Abstract
Characterizing the genetic alterations leading to the more aggressive forms of estrogen receptor positive (ER+) breast cancers are of critical significance in breast cancer management. Here we identify recurrent rearrangements between estrogen receptor gene ESR1 and its neighbor CCDC170, which are enriched in the more aggressive and endocrine-resistant luminal-B tumors, through large-scale analyses of breast cancer transcriptome and copy number alterations. Further screening of 200 ER+ breast cancers identifies eight ESR1-CCDC170 positive tumors. These fusions encode N-terminally truncated CCDC170 proteins (ΔCCDC170). When introduced into ER+ breast cancer cells, ΔCCDC170 leads to markedly increased cell motility and anchorage-independent growth, reduced endocrine sensitivity, and enhanced xenograft tumor formation. Mechanistic studies suggest that ΔCCDC170 engages Gab1 signalosome to potentiate growth factor signaling and enhance cell motility. Together, this study identifies neoplastic ESR1-CCDC170 fusions in a more aggressive subset of ER+ breast cancer, which suggests a new concept of ER pathobiology in breast cancer.
The crucial role of recurrent gene fusions in the development of solid tumors has been recently appreciated after several milestone discoveries1, 2. In particular, the discovery of an EML4-ALK fusion in ∼4% of lung cancer has led to development of an effective drug with stunning clinical impacts3. Recently, next-generation sequencing (NGS) has greatly enhanced gene fusion discovery in solid tumors, which has led to the identification of a VTI1A-TCF7L2 fusion in 3% of colon cancers4, a BCOR-CCNB3 fusion in 4% of bone sarcomas5, and a FGFR-TACC fusion in 3% of glioblastomas6. Although low in percentage, these neoplastic gene fusions will likely advance the genetic subtyping of solid tumors that may be curable by targeting them. In breast cancer, a recent RNA sequencing (RNAseq) study reported multiple fusions of the MAST and NOTCH family genes; whereas individual gene fusions appear to rarely recur7. Another genome sequencing study revealed a MAGI3-AKT3 gene fusion in 3% of breast cancers that is enriched in triple-negative tumors8. To date, the role of recurrent fusions in estrogen-receptor (ER) positive breast cancers is ill-understood. ER+ breast cancers can be classified into the “luminal A” and “luminal B” subtypes. While luminal A tumors can be effectively treated by endocrine therapy against the ER, the luminal B subtype tumors are more aggressive with a higher risk of early relapse with endocrine therapy9. It has been unclear what drives these tumors to be more aggressive, and there are limited options for treating this type of cancer. These issues may be effectively addressed by uncovering the genetic aberrations that drive the development of these tumors.
In this study, we develop an integrative pipeline called “Fusion Zoom” to detect recurrent gene fusions from RNAseq and genomic datasets. We postulate that the detection of pathological gene fusions would be greatly improved by applying more sensitive parameters to comprehensively capture the authentic fusion sequences from the RNAseq data, and by integrating distinct types of genomic data to prioritize the driving fusion events. Based on the observation that gene rearrangements are frequently associated with intragenic copy number aberrations (or “unbalanced” breakpoints), we previously formulated a fusion breakpoint principle to describe the characteristic intragenic copy number changes delineating recurrent fusion genes10, which empowers the bioinformatics analysis to catalog meaningful fusion genes from copy number data. To facilitate high-throughput biological interpretation of candidate fusions, we also developed a Concept Signature (ConSig) analysis that nominates biologically important genes underlying cancer by assessing their association with molecular concepts characteristic of cancer genes (http://consig.cagenome.org)10. Based on these principles, here we develop a pipeline that detects recurrent chimeras potentially encoding in-frame protein products from RNAseq data, catalogs the unbalanced breakpoints at the genomic loci of these fusion partner genes from copy number data, and prioritizes pathological gene fusions through the ConSig analysis (Fig. 1a, see Methods). We apply this approach to the RNAseq and copy number datasets from The Cancer Genome Atlas (TCGA), and identify neoplastic fusion events between the estrogen receptor gene ESR1 and the adjacent gene CCDC170 in a subset of ER+ breast cancers that is preferentially found in luminal B tumors.
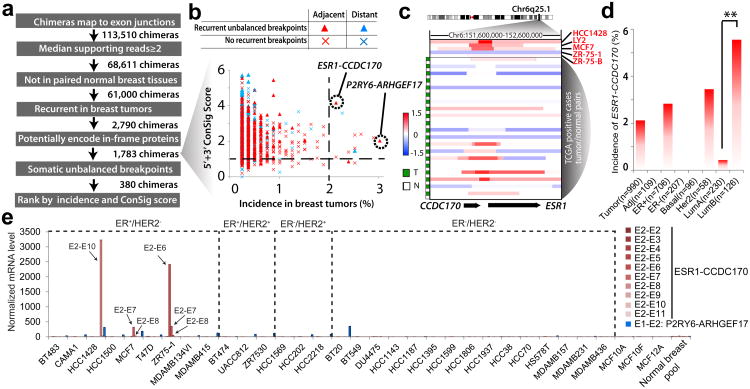
Figure 1. Discovering recurrent gene fusions in invasive breast cancer.
(a) The workflow of the Fusion Zoom pipeline. (b) Prioritizing fusion candidates by copy number breakpoints, ConSig score, and incidence in breast cancer. (c) Log2 transformed copy number data at the CCDC170/ESR1 locus for ESR1-CCDC170 positive cell lines and tumors (LY2 and ZR-75-B are derivative clones of MCF7 and ZR-75-1 respectively). T, tumor, N, normal blood from the same patient (in 3 cases, paired normal breast tissues are shown as blood tissues are not available). Copy number data for index breast cancer cell lines and tumors are from Heiser et al44 and TCGA49 respectively. (d) The incidence of ESR1-CCDC170 fusion in different breast cancer clinical subtypes. **p<0.01 (Fisher's exact test). (e) Nanostring analysis of 30 breast cancer cell lines reveals the presence of ESR1-CCDC170 in HCC1428, ZR-75-1, and MCF7 cells. E2-E2, E2-E3… E2-E11: exon 2 of ESR1 is fused to exon 2, 3 …11 of CCDC170. The exon numbers are based on reference sequence NM_001122742 for ESR1, NM_025059 for CCDC170, NM_176796 for P2RY6, and NM_014786 for ARHGEF17.
Results
Integrative analysis revealed a recurrent ESR1 rearrangement
Using the Fusion Zoom pipeline, we analyzed the RNAseq data of 795 invasive breast tumors and 107 paired normal breast tissues from TCGA. A total of 113,510 putative gene fusions were detected, among which 2,790 fusions were found to be tumor-specific and recurrent in this cohort of patients (present in ≥2 tumors). Among these recurrent fusion candidates, 1,783 are found to have the potential to encode in-frame protein products (see Methods). Interestingly, the vast majority of these recurrent chimeras were from genes that are less than 500 kb apart (Fig. 1b), whereas distant gene fusions rarely recurred in more than 1% of breast tumors (Fig. 1b). Chimeras from adjacent genes are generally considered as non-genomic transcription-induced chimeras (TICs) resulting from intergenic splicing11, 12. Interestingly our analysis of TCGA copy number data (SNP6.0) for 865 breast tumors (most of these tumors have matched RNAseq data) did reveal somatic unbalanced breakpoints within a subset of tumors expressing chimeras, suggesting that some of these fusions could be the consequence of DNA rearrangements. To further reveal the most frequent and pathologically relevant fusions, we classified the 1,783 fusion candidates based on the presence of recurrent unbalanced breakpoints (see Methods), and then prioritized these candidates based on their frequency of detection in breast tumors and by ConSig score of fusion genes (Fig. 1b). This analysis nominated two lead candidates, ESR1-CCDC170 and P2RY6-ARHGEF17, among which ESR1-CCDC170 is most frequently associated with unbalanced breakpoints among all candidates expressed in >1% of breast tumors (Fig. 1c).
Next, we examined the expression of both candidates in a panel of 30 breast cancer cell lines by Nanostring analysis, which applies nanoparticles to detect the abundance of target transcripts from total RNA13. This assay detected high levels of ESR1-CCDC170 expression in some but not all of these cell lines (Fig. 1e). The three ESR1-CCDC170-positive cell lines, MCF7, ZR-75-1, and HCC1428, are all ER+ and derived from metastatic breast tumors. The sizes of the fusion variants detected in these cell lines are different, suggesting that different genomic regions are involved in the fusion events (Fig. 1e and Supplementary Fig. 1a). In addition, none of the benign breast epithelial cell lines or pooled normal breast tissues harbors the ESR1-CCDC170 fusion. These data suggest that ESR1-CCDC170 may be an authentic recurrent gene fusion. P2RY6-ARHGEF17 was expressed at a modest level in many of the breast cancer cell lines analyzed (Fig. 1e and Supplementary Fig. 1b). We speculated that this is most likely a TIC and thus did not study it further.
ESR1-CCDC170 is expressed in more aggressive ER+ tumors
ESR1 encodes the estrogen receptor, whereas CCDC170 encodes a protein with unknown function. CCDC170 is broadly expressed at modest levels in human tissues, with the fallopian tube to be the highest expressing organ (Supplementary Fig. 2). In normal breast tissues, CCDC170 is expressed at a moderate level. To date there is no report about the role of CCDC170 in mammary gland biology. The observed fusions between ESR1 and CCDC170 joins the 5′-untranslated region of ESR1 upstream to the coding region of CCDC170, enabling the expression of truncated CCDC170 under the promoter of the ESR1 gene. To more accurately capture the ESR1-CCDC170 chimerical reads from RNAseq data, we reconstructed all possible variant sequences by randomly combining each of the exons of ESR1 with that of CCDC170. Aligning these putative sequences with the RNAseq data for 990 tumors (released to date by TCGA) revealed 21 ESR1-CCDC170 positive breast tumors (all of which are ER+, except one indeterminate case). About 55% of these positive tumors showed copy number gains between the ESR1 and CCDC170 loci as shown in Fig.1c (also see Supplementary Table 1). Analysis of clinicopathological data14 suggest that this fusion is preferentially present in the luminal B rather than the luminal A subtype (Fisher's exact test, p<0.01, see Fig. 1d). In contrast, wild-type (wt) CCDC170 is broadly expressed in ER+ breast tumors, with no significant difference between these two luminal subtypes (Supplementary Fig. 3). The expression of wtCCDC170 in breast cancer cell lines is almost exclusive to ER+ lines (Supplementary Fig. 1a). This is consistent with the previous report about its co-expression with ER15. We then tested the presence of ESR1-CCDC170 in 200 ER+ breast tumors by reverse transcription PCR (RT-PCR), using primers from exon 2 of ESR1 and exon 10 of CCDC170, which can detect most fusion variations. Among these tumors, eight showed strong expression of ESR1-CCDC170 (4%), which were verified by capillary sequencing (Fig. 2a and Supplementary Table 2). In contrast, no expression of these fusion variants was detected in the available paired adjacent normal breast tissues, suggesting that the fusion between ESR1 and CCDC170 is highly tumor-specific (Fig. 2b).
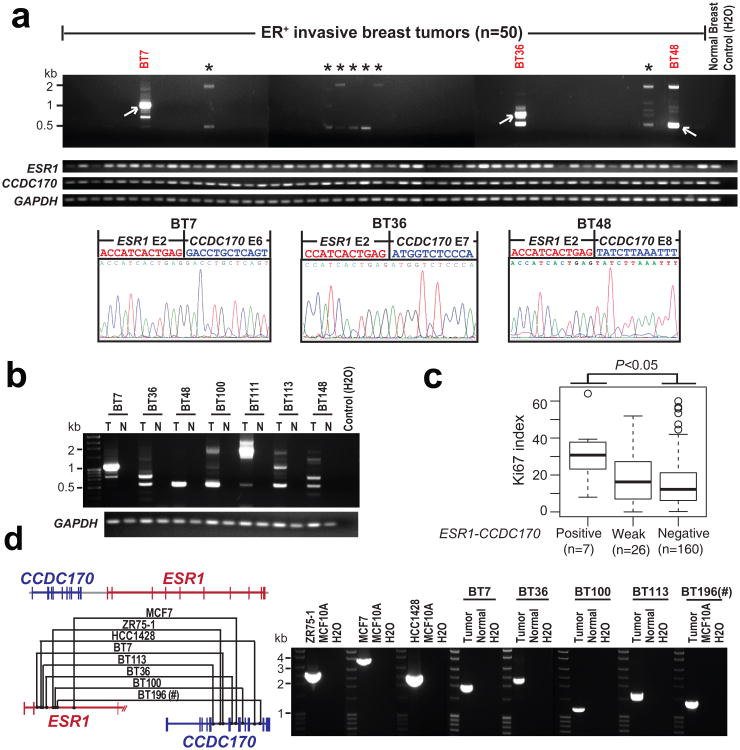
Figure 2. Characterization of the ESR1-CCDC170 fusion in breast cancer cell lines and tissues.
(a) Representative RT-PCR results of ESR1-CCDC170, wtESR1, and wtCCDC170 in ER+ breast cancer tissues. * Weak ESR1-CCDC170 transcripts detected by RT-PCR. (b) RT-PCR analysis of ESR1-CCDC170 in paired tumor (T) and adjacent normal tissues (N) from seven strong positive cases. (c) The Ki67 index for ESR1-CCDC170 positive, weak, and negative cases evaluated by IHC assay using available tissue sections for 193 ER+ cases assessed for ESR1-CCDC170 expression. P-value was determined by t-test. (d) Genomic PCR analysis of the positive cell lines and 5 strong positive tumor samples, confirming the ESR1-CCDC170 rearrangements. Left panel shows the schematic of identified genomic fusion points in different samples; right panel shows the gel image of ESR1-CCDC170 genomic PCR products. #Paired normal tissue is not available for BT196.
To examine the association of ESR1-CCDC170 with the luminal B subtype, we assayed Ki67 expression by immunohistochemistry (IHC) in the ER+ cases assessed by RT-PCR for ESR1-CCDC170. Ki67 is a proliferation biomarker, and a high Ki67 index has been used in the clinic to classify luminal B tumors (with a cutoff of 13∼15% positivity)16-18. Among the 200 ER+ cases, 193 cases had evaluable tissue sections for Ki67 IHC analysis. The IHC results showed that ESR1-CCDC170-positive cases have significantly higher Ki67 scores than negative cases (Fig. 2c, Supplementary Fig. 4). Using 15% positivity as cutoff, 80 tumors have high Ki67 index, among which 6 cases are fusion-positive (7.5%); among the 113 Ki67-low tumors, only one tumor is fusion-positive (0.9%). Fisher's exact test suggests a significant enrichment of fusion-positive cases in Ki67 high tumors (p=0.02). This data support the association of ESR1-CCDC170 with the more aggressive luminal B subtype.
ESR1-CCDC170 genomic rearrangements and protein products
ESR1 and CCDC170 are located 69 kb apart on chromosome 6 with CCDC170 positioned 5′ of ESR1. This placement prevents strong cis-splicing events that frequently happen between neighboring genes placed in forward order. To further verify the genomic origin of ESR1-CCDC170 in the cell lines and tumors showing strong expression of the chimeras, we carried out genomic PCR using tiling primers designed for the specific ESR1 or CCDC170 intron regions suspected to harbor the rearrangement based on the fusion variant in each index case revealed by RT-PCR (Supplementary Table 3), and the amplified products were further analyzed by capillary sequencing. Using this approach, the genomic fusion points in all 3 fusion-positive cell lines and 5 out of 8 strong fusion-positive tumors have been successfully identified (Fig. 2d). The sequencing results revealed distinct genomic fusion points in different cell lines and tumors. The MCF7 cells showed a duplication of the fusion junction, whereas the remaining cases showed 1-10 base-pair homology between the ESR1 and CCDC170 sequences at the rearrangement junctions (Supplementary Fig. 5).
We then examined the structure of the four major fusion variants (E2-E6, E2-E7, E2-E8, and E2-E10) detected in both breast cancer cell lines and tumors, in which exon 2 of ESR1 is fused to exon 6, 7, 8, or 10 of CCDC170. The common theme of these fusion variants appears to create different-sized N-terminally truncated CCDC170 proteins (ΔCCDC170), while ESR1 does not contribute to any of the fusion amino acid sequence (Fig. 3a). To identify the protein products of the four major fusion variants, we ectopically expressed the putative open-reading frames (ORFs) of these variants in MCF10A human breast epithelial cells (Supplementary Fig. 6a). Western blot analysis using a commercial polyclonal antibody against the C-terminus of CCDC170 detected the predicted 41kDa (E2-E6), 39kDa (E2-E7), 30kDa (E2-E8), or 14kDa (E2-E10) ΔCCDC170 bands specific to the transduced MCF10A cells (Fig. 3b). In addition, we expressed the E2-E7 and E2-E10 ORFs in the fusion-negative T47D breast cancer cells (ER+), and detected proteins of the same sizes. Next, we performed Western blot analysis to detect the endogenously expressed ΔCCDC170 proteins in the ESR1-CCDC170-positive cell lines. We were able to readily detect the 14kDa E2-E10 protein expressed by the HCC1428 cells, the identity of which has been verified by specific knockdown of the E2-E10 fusion using an siRNA against this fusion junction. We were unable to conclusively detect the endogenous proteins expressed by the ZR-75-1 or MCF7 cell lines presumably due to the presence of cross-reactive proteins or low expression levels respectively (Supplementary Fig. 7).
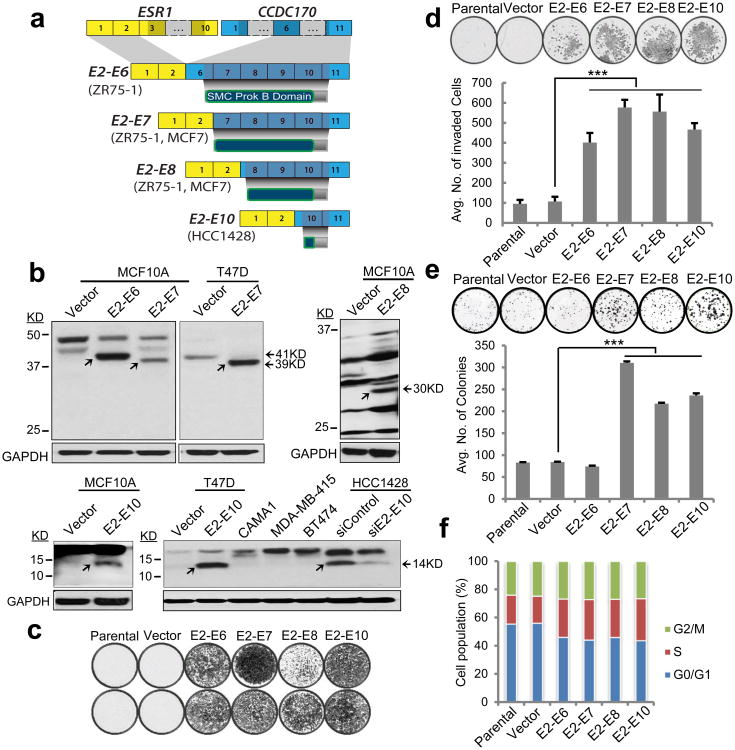
Figure 3. Characterization of ESR1-CCDC170 protein products and their transforming activity in MCF10A breast epithelial cells.
(a) Schematic of ESR1-CCDC170 fusion variants and encoded proteins identified in breast cancer cell lines. ORFs are depicted in dark shades. The exon numbers are based on reference sequence NM_001122742 for ESR1 and NM_025059 for CCDC170. (b) Immunoblot analysis of MCF10A and T47D cells expressing ΔCCDC170 ORFs, the fusion-positive HCC1428 cell line, and fusion-negative control cell lines, using an anti-CCDC170 polyclonal antibody. Arrows indicate the ΔCCDC170 bands. To enhance the detection of differentially sized ΔCCDC170 protein variants, the blot region pertaining to the molecular weight of each respective ΔCCDC170 variant was cut and then probed with the antibody. A longer exposure time is required to enhance the visualization of the E2-E8 fusion protein. The identity of the 14kD band detected in HCC1428 is verified by an siRNA against the E2-E10 fusion expressed by this line. Overexpression of ΔCCDC170 variants in MCF10A cells significantly enhances (c) cell migration, (d) matrigel invasion, and (e) clonal expansion. (f) Cell cycle analysis of the MCF10A cell models. Error bars represent the standard deviation of at least three replicate measurements per condition. The results shown are representative of experiments performed at least twice. *** P<0.001 (t-test). “Vector” indicates MCF10A cells transduced with pLenti7.3 vector containing an YFP ORF.
ESR1-CCDC170 endows more aggressive phenotypes
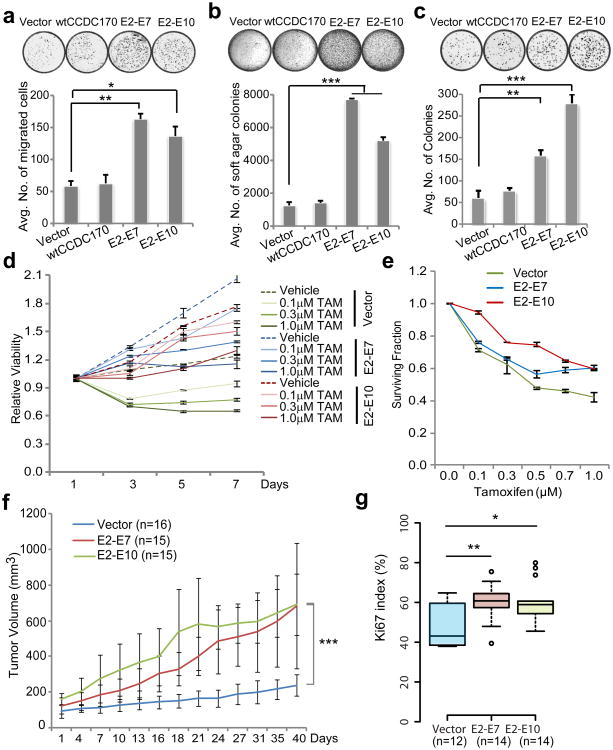
Next, we sought to examine the oncogenic potential of the ΔCCDC170 proteins generated by the four ESR1-CCDC170 fusion variants in the MCF10A breast epithelial cells. Impressively, ectopically expressing the ORF of each of these fusion variants dramatically increased the migration and invasion capabilities as shown by the Boyden chamber assay (Fig. 3 c-d). In addition, the E2-E7 and E2-E10 variants also induced a moderate but significant increase in cell proliferation and colony forming ability, as measured by MTT assay (Supplementary Fig. 6b) and clonogenic assay (Fig. 3e) respectively. Soft agar colony formation assays did not show an increase in anchorage-independent growth of the engineered MCF10A cells, whereas 3D culture of these cells in Matrigel revealed impaired acini morphogenesis (Supplementary Fig. 6c). Cell cycle analysis revealed an increase in S-G2/M phase cells, and a decrease in G0/G1 phase cells in all models (Fig. 3f). As MCF10A cells do not express wtCCDC170 (see Supplementary Fig. 1a), the observed changes are likely to be independent of wtCCDC170. To investigate the role of ESR1-CCDC170 in ER+ breast cancer cells, we examined the phenotypic changes of T47D breast cancer cells ectopically expressing the E2-E7 or E2-E10 fusions or the wtCCDC170 (as a control). T47D is a luminal breast cancer cell line that is highly dependent on estrogen19. Our data show that while both E2-E7 and E2-E10 fusions significantly increased cell motility, anchorage-independent growth, and colony forming ability of T47D cells, the wtCCDC170 did not (Fig. 4 a-c). Further, both the E2-E7 and E2-E10 variants rendered the T47D cells less sensitive to estrogen deprivation and 4-hydroxytamoxifen treatment (4-OHT, the active metabolite of tamoxifen used in vitro) (Fig. 4 d-e). Of note, T47D cells typically do not proliferate when deprived of estrogen, whereas ΔCCDC170 transduced T47D cells continue to grow in the absence of estrogen and irrespective of tamoxifen treatment (Fig. 4d). Moreover, ΔCCDC170 enhanced the ER transcriptional activity in the presence of estrogen but not with endocrine therapy (Supplementary Fig. 8a), suggesting that the fusion-mediated endocrine-sensitivity changes are unlikely due to the restoration of ER activity. To further examine the oncogenic potential of ΔCCDC170 in the in vivo context, we transplanted the T47D cells expressing the ΔCCDC170 variants or vector control into female athymic nude mice implanted with estradiol (E2) pellets. Impressively, in contrast to the slow growth kinetics of the tumor in the vector control group, a profound increase in tumor growth was observed in the transduced xenograft models expressing E2-E7 or E2-E10 ORFs (Fig. 4f and Supplementary Fig. 8b). In addition, immunostaining of tumor tissue arrays revealed that T47D xenograft tumors overexpressing ΔCCDC170 variants have a Ki67 index significantly greater than that of control T47D tumors (Fig. 4g).
Figure 4. ESR1-CCDC170 endows more aggressive phenotypes in T47D ER+ breast cancer cells.
Ectopic expression of ΔCCDC170 in T47D cells results in a significant increase in (a) cell motility, (b) anchorage independent growth, and (c) colony-forming ability. (d) Time-point changes in the proliferation of fusion-expressing T47D cells after tamoxifen treatment (4-OHT). (e) Surviving fraction of T47D cells expressing ΔCCDC170 after 7 days of tamoxifen treatment (4-OHT). In assay d-e, T47D cells are deprived of estrogen for tamoxifen treatment. Error bars represent the standard deviation of two replicate measurements per condition and results shown are representative of experiments performed at least twice. (f) The growth curve of xenograft tumors expressing vector, E2-E7, or E2-E10 ΔCCDC170 variants engrafted bilaterally in athymic nude mice (8 mice /group). Tumor volumes of deceased mice are not included after the day of death. Day 0 represents the first tumor measurement 7 days post tumor cell implantation. Data are presented as mean ± SD of indicated sample size. (g) Boxplots comparing the Ki67 scores in available xenograft tumor tissues expressing vector (n=12), E2-E7 (n=14) or E2-E10 (n=14). “Vector” indicates the pLenti7.3 vector control containing an YFP ORF. *p<0.05, **p<0.01, ***p<0.001 (the p-value for Fig. 4f is based on ANOVA, and others are all based on t-test).
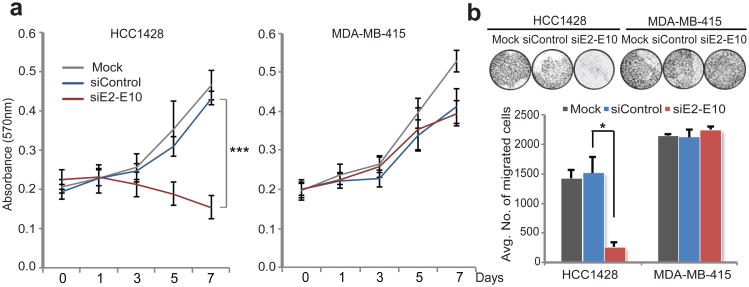
To further investigate the function of endogenous ESR1-CCDC170, we examined the consequence of specific knockdown of this fusion in HCC1428 cells harboring the E2-E10 variant. This cell line was chosen for the knockdown model as the E2-E10 variant is amenable to the design of fusion-specific siRNA and is the only variant expressed by this cell line. In addition, the protein product of this variant is readily detectable by the available antibody, which can be used to examine the knockdown efficiency. As shown in Fig. 3b and Supplementary Fig. 9, this siRNA effectively and specifically knocks down the E2-E10 fusion variant. MTT and Boyden chamber assays revealed that repression of the E2-E10 fusion by siRNA in HCC1428 cells potently inhibited their growth and diminished their migration toward the fibroblast attractant, while no significant effect was observed in the fusion-negative MDA-MB-415 cells (Fig. 5 a-b). To further exclude the siRNA off-target effects in HCC1428 cells, we performed rescue experiments by ectopically expressing the E2-E10 fusion variant in this line. Forced expression of E2-E10 variant rescued the knockdown effect of E2-E10 siRNA on proliferation of HCC1428 cells (Supplementary Fig. 10). This result further corroborated the role of the endogenous E2-E10 fusion expressed in HCC1428 cells.
Figure 5. Evaluating the function of the endogenous ESR1-CCDC170 fusion in HCC1428 breast cancer cells by genetic inhibition.
(a) Knockdown of ESR1-CCDC170 in HCC1428 cells impairs cell proliferation (MTT assay). Error bars represent the standard deviation of four replicate measurements per condition. *** P<0.001 (t-test based on day 7 data). (b). Knockdown of ESR1-CCDC170 in HCC1428 cells impairs cell motility as revealed by transwell migration assay. NIH 3T3 cells is used as chemo-attractant. The fusion-negative MDA-MB-415 cell line (ER+/Her2−) was used as negative control. Error bars represent the standard deviation of two replicate measurements per condition. The results shown are representative of experiments performed at least twice. *p<0.05 (t-test).
ESR1-CCDC170 engages Gab1 signalosome
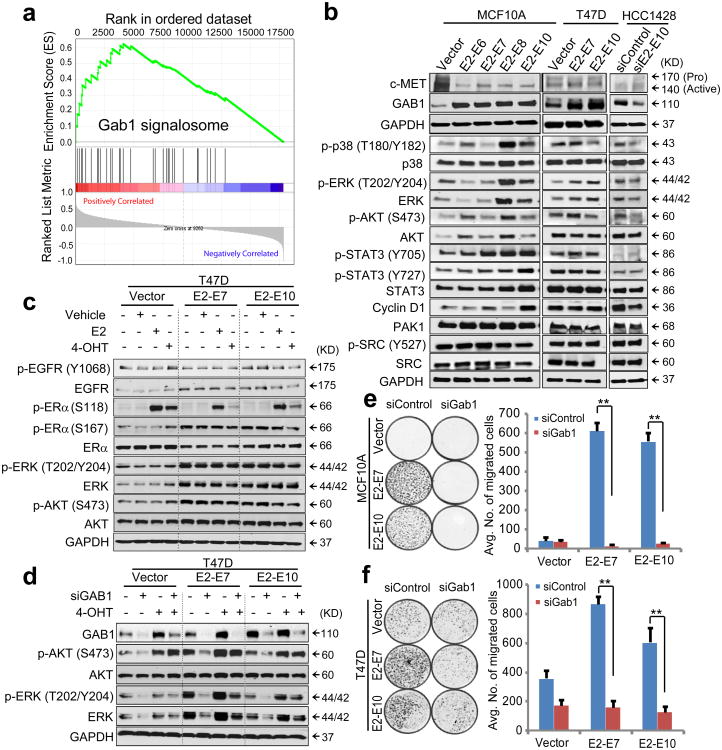
To investigate the key oncogenic pathways that characterize the ESR1-CCDC170 positive tumors, we performed Gene Set Enrichment Analyses (GSEA) using the matched Agilent gene profiling data from TCGA to select differentially expressed genes between fusion-positive and negative tumors. Among the top upregulated pathways in ESR1-CCDC170-positive tumors, the signaling gene sets along the c-Met/Gab1/PI3K-AKT axis appear to be most relevant to the observed phenotypes (Supplementary Fig. 11a). Of particular interest is the upregulation of the Gab1 signalosome (Fig. 6a). Gab1 is a key docking protein that enhances the downstream signaling of c-Met and many other receptor tyrosine kinases20, 21, and is also a key scaffold protein involved in the formation of invadopodia22. Further analysis revealed significant upregulation of Gab1 but not c-Met in the fusion-positive breast tumors (Supplementary Fig. 11b). Interestingly, when ΔCCDC170 variants were overexpressed in MCF10A or T47D cells, Gab1 was also upregulated; conversely, repression of ΔCCDC170 reduced Gab1 protein level in HCC1428 cells (Fig. 6b). In contrast, c-Met protein levels were not increased in the MCF10A and T47D cells expressing ΔCCDC170, and were not affected by E2-E10 knockdown in HCC1428 cells.
Figure 6. ESR1-CCDC170 may engage Gab1 to enhance cell motility and augment growth factor signaling.
(a) The representative enrichment plot of upregulated Gab1 signalosome genes in ESR1-CCDC170-positive breast tumors versus the same number of randomly selected luminal B tumors. (b) Western blot showing the alterations of signaling molecules in MCF10A or T47D cells overexpressing ΔCCDC170 variants, or following knockdown of the E2-E10 fusion in HCC1428 cells. (c) The impact of ΔCCDC170 expression on ERα, EGFR, AKT, and ERK levels and phosphorylations in T47D cells in the context of serum starvation and endocrine treatment. Cells were deprived of estrogen and serum, and then treated with vehicle, 1nM estrogen (E2), or 100nM 4-hydroxy tamoxifen (4-OHT) for 20 minutes. (d) Alterations of AKT and ERK activities following GAB1 knockdown in transduced T47D cells. Cells were deprived of estrogen, transfected with GAB1 siRNA for 72h, and treated with 100nM 4-OHT for 20 min. e-f, The impact of Gab1 knockdown on fusion-driven cell motility in MCF10A (e) and T47D (f) cells. Error bars represent the standard deviation of two replicate measurements per condition. The results shown are representative of experiments performed at least twice. **p<0.01 (t-test).
Next, we performed Western blot analysis to examine the impact of ΔCCDC170 expression on Gab1 downstream signaling molecules (Supplementary Fig. 11c). This revealed the positive correlation of phospho AKT, ERK, and p38 with ΔCCDC170 expression, the extent of which varies between different models (Fig. 6b). To test if ΔCCDC170 expression can result in hyperactive growth factor signaling irrespective of endocrine condition, the T47D cell models were deprived of estrogen for 48h and serum-starved for 24h, and then treated with vehicle, E2, or tamoxifen. Interestingly, sustained phosphorylation of AKT and ERK was observed in the T47D cells expressing ΔCCDC170 even after withdrawal of estrogen and serum, and this effect was not significantly altered by the administration of estrogen or tamoxifen (Fig. 6c). This suggests that the hyperactive growth factor signaling observed with ΔCCDC170 expression may not be attributed to the estrogen-regulated non-genomic ER activity known to modulate growth factor signaling23. Further, increased phosphorylation of the Serine 167 residue on ERα was observed with ΔCCDC170 expression. This site has been reported to be phosphorylated by both AKT and ERK, enhancing ERα transcriptional activity24. Gab1 silencing using a documented siRNA25 counteracted the enhanced AKT and ERK signaling driven by ΔCCDC170 (Fig. 6d, Supplementary Fig. 11d), suggesting that ESR1-CCDC170 may act through Gab1 to augment growth factor signaling. Of note, Gab1 repression cannot diminish AKT activation in the presence of tamoxifen, suggesting that tamoxifen may bypass Gab1 and engage some other mechanism to activate AKT, possibly through tamoxifen-activated non-genomic ER activity26. Further, Gab1 knockdown also diminished the fusion-driven cell motility in both MCF10A and T47D cells, supporting the importance of Gab1 signaling in the fusion-driven invasive program (Fig. 6 e-f).
Discussion
The genetic makeup and underlying biology contributing to the highly proliferative and aggressive phenotype of the luminal B breast tumors is not well understood. In this study, we have identified a recurrent genomic rearrangement event between the ESR1 and CCDC170 loci, and provided strong molecular and functional evidence that this fusion is enriched in luminal B tumors and promotes more aggressive oncogenic phenotypes. Our finding of ESR1-CCDC170 is an example of gain-of-function mutation, wherein CCDC170 is fused to ESR1 and utilizes the constitutively active promoter of ER to drive the expression of a truncated form of CCDC170 gene. The truncation of the CCDC170 protein resulting from this fusion may twist the biology of this protein and generate a phenotype distinct from the wild-type protein. Of note, ESR1-CCDC170 is also detected by two previous studies interrogating different RNAseq datasets as a candidate fusion in breast cancer which further support its recurrence7, 27. However, these studies did not provide any data on the genomic event underlying this fusion, or its pathobiology and clinical relevance in breast cancer. Our integrative bioinformatics analysis provided multiple clues to lock in on this fusion as a recurrent, pathological, genomic fusion event from the large number of putative fusions detected by RNAseq. We then validated the genomic rearrangements generating this fusion by genomic PCR, characterized its protein products, elucidated its pathological role and engaged mechanism, and verified its enrichment in the more aggressive luminal B subtype. While it remains to be answered whether such enrichment could be attributable to the increased genomic instability characteristic of luminal B tumors that may promote the formation of this fusion, our biological data show that ESR1-CCDC170 endows ER+ breast cancer cells with more aggressive phenotypes, such as enhanced cell migration, invasion, anchorage-independent growth, and reduced endocrine sensitivity. These properties are consistent with the behavior of luminal B tumors. In addition, we also observed markedly increased ki67, the luminal B marker, in the T47D xenograft tumors overexpressing ΔCCDC170 variants. Moreover, our “knockdown/rescue” studies of the E2-E10 fusion expressed in the HCC1428 cell line, which encodes the smallest truncated version of CCDC170 that are retained in all fusion variants, provided a proof of concept for the function of the endogenous ESR1-CCDC170 fusions expressed in breast cancers. Further mechanistic studies suggest that this fusion may engage Gab1 signaling to enhance cell motility and augment the downstream signaling of growth factor receptors28. More important, the enhancement of growth factor signaling driven by this fusion appears to be sustained even after withdrawal of serum, and is not affected by endocrine treatment. Together, these findings may shed light on the genetic aberrations underlying the more aggressive and fatal ER+ breast tumors.
Our RT-PCR analysis of ER+ breast tumor tissues revealed 8 out of 200 tumors as ESR1-CCDC170-positive cases with strong expression of this fusion (4%). Of note, besides these cases, we also observed weak expression of ESR1-CCDC170 in an additional 10% of ER+ breast tumors, which are distinguishable from the strong positives (see methods). These weak cases show a slightly increased Ki67 index comparing to fusion-negative breast tumors but this difference is not statistically significant (Fig. 2c). We speculate that these may be the result of random weak trans-splicing events between ESR1 and CCDC170, considering the vicinity of the two genes. In fact, such trans-splicing events are not unique to this fusion29. Oncogenic gene fusions resulting from distant translocations are often found to be expressed at a low level in normal tissues, such as the EML4-ALK30, NPM-ALK31, JAZF1-JJAZ132, and BCR-ABL1 fusions33. It is thought that high-level expressions coincide with gene rearrangements, whereas low-level expressions are likely to be trans-splicing events32. Nevertheless, we cannot exclude the possibility of ESR1-CCDC170 rearrangements in a small subset of cancer cells in rare cases. Thus further investigation is needed to elucidate their clinical significance.
Another interesting question about this fusion is how such cryptic rearrangements could be generated. As CCDC170 is located to the 5′ of ESR1 on the same DNA strand, it is unlikely that such rearrangements are generated by deletions or inversions, which would require CCDC170 to be at the 3′ of ESR1, or on the opposite strand, respectively. Considering the frequent duplications between the two genes in fusion-positive tumors, one possible mechanism of these cryptic rearrangements could be tandem duplication, which is defined as the occurrence of two identical sequences, one following the other, in a chromosome segment. Tandem duplication has been found to cause other gene fusions in cancer34-36. If this mechanism is responsible for ESR1-CCDC170 fusions, ESR1 expression would not be disrupted as such rearrangements are likely to retain a copy of the wild-type ESR1 while forming the fusion gene (Supplementary Fig. 12a), as reported for other gene fusions generated by tandem duplication37. Indeed, the expression of ESR1 in ESR1-CCDC170-positive tumors is similar to that of fusion-negative ER+ breast tumors (Supplementary Fig. 12b). In addition to tandem duplication, more complex mechanisms such as insertions may be responsible in the positive cases that do not exhibit duplications between the ESR1 and CCDC170 loci (Fig. 1c).
Chimeras from adjacent genes account for a vast majority of chimera sequences in cancer transcriptome38, 39. Our finding of the ESR1-CCDC170 gene fusions supports the possibility of chromosomal rearrangements between adjacent genes generating recurrent gene fusions. Such cryptic genetic changes are not generally detectable by conventional cytogenetic approaches, and the resulting chimeras are usually submerged in the overwhelming number of TICs38, 39. This finding suggests that special attention should be paid to the possible genomic origin of adjacent chimeras in the discovery of gene fusions from RNA sequencing data. To our knowledge, ESR1-CCDC170 could be the most important recurrent gene fusion yet reported in ER+ breast cancers. This discovery may shed new light on the special genetic aberrations underlying a subset of more aggressive ER+ breast cancers, and offers a new diagnostic strategy to identify this group of patients for more appropriate treatments. Further studies are needed to comprehensively investigate the oncogenic process initiated by the ΔCCDC170 proteins resulting from this fusion and elucidate their role in breast cancer endocrine resistance.
Methods
Analyses of RNAseq and copy number data
The copy number and RNAseq (Illumina HiSeq, paired-end) data for breast tumors used in this study were from TCGA (http://cancergenome.nih.gov/ and https://cghub.ucsc.edu). Paired-end RNA sequences for 795 breast tumors and 107 paired normal breast tumors were aligned to human genome build 19 using the Tophat 2.0 fusion junction mapper40. Using our Perl script pipeline called “Fusion Zoom”, the putative fusion junctions were mapped to human exons (derived from UCSC gene and Ensemble gene) to identify chimerical sequences. The putative gene fusions are required to be supported by a minimum of one read that maps to the exon junctions of the two fusion genes. This criterion was expected to filter out most artifactual gene fusions randomly ligated during the sequencing procedure. This is based on the fact that authentic gene fusion junctions are usually formed by exon boundaries of partnering genes, whereas the fusion junctions of these artifactual fusions are unlikely to coincide with exon boundaries41. Putative fusion sequences were then constructed and aligned against human genome and transcriptome using the accurate aligner BLAST. The chimeric sequences that can mostly align to a wild-type genomic or transcript sequence were disregarded. After such filtering, a total of 68,611 chimeras with >2 median number of reads across all tumors were identified. A total of 2790 putative fusions were identified as somatic and recurrent (present in more than one breast tumors). Among these, 1783 putative fusions were found to have the potential to encode in-frame protein products. Here the in-frame analysis detects a fusion that either results in an in-frame chimerical protein, or combines the untranslated 5′ UTR of the 5′ partner with the full-length ORF of the 3′ partner. This is computed based on the reading-frames of the respective UCSC and Ensemble wild-type transcripts. The ORF analysis based on the reading frames of exons of the partner genes cannot predict all the de novo ORFs generated by the fusions. Here we required the candidate fusion to present an in-frame fusion variant in at least one sample. This step filtered out about 1000 candidates that never present any in-frame variant in any single sample, which are less likely to be functionally relevant. Of note, this approach cannot detect a truncated ORF initiated by an internal ATG site (such as in ESR1-CCDC170 fusions). However, in rare cases, the TCGA positive tumor appears to express the ESR1-CCDC170 variant that involves more 5′ exons of ESR1, thus generating a reading frame with a small fragment of ESR1 ORF in-frame fused to truncated CCDC170 ORF. This triggers the program to consider this fusion as potentially encoding in-frame ORF.
The fusion candidates were then ranked by the incidence of fusion transcripts in breast tumors and the concept signature (ConSig) score (http://consig.cagenome.org, release 2)10. To assess the unbalanced breakpoints within candidate fusion genes, we obtained TCGA “level 3” Affymetrix SNP 6.0 copy number data for 865 breast tumors. These level 3 data are generated by circular binary segmentation42. The genomic position of each copy number transition was mapped with the genomic regions of all human genes. The genomic region of each human gene was designated as the starting of the transcript variant most approaching the 5′ of the gene, and the end of the variant most approaching the 3′ of the gene. The “broken” genes with intragenic copy number breakpoints were classified into candidate 5′ and 3′ partners based on the association of these unbalanced breakpoints with gene placements. 5′ amplified genes or 3′ deleted genes were considered as potential 5′ partners, while 5′ deleted or 3′ amplified genes were considered as potential 3′ partners according to the fusion breakpoint principle10. The copy number transitions within the ESR1/CCDC170 loci were manually assessed using segmented copy number data visualized with integrative genomics viewer (Fig. 1c)43. Copy number data for index breast cancer cell lines are from Heiser et al44. Fusion-associated copy-number gain (CNG) is defined as increased copy number in-between CCDC170 and ESR1 loci comparing to 5′CCDC170 and 3′ ESR1 regions (visually assessed based on segmented copy number data at ESR1/CCDC170 loci). Thus CNG also includes the case where both 5′CCDC170 and 3′ ESR1 regions have copy number loss. The 380 recurrent fusion candidates revealed by the above integrative analysis are provided in Supplementary Data 1.
To more accurately capture ESR1-CCDC170 chimerical reads, we reconstructed all putative fusion-variant transcripts by combining each of ESR1 exons with each of the CCDC170 exons. The resulting putative ESR1-CCDC170 variant sequences are provided in Supplementary Data 2. Using the Burrows-Wheeler Aligner (BWA), we aligned these ESR1-CCDC170 variant sequences with the RNAseq data for 990 breast tumors released to date by TCGA, allowing up to 3 mismatches. Using a Perl script, we processed the Bam output files to identify junction or encompassing chimerical reads. A series of filtering steps were performed to remove the false positives due to misalignments. The raw sequences of fusion reads identified after these filtering are provided in Supplementary Data 3. A breast tumor was considered as fusion-positive if BWA revealed a minimum of three chimerical reads with at least one read mapped to the fusion junction. To assure that the alignments are acceptable, paired reads supporting ESR1-CCDC170 were manually realigned with the respective putative variant sequences as well as the human transcriptome and genome reference sequences using BLAST or BLAT. For index tumors with <10 supporting fusion reads, all fusion reads were curated. For index tumors with ≥10 supporting fusion reads, all junction reads and at least 10 fusion mates (if available) were curated. The curation results are provided in Supplementary Data 3. PAM50-based clinical subtypes of breast cancer for TCGA samples were derived from the TCGA publication45. The clinical data for TCGA samples were obtained from UCSC Cancer Genome Browser46.
Gene expression data analysis
Gene set enrichment analysis (GSEA) was done by comparing the ESR1-CCDC170-positive breast tumors profiled by gene expression array (data from TCGA), with the same number of randomly chosen fusion-negative luminal B breast tumors, using a signal to noise ratio algorithm. The curated canonical pathways from the Molecular Signatures Database (MSigDb, http://www.broadinstitute.org/gsea/msigdb/) were used as the gene-set database. The process of randomly selecting fusion-negative luminal B samples and then performing GSEA analyses was repeated 100 times. The Normalized Enrichment Scores for each pathway were averaged and then ranked to identify consensus-enriched pathways (Supplementary Fig. 11a). Gene expression data for normal human tissues (Affymetrix U133 plus 2.0) are from the Human Body Index dataset (GSE7307), and are analyzed using Oncomine (www.oncomine.org).
Cell line and tissue collections
Breast cancer cell lines were obtained from American Type Culture Collection (ATCC) including the NCI-ATTC ICBP 43 cell line kit. All breast tumor tissues were obtained from the Tumor Bank of the Lester and Sue Smith Breast Center at Baylor College of Medicine. The total RNA for normal breast tissues (5 Donor Pool) was purchased from BioChain (R1234086-P).
Nanostring assay
The code sets for the ESR1-CCDC170 and P2RY6-ARHGEF17 fusion variants were designed by Nanostring Technologies based on the fusion junction sequences. Expressions of these fusion variants were quantified from 500ng total RNAs using the Nanostring nCounter Assay System following the manufacturer's instructions. Raw counts were normalized to the mRNA levels of the house-keeping genes TFRC, TBP, and PUM1.
RT-PCR and genomic PCR
Complementary DNA was generated from 1μg of total RNA using the Transcriptor First Strand cDNA Synthesis Kit (Roche) in the presence of both oligo (dT) and random primers. RT-PCR of the ESR1-CCDC170 fusion was performed with Platinum Taq High Fidelity (Invitrogen) and fusion-specific primers (Forward: 5′-CTGCGGTACCAAATATCAGCAC -3′; Reverse: 5′- CTTCTCCAGTTGGTCTCTGGAT-3′). To avoid contamination, a clean room was used for setting up PCR reactions which is separated from the areas used for thermal cycling and manipulation of PCR products. In addition, a special set of pipettes and tips with aerosol filters was used to set up the PCR reaction. All cDNA samples were subjected to 35 PCR cycles of 94°C for 30 sec, 56°C for 30 sec, and 68°C for 2 min. For semi-quantification of RT-PCR results, band intensities were quantified using ImageJ software (National Institutes of Health) and normalized to respective GAPDH controls. A relative value more than 0.8 was considered as positive for ESR1-CCDC170 fusion. All the weak cases had a relative value below 0.3. Genomic PCR was carried out with 200-300ng of genomic DNA from cell lines or tissues using the Expand Long Range PCR system (Roche) and primers listed in Supplementary Table 3. PCR products were gel purified for capillary sequencing (Lone Star Labs or Beckman Coulter Genomics). The ESR1-CCDC170 genomic fusion sequences revealed by capillary sequencing are provided in Supplementary Data 4.
Ki67 immunohistochemistry
Formalin-fixed paraffin-embedded whole tissue sections (FFPE) (for breast cancer tissues) or tissue microarrays (20 tissue cores/slide) (for T47D xenograft tumors) were stained using a mouse Ki67 monoclonal antibody (MIB1 clone, Dako) as previously described47. Briefly, microwave-assisted heat induced retrieval method for antigen epitopes was performed in Tris-HCl buffer, at pH 9.0 for 10 minutes. Endogenous peroxidase activity was blocked by incubation in a 3% hydrogen peroxide for 10 minutes. The primary antibody MIB1 at dilution of 1:200 was incubated for 1 hour at room temperature, followed by incubations with polymer labelled EnVision™+ HRP reagents (DAKO, #K4001) and DAB substrate (DAKO, #K3468). The slides were then counterstained with Harris' hematoxylin. Normal human tonsil was used as positive control. Immunostaining was evaluated by two pathologists who were blinded to the sample information, according to recommendations from the international Ki67 working group48. Briefly, the section was first scanned at low magnification (10-20×) to determine the most representative areas. The Ki67 index was calculated as the percentage of Ki67 positive cells among a total of 500 cells at 40× magnification. For heterogeneous cases with hot spots, the Ki67 index was calculated as the average percentage of Ki67 positive cells among a total of 250 cells in hot spots and 250 cells in other areas.
In vitro overexpression of ESR1-CCDC170 ORFs
The cDNA fragments of E2-E6, E2-E7, E2-E8, or E2-E10 fusion variants containing the full-length ORFs were amplified from ZR-75-1 or HCC1428 cDNAs using Phusion DNA polymerase (NEB) with the forward primer 5′-CCATGCTCCTTTCTCCTGCCCA-3′ from 5′ ESR1, and reverse primer 5′-TGTGCCATGTCTTATGGCCACCT-3′ from the 3′ untranslated region of CCDC170. The predicted ORFs of these four variants and YFP control were then cloned into the pLenti7.3 vector (Invitrogen). The ORF sequences of ESR1-CCDC170 fusion variants are provided in Supplementary Data 5. After verification by sequencing, these lentiviral constructs were infected into selected cell lines using the ViraPower™ Lentiviral Support Kit (Invitrogen). Cells with high GFP reporter expression were selected using flow cytometry.
siRNA knockdown experiments
The E2-E10-specific siRNA (5′-CAUCACUGAGAUUAAAACU-3′) and Gab1-specific siRNA (siGenome GAB1: 5′-GAGAGUGGAUUAUGUUGUU-3′) were purchased from Dharmacon. All siRNAs were transfected using Lipofectamine RNAi MAX Reagent (Invitrogen) according to manufacturer's instructions.
Western blot
Protein samples were separated in SDS-PAGE gel and transferred either onto 0.2μm PVDF membrane for detection of the E2-E10 protein product, or 0.2μm nitrocellulose membrane for other proteins. The dilutions of primary antibodies used were 1:250-1:1000 for rabbit anti-CCDC170 (GeneTex), 1:1000 for mouse anti-cMet and rabbit anti-Gab1 (Cell Signaling) and other antibodies. Antibodies were obtained from Santa Cruz (cyclin D1), Thermo Fisher (ERα), Abcam (PAK1), Millipore (Src), and Cell Signaling (all other molecules). To study the fusion-driven signaling in the condition of serum withdrawal and endocrine treatment, cells were maintained in phenol red-free medium for 48h, serum-starved for 24h, and then treated for 20 minutes with vehicle (Ethanol), 17β-estradiol (E2) (1nM) or Tam (100nM). 4-OH tamoxifen (Tam) and 17β-estradiol (E2) were obtained from Sigma-Aldrich.
Cell proliferation assay
Cell proliferation was measured by MTT assay using the Cell Proliferation kit I (Roche) according to manufacturer's instructions. For tamoxifen sensitivity studies, cells were estrogen-deprived (ED) for 48h using phenol red-free medium with charcoal-dextran-stripped FBS, seeded (1000-2500 cells/well) in 96-well plates, and exposed to varying doses of Tam (0.1-1.0 μM); cell proliferation was assessed after 7 days. The surviving fraction of cells was calculated by dividing the OD value from drug-treated wells by the OD value of vehicle-treated wells.
Clonogenic assay
Cells were seeded at a density of 300-500 cells/well in 6-well plate and incubated for 14-21 days. As ΔCCDC170 promotes the formation of large-sized colonies, the colonies > 350μm in diameter were counted for comparison, using GelCount (Oxford Optronix Ltd.).
Soft-agar colony formation assay
Cells were suspended in growth medium containing 0.35% SeaPlaque Agarose (Lonza), and plated at a density of 5000 cells/well in a 6-well plate containing 0.7% base agar in growth medium. The cells were then incubated for 14 days, and colonies ≥100μm in diameter were counted using GelCount.
Migration and invasion assay
Transwell migration and invasion assays were performed using Boyden chambers. Cells were serum-starved for 24h and seeded at a density of 5×104-2×105 in serum-free medium onto transwell inserts of 8μm pore size for migration assay, or onto transwell chambers coated with Matrigel (BD Biosciences) for invasion assay. To facilitate the migration of HCC1428 and MDA-MB-415 cells, NIH3T3 cells seeded in the bottom chamber served as chemoattractant. After 48-72 hours, the inserts were fixed in 4% formaldehyde and stained with hematoxylin and eosin.
ERE luciferase reporter assay
Cells were co-transfected with 1 μg of an ERE (estrogen transcriptional response element) luciferase reporter construct (ERE-TK-Luc) and 0.1 μg of pCMV β-galactosidase as an internal control for transfection efficiency in serum-free medium using XtremeGene HP (Roche). The luciferase levels were measured with a Luciferase Reporter Assay kit (Promega) in a luminometer and normalized to β-gal activity.
FACS analysis
For cell cycle analysis, propidium iodide-stained cells were analyzed in a LSRFortessa cell analyzer (BD Biosciences), and cell cycle phases were calculated using FlowJo (www.flowjo.com).
In vivo xenograft experiments
All animal work has been approved by the BCM Institutional Animal Care and Use Committee. 2×107 transduced T47D cells were resuspended in 20% Matrigel solution, and were transplanted bilaterally to 4-6 week old female athymic nude mice supplemented with 60-day-release 17β-estradiol pellets. Xenograft tumors of the T47D models were successfully engrafted in eight mice per group. The growth of the xenograft tumors was monitored twice per week and tumor volume was measured using the formula 1/2(length × width2).
Statistical analysis
For the in vivo study, statistical comparison of tumor volumes was performed using one-way ANOVA. The results of all in vitro experiments were analyzed by Student's t-tests, and all data are shown as mean ± standard deviation.
Supplementary Material
Acknowledgments
The results published here are in part based upon data generated by TCGA (dbGaP accession: phs000178.v6.p6). We thank Dr. Joe W. Gray for providing the genomic data for breast cancer cell lines. We thank Zhongqiu Guo and Sufeng Mao for help with Ki67 IHC assays, Sabrina Herrera for help with pathology analysis, and the Antibody-based Proteomics Core of the Dan L. Duncan Cancer Center (DLDCC) at BCM (supported by NCI P30-125123) for help with cell line extracts. The computational infrastructure was supported by the DLDCC Biostatistics and Informatics Shared Resource and the Rice University BlueBioU Computer Cluster (supported by a 2010 IBM Award and NIH grant NCRR S10RR02950). This study was supported by CDMRP grants W81XWH-12-1-0166 (X-S.W.), W81XWH-12-1-0167(R.S.), W81XWH-13-1-0201(X-X.C), and W81XWH-13-1-0431 (J.V.), Nancy Omens foundation (X-S.W), Susan G. Komen Foundation PDF12231561 (J. K.), NIH grant CA183976 (X-S.W.), P30-125123-06 (S.G.H.), and PG12221410 (S.G.H.). All breast tumor tissues were provided by the Tumor Bank of the BCM Breast Center.
Footnotes
Author Contributions: X-S.W. conceived and supervised the study, performed bioinformatics analysis, analyzed the data, and wrote the manuscript. J.V. designed and performed cell biology, mechanistic, and in vivo experiments, analyzed the data, and co-wrote the manuscript. Y.T. designed and performed molecular biology experiments, analyzed the data, and co-wrote the manuscript. X-X.C. performed cell biology experiments and pathology studies. J-A.K. helped develop stable overexpression cell lines. X.W. assisted in the in vivo studies. S.N.M. and L.J.N.C. helped perform Nanostring assay. D.P.E. provided breast cancer cell lines and RNA extracts. A.C. helped with pathology studies. S.G.H performed the statistical analysis. G.C.C., E.C., and R.S. revised the manuscript.
Competing Financial Interests: The authors declare no competing financial interests.
References
- 1.Tomlins SA, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 2.Soda M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 3.Koivunen JP, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14:4275–4283. doi: 10.1158/1078-0432.CCR-08-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bass AJ, et al. Genomic sequencing of colorectal adenocarcinomas identifies a recurrent VTI1A-TCF7L2 fusion. Nat Genet. 2011;43:964–968. doi: 10.1038/ng.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pierron G, et al. A new subtype of bone sarcoma defined by BCOR-CCNB3 gene fusion. Nat Genet. 2012;44:461–466. doi: 10.1038/ng.1107. [DOI] [PubMed] [Google Scholar]
- 6.Singh D, et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science. 2012;337:1231–1235. doi: 10.1126/science.1220834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson DR, et al. Functionally recurrent rearrangements of the MAST kinase and Notch gene families in breast cancer. Nature medicine. 2011;17:1646–1651. doi: 10.1038/nm.2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerji S, et al. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature. 2012;486:405–409. doi: 10.1038/nature11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sotiriou C, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10393–10398. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang XS, et al. An integrative approach to reveal driver gene fusions from paired-end sequencing data in cancer. Nat Biotechnol. 2009;27:1005–1011. doi: 10.1038/nbt.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parra G, et al. Tandem chimerism as a means to increase protein complexity in the human genome. Genome research. 2006;16:37–44. doi: 10.1101/gr.4145906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akiva P, et al. Transcription-mediated gene fusion in the human genome. Genome research. 2006;16:30–36. doi: 10.1101/gr.4137606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geiss GK, et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 2008;26:317–325. doi: 10.1038/nbt1385. [DOI] [PubMed] [Google Scholar]
- 14.Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunbier AK, et al. ESR1 is co-expressed with closely adjacent uncharacterised genes spanning a breast cancer susceptibility locus at 6q25.1. PLoS genetics. 2011;7:e1001382. doi: 10.1371/journal.pgen.1001382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheang MC, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. Journal of the National Cancer Institute. 2009;101:736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Voduc KD, et al. Breast cancer subtypes and the risk of local and regional relapse. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 18.Tran B, Bedard PL. Luminal-B breast cancer and novel therapeutic targets. Breast cancer research : BCR. 2011;13:221. doi: 10.1186/bcr2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dickson RB, Bates SE, McManaway ME, Lippman ME. Characterization of estrogen responsive transforming activity in human breast cancer cell lines. Cancer research. 1986;46:1707–1713. [PubMed] [Google Scholar]
- 20.Gu H, Neel BG. The “Gab” in signal transduction. Trends in cell biology. 2003;13:122–130. doi: 10.1016/s0962-8924(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Rohrschneider LR. The gift of Gab. FEBS Lett. 2002;515:1–7. doi: 10.1016/s0014-5793(02)02425-0. [DOI] [PubMed] [Google Scholar]
- 22.Rajadurai CV, et al. Met receptor tyrosine kinase signals through a cortactin-Gab1 scaffold complex, to mediate invadopodia. Journal of cell science. 2012;125:2940–2953. doi: 10.1242/jcs.100834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shou J, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. Journal of the National Cancer Institute. 2004;96:926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 24.de Leeuw R, Neefjes J, Michalides R. A role for estrogen receptor phosphorylation in the resistance to tamoxifen. Int J Breast Cancer. 2011;2011:232435. doi: 10.4061/2011/232435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin ZG, Wong C, Wu J, Berk BC. Flow shear stress stimulates Gab1 tyrosine phosphorylation to mediate protein kinase B and endothelial nitric-oxide synthase activation in endothelial cells. The Journal of biological chemistry. 2005;280:12305–12309. doi: 10.1074/jbc.M500294200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell RA, et al. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. The Journal of biological chemistry. 2001;276:9817–9824. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- 27.Sakarya O, et al. RNA-Seq mapping and detection of gene fusions with a suffix array algorithm. PLoS Comput Biol. 2012;8:e1002464. doi: 10.1371/journal.pcbi.1002464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodrigues GA, Falasca M, Zhang Z, Ong SH, Schlessinger J. A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Molecular and cellular biology. 2000;20:1448–1459. doi: 10.1128/mcb.20.4.1448-1459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gingeras TR. Implications of chimaeric non-co-linear transcripts. Nature. 2009;461:206–211. doi: 10.1038/nature08452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martelli MP, et al. EML4-ALK rearrangement in non-small cell lung cancer and non-tumor lung tissues. The American journal of pathology. 2009;174:661–670. doi: 10.2353/ajpath.2009.080755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maes B, et al. The NPM-ALK and the ATIC-ALK fusion genes can be detected in non-neoplastic cells. The American journal of pathology. 2001;158:2185–2193. doi: 10.1016/S0002-9440(10)64690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li H, Wang J, Mor G, Sklar J. A neoplastic gene fusion mimics trans-splicing of RNAs in normal human cells. Science. 2008;321:1357–1361. doi: 10.1126/science.1156725. [DOI] [PubMed] [Google Scholar]
- 33.Biernaux C, Loos M, Sels A, Huez G, Stryckmans P. Detection of major bcr-abl gene expression at a very low level in blood cells of some healthy individuals. Blood. 1995;86:3118–3122. [PubMed] [Google Scholar]
- 34.Jones DT, et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68:8673–8677. doi: 10.1158/0008-5472.CAN-08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lipson D, et al. Identification of new ALK and RET gene fusions from colorectal and lung cancer biopsies. Nature medicine. 2012;18:382–384. doi: 10.1038/nm.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng CK, et al. The role of tandem duplicator phenotype in tumour evolution in high-grade serous ovarian cancer. J Pathol. 2012;226:703–712. doi: 10.1002/path.3980. [DOI] [PubMed] [Google Scholar]
- 37.Varela I, et al. Somatic structural rearrangements in genetically engineered mouse mammary tumors. Genome Biol. 2010;11:R100. doi: 10.1186/gb-2010-11-10-r100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McPherson A, et al. deFuse: an algorithm for gene fusion discovery in tumor RNA-Seq data. PLoS Comput Biol. 2011;7:e1001138. doi: 10.1371/journal.pcbi.1001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maher CA, et al. Chimeric transcript discovery by paired-end transcriptome sequencing. Proc Natl Acad Sci U S A. 2009;106:12353–12358. doi: 10.1073/pnas.0904720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–1111. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hahn Y, et al. Finding fusion genes resulting from chromosome rearrangement by analyzing the expressed sequence databases. Proc Natl Acad Sci U S A. 2004;101:13257–13261. doi: 10.1073/pnas.0405490101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Olshen AB, Venkatraman ES, Lucito R, Wigler M. Circular binary segmentation for the analysis of array-based DNA copy number data. Biostatistics. 2004;5:557–572. doi: 10.1093/biostatistics/kxh008. [DOI] [PubMed] [Google Scholar]
- 43.Thorvaldsdottir H, Robinson JT, Mesirov JP. Integrative Genomics Viewer (IGV): high-performance genomics data visualization and exploration. Brief Bioinform. 2013;14:178–192. doi: 10.1093/bib/bbs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heiser LM, et al. Subtype and pathway specific responses to anticancer compounds in breast cancer. Proc Natl Acad Sci U S A. 2012;109:2724–2729. doi: 10.1073/pnas.1018854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koboldt DC, et al. Comprehensive molecular portraits of human breast tumours. Nature. 2012 doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu J, et al. The UCSC Cancer Genomics Browser. Nat Methods. 2009;6:239–240. doi: 10.1038/nmeth0409-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tham YL, et al. Clinical response to neoadjuvant docetaxel predicts improved outcome in patients with large locally advanced breast cancers. Breast Cancer Res Treat. 2005;94:279–284. doi: 10.1007/s10549-005-9020-z. [DOI] [PubMed] [Google Scholar]
- 48.Dowsett M, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103:1656–1664. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Itamochi H, et al. Checkpoint Kinase Inhibitor AZD7762 Overcomes Cisplatin Resistance in Clear Cell Carcinoma of the Ovary. Int J Gynecol Cancer. 2014;24:61–69. doi: 10.1097/IGC.0000000000000014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.