Abstract
Oral cancer is the sixth most common cancer worldwide, with a high prevalence in South Asia. Tobacco and alcohol consumption remain the most dominant etiologic factors, however HPV has been recently implicated in oral cancer. Surgery is the most well established mode of initial definitive treatment for a majority of oral cancers. The factors that affect choice of treatment are related to the tumor and the patient. Primary site, location, size, proximity to bone, and depth of infiltration are factors which influence a particular surgical approach. Tumors that approach or involve the mandible require specific understanding of the mechanism of bone involvement. This facilitates the employment of mandible sparing approaches such as marginal mandibulectomy and mandibulotomy. Reconstruction of major surgical defects in the oral cavity requires use of a free flap. The radial forearm free flap provides excellent soft tissue and lining for soft tissue defects in the oral cavity. The fibula free flap remains the choice for mandibular reconstruction.
Over the course of the past thirty years there has been improvement in the overall survival of patients with oral carcinoma largely due to the improved understanding of the biology of local progression, early identification and treatment of metastatic lymph nodes in the neck, and employment of adjuvant postoperative radiotherapy and chemoradiotherapy.
The role of surgery in primary squamous cell carcinomas in other sites in the head and neck has evolved with integration of multidisciplinary treatment approaches employing chemotherapy and radiotherapy either sequentially or concurrently. Thus, larynx preservation with concurrent chemoradiotherapy has become the standard of care for locally advanced carcinomas of the larynx or pharynx requiring total laryngectomy. On the other hand, for early staged tumors of the larynx and pharynx, transoral laser microsurgery has become an effective means of local control of these lesions. Advances in skull base surgery have significantly improved the survivorship of patients with malignant tumors of the paranasal sinuses approaching or involving the skull base. Surgery thus remains the mainstay of management of a majority of neoplasms arising in the head and neck area. Similarly, the role of the surgeon is essential throughout the life history of a patient with a malignant neoplasm in the head and neck area, from initial diagnosis through definitive treatment, post-treatment surveillance, management of complications, rehabilitation of the sequelae of treatment, and finally for palliation of symptoms.
Keywords: Oral Cancer, Oral Cancer Surgery, Head and Neck Cancer, Head and Neck Cancer Surgery, Oral Cancer Outcomes, Multi-Modality Treatment, Advanced Mouth Cancer, Mouth Cancer Treatment, Multidisciplinary Treatment Oral Cancer
Cancer of the head and neck is a relatively uncommon human cancer. The term “head and neck cancer” covers a large number of neoplasms with diverse natural history arising in one anatomic region. Under the common term of “head and neck cancer” are included; tumors of the mucosa of the upper aerodigestive tract including oral cavity, pharynx, larynx, and sinuses. Also included are tumors of the salivary glands, thyroid, soft tissue and bone tumors and skin cancers. This special issue of Oral Oncology is dedicated to “multidisciplinary approaches in head and neck cancer”. However, it will largely emphasize on oral cancer management. Thus, this article will address the role of surgery in the contemporary management of oral cancer, but will briefly include the role of surgery and the surgeon in other sites in the head and neck such as pharynx, larynx, sinuses, salivary glands, thyroid, as well as skin, soft tissue and bone tumors. While broad philosophical issues in the surgical management of these other sites will be discussed here, it is not possible to cover the details of the surgical aspects of management of neoplasia arising in these other sites in this manuscript.
Oral cancer is the sixth most common cancer worldwide. Lifestyle, habits and demographic as well as genetic factors influence geographic variations in the incidence of oral cancer.1 For example, oral cancer is the most common cancer in India and accounts for thirty-five percent of all newly diagnosed cancers in men. The etiology of oral cancer is well established in most instances with consumption of tobacco in any form and alcohol being the most common etiologic agents.2 Recently, however, exposure to the human papilloma virus has been implicated in young patients with oral carcinoma. The exact mechanism of carcinogenesis in this setting still remains to be elucidated.
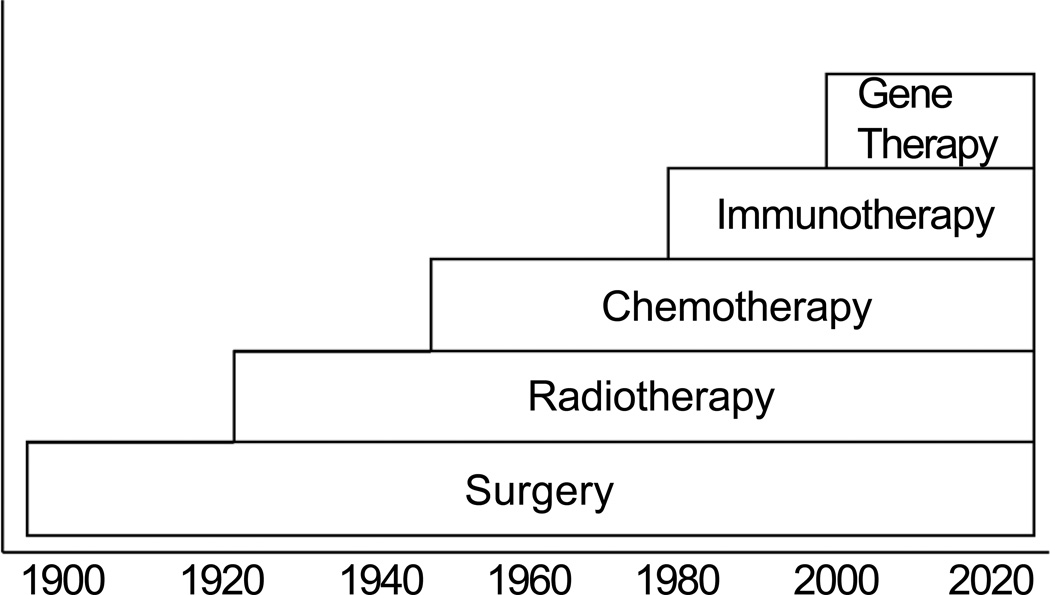
Surgery is the most well established mode of initial definitive treatment for a majority of oral cancers, with a longstanding history of being the accepted method of treatment for well over a century (Figure 1). Introduction of ionizing radiation, following the discovery of radium, became an important means of nonsurgical treatment of oral carcinoma. However, in the majority of patients with advanced cancer, radiotherapy is employed in conjunction with surgery, most often offered as post-operative treatment. Chemotherapy in the management of oral carcinoma was considered palliative in the 1950’s, 60’s and 70’s. However with the introduction of Cis-platinum, clinical trials of induction chemotherapy demonstrated that response to chemotherapy was observed in a significant number of patients. However, unlike other sites in the head and neck area, the response to induction chemotherapy did not translate into long term control of primary oral squamous cell carcinomas.3 Targeted therapies with EGFR inhibitors are an active area of investigation at this time. Immunotherapy and gene therapy are also areas of research where further work needs to be done.
Figure 1.
History of development of therapeutic modalities for cancer.
FACTORS AFFECTING CHOICE OF TREATMENT
The factors that influence the choice of initial treatment are those related to the characteristics of the primary tumor, those related to the patient and those related to the treatment team. Thus, they are categorized under (1) tumor factors, (2) patient factors; (3) physician factors. In selection of optimal therapy for oral carcinoma one should consider these three sets of parameters in initial treatment planning. The ultimate goal of treatment of cancer of the oral cavity is to eradicate the cancer, preserve or restore form and function, minimize the sequelae of treatment and finally prevent any subsequent new primary cancers. In order to achieve these goals, the currently available treatment modalities include surgery, radiotherapy, chemotherapy, combined modality treatments and primary and secondary prevention strategies including lifestyle changes as well as chemoprevention.
TUMOR FACTORS
The tumor factors that affect the choice of initial treatment of oral cancer are primary site, size (T Stage), location (anterior versus posterior), proximity to bone (mandible or maxilla), status of cervical lymph nodes, previous treatment, and histology (type, grade and depth of invasion).4
The biological behavior of primary cancers in the oral cavity is different at various sites. Lip cancer, for example, behaves in a fashion similar to skin cancer with an excellent potential for long term cure, and thus has a very favorable prognosis, stage for stage. Similarly squamous carcinoma of the hard palate and upper gum has a relatively indolent behavior with a low risk of regional lymph node metastases. On the other hand, cancers of the oral tongue, floor of the mouth and lower gum have a high risk of regional lymph node metastases with an adverse impact on prognosis. The size of the primary tumor clearly has a heavy impact on the decision regarding choice of initial treatment. Small and superficial primary tumors of the oral cavity are easily accessible for surgical resection through the open mouth. On the other hand, larger tumors require more extensive surgical approaches for exposure and excision. Certain primary sites in the oral cavity are easily amenable to initial treatment by radiotherapy, such as primary tumors of the tongue, in contrast to those which are situated in proximity to bone, such as lesions of the gum and hard palate. In addition, with increasing size, the risk of regional lymph node metastases increases bringing into consideration the need for elective treatment of the clinically negative neck, which is at risk of harboring micrometastases. Certain primary sites in the oral cavity have a higher risk of regional dissemination compared to other sites. For example, primary tumors of the oral tongue and floor of mouth have an increased risk of lymph node metastases compared to similar staged lesions of the hard palate or upper gum. Primary tumors located in the anterior part of the oral cavity have a lesser risk of dissemination to regional lymph nodes compared to similar staged lesions in the posterior part of the oral cavity or oropharynx.
The presence of clinically palpable cervical lymph node metastases requires a neck dissection as an integral part of surgical treatment. The extent of neck dissection, however, varies depending on the extent of nodal metastases and the location of palpable lymph nodes. The patterns of regional lymph node metastases from primary cancers of the oral cavity are well established and sequential progression of metastatic spread occurs from primary oral cancers.5 The first echelon lymph nodes for oral cancer are located at levels 1, 2, and 3 in the neck with a relatively infrequent dissemination to level 4. Skip metastases to level 5 does not occur. Therefore in planning elective dissection of regional lymph nodes for micrometastases, clearance of lymph nodes at level 5 is not necessary. On the other hand, when gross metastases are present in the anterior triangle of the neck, a comprehensive dissection of all five levels of lymph nodes is recommended.
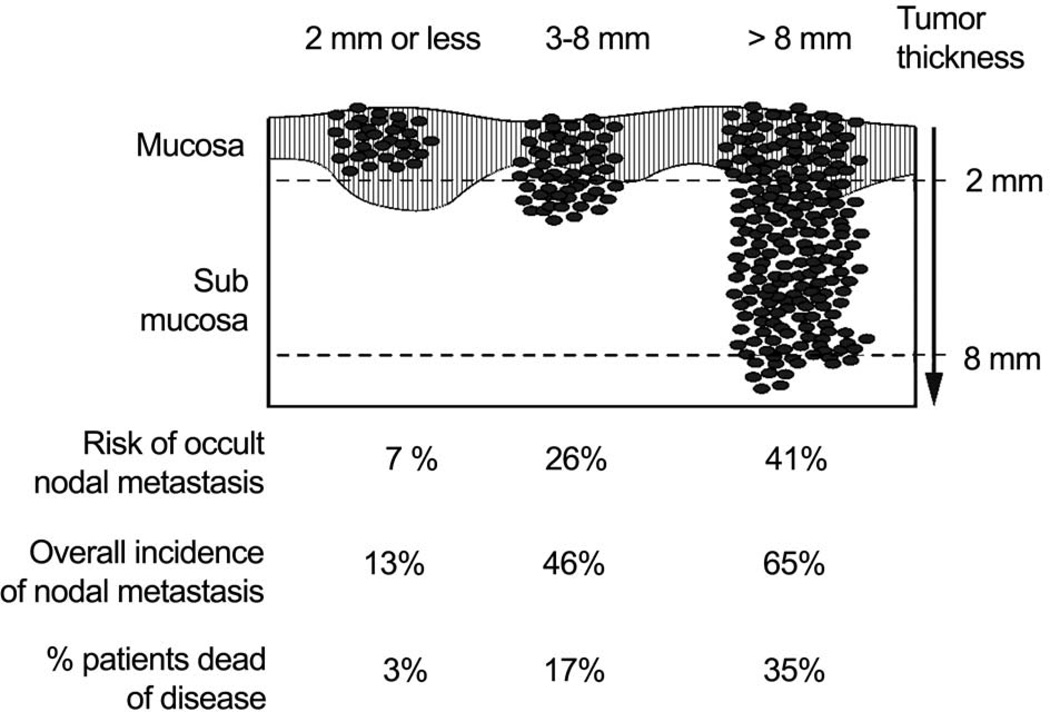
The histology of the primary tumor is an important parameter which influences selection of initial treatment. Squamous carcinomas greatly predominate in the oral cavity and account for over ninety percent of all primary malignant neoplasms in the oral cavity. The second most frequent histology is tumors of the minor salivary glands. Primary mucosal melanomas and tumors of the soft tissue are extremely rare. All other primary malignant tumors in the oral cavity except for lymphoma are treated by surgery. The histologic progression of squamous cell carcinoma from in situ to invasive carcinoma is demonstrated in a majority of primary squamous cell carcinomas. While histological grade of the lesion reflects the aggressiveness of the tumor, that, in itself has never been shown to be an independent parameter of prognosis on multivariate analysis. On the other hand, the most important histologic feature of the primary tumor which heavily impacts upon selection of treatment and eventual prognosis is its depth of invasion. In situ and superficially invasive lesions have a lower risk of regional lymph node metastases and they are highly curable. On the other hand, thicker lesions which are deeply infiltrating the underlying soft tissues have a significantly increased risk of lymph node metastases with its adverse impact on prognosis.6 The impact of the thickness of the primary tumor is well demonstrated in early stage (T1 and T2), carcinomas of the oral tongue and floor of mouth. (Figure 2). Thus it would be advantageous to know the exact thickness of the lesion prior to surgical intervention. However, it is clinically impractical to be able to have that information available prior to surgical excision of the primary tumor in many instances. In general, thickness of the lesion appreciated by palpation is a reasonably good indicator of deeply invasive lesions versus superficial lesions, to help decide upon the need for elective dissection of regional lymph nodes at risk in the clinically negative neck.
Figure 2.
Risk of nodal metastases and death in relation to thickness of primary squamous cell carcinomas of the tongue and floor of mouth. (Adapted from Spiro, et al.6)
Patients with advanced stage disease, that is those presenting with spread to regional lymph nodes or with large primary tumors (T3 and T4), are candidates for consideration of combined modality treatment.
PATIENT FACTORS
Several factors relative to patient characteristics are crucial in the selection of initial treatment for oral cancer. These are the patient’s age, general medical condition, tolerance of treatment, occupation of the patient, acceptance and compliance by the patient, lifestyle (smoking and drinking) and other socioeconomic considerations. In general, older age is not a contra-indicator for implementation of appropriate surgical treatment.7,8 However, advancing age, intercurrent disease and debility due to associated cardiopulmonary conditions increases the risk of morbidity and mortality with extensive surgical treatment. The ability of the patient to tolerate an optimal therapeutic program is similarly an important facet which influences the choice of initial treatment. The patient’s acceptance of and compliance with the proposed treatment are similarly important considerations in designing an optimal treatment program for the tumor. The patient’s lifestyle, with particular reference to smoking and alcohol consumption, should have a heavy impact on selection of treatment and tolerance to treatment. Thus, physical and lifestyle related co-morbidities play an important role in the patient’s ability to tolerate the recommended treatment and on eventual prognosis.9 Unwillingness on the part of the patient to give up smoking and drinking causes further complications of therapy and increases the risk of multiple primary tumors. Any previous treatment in the same area also influences the decision regarding selection of therapy. For example, radiation therapy previously delivered to the same area for a different lesion may not be available to treat a second tumor in the same area. On the other hand, surgical intervention in a previously treated region requires careful surgical planning, particularly with reference to wound closure and reconstructive surgery, with utilization of non-irradiated regional musculocutaneous flaps or free tissue transfer.
PHYSICIAN FACTORS
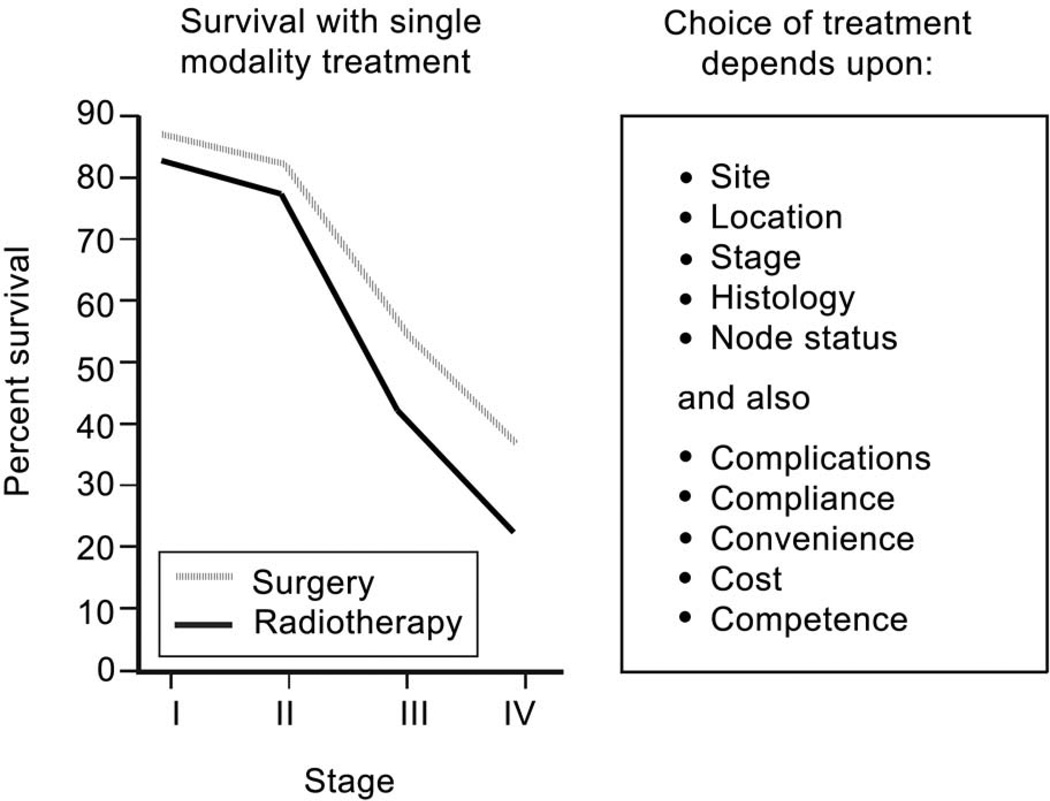
The factors related to the treatment delivery team are also important in making the selection of initial definitive treatment for oral cancer. Expertise in various disciplines including surgery, radiotherapy, chemotherapy, rehabilitation services, dental and prosthetic support, and psycho-social support are all crucial in bringing about a successful outcome of the therapeutic program. Management of cancer of the oral cavity is a multi-disciplinary team effort, and technical capabilities and support services from various disciplines are essential for a successful outcome. These factors that play an important role in selection of initial treatment are summarized in figure 3. Outcomes of early stage tumors treated either by surgery or radiotherapy as initial definitive treatment are comparable. In that setting, however, the sequelae of treatment will be an important consideration in selection of initial therapy. Thus for patients with advanced staged disease, the current preference for the sequence of combined modality treatment program is surgical resection with immediate appropriate reconstruction followed by post-operative radiation therapy or post-operative concurrent chemoradiotherapy. The observations from two prospective randomized trials of adjuvant chemoradiotherapy have shown that patients who have extracapsular extension of disease in metastatic cervical lymph nodes and those who have positive margins have a significant improvement in local regional control and disease free survival by addition of chemotherapy to post-operative radiation therapy compared to post-operative radiation therapy alone.10 However, the majority of patients treated with chemoradiation therapy develop grade 3 and 4 toxicity, which causes significantly more severe and prolonged morbidity.
Figure 3.
Outcomes with single modality treatment (surgery or radiotherapy) by stage of disease for squamous cell carcinomas of the oral cavity, and factors affecting the choice of treatment.
SURGICAL APPROACHES
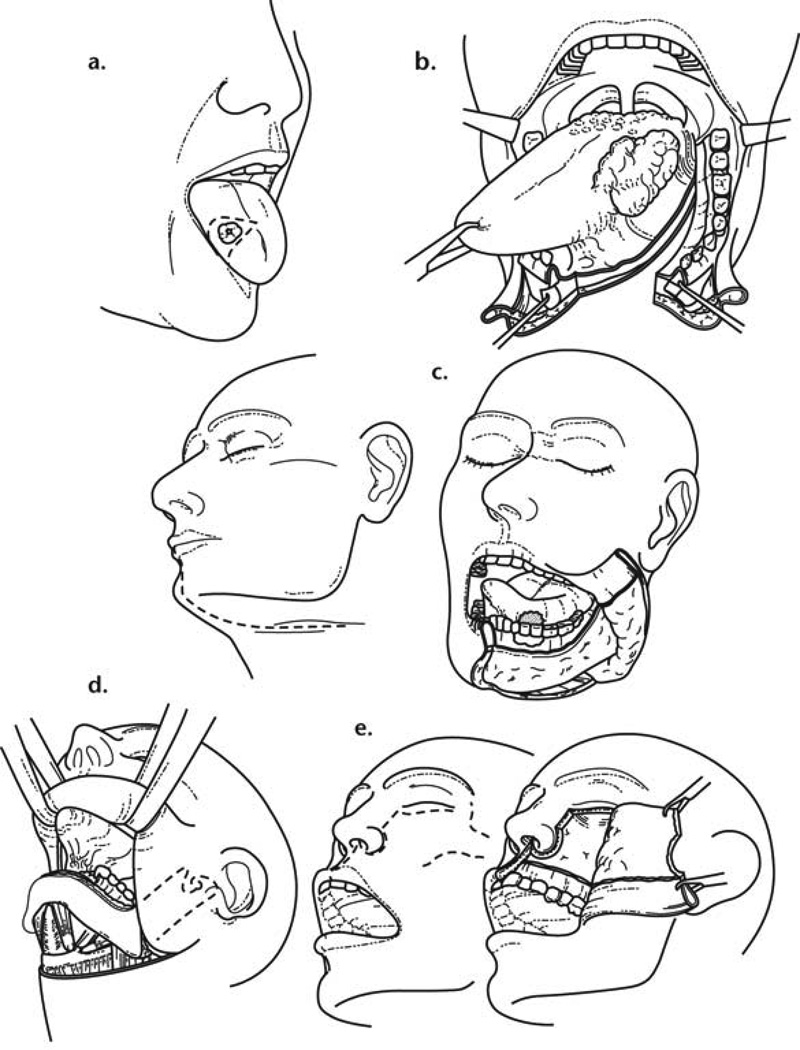
The factors that influence the choice of a particular surgical approach for primary tumors of the oral cavity are the size of the primary tumor, its depth of infiltration, the site of the primary tumor (that is anterior versus posterior location), and proximity of the tumor to mandible or maxilla. In addition to pre-operative clinical assessment of the primary tumor, examination under anesthesia is often indicated to accurately delineate the extent of the tumor. The proximity of the tumor to the maxilla or mandible mandates the need for adequate clinical and radiographic assessment to rule out the possibility of bone invasion. The most commonly employed imaging studies are a panoramic radiograph of the mandible (Orthopantomogram). In addition to this, a more detailed assessment of mandible invasion can be accomplished with a CT scan and a denta scan. On the other hand, magnetic resonance imaging provides a detailed assessment of the extent of tumor infiltration in the soft tissue. The most commonly employed surgical approaches for resection of primary oral cancer are peroral, mandibulotomy, lower cheek flap approach, visor flap approach or upper cheek flap approach. (Figure 4). The visor flap avoids a lower lip splitting incision and provides satisfactory exposure only for the anterior aspect of the oral cavity. It, however, produces numbness of the skin of the chin due to the necessity to divide both mental nerves. Similarly, a sublabial degloving approach avoids an upper lip splitting Weber-Ferguson incision for resection of tumors of the anterior part of the nasal cavity and the infrastructure of the maxilla. Detailed description of these surgical procedures for resection of primary oral carcinomas are beyond the scope of this article.
Figure 4.
Surgical approaches for oral cancer. (From Shah J and Patel S.4)
MANAGEMENT OF THE MANDIBLE
Adequate assessment of the mandible for invasion by primary tumors of the oral cavity is crucial to accurate surgical treatment planning. The mandible is considered at risk when the primary tumor overlies the mandible, is adherent to the mandible, or is in proximity to the mandible. The mechanism for spread of oral cancers to the mandible has been well studied.11 Primary carcinomas of the oral cavity extend along the floor of the mouth or the buccal mucosa to approach the attached lingual or buccal gingiva. From hereon the tumor does not extend directly through the intact periosteum and cortical bone toward the cancellous part of the mandible since the periosteum acts a significant protective barrier. Instead the tumor advances from the attached gingiva towards the alveolus. In patients with teeth, the tumor extends through the dental sockets into the cancellous part of the bone and invades the mandible. In edentulous patients the tumor extends up to the alveolar crest and then infiltrates through the dental pores in the alveolar process and extends to the cancellous part of the mandible. Thus in patients with very early invasion of the alveolar process, marginal mandibulectomy is feasible since the cortical part of the mandible inferior to the roots of the teeth remains uninvolved and can be safely spared.
In edentulous patients the feasibility of marginal mandibulectomy depends on the vertical height of the body of the mandible. With aging of the patient the alveolar process recedes and the mandibular canal comes closer and closer to the surface to the alveolar process. Thus resorption of the alveolar process eventually leads to a pipe stem mandible in very elderly patients. The ability to perform a marginal mandibulectomy in that setting is unlikely. Radiotherapy to the mandible causes significant endothelial damage to the endosteal blood vessels and therefore the feasibility of a marginal mandibulectomy in an edentulous mandible that has been previously irradiated is very hazardous. The probability of pathologic fracture in that setting is very high.
When there is extension of tumor to involve the cancellous part of the mandible, a segmental mandibulectomy must be performed. Segmental mandibulectomy may also be required in patients who have massive primary tumors with extensive soft tissue disease surrounding the mandible. On the other hand segmental mandibulectomy should not be considered simply to gain access to large primary cancers of the oral cavity which are not in the vicinity of the mandible. The concept of the “commando operation” needs to be redefined, since there are no lymphatic channels traversing through the mandible warranting the need to perform an in continuity composite resection of the uninvolved mandible.12 In such a setting to gain access to the large primary oral cancer a mandibulotomy can be performed without the need to sacrifice the normal intervening mandible.13 Segmental mandibulectomy should be considered only when there is gross invasion of the cancellous part of the bone by oral cancer, for primary bone tumors of the mandible, metastatic tumors to the mandible, invasion of inferior alveolar nerve or canal by tumor, and for massive soft tissue disease around the mandible.
RECONSTRUCTIVE SURGERY
Reconstructive surgery following resection for oral cancer is considered when there is functional or aesthetic loss of structures in the oral cavity. Thus loss of a significant part of the tongue, floor of mouth or buccal mucosa, and loss of a segment of the mandible following resection of the primary tumor would be indicators for reconstructive surgery. Superficial surgical defects of the mucosa and underlying soft tissues can be adequately reconstructed using simply a split thickness skin graft. On the other hand, larger defects of the tongue exceeding one half of the tongue or large surface areas of the floor of the mouth, gum and buccal mucosa require a free tissue transfer. A radial forearm free flap provides excellent tissue for resurfacing mucosal defects and underlying soft tissue deficiencies. The radial forearm flap is also an excellent choice for reconstruction of any substantial resection of the tongue.
Fibula free flap reconstruction is currently the choice of reconstruction for defects following segmental mandibulectomy in any part of the mandible. While other free flaps are available, the fibula provides the maximum length and bone stock to achieve a satisfactory reconstruction of the lower jaw.14 Other free flaps available for mandible reconstruction are those from the iliac crest, scapula, and the radial forearm osteocutaneous flap. The descending circumflex iliac artery flap (DCIA) provides bone as well as soft tissue and skin for reconstruction of composite defects of the mandible. Similarly, the scapula free flap provides separate components of bone, muscle and skin on the same vascular pedicle in any combination. The choice of a particular free flap depends upon the location and the length of mandible reconstruction, as well as the need for soft tissue and mucosal lining or skin coverage at the site of resection.
Resection of small parts of the upper gum and palate rarely require major reconstructive effect. Such defects are easily rehabilitated by a maxillofacial prosthetic device (dental obturator). On the other hand, larger defects of the upper gum and hard palate may be considered for immediate reconstruction using an osteocutaneous free flap from fibula, iliac crest or scapula.15 In the elderly edentulous patient, repair of the large surgical defect of the upper gum and hard palate can be accomplished with a soft tissue free flap, such as a rectus abdominus or anterolateral thigh flap.
OSSEOINTEGRATED DENTAL IMPLANTS
The optimal rehabilitation of patients undergoing reconstructive surgery of the mandible following resection for oral cancer is restoration of permanent teeth. Osseointegrated dental implants can be considered in reconstruction of the lower or upper jaw with an osseus or osteocutaneous free flap. The selection of immediate insertion of implants or delayed placement of implants is a matter of personal preference of the surgical team. The pros and cons of both methods are heavily debated in the literature. We prefer secondary placement of implants after adequate bone healing of the free tissue transfer has taken place.16
OUTCOMES OF SURGICAL TREATMENT OF ORAL CANCER
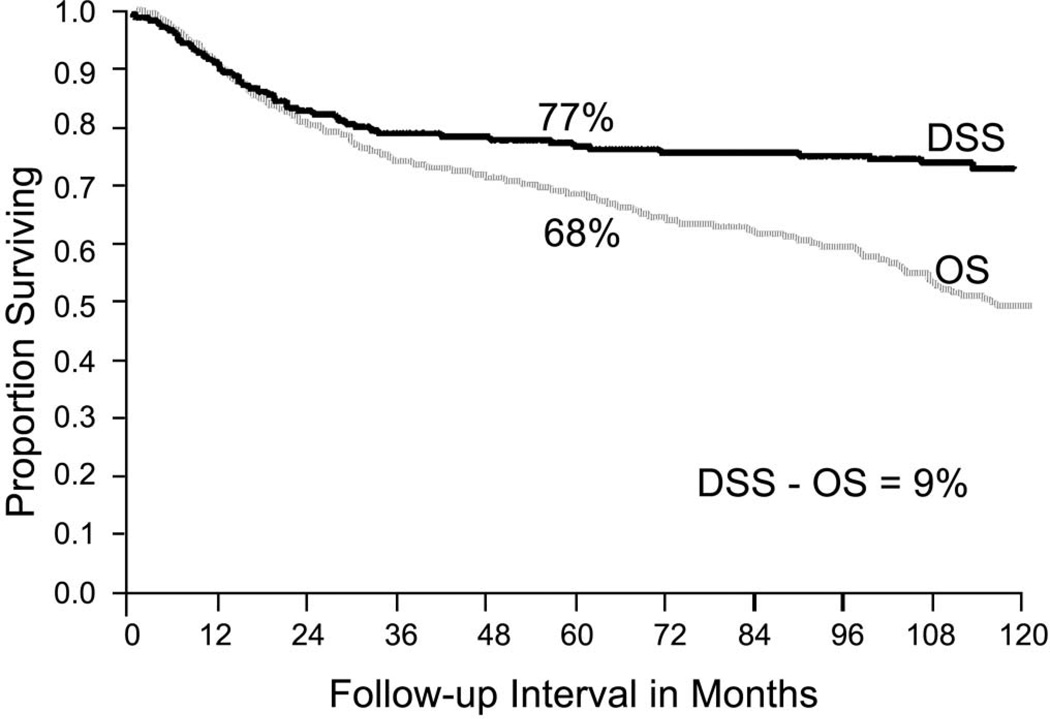
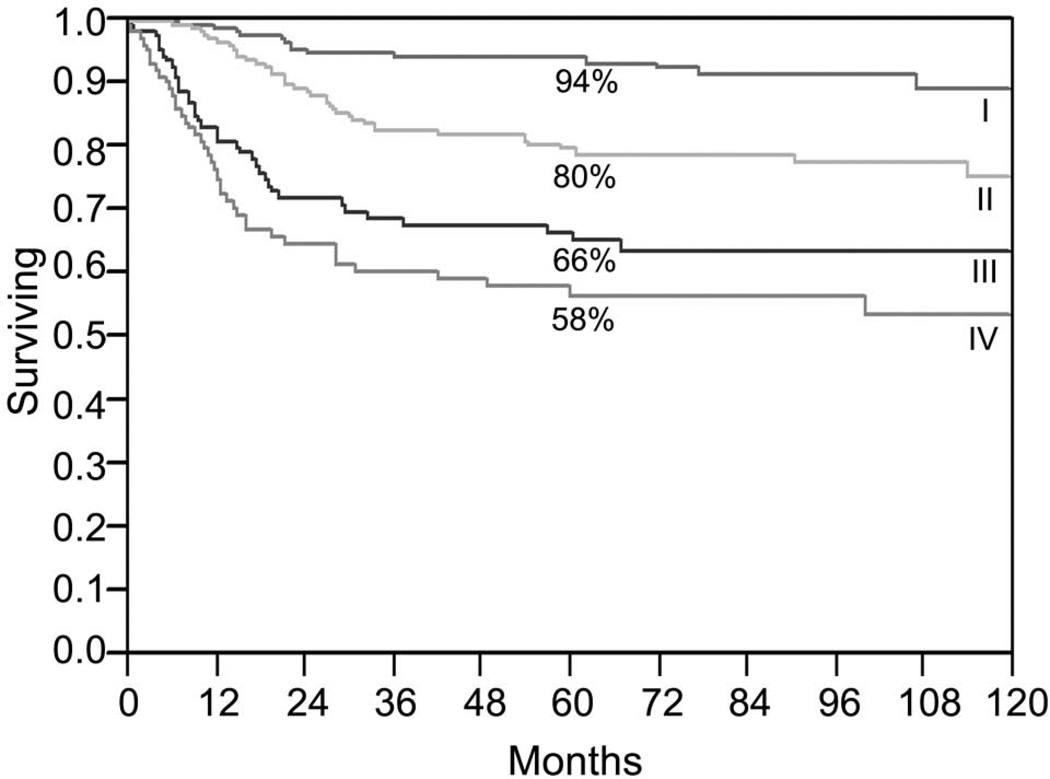
The most important factor which affects long term outcome following initial treatment of cancer of the oral cavity is the stage of disease at the time of presentation. Early staged tumors offer excellent cure rates, however once regional lymph node metastases have taken place a significant drop in the cure rate is to be expected. The five year overall and cause specific survival rates for squamous cell carcinomas of the oral cavity by stage of disease are depicted in figure 5.
Figure 5.
Overall and disease specific survival for patients with squamous cell carcinomas of the oral cavity treated at MSKCC (1986–1995). (Adapted from Shah JP, Johnson NW, et al.26)
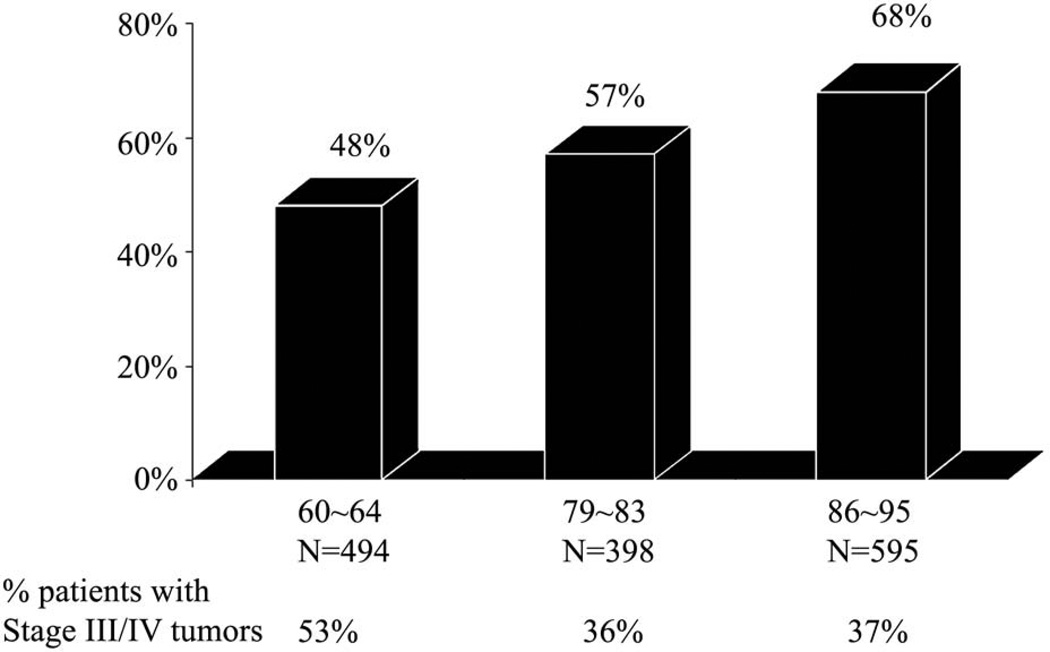
Early diagnosis and implementation of appropriate surgical treatment based on tumor and patient factors, selective management of regional lymph node metastases at risk, and involvement of multidisciplinary teams for implementation of adjuvant radiotherapy or chemoradiotherapy, have all contributed to improvements in survival of patients with oral cancers. (Fig. 6) Contemporary surgical techniques of tumor resection and reconstruction are essential to improve the quality of life of patients following surgical resection of oral cancer.
Figure 6.
Overall survival of patients with advanced stage oral cancer in three different time frames at MSKCC. New York. (From Shah J and Patel S.4)
OTHER SITES
The role of surgery in other sites in the head and neck has evolved with an increasing role for combined modality treatments using chemo- and radiotherapy. A combination of targeted therapies with radiation has further opened new options of definitive treatment in certain sites. A broad outline of current preferences and the role of surgery in the management of various mucosal and non-mucosal malignant tumors follows.
PHARYNX
The pharynx is anatomically that part of the upper aerodigestive tract which extends from the superior surface of the vault of the nasopharynx up to the lower border of the cricoid cartilage at the pharyngoesophageal junction. The pharynx is anatomically divided into the nasopharynx, oropharynx and hypopharynx. Management of tumors arising in these locations require individual considerations.
NASOPHARYNX
In general, epithelial neoplasms of the nasopharynx are treated with a combination of radiation therapy with chemotherapy consisting of cytotoxic drugs or anti-EGFR targeted drugs. Surgery is very rarely employed for management of localized resistant or recurrent nasopharyngeal carcinomas, malignant tumors of nonsquamous origin and for surgical salvage of recurrent or persistent metastatic carcinoma to cervical lymph nodes.
OROPHARYNX
The oropharynx consists of four sites, the soft palate, tonsil, base of the tongue and pharyngeal wall. The survival outcomes of therapy for these tumors remain essentially the same regardless of the treatment combination employed. In the past, surgery followed by radiotherapy was the standard of care. However at present, concurrent chemoradiotherapy appears to be the preferred choice of therapy. Surgical intervention would be considered for tumors of minor salivary gland origin or squamous cell carcinoma which remains persistent after chemoradiotherapy or recurs after chemoradiotherapy. Surgical access to neoplasms of the oropharynx can be obtained via a mandibulotomy, lateral pharyngotomy or transoral robotic surgery (TORS).17
HYPOPHARYNX
The hypopharynx is divided into three subsites, the pyriform sinus, pharyngeal wall and post cricoid region. The pyriform sinus by far is the most common primary site in the hypopharynx. Early stage tumors of the hypopharynx (T1 & T2) can be managed by transoral laser microsurgery,18 open partial laryngopharyngectomy or radiation therapy with or without chemotherapy. On the other hand, advanced stage tumors (T3, T4 or Stage III, Stage IV) require a treatment combination of chemotherapy and radiation therapy. At present, concurrent chemoradiotherapy is preferred over sequential treatment. The very advanced carcinomas of the hypopharynx with invasion of the larynx and destruction of cartilage require primary surgery. The surgical procedure in that setting would be pharyngolaryngectomy with partial pharyngeal resection or total circumferential pharyngolaryngectomy. The important issue in these surgical procedures is reconstruction of the pharyngoesophageal defect. Regional myocutaneous flaps, such as a pectoralis major myocutaneous flap, or free flap reconstruction with radial forearm free flap or anterolateral thigh free flap remain the preferred modalities of reconstruction for partial pharyngeal defects. For total circumferential pharyngolaryngectomy, gastric transposition, free jejunal flap or a tubed radial forearm free flap remain the current choices of reconstructive methods. A majority of the patients with squamous cell carcinoma in the hypopharynx have involvement of regional cervical lymph nodes. Therefore, surgical clearance of lymph nodes in the neck becomes an integral part of overall surgical strategy.
LARYNX
The anatomic regions of the larynx are divided into supraglottic, glottic and subglottic. Primary tumors of the subglottic larynx are exceedingly rare. Carcinomas of the supraglottic larynx have a higher proclivity for regional lymph node metastases compared to primary glottic cancers. Early stage tumors of the supraglottic or glottic larynx can be managed either by surgery or radiotherapy. Long term tumor control with either modality of treatment is comparable. Early stage (T1 & T2) carcinomas of the supraglottic larynx can be managed very effectively by transoral laser microsurgery.19 Open supraglottic partial laryngectomies are required under rare circumstances. Functional outcome following therapy for early stage glottic cancers is superior with radiotherapy compared to surgery. Therefore, transoral laser microsurgery or open partial laryngectomy for untreated early stage glottic carcinomas is generally not preferred. On the other hand, salvage partial laryngectomy for recurrent tumors after previous radiotherapy is technically feasible and offers respectable tumor control rates.20 Management of advanced cancers of the larynx requiring total laryngectomy was revolutionized by the results of the VA Larynx Preservation Trial.21 Advanced cancers of the larynx (T3 & T4) are currently treated by a larynx preservation treatment program of concurrent chemoradiotherapy.22 Total laryngectomy is reserved for treatment failures following previous chemoradiotherapy or for very advanced laryngeal carcinomas with thyroid or cricoid cartilage invasion.
SINUSES AND SKULL BASE
Tumors of the nasal cavity, paranasal sinuses, and tumors that approach or involve the skull base require surgery as the initial definitive treatment for most histological varieties of tumors arising in these locations. Technical details of various types of surgical procedures for excision of tumors of the nasal cavity or maxillary antrum is beyond the scope of this paper. Tumors which approach or involve the anterior skull base require craniofacial resection for adequate excision. Significant experience in craniofacial surgery is accumulated worldwide with reproducible results.23 The role of endonasal endoscopic surgery for limited tumors of the nasal cavity approaching the skull base is currently an area of interest and is being employed under select circumstances.24 Adjuvant treatment following adequate surgical excision is usually employed with post operative radiotherapy or chemoradiotherapy.
THYROID GLAND
Differentiated carcinoma of the thyroid gland is primarily a surgical disease. The extent of surgery is decided upon by risk group stratification of the patient and the tumor.25 A majority of patients with differentiated carcinoma of the thyroid gland fall in the low risk category and require thyroid lobectomy or total thyroidectomy with or without adjuvant post operative radioiodine therapy. Locally aggressive thyroid carcinomas and poorly differentiated cancers require extensive surgery, which may include portions of the larynx, trachea, esophagus or adjacent soft tissues. These tumors may not be iodine avid and will require postoperative external radiation therapy.
SALIVARY GLANDS
Surgery remains the mainstay of initial definitive treatment for nearly all tumors of the major and minor salivary glands. Three-quarters of salivary gland tumors arise in the major salivary glands, (parotid, submandibular and sublingual), with a small proportion arising in minor salivary glands of the nasal cavity, sinuses, oral cavity, pharynx and larynx. Regional lymph node metastases occur infrequently and therefore elective dissection of regional lymph nodes is not recommended. Management of the facial nerve in surgery for tumors of the parotid gland is a crucial issue and requires significant judgment and technical dexterity in preserving the nerve or resecting and reconstructing the facial nerve with a nerve graft. For malignant tumors in general, the affected gland is removed in toto, with preservation of the facial nerve if technically feasible. Surgery for malignant tumors of the minor salivary glands is site dependent and the general principles of surgery at such locations are similar to that for squamous cell carcinoma.
SKIN, SOFT TISSUE, AND BONE TUMORS
Primary surgical excision of malignant neoplasms of the skin, soft tissues and bone remains the mainstay of initial definitive treatment. The details of surgical procedures arising in these tissues are so wide that it is not possible to cover the entire spectrum of these neoplasms in this manuscript. In general, however, whenever feasible a wide three-dimensional resection securing negative margins remains the mainstay of appropriate surgery. When indicated, immediate reconstruction is embarked upon, particularly for large soft tissue and skin defects and for bony defects affecting the framework of the face. In general regional myocutaneous flaps and free tissue transfer remain the essential modalities for immediate reconstruction of such defects.
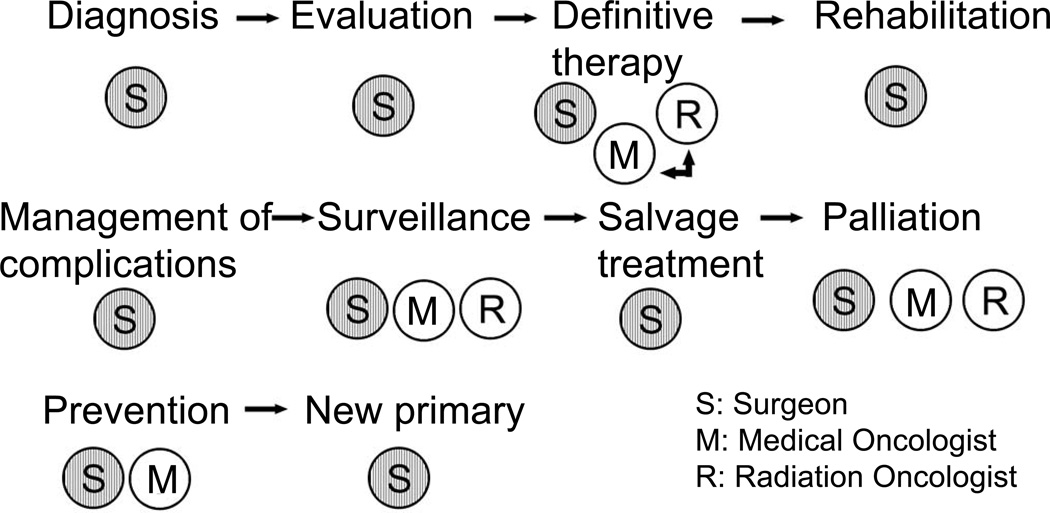
Surgery thus remains the mainstay of management of a majority of neoplasms arising in the head and neck area. The role of the surgeon begins from making the initial clinical diagnosis, assessing the extent of the tumor for proper staging and obtaining tissue by biopsy for confirmation of histologic diagnosis. Surgical expertise is required for endoscopic evaluation of lesions of the pharynx, larynx, nasal cavity and sinuses, and a high degree of technical skill and judgment are required for major head and neck surgical procedures. (Fig. 7) It is imperative to emphasize at this point that head and neck surgery is a specialty which requires dedicated additional training following basic surgical training in general surgery, otolaryngology, plastic surgery or maxillofacial surgery. Thus, a head and neck surgical oncologist is an individual who has surgical expertise along with knowledge and familiarity with the biological behavior of the cancer and the appreciation of the importance of multidisciplinary treatment.
Figure 7.
Role of various specialists during the life of a patient with head and neck cancer.
The role of the surgeon is again required following initial definitive surgery for clinical surveillance, early diagnosis of recurrent tumor or a new primary tumor and for its subsequent management. Surgical expertise is also required for management of complications of therapy, such as management of non-healing ulcerations and strictures following radiotherapy or radiochemotherapy. Surgical expertise is required for rehabilitation of functional and aesthetic defects created by initial treatment of the cancer. Examples of this are rehabilitation of the paralyzed vocal cord, management of airway and esophageal obstructions and management of the paralyzed face. Services of a surgeon will also be essential for palliation of symptoms such as relief of pain and management of advanced fungating tumors with ulceration, bleeding, asphyxia and inanition. Thus, surgery and the services of a surgeon remain central to the management of head and neck neoplasms in general, and oral cancer, in particular throughout the life of the patient.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
None declared.
References
- 1.Moore SR, Johnson NW, Pierce AM, Wilson DF. The epidemiology of mouth cancer: a review of global incidence. Oral Dis. 2000;6:65–74. doi: 10.1111/j.1601-0825.2000.tb00104.x. [DOI] [PubMed] [Google Scholar]
- 2.Sankaranarayanan R. Oral cancer in India: a clinical and epidemiological review. Oral Surg Oral Med Oral Pathol. 1990;69:325–330. doi: 10.1016/0030-4220(90)90294-3. [DOI] [PubMed] [Google Scholar]
- 3.Endicott N, Jensen R, Lyman G, et al. Adjuvant chemotherapy for advanced head and neck squamous carcinoma. Cancer. 1987 Jun;60:301–311. doi: 10.1002/1097-0142(19870801)60:3<301::aid-cncr2820600306>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 4.Shah JP, Patel SG. Head and Neck Surgery and Oncology. 3rd Edition. Edinburgh, London, New York: Mosby; 2003. [Google Scholar]
- 5.Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg. 1990 Oct;160(4):405–409. doi: 10.1016/s0002-9610(05)80554-9. [DOI] [PubMed] [Google Scholar]
- 6.Spiro RH, Huvos AG, Wong GY, Spiro JD, Gnecco CA, Strong EW. Predictive value of tumor thickness in squamous carcinoma confined to the tongue and floor of the mouth. Am J Surg. 1986 Oct;152(4):345–350. doi: 10.1016/0002-9610(86)90302-8. [DOI] [PubMed] [Google Scholar]
- 7.Jun MY, Strong EW, Saltzman EI, Gerold FP. Head and neck cancer in the elderly. Head Neck Surg. 1983 May-Jun;5(5):376–382. doi: 10.1002/hed.2890050503. [DOI] [PubMed] [Google Scholar]
- 8.Friedlander PL, Schantz SP, Shaha AR, Yu G, Shah JP. Squamous cell carcinoma of the tongue in young patients: a matched-pair analysis. Head Neck. 1998 Aug;20(5):363–368. doi: 10.1002/(sici)1097-0347(199808)20:5<363::aid-hed1>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 9.Piccirillo JF. Inclusion of comorbidity in a staging system for head and neck cancer. Oncology (Williston Park) 1995 Sep;9(9):831–836. discussion 841–845–8. Review. [PubMed] [Google Scholar]
- 10.Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, Ozsahin EM, Jacobs JR, Jassem J, Ang KK, Lefèbvre JL. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501) Head Neck. 2005 Oct;27(10):843–850. doi: 10.1002/hed.20279. [DOI] [PubMed] [Google Scholar]
- 11.McGregor AD, MacDonald DG. Routes of entry of squamous cell carcinoma to the mandible. Head Neck Surg. 1988 May-Jun;10(5):294–301. doi: 10.1002/hed.2890100502. [DOI] [PubMed] [Google Scholar]
- 12.Marchetta FC, Sako K, Murphy JB. The periosteum of the mandible and intraoral carcinoma. Am J Surg. 1971 Dec;122(6):711–713. doi: 10.1016/0002-9610(71)90432-6. [DOI] [PubMed] [Google Scholar]
- 13.Spiro RH, Gerold FP, Shah JP, Sessions RB, Strong EW. Mandibulotomy approach to oropharyngeal tumors. Am J Surg. 1985 Oct;150(4):466–469. doi: 10.1016/0002-9610(85)90155-2. [DOI] [PubMed] [Google Scholar]
- 14.Hidalgo DA. Fibula free flap mandibular reconstruction. Clin Plast Surg. 1994 Jan;21(1):25–35. [PubMed] [Google Scholar]
- 15.Brown JS, Magennis P, Rogers SN, Cawood JI, Howell R, Vaughan ED. Trends in head and neck microvascular reconstructive surgery in Liverpool (1992–2001) Br J Oral Maxillofac Surg. 2006 Oct;44(5):364–370. doi: 10.1016/j.bjoms.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Teoh KH, Huryn JM, Patel S, Halpern J, Tunick S, Wong HB, Zlotolow IM. Implant prosthodontic rehabilitation of fibula free-flap reconstructed mandibles: a Memorial Sloan-Kettering Cancer Center review of prognostic factors and implant outcomes. Int J Oral Maxillofac Implants. 2005 Sep-Oct;20(5):738–746. [PubMed] [Google Scholar]
- 17.Weinstein GS, O'Malley BW, Jr, Snyder W, Hockstein NG. Transoral robotic surgery: supraglottic partial laryngectomy. Ann Otol Rhinol Laryngol. 2007 Jan;116(1):19–23. doi: 10.1177/000348940711600104. [DOI] [PubMed] [Google Scholar]
- 18.Steiner W, Ambrosch P, Hess CF, Kron M. Organ preservation by transoral laser microsurgery in piriform sinus carcinoma. Otolaryngol Head Neck Surg. 2001 Jan;124(1):58–67. doi: 10.1067/mhn.2001.111597. [DOI] [PubMed] [Google Scholar]
- 19.Ambrosch P, Kron M, Steiner W. Carbon dioxide laser microsurgery for early supraglottic carcinoma. Ann Otol Rhinol Laryngol. 1998 Aug;107(8):680–688. doi: 10.1177/000348949810700810. [DOI] [PubMed] [Google Scholar]
- 20.Shah JP, Loree TR, Kowalski L. Conservation surgery for radiation-failure carcinoma of the glottic larynx. Head Neck. 1990 Jul-Aug;12(4):326–331. doi: 10.1002/hed.2880120409. [DOI] [PubMed] [Google Scholar]
- 21.The Department of Veterans Affairs Laryngeal Cancer Study Group. Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. N Engl J Med. 1991 Jun;324(24):1685–1690. doi: 10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 22.Forastiere AA, Goepfert H, Maor M, et al. Concurrent Chemotherapy and Radiotherapy for Organ Preservation in Advanced Laryngeal Cancer. N Engl J Med. 2003 Nov;349(22):2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 23.Patel SG, Singh B, Polluri A, Bridger PG, Cantu G, Cheesman AD, deSa GM, Donald P, Fliss D, Gullane P, Janecka I, Kamata SE, Kowalski LP, Kraus DH, Levine PA, dos Santos LR, Pradhan S, Schramm V, Snyderman C, Wei WI, Shah JP. Craniofacial surgery for malignant skull base tumors: report of an international collaborative study. Cancer. 2003 Sep 15;98(6):1179-8. doi: 10.1002/cncr.11630. [DOI] [PubMed] [Google Scholar]
- 24.Kassam AB, Gardner P, Snyderman C, Mintz A, Carrau R. Expanded endonasal approach: fully endoscopic, completely transnasal approach to the middle third of the clivus, petrous bone, middle cranial fossa, and infratemporal fossa. Neurosurg Focus. 2005 Jul 15;19(1):E6. [PubMed] [Google Scholar]
- 25.Shaha AR, Shah JP, Loree TR. Risk group stratification and prognostic factors in papillary carcinoma of thyroid. Ann Surg Oncol. 1996 Nov;3(6):534–538. doi: 10.1007/BF02306085. [DOI] [PubMed] [Google Scholar]
- 26.Shah JP, Johnson NW, Batsakis JG. Oral Cancer. London: Martin Dunitz; 2003. pp. 387–394. [Google Scholar]