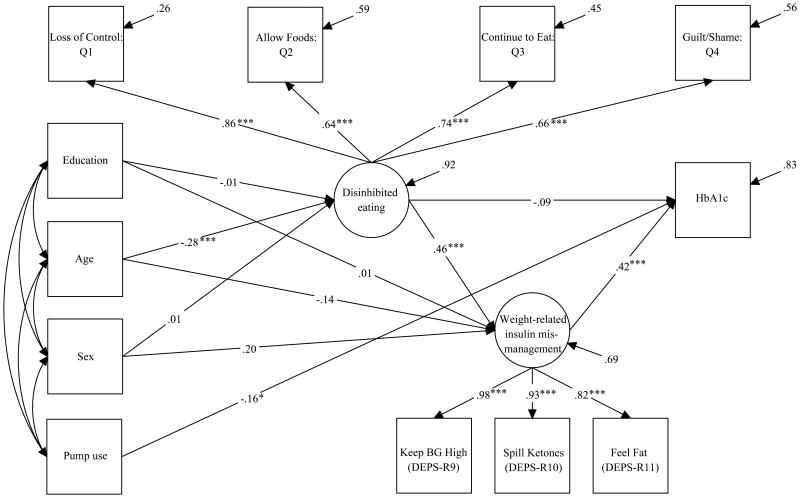
Model fit indices exhibited excellent fit, showing that the hypothesized model fit these data well (χ2(37, N=275)= 61.588, p=.033; CFI=.963; RMSEA=.040 (90% CI [0.012, 0.061]); WRMR=.784). Table 2 and Figure 2 show estimated model parameters. There was a significant effect of Disinhibited Eating on Weight-Related Insulin Mismanagement (B=0.528, SE=0.098, p<.001, 95% CI [0.337, 0.719], β=0.457). There was a significant effect of Weight-Related Insulin Mismanagement on HbA1c (B= 0.432, SE= 0.087, p<.001, 95% CI [0.262, 0.602], β= 0.417). Disinhibited Eating did not significantly predict HbA1c (B=-0.105, SE= 0.109, p=.339, 95% CI [-0.318, 0.108], β= -0.088). The model explained 31.3% of the variance in the Weight-Related Insulin Mismanagement latent variable and 16.8% of the variance in HbA1c. Older age was negatively associated with Disinhibited Eating (B=-0.022, SE=0.005, p<.001, 95% CI [-0.031, -0.012], β= -0.136), and pump use was negatively associated with HbA1c (B= -0.429, SE= 0.182, p <.05, 95% CI [-0.785, -0.072], β= -0.158).
Table 2. Disinhibited Eating, Weight-Related Insulin Mismanagement and HbA1c.
| Parameter Estimate | Unstandardized (SE) | Standardized |
|---|---|---|
| Covariates | ||
| Age (yrs, centered at 43yrs) → Disinhibited Eating | -0.022*** (0.005) | -0.285 |
| Sex (0=Male) → Disinhibited Eating | -0.019 (0.148) | 0.008 |
| Education (0=Some college or less) → Disinhibited Eating | -0.021 (0.147) | -0.009 |
| Age (yrs, centered at 43yrs) → Weight-Related Insulin Mismanagement | -0.012 (0.009) | -0.136 |
| Sex (0=Male) → Weight-Related Insulin Mismanagement | 0.510 (0.285) | 0.197 |
| Education (0=Some college or less) → Weight-Related Insulin Mismanagement | 0.028 (0.242) | 0.010 |
| Pump use (0=No) → HbA1c | -0.429* (0.182) | -0.158 |
| Measurement Model Estimates | ||
| Disinhibited Eating → “Loss of Control: Q1” | 1.164*** (0.104) | 0.861 |
| Disinhibited Eating → “Allow Foods: Q2” | 0.949*** (0.112) | 0.639 |
| Disinhibited Eating → “Continue to Eat: Q3” | 1.013*** (0.095) | 0.743 |
| Disinhibited Eating → “Guilt/Shame: Q4” | 0.991*** (0.140) | 0.661 |
| Weight-Related Insulin Mismanagement → DEPS-R9 | 0.868*** (0.057) | 0.984 |
| Weight-Related Insulin Mismanagement → DEPS-R10 | 0.817*** (0.080) | 0.932 |
| Weight-Related Insulin Mismanagement → DEPS-R11 | 0.709*** (0.059) | 0.819 |
| Structural Model Estimates | ||
| Disinhibited Eating → Weight-Related Insulin Mismanagement | 0.528*** (0.098) | 0.457 |
| Weight-Related Insulin Mismanagement → HbA1c | 0.432*** (0.087) | 0.417 |
| Disinhibited Eating → HbA1c | -0.105 (0.109) | -0.088 |
| Residual Variances | ||
| Residual variance in “Loss of Control: Q1” | 0.517*** (0.132) | 0.259 |
| Residual variance in “Allow Foods: Q2” | 1.419*** (0.160) | 0.591 |
| Residual variance in “Continue to Eat: Q3” | 0.907*** (0.110) | 0.448 |
| Residual variance in “Guilt/Shame: Q4” | 1.381*** (0.129) | 0.563 |
| Residual variance in Weight-Related Insulin Mismanagement | 1.000 | 0.687 |
| Residual variance in HbA1c | 1.301*** (0.103) | 0.832 |
Notes:
p<.05.
p < .001.
Loss of Control: Q1 = Frequency at which participants reported this experience occurred: “Do you feel like you lose control over your eating when your blood sugar is low?”; Allow Foods: Q2 = Frequency at which participants reported this behavior occurred: “When you think your blood sugar is low, how often do you eat foods that you do not normally ‘allow’ yourself to have (e.g., chips, candy etc)”; Continue to Eat: Q3 = Frequency at which participants reported this behavior occurred: When you think your blood glucose is low, how often do you continue to eat until you feel better, rather than waiting 15 minutes or so between servings to see if your symptoms remit?”; Guilt/Shame: Q4 = Frequency at which participants reported this experience occurred: “Does eating in a way that is out of your normal routine, for example, having a snack in between meals, when your blood sugar is low, make you feel guilty, shameful or regretful?”; Keep BG High (DEPS-R9) = Diabetes Eating Problems Survey-Revised item 9: “I try to keep my blood sugar high so that I will lose weight,”; Spill Ketones (DEPS-R10) = Diabetes Eating Problems Survey-Revised item 10: “I try to eat to the point of spilling ketones in my urine”; Feel Fat (DEPS-R11) = Diabetes Eating Problems Survey-Revised item 11: “I feel fat when I take all of my insulin.”
Figure 2. Structural equations model and estimated model parameters (standardized).
Notes: *p<.05. ***p < .001. Loss of Control: Q1 = Frequency at which participants reported this experience occurred: “Do you feel like you lose control over your eating when your blood sugar is low?”; Allow Foods: Q2 = Frequency at which participants reported this behavior occurred: “When you think your blood sugar is low, how often do you eat foods that you do not normally ‘allow’ yourself to have (e.g., chips, candy etc)”; Continue to Eat: Q3 = Frequency at which participants reported this behavior occurred: When you think your blood glucose is low, how often do you continue to eat until you feel better, rather than waiting 15 minutes or so between servings to see if your symptoms remit?”; Guilt/Shame: Q4 = Frequency at which participants reported this experience occurred: “Does eating in a way that is out of your normal routine, for example, having a snack in between meals, when your blood sugar is low, make you feel guilty, shameful or regretful?”; Keep BG High (DEPS-R9) = Diabetes Eating Problems Survey-Revised item 9: “I try to keep my blood sugar high so that I will lose weight,”; Spill Ketones (DEPS-R10) = Diabetes Eating Problems Survey-Revised item 10: “I try to eat to the point of spilling ketones in my urine”; Feel Fat (DEPS-R11) = Diabetes Eating Problems Survey-Revised item 11: “I feel fat when I take all of my insulin.”
We observed that Weight-Related Insulin Mismanagement mediated the relation between Disinhibited Eating and HbA1c. First, greater Disinhibited Eating was associated with greater Weight-Related Insulin Mismanagement (B= 0.528, SE= 0.098, p <0.001, 95% CI [-0.337, 0.719], β= 0.457), and greater Weight-Related Insulin Mismanagement was linked with higher HbA1c (B= 0.432, SE= 0.087, p<0.001, 95% CI [0.262, 0.602], β= 0.417). We observed a nonsignificant positive effect of Disinhibited Eating on HbA1c (i.e., the total effect; B=0.123, SE=0.077, p=0.111, β=.103). After accounting for the effect of Weight-Related Insulin Mismanagement, the coefficient of Disinhibited Eating on HbA1c (i.e., the direct effect) remained nonsignificant, but became negative (B=-0.105, SE=0.109, p=0332, 95% CI [-0.318, 0.108], β=-.088). Finally, there was formal evidence of mediation, with a statistically significant indirect effect of Disinhibited Eating on HbA1c through Weight-Related Insulin Mismanagement (B=0.228, SE=0.070, p=.001, β=191).
Secondary analyses
We next examined whether one or more of the Disinhibited Eating indicators were differentially linked to covariates, Weight-Related Insulin Mismanagement, or HbA1c. In order to do so, we fit four additional exploratory models where we substituted each individual Disinhibited Eating indicator for the latent variable. As shown in Supplementary Tables S1-S4, the relations among each of the Disinhibited Eating indicators with other variables in the model were slightly attenuated, but were not substantially different from those observed when Disinhibited Eating was modeled as an exogenous latent variable.
The majority of our sample endorsed eating in an unrestrained manner when they think their BG is low and feeling badly about it at least once in a while. Given this, we were curious as to whether this could be considered normative among individuals with type 1 diabetes, or if disinhibited eating experiences occur more frequently among individuals who also have clinically significant eating disorder symptomatology. To test this, we divided our sample into two groups – individuals with DEPS-R scores of at least 20 or above (n=67), and those with DEPS scores below 20 (n=206) – and then examined group differences on each of the four Disinhibited Eating indicators. Results of independent t-tests indicated that when compared to individuals with DEPS-R scores below 20, individuals with DEPS-R scores of 20 or greater reported significantly greater frequency of all four Disinhibited Eating behaviors. Specifically, participants in the high DEPS-R group reported engaging in the following behaviors more frequently: eating foods they do not typically allow when they think their BG is low (t(269) = -4.26, p< .001), continuing to eat rather than waiting for symptoms to remit (t(266) = -5.30, p<.001), feeling a loss of control over eating (t(267) = -7.16, p< .001), and experiencing guilt, shame and regret for breaking routine (t(265) = -7.10, p<.001).
Discussion
Intentional insulin omission is a serious problem for diabetes self-care, yet little is known about the factors associated with this dangerous behavior. Although a few studies have indicated weight dissatisfaction is an important variable associated with insulin omission [47], weight concerns are also common among both the general population and among patients with type 1 diabetes who do not omit insulin [47-49]. Thus, this construct offers an incomplete explanation for intentional insulin omission. The current study tested one proposed pathway to weight-related insulin mismanagement: uncontrolled eating when BG is thought to be low which leads to withholding insulin to re-establish control and make up for calories consumed.
Ninety-eight percent of our sample reported that they eat in a disinhibited manner when they think their BG is low and feel badly about it at least once in a while. The frequency of disinhibited eating was associated with indicators of weight-related insulin omission. Weight-related insulin mismanagement, in turn, predicted higher HbA1c. Although omitting insulin when one is eating to circumvent or address actual low BG is appropriate, the current study suggests that eating in a disinhibited manner increases the likelihood of omitting for weight purposes; an inappropriate adaptation of insulin regimen.
Eating more freely when experiencing low blood sugar is adaptive and in part physiologically driven [50-51]. However, this study suggests that when it is experienced as disinhibition—with a loss of control, feelings of guilt, shame and regret, this may impact an individual's ability to healthfully manage their diabetes. As can be seen in the supplementary tables, guilt and shame accounted for the greatest variance in weight-related insulin omission; followed by the feeling of a loss of control. Thus, the actual eating behaviors (such as eating foods that are not typically included in one's diet), while greater among those with clinically significant disordered eating (DEPS-R > 20), is somewhat less important than how one experiences their eating behavior; as uncontrolled and as something for which to feel guilty or shameful.
The current study does not differentiate between eating in a disinhibited manner during actual hypoglycemia from eating in a disinhibited manner during perceived hypoglycemia or when BG is only relatively low or declining within normal range. As noted earlier, participants were not asked whether they check their BG at the times when they eat in a disinhibited manner. Symptoms of hypoglycemia may be experienced at higher levels (above 70mg/dL) among individual who are chronically hyperglycemic [52]. If eating in a disinhibited manner is also occurring when BG is declining or only relatively low (i.e., low compared to the individual's typical BG range), then this could have significant implications. Specifically, it could increase the frequency of withholding insulin and further negatively impact glycemic control.
In the current study, insulin mismanagement mediated the relation between disinhibited eating and HbA1c, such that the experience of disinhibited eating was associated with greater insulin mismanagement, which was, in turn, linked with higher HbA1c levels. We did not observe total or direct effects of disinhibited eating on HbA1c. Recently reviewed work evaluating detection of mediation in behavioral sciences found significant total or direct effects are not necessary to observe mediation [53-55]. In fact, a lack of significant total and direct effects is not unexpected when examining the mediators of distal outcomes [54]. Future work should investigate the impacts of disinhibited eating and insulin management on more proximal indicators of blood sugar levels (e.g., interstitial glucose).
Findings from the current study should be taken in light of its limitations. Importantly, the current study was cross-sectional. Thus, directionality of associations cannot be assumed and the study thus precludes causal inferences. Relevant variables were also assessed by self-report, and factors such as appetite (which is impacted by insulin administration) and medical complications were not assessed or controlled in the analyses. Future studies should assess eating, insulin administration, and glucose levels using ecological momentary assessment methods that allow for experience sampling in real time, include hunger ratings, and employ laboratory-based tests of HbA1c. Such methodology would be a stronger test of these associations, addressing possible confounds such as memory or reporting biases in self-report, and the impact of hunger on eating behavior or insulin omission.
It may be noted that while the items that assessed the behavioral indicators of disinhibited eating explicitly specified perceived low BG (“Do you eat foods you typically do not allow when you think your BG is low?); the emotional indicators did not (“Do you lose control over your eating when your BG is low?”). However, empirically, these items were found to be non-distinguishable, forming a single factor with good reliability. Additional research employing ecological momentary assessment methods may further specify the BG parameters (e.g., level, rate of decline) individuals with type 1 diabetes may respond to with disinhibited eating.
Importantly, it could be the case that individuals who report disinhibited eating when BG is low are alike in some other way not identified here (e.g., perhaps they eat in a disinhibited manner at other times as well or in response to feelings of anxiety which mimic hypoglycemia). Thus, it may be that some other variable is equally or even more important for weight-related insulin omission. Additional research should test alternative models, as well as include independent measures of weight concerns and glycemic control.
The current study also utilized a moderate sample size; and while less than ideal, the sample size is sufficient given the number of tested pathways [56]. The sample has limitations in generalizability, with a greater proportion of Caucasian, higher education and insulin pump users. Validating the construct scales and outcomes in samples that are diverse in ethnic and socioeconomic status compositions would further solidify study findings.
To date, there are no interventions that have demonstrated effectiveness in improving HbA1c among individuals with type 1 diabetes who omit insulin for weight purposes or have a comorbid eating disorder. The current study suggests intervening upon: 1) antecedents to disinhibited eating, including unhelpful attempts to maintain tight dietary control and maladaptive reactivity to BG decline, and 2) how individuals respond emotionally to uncontrolled eating, may decrease risk of insulin mismanagement. If the study results are replicated, incorporating these novel targets could have a significant impact on clinical practice and improve outcome for the patients with type 1 diabetes most at risk for complications associated with chronic hyperglycemia.
Supplementary Material
Highlights.
Disinhibited eating when blood sugar is thought to be low was common in type 1 diabetes participants
The frequency of disinhibited eating predicted weight-related insulin omission
Weight-related insulin omission was a significant predictor of metabolic control
Disinhibited eating when one thinks their blood sugar is low may be an important therapeutic target
Acknowledgments
The authors wish to thank James Lane, PhD and Mark Feinglos, MD (Duke University Medical Center) for their edits and review; and John Buse, MD, PhD and Michelle Duclos, MPH, CCRC (University of North Carolina School of Medicine) for their assistance with recruitment.
Sources of Funding: Investigators received salary support from the National Institute of Diabetes and Digestive and Kidney Diseases grant (R01 DK089329; Merwin) and National Institute on Aging (5T32 AG00029-35; Cohen).
Author note: This research was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK089329; Merwin).
Footnotes
Conflicts of Interest: No conflicts of interest were declared.
Contributions: Rhonda M. Merwin, PhD, of Duke University Medical Center (DUMC), developed the idea, led the study team, and wrote the majority of the manuscript. Dr. Rhonda M. Merwin is the guarantor of this work. She had full access to all study data and takes responsibility for the integrity of the data and the accuracy of the data analysis. Other author contributions are as follows: Ashley A. Moskovich, MA, of Duke University, participated in the development of the idea, assisted with compiling the data and participated in the writing of the manuscript. Natalia O. Dmitrieva, PhD, of DUMC, performed the data analysis and participated in the writing of the manuscript. Carl F. Pieper, DPH, consulted and assisted in the preparation of the data analysis plan and results. Lisa K. Honeycutt, MA, of DUMC, participated in development of the idea and assisted with data gathering. Nancy L. Zucker, PhD, of Duke University and DUMC, participated in development of the idea and editorial review. Richard S. Surwit, PhD, of Duke University and DUMC, participated in development of the idea and editorial review. Lori Buhi, BS, of Duke University, participated in development of the idea and analytic strategy. No conflicts of interest exist for any of the study's authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Morris AD, et al. Adherence to insulin treatment, glycaemic control, and ketoacidosis in insulin-dependent diabetes mellitus. The Lancet. 1997;350(9090):1505–1510. doi: 10.1016/s0140-6736(97)06234-x. [DOI] [PubMed] [Google Scholar]
- 2.Zisser H, Rivera SC, Lane J. Zolpidem-induced sleep-eating resulting in significant hyperglycemia in a subject with type 1 diabetes discovered via continuous glucose monitoring. Clinical Diabetes. 2013;31(3):133–135. [Google Scholar]
- 3.Takii M, et al. The duration of severe insulin omission is the factor most closely associated with the microvascular complications of type 1 diabetic females with clinical eating disorders. International Journal of Eating Disorders. 2008;41(3):259–264. doi: 10.1002/eat.20498. [DOI] [PubMed] [Google Scholar]
- 4.Goebel-Fabbri AE, et al. Insulin restriction and associated morbidity and mortality in women with type 1 diabetes. Diabetes Care. 2008;31(3):415–419. doi: 10.2337/dc07-2026. [DOI] [PubMed] [Google Scholar]
- 5.Peyrot M, et al. Correlates of insulin injection omission. Diabetes Care. 2010;33(2):240–245. doi: 10.2337/dc09-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schober E, et al. Prevalence of intentional under- and overdosing of insulin in children and adolescents with type 1 diabetes. Pediatric Diabetes. 2011;12(7):627–631. doi: 10.1111/j.1399-5448.2011.00759.x. [DOI] [PubMed] [Google Scholar]
- 7.Goebel-Fabbri AE, Fikkan J, Franko DL, Pearson K, Anderson BJ, Weinger K. Insulin restriction and associated morbidity and mortality in women with type 1 diabetes. Diabetes Care. 2008;31(3):415–419. doi: 10.2337/dc07-2026. [DOI] [PubMed] [Google Scholar]
- 8.Young-Hyman DL, Davis CL. Disordered eating behavior in individuals with diabetes importance of context, evaluation, and classification. Diabetes Care. 2010;33(3):683–689. doi: 10.2337/dc08-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nielsen S. Eating disorders in females with type 1 diabetes: An update of a meta-analysis. European Eating Disorders Review. 2002;10:251–254. [Google Scholar]
- 10.Jones J, et al. Eating disorders in adolescent females with and without type 1 diabetes: Cross sectional study. British Medical Journal. 2000;320:1563–1566. [PMC free article] [PubMed] [Google Scholar]
- 11.Consultation, W. Definition, diagnosis and classification of diabetes mellitus and its complications. 1999;1 Part. [Google Scholar]
- 12.Forman BH, Goldstein PS, Genel M. Management of juvenile diabetes mellitus: usefulness of 24-hour fractional quantitative urine glucose. Pediatrics. 1974;53(2):257–263. [PubMed] [Google Scholar]
- 13.Kelly SD, et al. Disordered eating behaviors in youth with type 1 diabetes. The Diabetes Educator. 2005;31(4):572–583. doi: 10.1177/0145721705279049. [DOI] [PubMed] [Google Scholar]
- 14.Pinhas-Hamiel O, et al. Detecting intentional insulin omission for weight loss in girls with type 1 diabetes mellitus. International Journal of Eating Disorders. 2013;46:819–825. doi: 10.1002/eat.22138. [DOI] [PubMed] [Google Scholar]
- 15.Goebel-Fabbri AE, et al. Improvement and emergence of insulin restriction in women with type 1 diabetes. Diabetes Care. 2011;34(3):545–550. doi: 10.2337/dc10-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goebel-Fabbri AE. Disturbed eating behaviors and eating disorders in type 1 diabetes: Clinical significance and treatment recommendations. Current Diabetes Reports. 2009;9:133–139. doi: 10.1007/s11892-009-0023-8. [DOI] [PubMed] [Google Scholar]
- 17.Rodin G, et al. Eating disorders in young women with type 1 diabetes mellitus. Journal of Psychosomatic Research. 2002;53(4):943–949. doi: 10.1016/s0022-3999(02)00305-7. [DOI] [PubMed] [Google Scholar]
- 18.Olmsted MP, et al. The effects of psychoeducation on disturbed eating attitudes and behavior in young women with type 1 diabetes mellitus. International Journal of Eating Disorders. 2002;32(2):230–239. doi: 10.1002/eat.10068. [DOI] [PubMed] [Google Scholar]
- 19.Polivy J, Herman CP. Dieting and binging: a causal analysis. American Psychologist. 1985;40(2):193. doi: 10.1037//0003-066x.40.2.193. [DOI] [PubMed] [Google Scholar]
- 20.Grilo C, Shiffman S. Longitudinal investigation of the abstinence violation effect in binge eaters. Journal of Consulting and Clinical Psychology. 1994;62(3):611–619. doi: 10.1037//0022-006x.62.3.611. [DOI] [PubMed] [Google Scholar]
- 21.Collins SE, Witkiewitz K. Encyclopedia of Behavioral Medicine. Springer; 2013. Abstinence violation effect; pp. 8–9. [Google Scholar]
- 22.Urbszat D, Herman CP, Polivy J. Eat, drink, and be merry, for tomorrow we diet: Effects of anticipated deprivation on food intake in restrained and unrestrained eaters. Journal of Abnormal Psychology. 2002;111(2):396. doi: 10.1037//0021-843x.111.2.396. [DOI] [PubMed] [Google Scholar]
- 23.Stice E, et al. Negative affect moderate the relation between dieting and binge eating. International Journal of Eating Disorders. 2000;27(218-229) doi: 10.1002/(sici)1098-108x(200003)27:2<218::aid-eat10>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 24.Kovacs M, et al. Prevalence and predictors of pervasive noncompliance with medical treatment among youths with insulin-dependent diabetes mellitus. Journal of the American Academy of Child & Adolescent Psychiatry. 1992;31(6):1112–1119. doi: 10.1097/00004583-199211000-00020. [DOI] [PubMed] [Google Scholar]
- 25.Nathan DM. Long-term complications of diabetes mellitus. New England Journal of Medicine. 1993;328(23):1676–1685. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- 26.Group DS. Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ: British medical journal. 2002;325(7367):746. doi: 10.1136/bmj.325.7367.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Association AD. Standards of medical care in diabetes—2011. Diabetes Care. 2011;34(Supplement_1):S11. [Google Scholar]
- 28.Markowitz J, et al. Brief screening tool for disordered eating in diabetes. Internal consistency and external validity in a contemporary sample of pediatric patients with type 1 diabetes. Diabetes Care. 2010;33(495-500) doi: 10.2337/dc09-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stein RI, et al. What's driving the binge in binge eating disorder?: A prospective examination of precursors and consequences. International Journal of Eating Disorders. 2007;40(3):195–203. doi: 10.1002/eat.20352. [DOI] [PubMed] [Google Scholar]
- 30.Bollen K. Structural Equations with Latent Variables. New York: John Wiley & Sons; 1989. [Google Scholar]
- 31.Byrne BM. Structural equation modeling with Mplus: Basic concepts, applications, and programming. Routledge Academic; New York: 2011. [Google Scholar]
- 32.Schreiber JB, et al. Reporting structural equation modeling and confirmatory factor analysis results: A review. The Journal of Educational Research. 2006;99(6):323–338. [Google Scholar]
- 33.Muthén LK, Muthén BO. Mplus User's Guide. Los Angeles, CA: Muthén & Muthén; 2007. [Google Scholar]
- 34.Bollen K, Lennox R. Conventional wisdom on measurement: A structural equation perspective. Psychological bulletin. 1991;110(2):305. [Google Scholar]
- 35.Raykov T. Evaluation of scale reliability for unidimensional measures using latent variable modeling. Measurement and Evaluation in Counseling and Development. 2009;42(3):223–232. [Google Scholar]
- 36.Asparouhov T, Muthén B. Weighted least squares estimation with missing data. Mplus Technical Appendix. 2010:1–10. [Google Scholar]
- 37.Flora DB, Curran PJ. An empirical evaluation of alternative methods of estimation for confirmatory factor analysis with ordinal data. Psychological methods. 2004;9(4):466. doi: 10.1037/1082-989X.9.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muthén B. A general structural equation model with dichotomous, ordered categorical, and continuous latent variable indicators. Psychometrika. 1984;49(1):115–132. [Google Scholar]
- 39.Muthén B, du Toit SH, Spisic D. Robust inference using weighted least squares and quadratic estimating equations in latent variable modeling with categorical and continuous outcomes. Psychometrika. 1997;75 [Google Scholar]
- 40.Nussbeck FW, Eid M, Lischetzke T. Analysing multitrait–multimethod data with structural equation models for ordinal variables applying the WLSMV estimator: What sample size is needed for valid results? British Journal of Mathematical and Statistical Psychology. 2006;59(1):195–213. doi: 10.1348/000711005X67490. [DOI] [PubMed] [Google Scholar]
- 41.Rhemtulla M, Brosseau-Liard PÉ, Savalei V. When can categorical variables be treated as continuous? A comparison of robust continuous and categorical SEM estimation methods under suboptimal conditions. Psychological methods. 2012;17(3):354. doi: 10.1037/a0029315. [DOI] [PubMed] [Google Scholar]
- 42.Pickup J, Mattock M, Kerry S. Glycaemic control with continuous subcutaneous insulin infusion compared with intensive insulin injections in patients with type 1 diabetes: meta-analysis of randomised controlled trials. BMJ: British Medical Journal. 2002;324(7339):705. doi: 10.1136/bmj.324.7339.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoek HW, Van Hoeken D. Review of the prevalence and incidence of eating disorders. International Journal of eating disorders. 2003;34(4):383–396. doi: 10.1002/eat.10222. [DOI] [PubMed] [Google Scholar]
- 44.Davis C, et al. Immediate pleasures and future consequences. A neuropsychological study of binge eating and obesity. Appetite. 2010;54(1):208–213. doi: 10.1016/j.appet.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 45.Peyrot M, et al. Correlates of insulin injection omission. Diabetes Care. 2010;33:240–245. doi: 10.2337/dc09-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bryden KS, et al. Eating habits, body weight, and insulin misuse. A longitudinal study of teenagers and young adults with type 1 diabetes. Diabetes Care. 1999;22(12):1956–1960. doi: 10.2337/diacare.22.12.1956. [DOI] [PubMed] [Google Scholar]
- 47.Khan Y, Montgomery A. Eating attitudes in young females with diabetes: Insulin omission identifies a vulnerable subgroup. British journal of medical psychology. 1996;69(4):343–353. doi: 10.1111/j.2044-8341.1996.tb01877.x. [DOI] [PubMed] [Google Scholar]
- 48.Cash T, Henry P. Women's body images: The results of a national survey in the U.S.A. Sex Roles. 1995;33(1-2):19–28. [Google Scholar]
- 49.Neumark-Sztainer D, Patterson J, Mellin A, Ackard DM, Utter J, Story M, Sockalosky J. Weight control practices and disordered eating behaviors among adolescent females and males with type 1 diabetes: associations with sociodemographics, weight concerns, familial factors, and metabolic outcomes. Diabetes Care. 2002;25(8):1289–1296. doi: 10.2337/diacare.25.8.1289. [DOI] [PubMed] [Google Scholar]
- 50.Campfield LA, et al. Human eating: evidence for a physiological basis using a modified paradigm. Neuroscience & Biobehavioral Reviews. 1996;20(1):133–137. doi: 10.1016/0149-7634(95)00043-e. [DOI] [PubMed] [Google Scholar]
- 51.Ciampolini M, B R. Training to estimate blood glucose and to form associations with initial hunger. Nutrition & Metabolism. 2006;3(42) doi: 10.1186/1743-7075-3-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gjedde A, Crone C. Blood-brain glucose transfer: repression in chronic hyperglycemia. Science. 1981;214(4519):456–457. doi: 10.1126/science.7027439. [DOI] [PubMed] [Google Scholar]
- 53.Rucker DD, et al. Mediation analysis in social psychology: Current practices and new recommendations. Social and Personality Psychology Compass. 2011;5(6):359–371. [Google Scholar]
- 54.Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychological methods. 2002;7(4):422. [PubMed] [Google Scholar]
- 55.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annual review of psychology. 2007;58:593. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bentler PM, Chou CP. Practical issues in structural modeling. Sociological Methods & Research. 1987;16(1):78–117. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.