Abstract
Purpose of the review
To present the background of liver fluke-associated cholangiocarcinoma (CCA) in Thailand focusing on recent epidemiological data and pathogenesis of this bile duct cancer.
Recent findings
More systematic tumor registration in Thailand nowadays uncovers new high incidence areas that do not confine to only in the northeastern part but also some provinces in the northern Thailand. The link between the liver fluke, Opisthorchis viverrini, and CCA, particularly in the cellular and molecular pathogenesis is more elucidated.
Summary
Thailand is still the country with highest incidence of CCA in the world. Liver fluke induces chronic inflammation leading to oxidative DNA damage of the infected biliary epithelium and malignant transformation. Eradication of the fluke and identification of high risk population are urgently needed.
Keywords: Cholangiocarcinoma, liver fluke, epidemiology, pathogenesis, Thailand
Introduction
Primary liver cancer comprises two major types of cancer with distinguished histological characteristics and its origin – hepatocellular carcinoma (HCC) derives from hepatocytes, and cholangiocarcinoma (CCA). HCC, the most common liver cancer has stronger link to Hepatitis B and C infection. CCA is a rare tumor worldwide but it is highly prevalent in Thailand. Both epidemiological and experimental evidence implicate the carcinogenic liver fluke, Opisthorchis viverrini which is endemic in this region, as a major risk factor of CCA. Clinical manifestations, diagnosis and treatment are relatively similar to those of non-liver fluke associated CCA which have been recently reviewed [1–2]. We therefore highlight recent epidemiological data and the pathogenesis of Opisthorchis –induced CCA which show rapidly progress during the past few years.
Cholangiocarcinoma worldwide
By 2002, liver cancer (HCC, CCA and others) was the 6th most common cancer worldwide with 626,241 new cancer cases per year (442,149 in men and 184,092 in women; male to female ration ~ 2.5) [3••]. Because of the universally poor prognosis, the number of deaths per year was not far short of the number of new cases (598,412) [3••]. Between 1973 and 1997, incidence rates of liver and bile duct cancers increased in all part of the world, except Singapore [3••]. Geographic distribution of liver cancer is not uniform. In both male and female, liver cancer incidence rates are higher in less-developed than more developed regions [3••,4•]. The highest incidence rates are observed in sub-Saharan Africa, Eastern and South-Eastern Asia and some regions within the pacific islands (known as Melanesia) [3••,5].
CCA accounts around 10–25% among the primary liver cancers in most parts of the world with age-standardized incidence rates (ASR) between 0.3 and less than 1.5 per 100,000 populations in Western countries [3••, 6, 7]. Increasing trend of CCA incidence has been reported in many parts of these countries. For example, CCA increased around 16-fold and became the most common type of liver cancer in females in England and Wales during 1971–2001 [8]. Moreover, CCA rates are increased more than 120 % in Black and White American men recruited in the Surveillance, Epidemiology and End Results (SEER) program (1976–2000) [9]. In Eastern Asia, the incidence of CCA is also low such as Korea and Japan (ASR less than 1.5) [10••]. However, the CCA incidence in Thailand is exceedingly high with ASR of 33.4 per 100,000 in men, 12.3 per 100,000 in women [11••].
Hepatitis C virus (HCV) infection and liver cirrhosis, in addition to established risk factors (choledochal cysts, cholangitis, inflammatory bowel disease), have been suggested as potential risk factors for CCA in some large population-based/hospital-based case-control studies in Europe and the United States [12–14]. In Eastern Asia, a case–control study from Korea reported that patients with CCA were more likely to have anti-HCV antibodies but not hepatitis B surface antigen (HBsAg) compared with controls [15]. Similarly, a cohort study from Japan showed a relatively high incidence of CCA among 600 patients with HCV-related cirrhosis compared with the expected incidence in the general population [16]. The liver flukes, Clonorchis sinensis and O. viverrini, have been largely described to be risk factors for CCA in the Far East and Southeast Asia [15,17,18••]. O. viverrini has also been classified as Class I carcinogen by the World Health Organization and the International Agency for Research on Cancer [19••].
Cholangiocarcinoma in Thailand
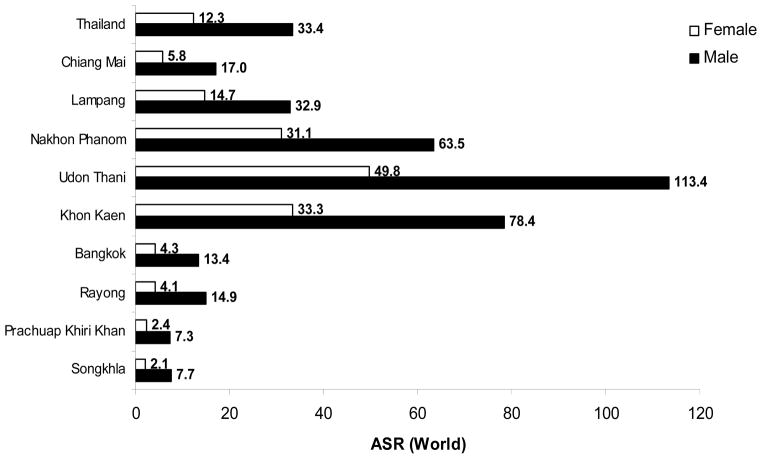
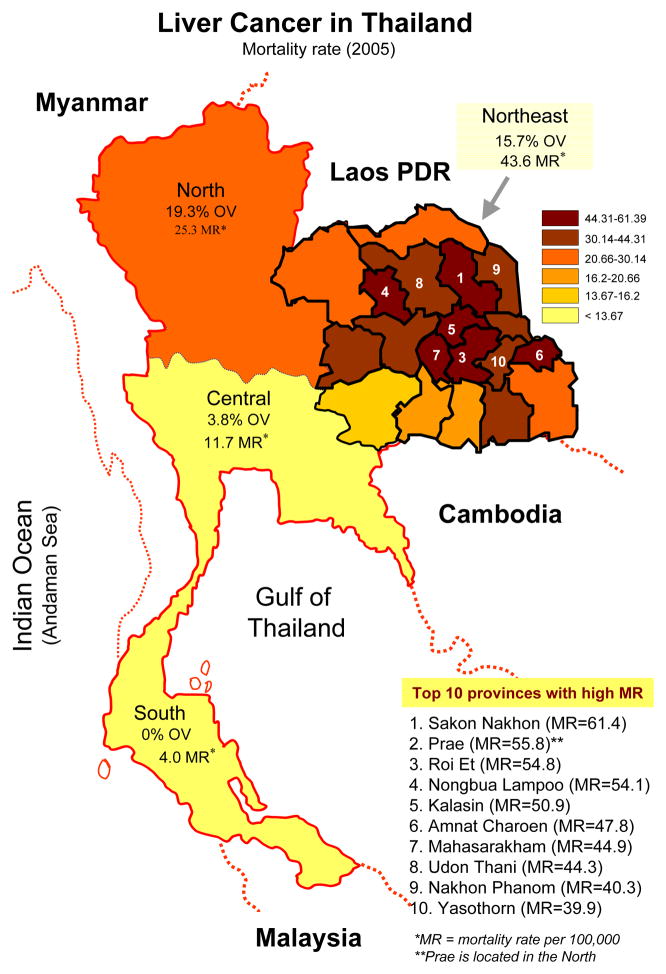
Primary liver cancer is the leading cancer in Thailand in men (ASR = 33.4/100,000 population) and the third in women (ASR = 12.3/100,000) in the year 1998–2000 [11••]. This makes the liver cancer a major cancer in men for the whole country, with an estimated 8,298 new cases in the year 1999 [11••]. Together with lung cancer which ranks number two of the top 10 cancer list, the cancer from these two sites account for 41.9 % of all cancer in men. There is a marked regional variation of liver cancer, with the highest incidence in the Northeast (Fig. 1). The incidence rate reported from several tumor registries in Thailand ranged between ASR = 7.3 (Prachuap Khiri Khan, upper South) and ASR = 113.4 (Udon Thani, upper Northeast) in male and from ASR = 2.1 (Songkla, lower South) and ASR = 49.8 (Udon Thani, upper Northeast) in female [11••]. This raises the new high incidence rate in the province other than Khon Kaen which has been reported the highest incidence in the world with the ASR of 94.8 and 39.4 in the year 1998–91 in male and female, respectively [20]. However, the highest mortality rate (MR) of liver and bile duct cancer in the year 2005 was in Sakol Nakhon (upper Northeast) with MR = 61.4 per 100,000 population while that of Udon Thani was only MR = 44.3 per 100,000 population (Fig. 2) [21••]. Therefore, there may be other provinces in Northeast Thailand, where there is no tumor registry, with higher liver and bile duct cancer incidence than in Udon Thani.
Figure 1.
Age-standardized incidence rates (ASR) of liver and bile duct cancer in male and female in different regions of Thailand (1998–2000)
Figure 2.
Mortality rate of liver cancer and O. viverrini infection rate in different regions of Thailand (2004). Note Northeast Thailand shows different high mortality rate particularly in the upper part and rank within the top ten provinces with high incidence in Thailand. Figure modified from [18•, 21•]
As described above, primary liver cancer in adult consists of 2 main histologic types: HCC and CCA. In Thailand, there is a considerable geographical variation in the histologic types of the primary liver cancer, with a striking occurrence of CCA which varies more than 8.5 folds among the regions, i.e. Nakhon Phanom (upper Northeast, CCA 85.5 %) and Prachuap Khiri Khan (upper South, CCA = 10.0 %) in men. Since majority of liver cancer are found in the North and Northeast which CCA accounts more than a half, therefore, CCA is the predominant type of liver cancer in Thailand. In contrast, the frequency of HCC is more or less constant in different parts of the country [11••].
Primary liver cancer is the fatal neoplasias, and liver and bile duct cancer ranks at the top five in the list of diseases in Thailand that cause high mortality rates in both male and female in total of 28,000 deaths for the year 2004 (Table 1) [22•]. Moreover, the liver and bile duct cancer cause the highest number of disability adjusted life years (DALYs) among other cancers (280,000 DALYs for male and 124,000 DALYs for female in the year 2004) [22•]. Mortality rates of liver and bile duct cancer in different region of Thailand and, particularly in all provinces of the Northeast are shown in Fig. 2 [21••]. High mortality rates are observed in the Northeast where CCA accounted for more than 70% of the primary liver cancer and the liver fluke is highly endemic (Fig. 2) [11••,23•]. This area has also been known as the poor and poorest region of Thailand.
Table 1.
Top 10 diseases with high mortality in Thai population stratified by sex for the year 2004 [22•].
| Rank | Male | Female | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Disease | Deaths (‘000) | % | % | Deaths (‘000) | Disease | |
| 1 | HIV/AIDS | 26 | 11.1 | 14.7 | 26 | Stroke |
| 2 | Stroke | 23 | 10.0 | 8.0 | 14 | Diabetes |
| 3 | Traffic accidents | 23 | 9.9 | 6.6 | 12 | Ischemic heart disease |
| 4 | Liver and bile duct cancer | 19 | 8.0 | 6.2 | 11 | HIV/AIDS |
| 5 | COPD | 14 | 5.9 | 4.9 | 9 | Liver and bile duct cancer |
| 6 | Ischemic heart disease | 13 | 5.6 | 3.8 | 7 | Lower respiratory tract infections |
| 7 | Bronchus and lung cancer | 9 | 3.7 | 3.4 | 6 | COPD |
| 8 | Diabetes | 8 | 3.5 | 3.2 | 6 | Nephritis & nephrosis |
| 9 | Cirrhosis | 8 | 3.4 | 2.9 | 5 | Traffic accidents |
| 10 | Lower respiratory tract infections | 7 | 2.9 | 2.5 | 4 | Cervix uteri cancer |
Liver fluke infection and cholangiocarcinoma in Thailand
Human liver fluke infection caused by Clonorchis sinensis and O. viverrini remains a major public health problem in Eastern and Southeast Asia. More than 600 million are at risk of these infections [24]. C. sinensis is endemic in south China, northern Vietnam, Taiwan and Korea while O. viverrini is found mainly in Thailand, Lao People’s Democratic Republic, Cambodia and central Vietnam. No stronger link between a human malignancy and a parasitic infection occurs than that between CCA and infection with the liver fluke, O. viverrini. In Thailand, CCA was observed in a high percentage of liver cancers from patients living in Northeast Thailand where the prevalence of O. viverrini infection is higher than elsewhere in this country as described above.
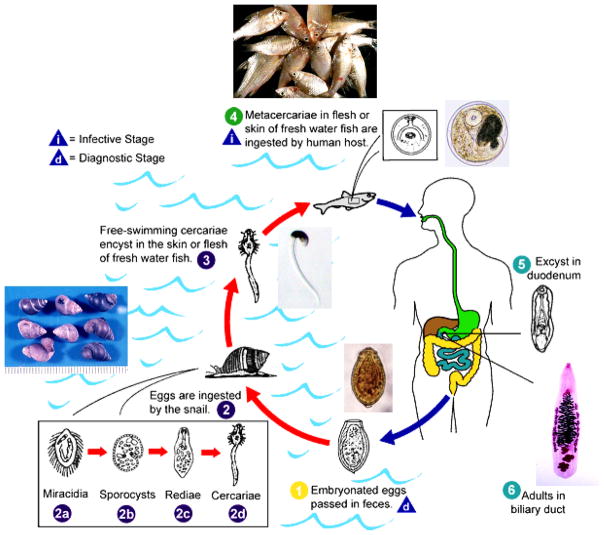
People get infection by eating uncooked cyprinoid fish harboring infective metacercariae of the fluke. Due to poor sanitation practices, infected people pass parasite eggs in their feces into natural water reservoirs, where the parasite eggs are eaten by snails of the genus Bithynia, the first intermediate host of the O. viverrini life cycle (Fig. 3). After several developmental stages in the snail, the parasite releases as free swimming cercariae from the infected snails and locates cyprinoid fishes, encyst in the fins, skin and muscles of the fish, and become metacercariae. Infection is accomplished when people ingest raw or undercooked fish in dishes such as koi pla and in turn the parasite’s life cycle is completed. An estimated 6 million people are infected with O. viverrini in Thailand. However, geographical pattern of the liver fluke infection is not uniform, with greatest prevalence in the North (19.3%), and Northeast (15.7%) compared with the Central (3.8%) and southern regions (0%) (Fig 2) [23•]. Despite widespread chemotherapy with praziquantel that has been implemented in the past, the prevalence of O. viverrini in some endemic areas approaches 70%.
Figure 3.
Life cycle of Opisthorchis viverrini. Embryonated eggs are discharged in the biliary ducts and in the stool
 Eggs are ingested by a suitable snail intermediate host
Eggs are ingested by a suitable snail intermediate host
 there are more than 100 species of snails that can serve as intermediate hosts. Each egg releases a miracidium
there are more than 100 species of snails that can serve as intermediate hosts. Each egg releases a miracidium
 which goes through several developmental stages (sporocyst
which goes through several developmental stages (sporocyst
 redia
redia
 and cercaria
and cercaria
 ). The cercaria is released from the snail and after a short period of free-swimming time in water, it comes into contact with and penetrates the flesh of a freshwater fish, such as Cyclocheilichthys armatus or Puntius leiacanthus, where it encysts as a metacercaria
). The cercaria is released from the snail and after a short period of free-swimming time in water, it comes into contact with and penetrates the flesh of a freshwater fish, such as Cyclocheilichthys armatus or Puntius leiacanthus, where it encysts as a metacercaria
 Infection of humans occurs by ingestion of undercooked, salted, pickled, or smoked freshwater fishes
Infection of humans occurs by ingestion of undercooked, salted, pickled, or smoked freshwater fishes
 . After ingestion, the metacercaria excysts in the duodenum
. After ingestion, the metacercaria excysts in the duodenum
 and ascends the biliary tract through the ampulla of Vater. Maturation to adults, Opisthorchis viverrini
and ascends the biliary tract through the ampulla of Vater. Maturation to adults, Opisthorchis viverrini
 takes approximately 1 month. The adult flukes (measuring 10 to 15 mm by 3 to 5 mm) reside in the small and medium sized biliary ducts. In addition to humans, carnivorous animals can serve as reservoir hosts. (Adapted from http://www.dpd.cdc.gov/DPDx/HTML/Opisthorchiasis.htm)
takes approximately 1 month. The adult flukes (measuring 10 to 15 mm by 3 to 5 mm) reside in the small and medium sized biliary ducts. In addition to humans, carnivorous animals can serve as reservoir hosts. (Adapted from http://www.dpd.cdc.gov/DPDx/HTML/Opisthorchiasis.htm)
Many correlation studies have shown that the incidence of CCA correlate strongly with the prevalence of O. viverrini infection, as measured by anti-O. viverrini antibody titers in the general population or parasite eggs in the feces [18••]. Sriamporn et al. [25••] reported a 10 year cohort study in this north-eastern province. Of 24,723 participants, 18,393 aged 35–69 years from 20 districts were tested for O. viverrini infection by stool examination, and the incidence of liver cancer in 1990–2001 was obtained for each district from the Tumor Registry of Khon Kaen University. The prevalence of O. viverrini infection in the sample subjects was 24.5%, ranging from 2% to 71% in different districts. Truncated age-standardized incidence of CCA at ages >35 years varied three-fold among districts, from 94 to 318 per 100,000 person-years with an average of 188. After adjustment for age, sex and period of sampling, a positive association was evident between prevalence of O. viverrini infection and incidence of CCA at the population level. Moreover, follow-up of the cohort by record-linkage to the provincial cancer registry revealed 363 liver and bile duct cancer cases, while the second common was cervical cancer (44 cases), of the 24,528 subjects by the year 2003 [26•]. In addition, 4,154 subjects of this cohort were randomly ultrasound screened and 21 (0.5%) showed suspected CCA [27•]. From these data it means that there may be 100,000 asymptomatic, suspected CCA cases in general population of the 20 million in Northeast Thailand.
Clinical manifestations of opisthorchiasis-associated CCA
Clinical manifestations of opisthorchiasis (that caused by O. viverrini infection) and its associated CCA have been recently reviewed [18••,28••,29•] Most subjects with opisthorchiasis have no symptoms. Only 5–10% of the (heavily infected) subjects have non-specific symptoms such as right upper quadrant abdominal pain, flatulence, fatigue, and a hot sensation over the abdomen.
Enlargement of the gall bladder can be detected by ultrasonography, and is reversed after elimination of flukes by praziquantel [28••]. By contrast, jaundice is the main clinical manifestation that accounts for about 60% of hospital cases of opisthorchiasis-related CCA in Northeast Thailand [29•]. The patients may present with (a) obstructive jaundice alone, (b) obstructive jaundice with fever, or (c) obstructive jaundice with acute abdominal complications, such as cholangitis, acalculous cholecystitis and generalized bile peritonitis. Non-jaundiced patients may present with dyspeptic pain, anorexia, weight loss, right upper abdominal mass and distant metastasis [29•].
CCA is generally accepted as a fatal tumor with extremely poor prognosis including Thai cases. The prognosis of CCA depends on a number of factors, such as tumor types, staging, free surgical margin, distant metastasis, etc. Similar to CCA in other series, Thai patients with intraductal growth type CCA have longer survival while the median survival time in diffuse type CCA (where tumor mass occurs in both left and right lobe) is only 65 days [30].
Pathology of opisthorchiasis and its associated CCA
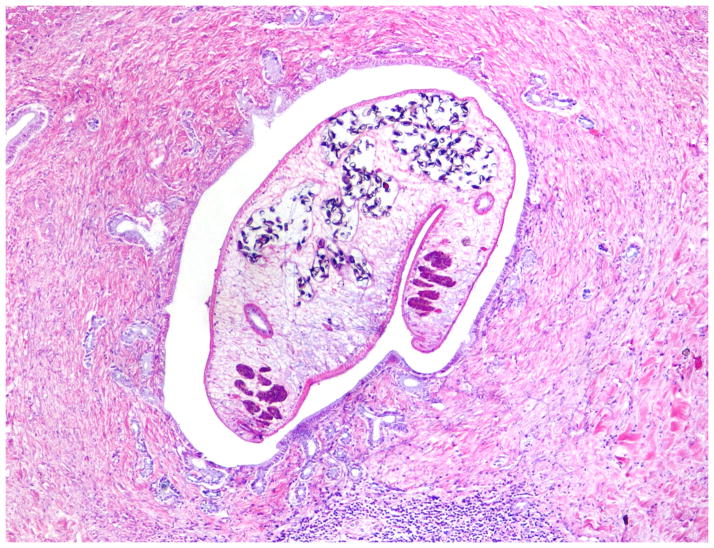
Pathological consequences of O. viverrini infection occur mainly in the liver, extrahepatic bile ducts and gallbladder, and have been described in both humans and animal models. The severity of the pathology appears to be associated with both intensity and duration of infection [28••]. The main histopathologic features of opisthorchiasis both in man and model mammals are inflammation, epithelial desquamation, epithelial and adenomatous hyperplasia, goblet cell metaplasia, periductal fibrosis and granuloma formation (Fig. 4).
Figure 4.
Histopathology of O. viverrini infected human liver showing periportal inflammation, adenomatous hyperplasia and periductal fibrosis. The adult parasite is seen in the bile duct lumen.
CCA originates from the surface epithelium of the bile duct. Atypical hyperplasia and dysplasia that lie adjacent to CCA are potential precancerous lesions which have been reported in both fluke-related CCA as well as CCA associated with other causes [31]. Comparison of CCA from Thai and Japanese cases revealed marked differences in non-cancerous areas; chronic cholangitis, adenomatous hyperplasia and cholangiofibrosis were more pronounced in Thai cases [32,33•]. However, a similar spectrum of tumor phenotypes was evident; 78–80% of CCA cases were tubular adenocarcinoma. These observations imply that CCA with different etiological backgrounds may have some common histogenesis. In addition, gene expression profiles of liver fluke and non-liver fluke associated CCA are different, particularly in genes involved in xenobiotic metabolisms [34••]. Another comparison of liver fluke-associated CCA from Thai patients and sporadic Australian CCA cases revealed that most cases of dysplastic biliary epithelium and CCA display a Brunner or pyloric gland cell phenotype and a gastric foveolar cell phenotype [35•]. The expression of D10 (a marker for Brunner or pyloric gland metaplasia cell phenotype) in hyperplastic and dysplastic epithelium and in CCA is consistent with a dysplasia-carcinoma sequence. Many more fluke-associated CCA than sporadic CCA display an intestinal goblet cell phenotype, indicating differences in the aetiopathology of the cancers in the two groups of patients [35•]. For Opisthorchis-associated CCA, dysplastic biliary epithelium or carcinoma in situ is commonly found in bile ducts in the non-tumorous liver. All of the above pathologies of the liver are considered to be predisposing lesions for CCA development.
Pathogenesis of Opisthorchis-associated CCA
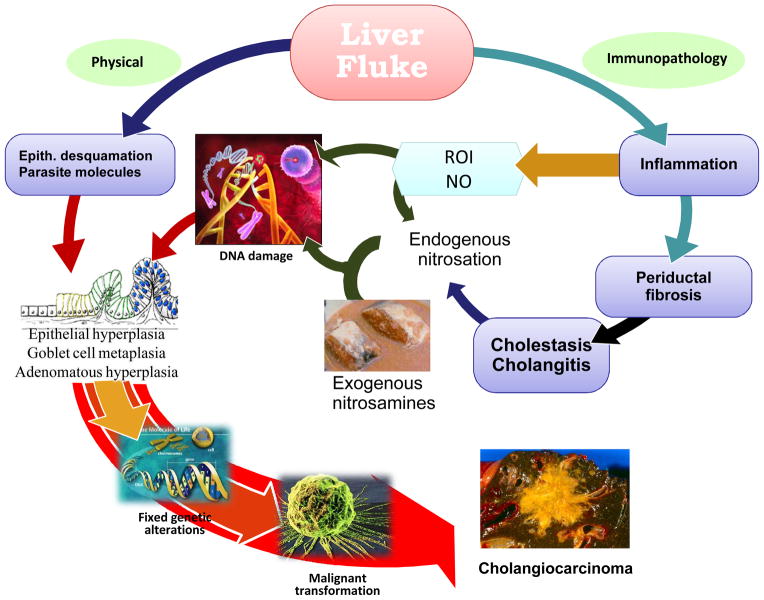
Pathogenesis of opisthorchiasis and its associated CCA have been extensively reviewed [18••,28••]. Similar to other infection-associated cancer, Opisthorchis-induced CCA is a result of chronic inflammation. Several mechanisms by which O. viverrini infection may enhance cholangiocarcinogenesis have been proposed (Fig. 5). Biliary epithelial desquamation, may be due to mechanical irritation caused by the liver fluke and/or its metabolic products. However, immunopathological processes may contribute to the long standing hepatobiliary damage. With liver fluke infection, inflammation, periductal fibrosis and proliferative responses including epithelial hyperplasia, goblet cell metaplasia and adenomatous hyperplasia may represent predisposing lesions that enhance susceptibility of DNA to carcinogens. Several N-nitroso compounds and their precursors occur at low levels in fermented food such as preserved mud fish paste, pla-ra that is a common food additive in Northeast Thailand. Moreover, endogenous nitrosation caused by Opisthorchis infection and inflammation has been observed in animals and humans. Both exogenous and in situ nitrosamine formation may lead to DNA alkylation and deamination in predisposed and inflamed tissues [18••]. Moreover, excess NO and other reactive oxygen intermediates produced by inflammatory cells during infection might exert direct cytotoxic and mutagenic effects and increased cell proliferation. Interestingly, diffuse oxidative DNA damage (8-nitroguanine and 8-oxodG) in biliary epithelium of hamsters infected with O. viverrini has been reported [36••,37•], and moreover, that repeated infections with the liver fluke result in enhanced biliary DNA damage [38••]. With the less apoptosis of the infected biliary epithelium, after several successions of replication, genetic alterations may become fixed which can lead to malignant transformation [18••].
Figure 5.
Proposed mechanisms of Opisthorchis induced cholangiocarcinoma. The liver fluke induces damage to the bile duct tissue at least by 2 pathways; mechanical (by parasite sucker/excretory-secretory products) and immunopathology to parasite antigens. Inflammation induced oxidative DNA damage occurs in concurrent with biliary epithelial proliferation driving by parasite molecules. Damaged DNA/genes after successive replication became fixed lead to malignant transformation.
Conclusion
Thailand has been reported the highest incidence of CCA in the world until nowadays because of the key risk factor, the liver fluke O. viverrini, is still endemic. Therefore, the best way to reduce or even eliminate the burden of disease attributable to the parasite is to stop the infection immediately, i.e. mass anthelmintic therapy- could be targeted to the infected individuals in particular along with intensive health education. In addition, we anticipate that new research endeavors focusing on mechanisms of host immune/inflammatory responses which induce DNA damage, including their polymorphisms, would lead the way towards elucidation of the links between O. viverrini infection and CCA and identification of high risk population.
Acknowledgments
We gratefully acknowledge support from the Thailand Research Fund (TRF grant no. BRG4580016) and the NIH-NIAID (award number 1UO1 AI065871).
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as
• of special interest
•• of outstanding interest
- 1.Patel T, Singh P. Cholangiocarcinoma: emerging approaches to a challenging cancer. Curr Opin Gastroenterol. 2007;23:317–323. doi: 10.1097/MOG.0b013e3280495451. [DOI] [PubMed] [Google Scholar]
- 2.Khan SA, Miras A, Pelling M, et al. Cholangiocarcinoma and its management. Gut. 2007;56:1755–1756. doi: 10.1136/gut.2007.138859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3••.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. This report intensively summarizes the global cancer profiles and highlights eight common cancers and associated important risk factors, cancer control and prevention measure. [DOI] [PubMed] [Google Scholar]
- 4•.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. This report describes the cancer statistics on incidence, mortality rate and survival in the United States. [DOI] [PubMed] [Google Scholar]
- 5.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 6.Jepsen P, Vilstrup H, Tarone RE, et al. Incidence rates of intra- and extrahepatic cholangiocarcinomas in Denmark from 1978 through 2002. J Natl Cancer Inst. 2007;99:895–897. doi: 10.1093/jnci/djk201. [DOI] [PubMed] [Google Scholar]
- 7.McLean L, Patel T. Racial and ethnic variations in the epidemiology of intrahepatic cholangiocarcinoma in the United States. Liver Int. 2006;26:1047–1053. doi: 10.1111/j.1478-3231.2006.01350.x. [DOI] [PubMed] [Google Scholar]
- 8.West J, Wood H, Logan RF, et al. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971–2001. Br J Cancer. 2006;94:1751–1758. doi: 10.1038/sj.bjc.6603127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGlynn KA, Tarone RE, El-Serag HB. A comparison of trends in the incidence of hepatocellular carcinoma and intrahepatic cholangiocarcinoma in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15:1198–1203. doi: 10.1158/1055-9965.EPI-05-0811. [DOI] [PubMed] [Google Scholar]
- 10••.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. This paper describes the burden of infection-associated cancer and presents cancer statistics in comprehensive. [DOI] [PubMed] [Google Scholar]
- 11••.Khuhaprema T, Srivatanakul P, Sriplung H, et al. Cancer in Thailand. Volume IV 1998–2000. Bangkok Medical Publisher; Bangkok: 2007. p. 132. This latest version of the book series on Cancer in Thailand reports all cancer statistics in Thailand during 1998–2000. [Google Scholar]
- 12.Welzel TM, Graubard BI, El-Serag HB, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: a population-based case-control study. Clin Gastroenterol Hepatol. 2007;5:1221–1228. doi: 10.1016/j.cgh.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welzel TM, Mellemkjaer L, Gloria G, et al. Risk factors for intrahepatic cholangiocarcinoma in a low-risk population: a nationwide case-control study. Int J Cancer. 2007;120:638–641. doi: 10.1002/ijc.22283. [DOI] [PubMed] [Google Scholar]
- 14.Shaib YH, El-Serag HB, Nooka AK, et al. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a hospital-based case-control study. Am J Gastroenterol. 2007;102:1016–1021. doi: 10.1111/j.1572-0241.2007.01104.x. [DOI] [PubMed] [Google Scholar]
- 15.Shin HR, Lee CU, Park HJ, et al. Hepatitis B and C virus, Clonorchis sinensis for the risk of liver cancer: A case–control study in Pusan, Korea. Int J Epidemiol. 1996;25:933–940. doi: 10.1093/ije/25.5.933. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi M, Ikeda K, Saitoh S, et al. Incidence of primary cholangiocellular carcinoma of the liver in Japanese patients with hepatitis C virus-related cirrhosis. Cancer. 2000;88:2471–2477. doi: 10.1002/1097-0142(20000601)88:11<2471::aid-cncr7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 17.Lim MK, Ju YH, Franceschi S, et al. Clonorchis sinensis infection and increasing risk of cholangiocarcinoma in the Republic of Korea. Am J Trop Med Hyg. 2006;75:93–96. [PubMed] [Google Scholar]
- 18••.Sripa B, Kaewkes S, Sithithaworn P, et al. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4:e201. doi: 10.1371/journal.pmed.0040201. Up to date on epidemiology, clinicopathology and pathogenesis of liver fluke-induced cholangiocarcinoma at cellular and molecular levels are extensively reviewed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.IARC. IARC Monogr Eval Carcinog Risks Hum; Schistosomes, liver flukes and Helicobacter pylori IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; Lyon. 7–14 June 1994; 1994. pp. 1–241. [PMC free article] [PubMed] [Google Scholar]
- 20.Vatanasapt V, Martin N, Sriplung H, et al. Cancer incidence in Thailand, 1988–1991. Cancer Epidemiol Biomarkers Prev. 1995;4:475–483. [PubMed] [Google Scholar]
- 21••.Health Information System Development Office, Thai Health Promotion Foundation. Provincial mortality rates, 2005. [Accessed 6 November 2007];Thailand Health Information. 2006 Nov;2(18) (in Thai) Available: http://www.hiso.or.th/hiso/picture/bro/PDF/lesson18.pdf. This latest information from government organization on provincial mortality rate of common cancers in Thailand. [Google Scholar]
- 22•.International Health Policy Program, Ministry of Public Health. Burden of Diseases and Injuries in Thailand. [Accessed 3 November 2007];Medium-term Report. 2007 (in Thai) Available: http://www.ihpp.thaigov.net/bod/index.html. Burden of diseases including cancers have been described in this update version.
- 23.Jongsuksuntigul P, Imsomboon T. Opisthorchiasis control in Thailand. Acta Trop. 2003;88:229–232. doi: 10.1016/j.actatropica.2003.01.002. [DOI] [PubMed] [Google Scholar]
- 24.Keiser J, Utzinger J. Emerging foodborne trematodiasis. Emerg Infect Dis. 2005;11:1507–1514. doi: 10.3201/eid1110.050614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sriamporn S, Pisani P, Pipitgool V, et al. Prevalence of Opisthorchis viverrini infection and incidence of cholangiocarcinoma in Khon Kaen, Northeast Thailand. Trop Med Int Health. 2004;9:588–594. doi: 10.1111/j.1365-3156.2004.01234.x. [DOI] [PubMed] [Google Scholar]
- 26.Sriamporn S, Parkin DM, Pisani P, et al. A prospective study of diet, lifestyle, and genetic factors and the risk of cancer in Khon Kaen Province, northeast Thailand: description of the cohort. Asian Pac J Cancer Prev. 2005;6:295–303. [PubMed] [Google Scholar]
- 27•.Mairiang E, Chaiyakum J, Chamadol N, et al. Ultrasound screening for Opisthorchis viverrini-associated cholangiocarcinomas: experience in an endemic area. Asian Pac J Cancer Prev. 2006;7:431–433. This is the largest series of ultrasound survey of normal population in rural communities in Northeast Thailand. [PubMed] [Google Scholar]
- 28.Sripa B. Pathobiology of opisthorchiasis: an update. Acta Trop. 2003;88:209–220. doi: 10.1016/j.actatropica.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Mairiang E, Mairiang P. Clinical manifestation of opisthorchiasis and treatment. Acta Trop. 2003;88:221–227. doi: 10.1016/j.actatropica.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 30.Uttaravichien T, Bhudhisawasdi V, Pairojkul C, et al. Intrahepatic cholangiocarcinoma in Thailand. J Hepatobiliary Pancreat Surg. 1999;6:128–135. doi: 10.1007/s005340050095. [DOI] [PubMed] [Google Scholar]
- 31.Shimonishi T, Sasaki M, Nakanuma Y. Precancerous lesions of intrahepatic cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2000;7:542–550. doi: 10.1007/s005340070002. [DOI] [PubMed] [Google Scholar]
- 32.Shirai T, Pairojkul C, Ogawa K, et al. Histomorphological characteristics of cholangiocellular carcinomas in northeast Thailand, where a region infection with the liver fluke, Opisthorchis viverrini is endemic. Acta Pathol Jpn. 1992;42:734–739. doi: 10.1111/j.1440-1827.1992.tb03223.x. [DOI] [PubMed] [Google Scholar]
- 33.Suzuki H, Isaji S, Pairojkul C, et al. Comparative clinicopathological study of resected intrahepatic cholangiocarcinoma in northeast Thailand and Japan. J Hepatobiliary Pancreat Surg. 2000;7:206–211. doi: 10.1007/s005340050177. [DOI] [PubMed] [Google Scholar]
- 34••.Jinawath N, Chamgramol Y, Furukawa Y, et al. Comparison of gene-expression profiles between Opisthorchis viverrini- and non-Opisthorchis viverrini-associated intrahepatic cholangiocarcinoma. Hepatology. 2006;44:1025–1038. doi: 10.1002/hep.21330. Genome wide gene expression profiles of fluke and non-fluke associated cholangiocarcinoma is extensive categorized. [DOI] [PubMed] [Google Scholar]
- 35•.Hughes NR, Pairojkul C, Royce SG, et al. Liver fluke-associated and sporadic cholangiocarcinoma: an immunohistochemical study of bile duct, peribiliary gland and tumour cell phenotypes. J Clin Pathol. 2006;59:1073–1078. doi: 10.1136/jcp.2005.033712. Comparative study on pathology and pathogenesis of Australian and Thai cholangiocarcinomas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinlaor S, Hiraku Y, Ma N, et al. Mechanism of NO-mediated oxidative and nitrative DNA Damage in hamsters infected with Opisthorchis viverrini: A model of inflammation-mediated carcinogenesis. Nitric Oxide. 2004;11:175–183. doi: 10.1016/j.niox.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 37•.Kawanishi S, Hiraku Y, Pinlaor S, et al. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol Chem. 2006;387:365–372. doi: 10.1515/BC.2006.049. Infection/inflammation induced oxidative/nitrative DNA damage in animals and humans are reviewed. [DOI] [PubMed] [Google Scholar]
- 38.Pinlaor S, Ma N, Hiraku Y, et al. Repeated infection with Opisthorchis viverrini induces accumulation of 8-nitroguanine and 8-oxo-7, 8-dihydro-2′deoxyguanine in the bile duct of hamster via inducible nitric oxide synthase. Carcinogenesis. 2004;25:1535–1542. doi: 10.1093/carcin/bgh157. [DOI] [PubMed] [Google Scholar]